First record of Pseudaeginella arraialensis (Amphipoda: Caprellidea) from the Gulf of Mexico
Ignacio Winfield a, *, José M. Guerra-García b
a Laboratorio de Crustáceos, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Av. de los Barrios 1, Los Reyes Iztacala, 54090 Tlalnepantla, Estado de México, Mexico
b Laboratorio de Biología Marina, Departamento de Zoología, Facultad de Biología, Universidad de Sevilla, Av. Reina Mercedes 6, 41012 Sevilla, Spain
*Corresponding author: ignacioc@unam.mx (I. Winfield)
Received: 20 July 2019; accepted: 5 February 2020
Abstract
During a sampling campaign in Puerto Progreso, Yucatán, Mexico, several specimens of the caprellid Pseudaeginella arraialensis were collected. They were attached to buoys as part of fouling communities between 0.5-12 m depth. The present study represents the first record of P. arraialensis for the Gulf of Mexico, increasing its geographical distribution range 7,500 km northwards, from Arraial do Cabo, Brazil to Puerto Progreso, Mexico, SW Gulf of Mexico.
Keywords: Amphipods; Caprellidae; Artificial structure
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Primer registro de Pseudaeginella arraialensis (Amphipoda: Caprellidea) del golfo de México
Resumen
Durante una campaña de muestreo en Puerto Progreso, Yucatán, México, fueron recolectados 124 ejemplares del caprélido Pseudaeginella arraialensis asociados a boyas como parte de las comunidades incrustantes entre los 0.5-12 m de profundidad. El presente estudio corresponde al primer registro de P. arraialensis para el golfo de México, incrementando su intervalo de distribución geográfica 7,500 km hacia el norte, desde Arraial do Cabo, Brasil hasta Puerto Progreso, México, SO del golfo de México.
Palabras clave: Anfípodos; Caprellidae; Estructura artificial
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Pseudaeginella Mayer, 1890 is a genus distributed world-wide, mainly in tropical and subtropical habitats (Iwasa-Arai et al., 2019), associated with algae mats, coral rubble, artificial floating structures as biofouling components, and deep-sea soft bottoms (Lacerda & Masunari, 2014; Mauro & Serejo, 2015). Currently, Pseudaeginella includes 14 species: P. antiquae Barnard, 1932 (nomen dubium); P. arraialensis Ros, Lacerda & Guerra-García, 2017; P. biscaynensis (McCain, 1968); P. campbellensis Guerra-García, 2003; P. colombiensis Guerra-García, Krapp-Schickel & Müller, 2006; P. freirei Siqueira & Iwasa-Arai, 2019; P. hormozensis Momtazi & Sari, 2013; P. inae Krapp-Schickel & Guerra-García, 2005; P. montoucheti (Quitete, 1971); P. polynesica (Müller, 1990); P. sanctipauli Laubitz, 1995; P. telukrimau Lim, Azman, Takeuchi & Othman, 2017; P. tristanensis (Stebbing, 1888), and P. vaderi Guerra-García, 2004.
According to Winfield and Ortiz (2013), P. biscaynensis is the only species of the genus inhabiting the southern sector of the Gulf of Mexico, as an epibiotic component associated with seagrasses and algal mats. During a general survey focused on biodiversity, invasive species, and biofouling fauna assemblages of benthic amphipods attached to buoys and artificial structures of Puerto Progreso, Yucatán, SW Gulf of Mexico (Winfield et al., 2015), several specimens of Pseudaeginella were collected on the algae Caulerpa cupressoides (Vahl) C. Agardh, 1817. The present study contributes to the knowledge about caprellids from the Gulf of Mexico, documenting the first record of P. arraialensis, increasing its geographical distribution range 7,500 km northwards.
Materials and methods
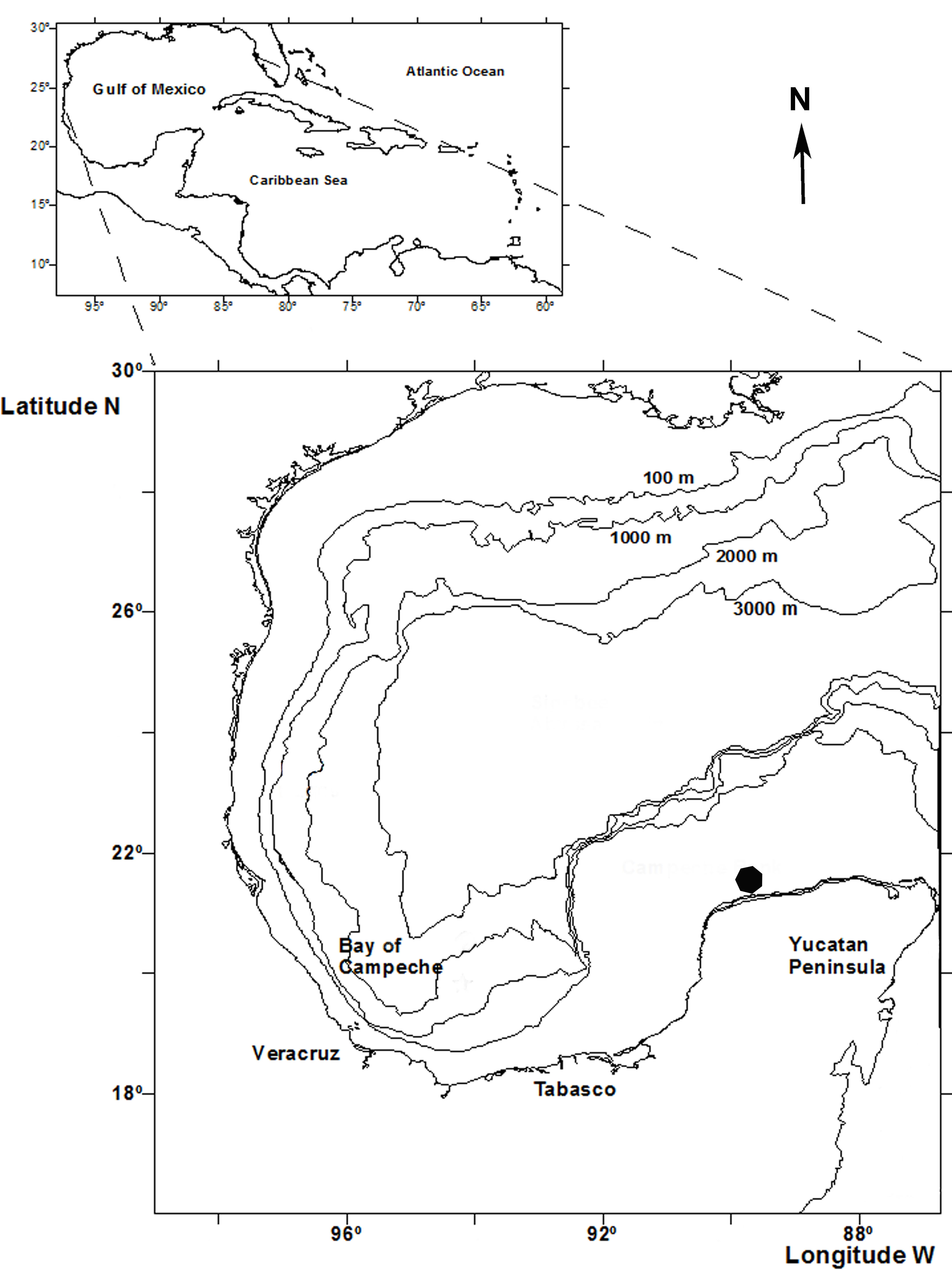
The Integral Port Administration (API), Progreso represents the second most important Mexican port in the Gulf of Mexico, based on its cultural and commercial exchange with Europe, South America, the USA, and many countries around the Caribbean Sea. The port has a 7.5 km long causeway, with customs offices, marine terminals and docks for oil tankers, and commercial and tourist services. API-Progreso is located on the northern coast of the Yucatán Peninsula (21°20’ N, 89°41’ W, 21°17’ N, 89°39’ W) (Fig. 1). The coastal zone has been characterized by its regime of mixed tide, extreme wave events throughout the year, tropical storms and hurricanes, sandy sea bottoms of calcareous origin, and several coastal lagoons (Appendini et al., 2012).
Samples were taken manually using SCUBA equipment, between 0.5 and 12 m depths on 4 buoys in the access to the port area during 2015. Macroalgae were collected from each buoy, chain, and base, according to the permission granted by API-Progreso, Armada de México and Sagarpa (DGOPA.01024.110213.0235) (Fig. 2). Underwater, the samples were collected with the help of a knife and deposited in plastic bags. Afterwards, samples were processed and separated on board. Each sample was filtered with a 500 µm sieve and stored in 70% ethanol. The caprellids were separated and following the original description of Ros et al. (2017) identified to species using a Leica M205C stereo microscope and Leica DM1000 optical microscopes at the Laboratory of Marine Biology, University of Sevilla, Spain.
Results
Order Amphipoda Latreille, 1816
Suborder Senticaudata Lowry & Myers, 2013
Superfamily Caprelloidea Leach, 1814
Family Caprellidae Leach, 1814
Subfamily Caprellinae Leach, 1814
Genus Pseudaeginella Mayer, 1890
Species of this genus are characterized by head and pereonite 1 partially fused; antenna 1 flagellum multi-articulate; antenna 2 flagellum 2-articulate, mandibular palp 3-articulate, molar small or absent, setal formula 1-x-1; inner plate of maxilliped smaller than outer plate; gills present on pereonites 3 and 4; pereonites 6 and 7 separated (not fused); pereopods 3 and 4 uni-articulate, minute; pereopods 5-7, 6-articulate; abdomen without appendages (modified from Ros et al., 2017).
Pseudaeginella arraialensis Ros, Lacerda & Guerra-García, 2017
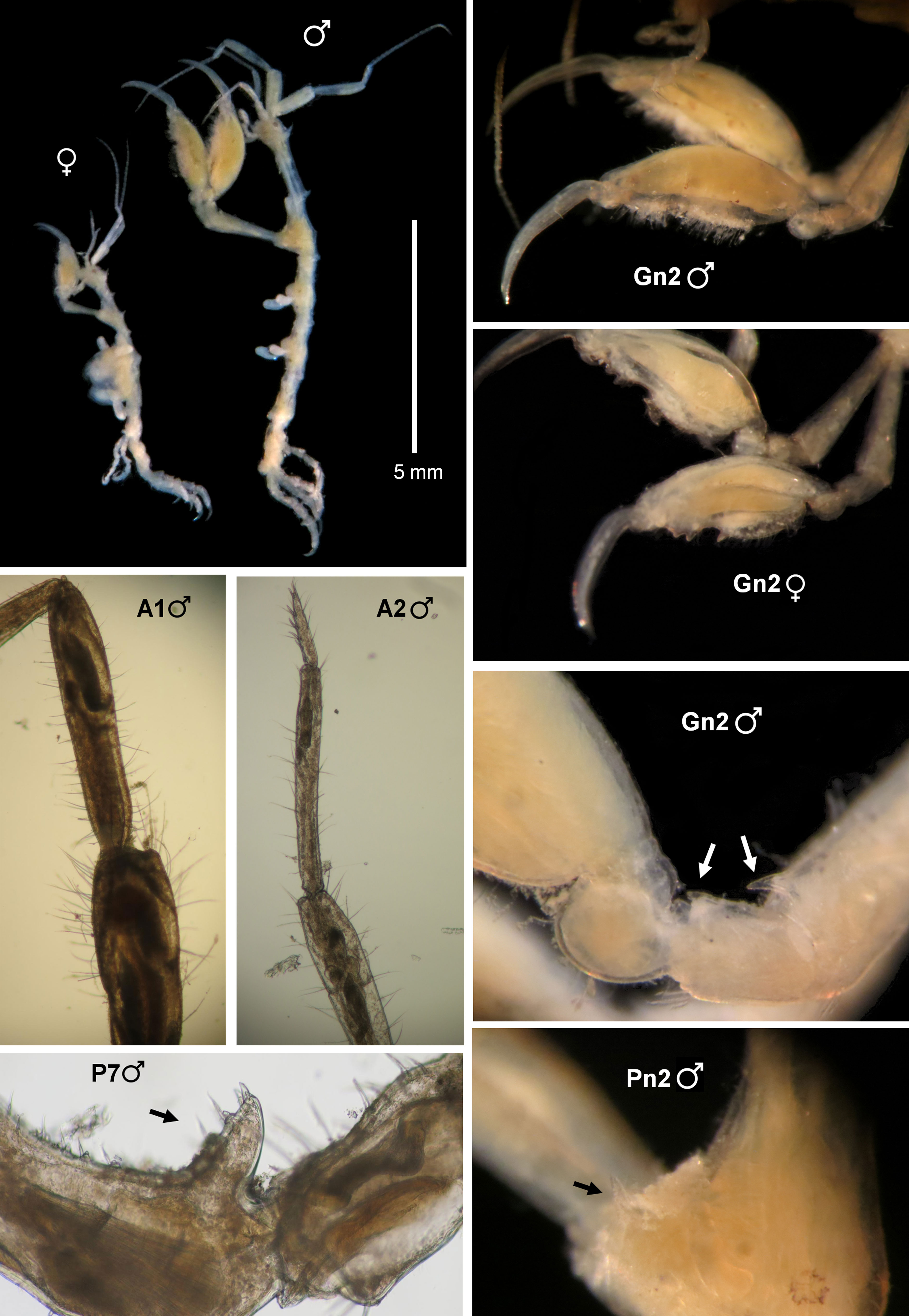
(Fig. 3)
Taxonomic summary
Material examined: 47 mature males, 52 females (15 inmature, 37 mature), 23 juveniles; 1 male and 1 female (both mature) are deposited in the Museo Nacional de Ciencias Naturales, Madrid, Spain (MNCN 20.04/12013).
Habitat: all specimens of P. arraialensis (124 ind.) were collected from the macroalga Caulerpa cupressoides, associated with floating buoys and the chain where the buoys are anchored to the bottom (Fig. 2), between 0.5 and 12 m depths at Puerto Progreso, Yucatán, Mexico. Additionally, 45 specimens of Paracaprella pusilla Mayer, 1890 were collected from the same substratum, occurring together with P. arraialensis.
New record: Puerto Progreso, Yucatán, Mexico; SW Gulf of Mexico.
Type locality: Praia do Forno (22º57’58” S, 42º00’27” W), Arraial do Cabo, Rio de Janeiro, Brazil, 0.5-2 m depth. Associated with the hydroids Eudendrium sp. and Ectopleura sp.; and on the macroalgae Corallina sp., Ceramium sp. and Chylocladia sp., both in fouling communities of artificial floating structures (floating restaurant and oyster culture) and nearby natural habitat (rocky shore).
Discussion
The morphology of the specimens collected in Mexico is in total agreement with the original description of Ros et al. (2017), based on material from Brazil. Head and pereonite 1 have a dorsal acute projection, bent forward. Pereonite 2 has also a dorsal projection medially and a marked hump distally and lateral acute projections (1 on each side) near the coxa of gnathopod 2 (Fig. 3, Pn2). The distal projections on the basis and ischium of gnathopod 2 are also present (Fig. 3, Gn2). Furthermore, peduncles and article 1 of antennae 1 and 2 (Fig. 3, A1), and propodus of gnathopod 2 (Fig. 3, Gn2) are very setose in males. Propodus of pereonite 7 with a proximal triangular projection provided with 2 grasping spines (Fig. 3, P7) as figured by Ros et al. (2017) based on material from Brazil. The abdomen has also been observed in several Mexican individuals and it is identical to those of Brazilian specimens, lacking appendages and with lateral lobes with setae and a single dorsal lobe with 2 plumose setae. Mexican specimens were also dissected for careful examination of mouthparts, and all the characters were in agreement with those reported in the Brazilian material. The mandibles have no sign of mandibular molar, and the palp has a setal formula 1-x-1, with x being 7-9. Maxilla 2 outer lobe always bears 6 serrated spines. The shape of the gills is also similar in the Mexican and Brazilian specimens, and pereonites 3 and 4 are also thickened ventrally in the specimens of both localities. Although the length of specimens is also similar in Mexico and Brazil, specimens from Mexico seem to be more elongate than Brazilian ones, which are more robust. But these differences could be due to different wave exposure, currents or other ecological conditions. Although both localities, the type locality and the site of the new record, are separated by more than 7,000 km we consider that the differences in robustness are not enough to justify the erection of a new species. Considering that the species has been found associated with man-made structures (Ros et al., 2017) in ports with intensive commercial and tourist traffic and it is prone to inhabit fouling communities, we could not rule out a continuous distribution of the species along the Atlantic coast from South America to the Gulf of Mexico, including the Caribbean Sea.

The species reaches locally high abundances in the 2 sites where it has been found so far, in Brazil and Mexico. Despite the numerous studies conducted in both regions (Mauro & Serejo, 2015; Serejo, 1998; Winfield & Ortiz, 2013; Winfield et al., 2015), the species was not described until 2017 (Ros et al., 2017). It could have been overlooked; however, it has a large size and it is conspicuous so we cannot discard that it could have been introduced recently attached to boats or vessels from other unexplored areas and it is presently spreading along the Western Atlantic. Further, morphological and molecular studies are necessary to explore if the species has a wider distribution and to find out about its origin.
With the new record documented in this study, 18 species of caprellids occur in the Gulf of Mexico belonging to 9 gen-era distributed from the shoreline to the deep-sea (ca. 3,800 m depth): Caprella Lamarck, 1801 (5 species), Deutella Mayer, 1890 and Paracaprella (3 species each); Pseudaeginella Mayer, 1890 (2 species); Hemi-aegina Mayer, 1890, Hemiproto McCain, 1968, Metaprotella Mayer, 1890, Phtisica Slabber, 1769 and Mayerella Huntsman, 1915 (1 species each) (Winfield & Ortiz, 2013; Paz-Ríos et al., 2014).
Finally, the caprellid amphipods that inhabit the shoreline, continental shelf, and deep areas of the Gulf of Mexico probably remain partially unknown based on the few oceanographic cruises that have included specific caprellid samplings. The number of recorded Caprelloidea species has remained low since the 19th century with some new records (Escobar-Briones & Winfield, 2003), a new species (Winfield & Ortiz, 2013), and redescription of 2 species (Paz-Ríos et al., 2014). Future exploratory efforts will probably lead to more records and discovery of new species in this large marine ecosystem.
Acknowledgements
We thank the “PASPA-DGAPA-UNAM, 2019” Program for the financial support awarded to the first author to travel to Universidad de Sevilla, Spain. Also, thanks are due to Comisión Nacional de Acuacultura y Pesca (Conapesca-DGOPA) and API-Progreso for the collecting license, and to Miguel A. Lozano Aburto (IIMyP-UV-Boca del Río) for his support during the sampling and sorting of species.
References
Appendini, C. M., Salles, P., Mendoza, E. T., López, J., & Torres-Freyermuth, A. (2012). Longshore sediment transport on the northern coast of the Yucatán Peninsula. Journal of Coastal Research, 28, 404–1417. https://doi.org/10.2112/jcoastres-d-11-00162.1
Escobar-Briones E., & Winfield, I. (2003). Checklist of benthic Gammaridea and Caprellidea (Crustacea: Peracarida: Amphipoda) from the Gulf of Mexico continental shelf and slope. Belgian Journal of Zoology, 113, 37-–44. https://doi.org/10.3989/scimar.2006.70n199
Iwasa-Arai, T., Leite-Siqueira, S. G., De Oliveira-Machado, G. B., & Pereira-Leite, F. P. (2019). Phylogenetic reconstruction of the genus Pseudaeginella (Amphipoda: Caprellidae), with the description of a new species from Brazil. Systematics and Biodiversity, 17, 179–189. https://doi.org/10.1080/14772000.2019.1572668
Lacerda, M. B., & Masunari, S. (2014). A new species of Paracaprella Mayer, 1890 (Amphipoda: Caprellida: Caprellidae) from southern Brazil. Zootaxa, 3900, 437–445. https://doi.org/10.11646/zootaxa.3900.3.7
Mauro, F. M., & Serejo, C. S. (2015). The family Caprellidae (Amphipoda: Caprelloidea: Caprellidae) from Campos Basin, Southwestern Atlantic, with a key of species occurring in Brazil. Zootaxa, 4006, 103–127. https://doi.org/10.11646/zootaxa.4006.1.5
Paz-Ríos, C., Guerra-García, J. M., & Ardisson, P. L. (2014). Caprellids (Crustacea: Amphipoda) from the Gulf of Mexico, with observations on Deutella mayeri, redescription of Metaprotella hummelincki, a taxonomic key and zoogeographical comments. Journal of Natural History, 48, 2517–2578. https://doi.org/10.1080/00222933.2014.931481
Ros, M., Lacerda, M. B., & Guerra-García, J. M. (2017). A new caprellid species (Crustacea: Amphipoda: Senticaudata) from Brazil. Zootaxa, 4258, 388–400. https://doi.org/10.11646/zootaxa.4258.4.6
Serejo, C. (1998). Gammaridean and caprellidean fauna (Crustacea) associated with the sponge Dysidea fragilis Johnston at Arraial do Cabo, Rio de Janeiro, Brazil. Bulletin of Marine Science, 63, 63–85.
Winfield, I., & Ortiz, M. (2013). The Caprellidea (Crustacea: Peracarida: Amphipoda) from the Gulf of Mexico with a description of a new species of Paracaprella. Scientia Marina, 77, 161–168. https://doi.org/10.3989/scimar.03753.26c
Winfield, I., Muciño-Reyes, M. R., Ortiz, M., Cházaro-Olvera, S., & Lozano-Aburto, M. A. (2015). Biodiversidad de los anfípodos bentónicos (Peracarida: Amphipoda) asociados a macroalgas de Puerto Progreso, Yucatán, México. Revista Mexicana de Biodiversidad, 86, 613–619. https://doi.org/10.1016/j.rmb.2015.05.002



