Margarita Ojeda, Ricardo Iglesias, José G. Palacios-Vargas*
Universidad Nacional Autónoma de México, Facultad de Ciencias, Departamento de Ecología y Recursos Naturales, Laboratorio de Ecología y Sistemática de Microartrópodos, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
*Corresponding author: troglolaphysa@hotmail.com (J.G. Palacios-Vargas)
Received: 22 September 2021; accepted: 12 August 2022
Abstract
Ptychoid mites from Mexico are diverse, represented by 60 species of 17 genera. Data includes 6 genera and 21 species of the family Steganacaridae. Currently 5 species of the genus Atropacarus (Hoplophorella) are recorded in the country. Herein we present redescriptions and new records of A. (H.) singularis from Quintana Roo and A. (H.) hamatus from Puebla and Guerrero. New records for the country are given and a brief discussion on distribution is presented. A key to all the known species of Atropacarus (Hoplophorella) in Mexico is given.
Keywords: Ptyctimous mites; Box mites; Quintana Roo; Atropacarus; Hoplophorella
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Ácaros pticoides Steganacaridae (Oribatida), redescripciones, registros nuevos y clave de identificación para las especies mexicanas
Resumen
Los ácaros pticoides de México son diversos, representados por 60 especies de 17 géneros. Los datos incluyen 6 géneros y 21 especies de la familia Steganacaridae. Actualmente, del género Atropacarus (Hoplophorella), se han registrado 5 especies en el país. Aquí presentamos las redescripciones de Atropacarus (H.) singularis de Quintana Roo y A. (H.) hamatus de Puebla y Guerrero. Se dan registros nuevos para el país y se discute brevemente su distribución. Se incluye una clave para todas las especies de Atropacarus (Hoplophorella) conocidas en México.
Palabras clave: Ácaros ptyctimous; Ácaros en caja; Quintana Roo; Atropacarus; Hoplophorella
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Ptyctimous mites are important representatives of Oribatida and a very species rich group studied worldwide. More than 1,400 species in 40 genera and subgenera are described, nearly 13% of the known mite species (Subías, 2004). They have the ability to fold their bodies (= ptychoidy) as protection against predators, so they are known as ptychoids. This particular strategy it seems has appeared in the evolution of the group at least 3 times (Sanders & Norton, 2004), as a convergent feature and the group of oribatids that present it are heterogeneous and belong to 2 separate supercohorts: Enarthronotides (Protoplophoridae and Mesoplophoridae) and Mixonomatides (Euphthiracaroidea and Phthiracaroidea known as Ptyctima, a monophyletic group [Balogh & Balogh, 1992; Grandjean, 1954, 1967]). They are known as “box mites” and constitute one of the most important groups of soil microarthropods; they are macro-phytophagous and feed on dead plant material, being most of them xylophagous. They live in litter and dead leaves, where immature stages build irregular galleries and cavities in decayed wood. They take an important part in the mechanical fragmentation of the organic matter, as well as in the decomposition of organic materials, humification and nutrient cycling processes in soil (Norton & Behan-Pelletier, 2009).
Classification of this heterogeneous group is still under discussion. Niedbała (1986) proposed a classification of the higher taxa of Phthiracaroidea, and his work has served as a reference in the following years. Recently, Niedbała and Liu (2018) presented a list of all the worldwide species of ptyctimous mites known.
Superfamily Phthiracaroidea is one of the most species rich oribatid mite superfamilies, with nearly 750 species in 20 genera (Norton & Behan-Pelletier, 2009; Subías et al., 2012). The number of families and the lower rank classification of the group is under discussion. Norton and Behan-Pelletier (2009), Woas (2002) and other authors recognized a single cosmopolitan family Phthiracaridae. Niedbała (1986) gave a new hypothesis on the phylogeny of the Phthiracaroidea with cladistic theory, and proposed some new taxa, recognizing 2 families: Phthiracaridae Perty, 1841 and Steganacaridae Niedbala, 1986, the last with 2 subfamilies, Steganacarinae Niedbala, 1986 and Atropacarinae Niedbala, 1986. Atropacarinae composed by 5 genera: Austrophthiracarus Balogh & Mahunka, 1978, Calyptophthiracarus Niedbała, 1994, Protophthiracarus Balogh, 1972, Notophthiracarus Ramsay, 1966 and Atropacarus Ewing, 1917. Atropacarus formed by 2 subgenera: Atropacarus (Atropacarus) Ewing, 1917 with Hoplophora stricula C. L. Koch, 1936 as its type species and Atropacarus (Hoplophorella) Berlese, 1923 with Hoplophorella cucullatus Ewing, 1909 as its type species. The genus is characterized by 15 pairs of notogastral setae and only 2 pairs of lyrifissures, ia and im present; 9 pairs of genital setae arranged in a single row and 5 pairs of anoadanal setae present; setae ad2 remote from the paraxial margin; setae d of tibiae IV minute and coupled with solenidia (Niedbała, 1992, 2004; Niedbała & Liu, 2018).
Table 1
Ptyctimous mites (Acari: Oribatida) reported from Mexico (compiled by Niedbała, 2004; Niedbała & Liu, 2018).
| Family | No. of genera | No. of species |
| Protoplophoridae | 4 | 5 |
| Phthiracaridae | 1 | 7 |
| Euphthiracaridae | 3 | 18 |
| Oribotritiidae | 2 | 8 |
| Steganacaridae | 6 | 21 |
| Mesoplophoridae | 1 | 1 |
| Total | 17 | 60 |
Knowledge of Mexican ptyctimous mites is broad, most of the known taxa are endemic and have been recorded from few regions in the country (Niedbała, 2004; Palacios-Vargas & Iglesias, 2004; Vázquez & Prieto-Trueba, 2001). Records of 60 species from Mexico have been reported (Niedbała, 2004; Niedbała & Liu, 2018), which include 17 genera from 6 families. Steganacaridae is represented by the highest number (6 genera; 21 spp.) and Mesoplophoridae with just 1 (Table 1). Until now 7 species belonging to Atropacarus are known from Mexico, 5 included in the subgenus Hoplophorella: A. (H.) brachys, Niedbała 2004, A. (H.) brevipilosus, Niedbala, 2004, A. (H.) cucullatus (Ewing, 1909), A. (H.) hamatus (Ewing, 1909) and A. (H.) vitrinus, (Berlese, 1913) and 2 in Atropacarus: A. (A.) plumatus Niedbala, 2004 and A. (A.) striculus (C. L. Koch, 1836). For the Yucatan Peninsula records of 2 species are known: A. (H.) vitrinus Niedbała, 2001 and A. (H.) hamatus (Ewing, 1909) from Cozumel Island, Quintana Roo (Niedbała & Ermilov, 2017) (Table 2).
In this contribution we present new records for 2 species of A. (Hoplophorella), collected recently from litter and soil samples in continental Quintana Roo state, Mexico. We add new localities for A. (H.) hamatus from material previously collected from different localities and states and identified as Hoplophorella sp. 3 by Ojeda (1983). We added complementary information to the description of each species and a key to all known species of Atropacarus (Hoplophorella) in Mexico.
Table 2
Records of the species of Atropacarus known from Mexico.
| Species | World distribution | Mexican records | Habitat of Mexican records | References |
| A. (Hoplophorella) brachys Niedbała, 2004 | Neotropical, Mesoamerica only
Neotropic: México, Antilles (Cuba) |
Quintana Roo: Cozumel Island | Litter of medium-high Tropical Forest | Niedbała, 2004
Niedbała & Ermilov, 2017 Niedbała & Liu, 2018 |
| A. (Hoplophorella) brevipilosus Niedbała, 2004 | Neotropical, Mesoamerica only
Neotropic: México, Antilles (Cuba) |
Oaxaca: Carretera Tuxtepec-Oax., Santiago Comaltepec | Rain forest | Niedbała 2004
Niedbała & Liu, 2018 |
| A. (Hoplophorella) cucullatus (Ewing,1909) | Semicosmopolitan
Neotropic: Cocos island, México, Guatemala, Belize, Costa Rica, Panamá, Galápagos Island, Greater & lesser Antilles, Trinidad, Venezuela, Ecuador, Bolivia, Perú, Brazil, Argentina |
Guerrero: Río Colotlipa | Litter | Niedbała, 2004
Niedbała & Liu, 2018 |
| A. (Hoplophorella) hamatus (Ewing, 1909) | Semicosmopolitan
Neotropic: Cocos island, México, Guatemala, Belize, Costa Rica, Panama, Galápagos island, Greater & Lesser Antilles, Trinidad, Venezuela, Ecuador, Bolivia, Perú, Brazil, Argentina |
Jalisco: Puerto Vallarta, Chamela
Veracruz: Palma Sola, Cabañas Guerrero: Taxco, Aguacachil cave, Sótano Tilaco Oaxaca: Carretera Tuxtepec-Oax. Santiago Comaltepec Puebla: Yohualichán Yucatán: Mérida |
Litter from mixed forest, coastal zone; palm´s litter;
Tropical forest, rain forest, litter of coffee plantation; litter of Pinus oocarpa; tree logs, cave soil |
Niedbała, 2004
Niedbała & Liu, 2018 |
| A. (Atropacarus) plumatus Niedbała, 2004 | Neotropical, México, endemic | San Luis Potosí: Xilitla. | Leaf and log litter | Niedbała, 2004
Niedbała & Liu, 2018 |
| A. (Atropacarus) striculus (C.L. Koch, 1836) | Semicosmopolitan
Neotropic: México, Guatemala (introduced?) |
México City
Oaxaca Nuevo León: Monterrey |
Litter of Rain forest; litter near cave; sifted cloud forest litter | Niedbała, 2004
Niedbała & Liu, 2018 |
| A. (Hoplophorella) vitrinus (Berlese, 1913) | Semicosmopolitan
Neotropic: Cocos island, México, Guatemala, Belize, Costa Rica, Panamá, Galápagos island, Greater & lesser Antilles, Trinidad, Venezuela, Ecuador, Bolivia, Perú, Brazil, Argentina |
Jalisco: Puerto Vallarta, Chamela
Veracruz: Palma Sola, Cabañas Guerrero: Taxco, Aguacachil cave, Sótano Tilaco Oaxaca: Carretera Tuxtepec-Oax. Santiago Comaltepec Puebla: Yohualichán Yucatán: Mérida Quintana Roo: Cozumel Island |
Litter from mixed forest, coastal zone; palm`s litter;
Tropical forest, rain forest, litter of coffee plantation; litter of Pinus oocarpa; logs, cave soil |
Niedbała 2004
Niedbała & Ermilov, 2017 Niedbała & Liu, 2018 |
Materials and methods
During a collecting trip to the region by JGP-V in 2017, samples of litter at the Environmental Management Unit (UMA in Spanish) “Tepez”, located 7 km from the town of Raudales, were obtained and placed in plastic bags for transportation to the laboratory of Dr. Magdalena Vázquez, Universidad de Quintana Roo, Chetumal, Quintana Roo. They were processed by Berlese-Tullgren funnels, and specimens preserved in 70% ethanol. For identification, the mites were macerated in lactic acid, and then mounted in Hoyer’s solution. Observations, measurements and illustrations were made using a Zeiss phase contrast microscope equipped with a camera lucida. Terminology follows Niedbała (1992, 2004). All measurements are given in micrometers (μm). All specimens were collected under the scientific collector’s license issued to J. G. Palacios-Vargas, and are deposited in the collection of Laboratorio de Ecología y Sistemática de Microartrópodos, Facultad de Ciencias, UNAM (LESM).
Results
Herein we present new records of A. (Hoplophorella) singularis and A. (H.) hamatus for Mexico, giving a complementary description of each species, and an identification key to all the known species of Atropacarus (Hoplophorella) for the country.
Phthiracaroidea Perty, 1841
Steganacaridae Niedbala, 1986
Atropacarus Ewing, 1917
Type species: Atropacarus (Atropacarus) striculus C. L. Koch, 1936.
Diagnostic characters. Body surface with concavities. Posterior furrows of prodorsum present, lamellar setae minute (length ratio of lamellar setae/prodorsum < 0.18). Ventral region: genital setae in a row or nearly so, distance between setae g6 and g5 longer than g5 and g4, setae ad1 always close to paraxial margin, in a row with anal setae; setae ad2 remote from paraxial margin or close to it. Legs: setae v´ on femora I minute (length ratio v”/v’ < 2.25); 15 pairs of notogastral setae, 9 pairs of genital setae and 5 pairs of anoadanal setae. Seta ad2 slightly away from paraxial margin
(Niedbała, 2004).
Atropacarus (Hoplophorella) Berlese, 1923
Type species: Atropacarus (Hoplophorella) cucullatus (Ewing, 1909).
Diagnostic characters. Fifteen pairs of notogastral setae, as a rule only 2 pairs of lyrifissures, ia and im present. Ventral region, 9 pairs of genital setae and 5 pairs of ano-adanal setae present on ano-adanal plate; setae ad2 remote from paraxial margin. Legs: setae ft” on tarsi I normal (Niedbała, 1994).
Atropacarus (Hoplophorella) singularis (Sellnick, 1959)
(Figs. 1-10)
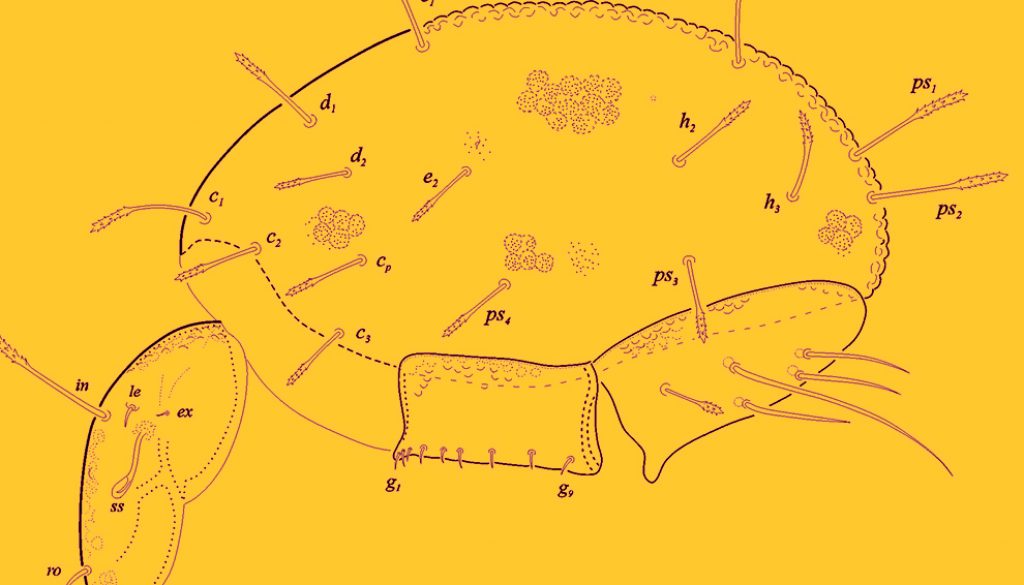
Size. Measurements: prodorsum: length 318, width 228.2; notogaster: length 541.6, width 278.4; genito- aggenital plate151 × 111; ano-adanal plate 192 × 141. Color cuticule: light to medium brown. Surface of body covered with round to irregular concavities – foveolae (Fig. 1), finely punctuated along the entire surface and inside concavities.
Prodorsum. Length 279 (N = 5): 246.5 – 384.5; width 229 (182.9 – 254.2) (Fig. 2). Prodorsum oval in shape, rostrum with rounded tip, sigillar fields with medium-deep sinus between rostral setae, 1 pair of strong median cristae present. Lateral carinae and posterior furrows well marked. Seta ro acicular, smooth and inserted almost in 1/3 from rostral apex (35). Seta in (120) bearing small barbs in distal half at both sides. Seta le small, smooth and spiniform (19) inserted close to bo (distance between bo and in, 19); in > ro > le > ex. Seta ex, located just down to botridium; small, fine and setiform. Sensillus elongated with a club-like head (Figs. 1, 2). Surface of prodorsum with prominent irregular concavities (foveolae) towards anterior one-third of tip with dense and fine punctuations towards the whole prodorsum surface and inside
foveolae (Fig. 2).
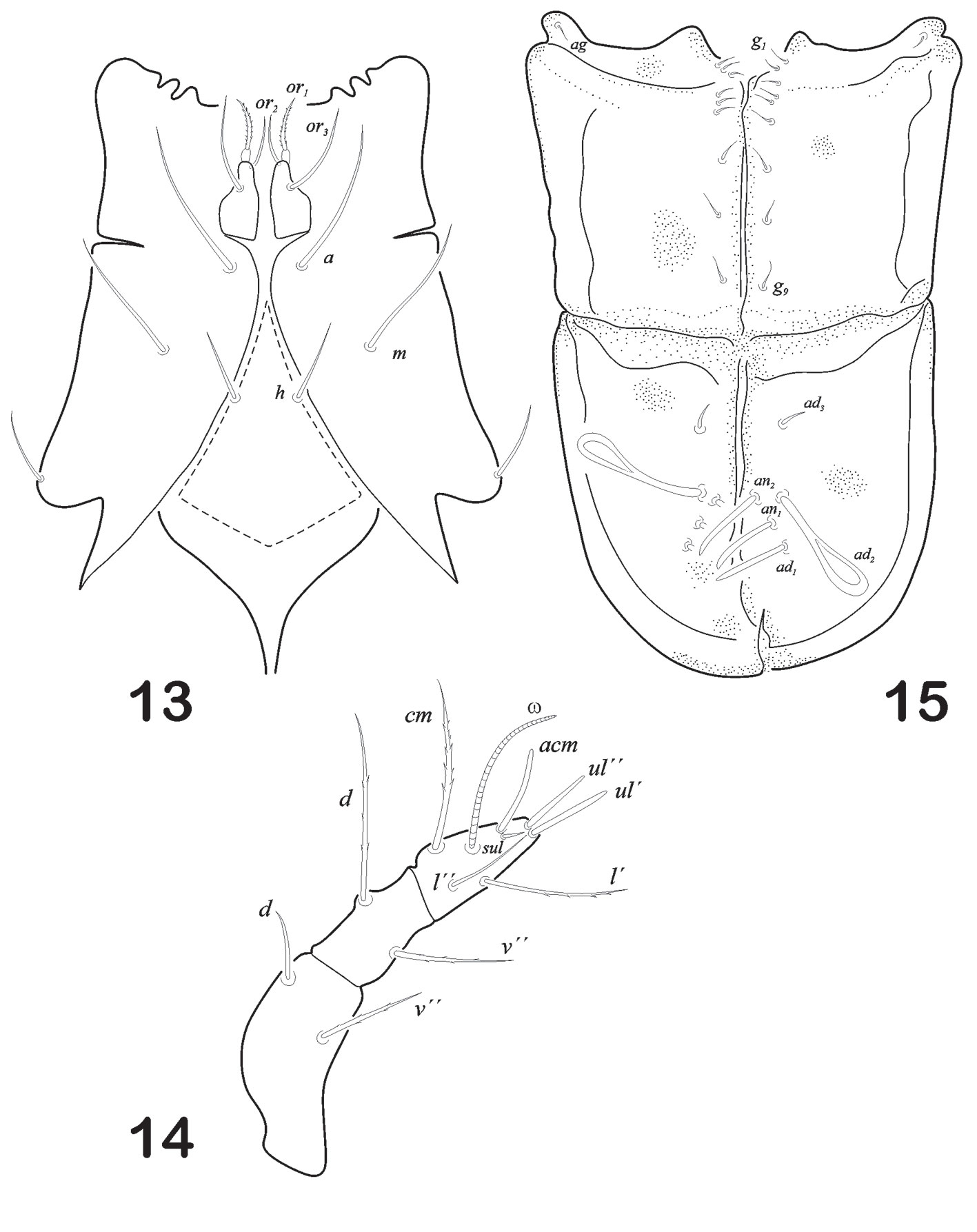
Mouthparts. Infracapitulum with rutellum well developed and anterior (or1) and posterior (or2) adoral setae are ciliated. Posteriorantiaxial setae (or3) setiform. Anterior (a) and median (m) smooth and setiform. A pair of minute setiform setae (h) (Fig. 3). Chelicera chelate-dentate type, cha and chb setae on fixed digit; with few small spines on antiaxial surface (Fig. 4). Pedipalp is 3 segmented with setal formula 2-2-7. Solenidion and a euphatidial seta (sul) close to solenidion base on tarsus (Fig. 5).
Notogaster (Fig. 1). Length (N = 5) 526 – (range 453 – 601); width 295 – (range: 246 -74). Notogaster with a globular shape and a rounded posterior end. Surface ornamentation with very prominent, round and deep foveolae. Fifteen pairs of notogastral setae which are rigid, almost erect, c1< c1 – d1, covered with small barbs on both sides at distal third. Setae c1, c2 and c3 close to anterior margin. Setae d1, d2, e2, h3 < c1, c2, c3, e1. Setae ps1, ps2 and h1 longest. Vestigial seta f1 anteriorly of h1.
Two pairs of lyrifissures ia and im present.
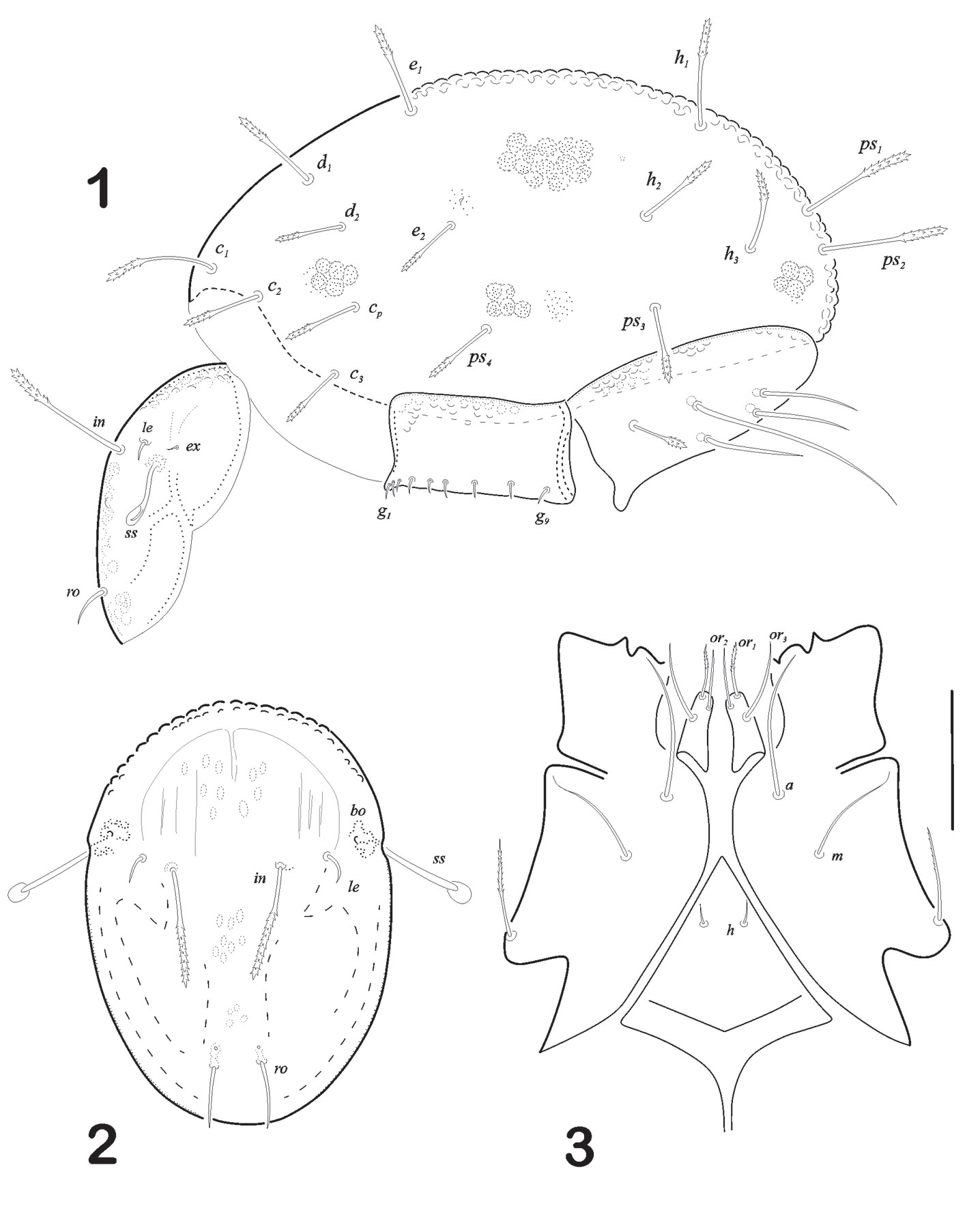
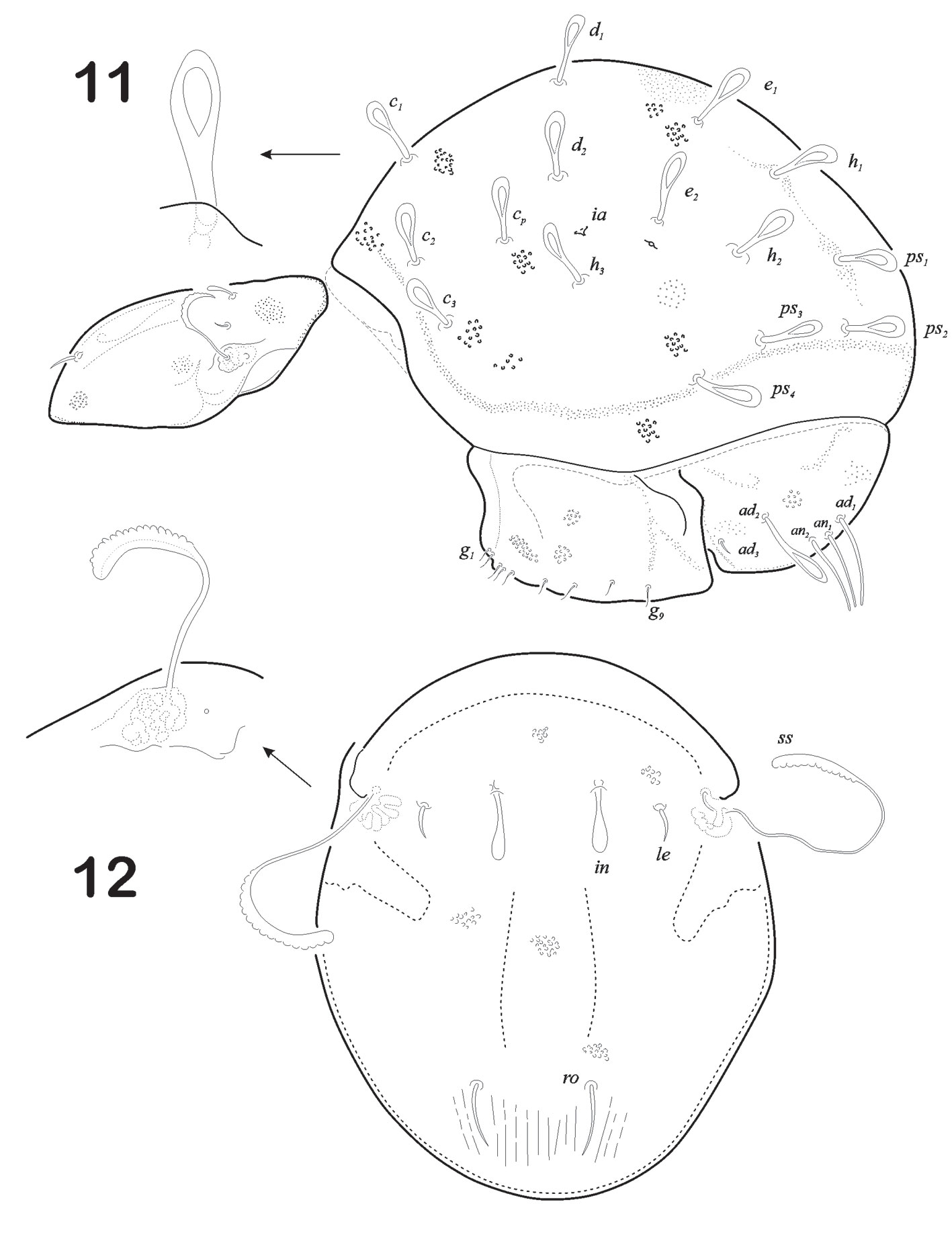
Anogenital region (Fig. 6). Each genital plate rectangular in shape, carrying broad and short aggenital plate with a minute seta ag at its outer corner. Genital setae g1 – g9 visible, arranged in a row towards the inner margin, g1-g3 smaller than others (measuring 5) and very closely arranged. Genital plates ornamented with deep concavities “foveolae”, more visible on external border. Ano-adanal plates are broader towards the middle region and rounded in posterior border. Three pairs of adanal and 2 pairs of anal setae present. Anal setae setiform, thin and pointed tip. Ad2 is much longer than ad1 and ad3 (146/ 59 respectively). Seta ad3 with 1/3 distal end medium-size barbs on both sides. Integument with foveolae as well as fine punctuations along all plates (Fig. 6).
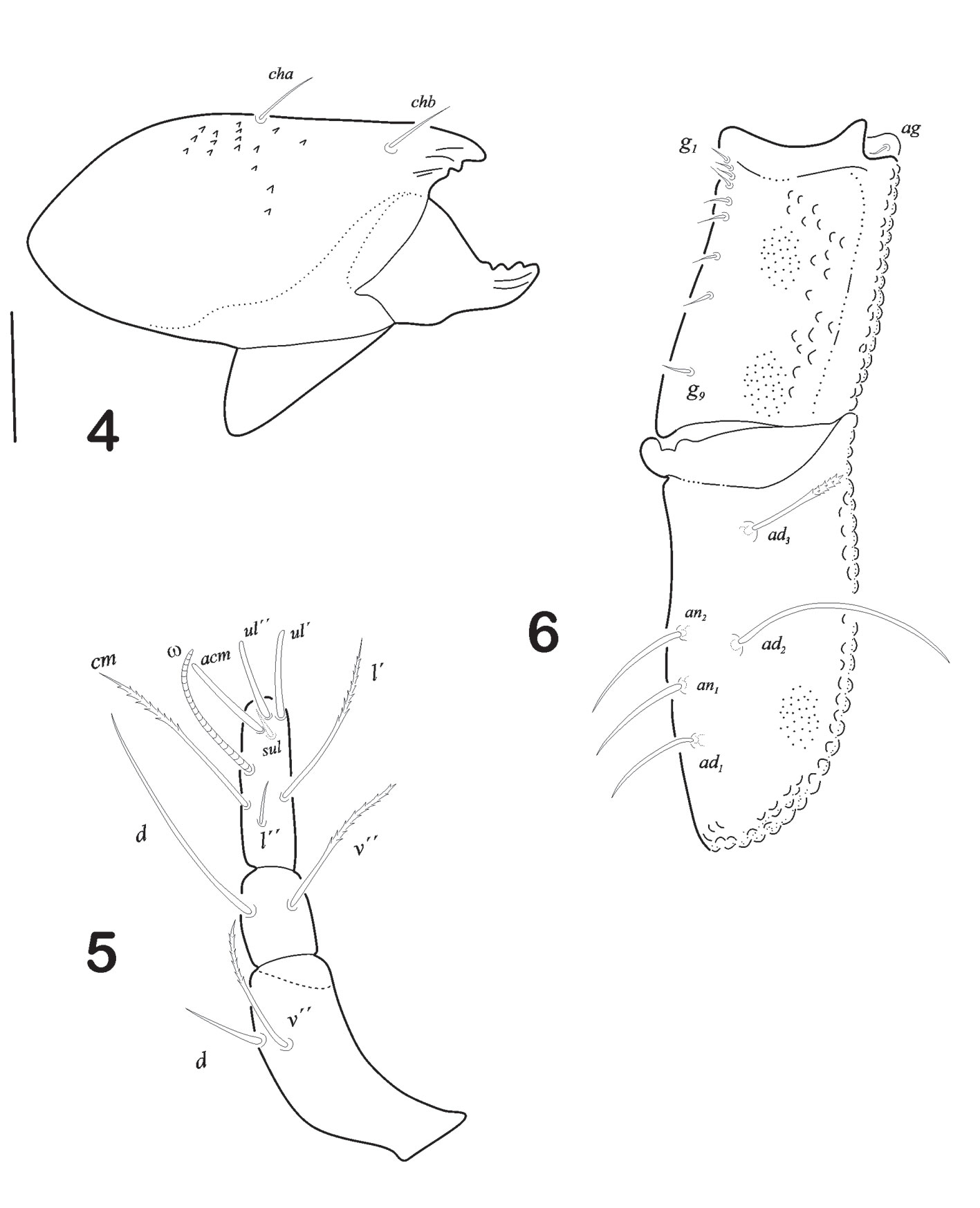
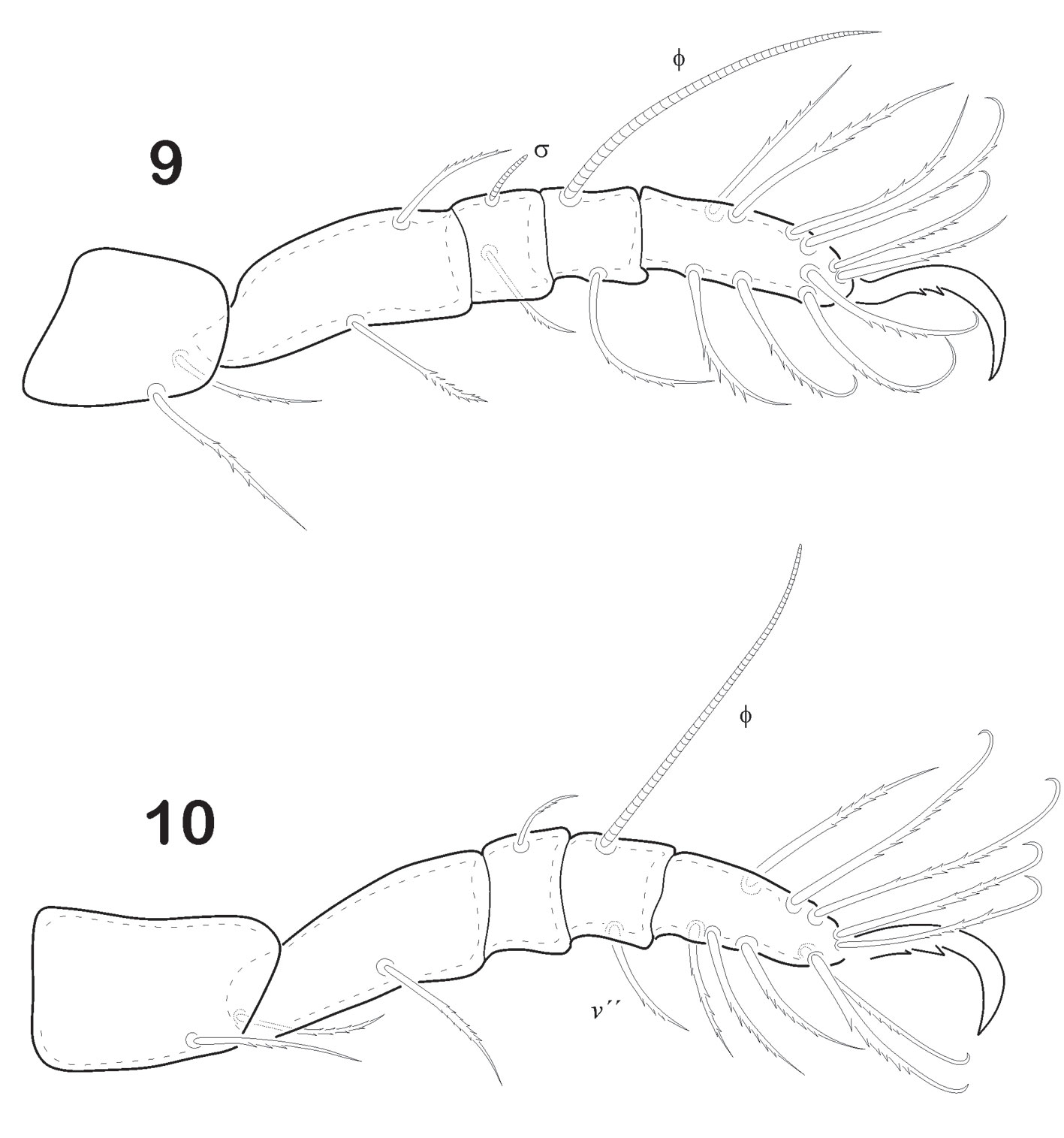
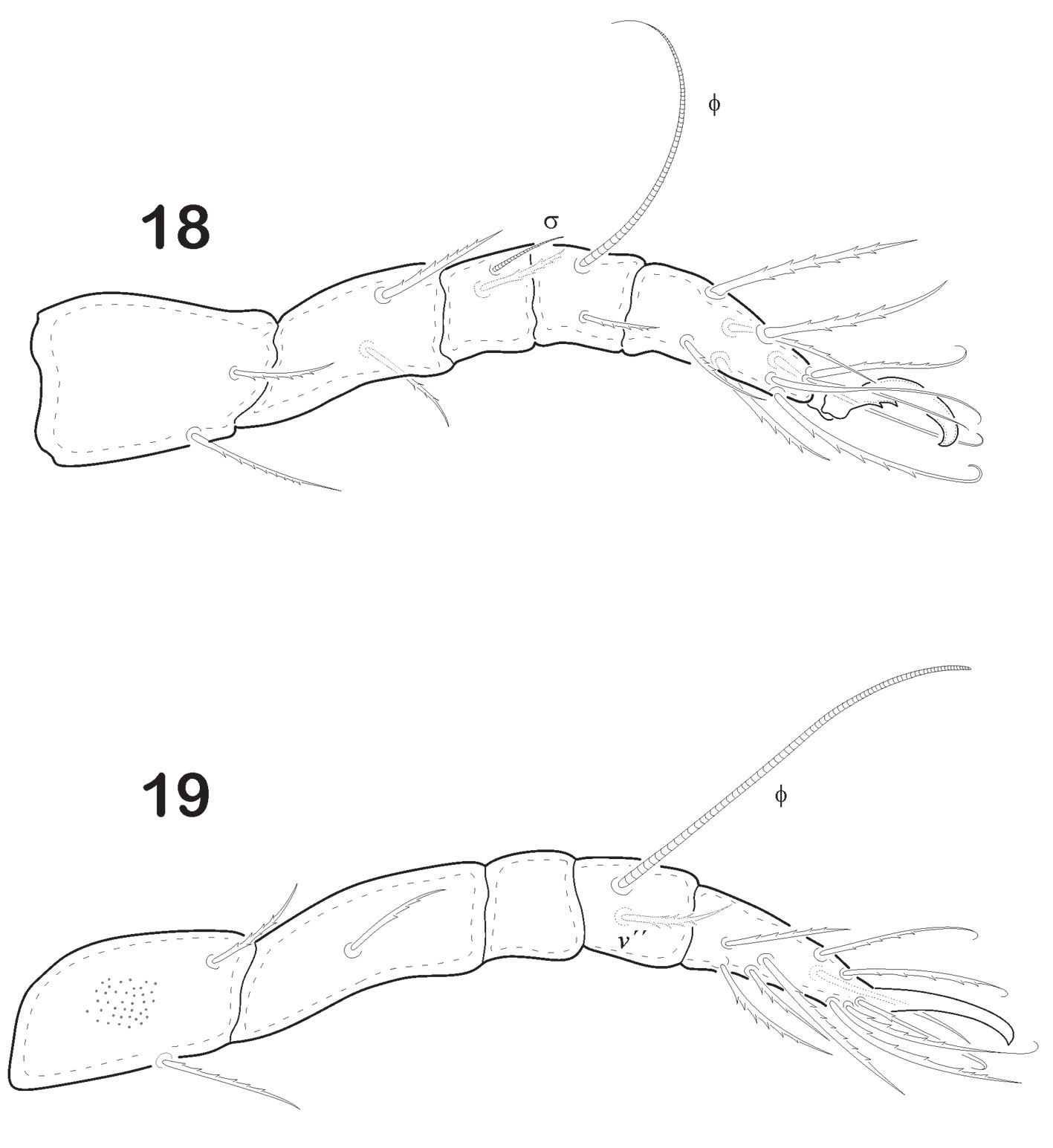
Legs (Figs. 7-10). Leg I longest of all. Tiny pores on either trochanter or femur or both. Tarsus of each leg monodactyl. Each claw strongly developed and bidentate midventrally. Anterior tooth is always more developed than the posterior. Formulae of setae “incomplete type”. I (1-4-1-4-16), II (1-3-2-2-12), III (2-2-1-1-10), IV (2-1-1-1-10). Ratio length of v “ to v ‘ on femur I is 2.25. Solenidiotaxy on the genu, tibia and tarsus I (2-1-3), II (1-1-2), III (1-1-0), IV (0-1-0) typical for all Phthiracaroidea. Seta d on femora I stout, bent and not bifurcated, situated at distal end of article; seta l’ on genua IV present and slightly barbulate.
New record. Material examined: Mexico: Quintana Roo: Municipality Othón O. Blanco, Chetumal: Puente Xul-ha, 18°33’12” N, 88°27’30” W, 6 m asl, ex litter. 27-VII-2017, J. G. Palacios-Vargas, col.
Atropacarus (Hoplophorella) hamatus (Ewing, 1909)
(Figs. 11-19)
Size. Measurements: prodorsum: length 279, width 229; notogaster: length 526, width 225; genito- aggenital plate 777 × 156; anoadanal plate 92 × 141 (Fig. 11). Color cuticule: light to medium brown.
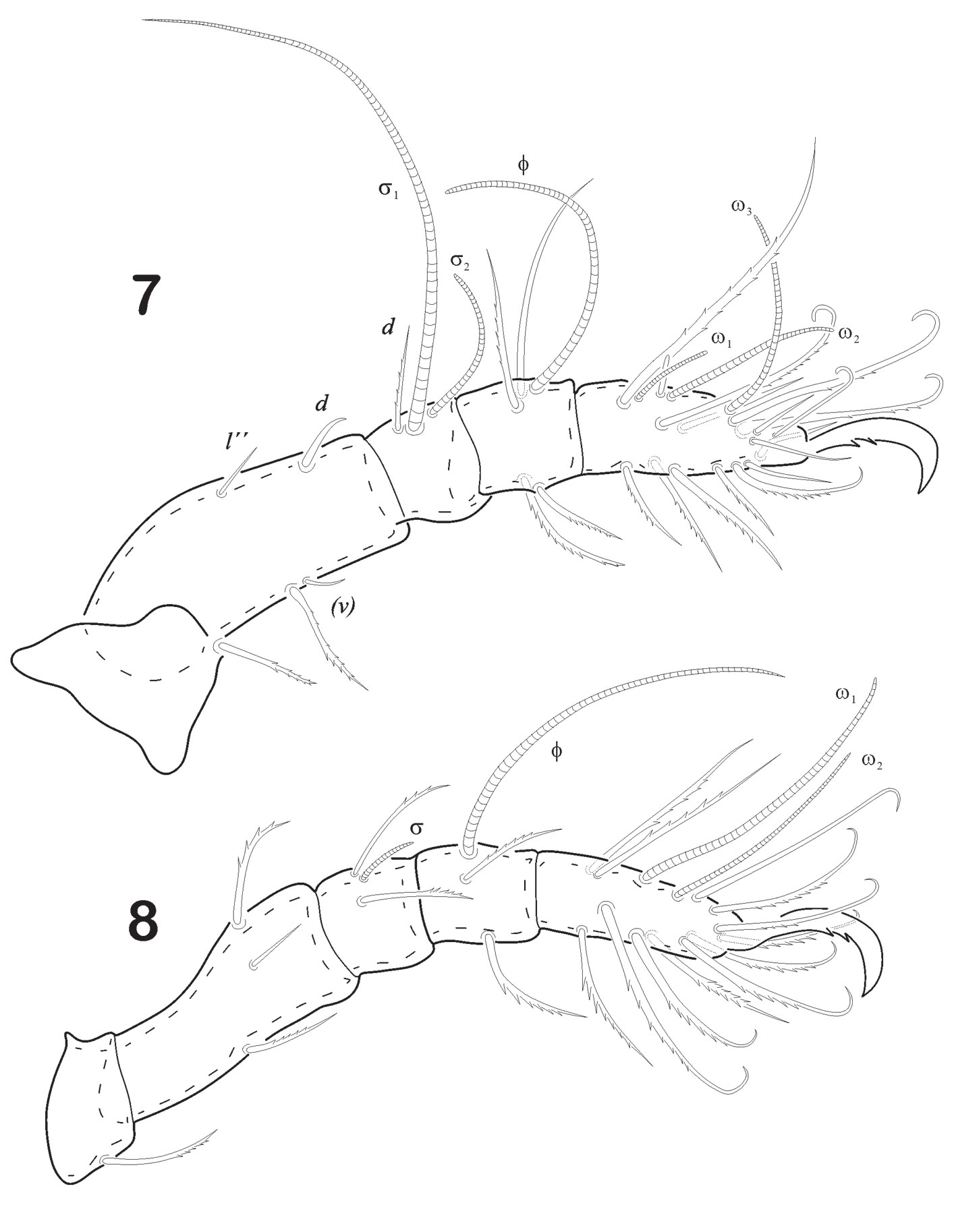
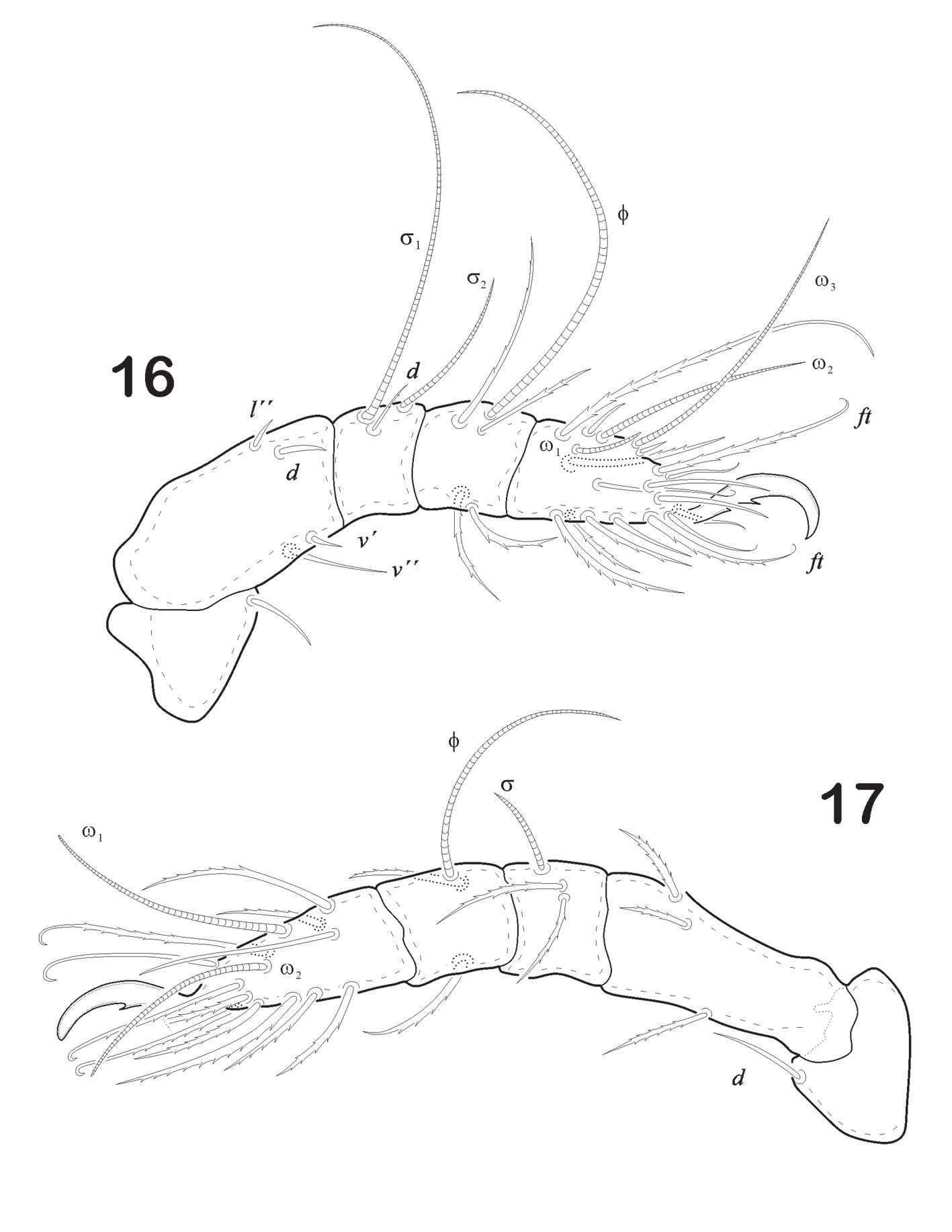
Prodorsum. Length (n = 5): 279 (range: 246 – 384); width: 229 (range: 182.9 – 254.2). Prodorsum oval in shape, rostrum with slightly pointed tip in lateral view (Fig. 11). Seta ro stiff, slightly bent forward, smooth and acicular, inserted in the last 1/4 of rostral apex, measuring 35 in length. Seta in clavate, almost 11/2 times le. Seta le small, acicular and smooth (16), distance between bo and in, 19. Seta ex vestigial. in > ro > le. Sensillus elongated, narrow, sickle-shape with small spine-like ondulate serrations on one side at distal end (Fig. 12). Prodorsum with fine punctuations towards anterior one-third of tip and whole prodorsum surface.
Mouthparts. Infracapitulum stenarthrous type. Rutellum well developed; anterior (or1) adoral setae ciliated; setae (or3) setiform. Anterior (a) and median (m) smooth setae. Posteriorly, setae h half size of a and m, setiform (Fig. 13). Chelicera, chelate-dentate type, bearing dorsal (cha) and lateral (chb) setae on fixed digit. Pedipalp 3-segmented with setal formula 2-2-8. There is a solenidion (ω) and 1 euphatidial seta (sul) close to solenidion base on tarsus (Fig. 14).
Notogaster. Length 526 – (Range 453.5 – 601.4); width 295.8 (Range 246.5 – 374.7). Notogaster globular shaped, posterior end rounded (Fig. 11). Ornamentation represented by fine punctuations along the whole body. Anterior collar absent. 15 pairs of leaf- shape notogastral setae swollen distally, very conspicuous, bent backwards and spatulate in shape (Fig. 11). Number and arrangement of setae typical of subgenus Hoplophorella. Seta c1 originates further (from the collar line) than c2 and c3. Seta c1 < c1 – d1. Lyrifissures ia located between d2 and e2 setae; setae h1 and ps1 arising from a protuberance.
Ano-genital region. Plates clearly visible in lateral view (Fig. 11). Ano-adanal plates somewhat rectangular in shape (Fig. 15). Proximal margin projects anteriorly to form an interlocking device with posterior corner of aggenito-genital plate and all along anterior margin, there are overlapping chitinized lobes on right and left adano-anal plates. Each genital plate is rectangular in shape, carrying broad and short aggenital plate, with a minute seta ag at its outer corner. Genital setae g1 – g9 visible, arranged in a row towards the inner margin, g1-g3 smaller than others (measuring 5 and very closely arranged (formula 6:3). Genital plates ornamented with fine punctuations. Ano-adanal plates are a little broader towards the middle region and rounded in posterior border. Three pairs of adanal and 2 pairs of anal setae present. Anal setae setiform, thick and pointed tip; ad2 as longer as ad1 and ad3; ad2 resembles notogastral setae leaf – shape; ad1 resembles anal setae; ad3 short, smooth and aciculate. Integument with fine punctuations more visible in the middle part of plates.
Legs (Figs. 16-19). Leg I longest of all. Each leg has 5 segments. Tiny pores similar to ornamentation on epimers are seen on trochanter, femur or both. Tarsus monodactyl. Each claw is strongly developed and with well-developed teeth midventral. Leg chaetotaxy “incomplete type”, as follows: I (1-4-1-4-16), II (1-3-2-2-12), III (2-2-1-1-10), IV (2-1-0-1-10). Seta d on femora IV remote from distal end of article; seta l´ on genua IV absent. Solenidiotaxy on genu, tibia and tarsus I (2-1-3), II (1-1-2), III (1-1-0), IV (0-1-0) typical for all Phthiracaroidea.
Material examined. Mexico: Quintana Roo: Raudales UMA El Tepez 18°32’17” N, 88°17’08” W, 7 m asl: 25-vii-2017. J.G. Palacios-Vargas leg. Atropacarus (Hoplophorella) hamatus correspond to the same species cited and preliminary described as Hoplophorella sp. 1 in Ojeda (1983). With this work we start the formal naming and publication of ptychoid mites reported by the first author.
Previous records for Mexico (Niedbała, 2004). Jalisco: Puerto Vallarta, above Mismaloya, litter from mixed forest, coastal zone, 2 X 1995, leg. W. Niedbała – (1 specimen); Puerto Vallarta, palm litter near the beach of Hacienda Buenaventura, 26 IX 1995, leg. W. Niedbała – (1 specimen.); Puerto Vallarta, Quimixto, waterfalls, litter from mixed forest near cascade, 27 IX 1995, leg. W. Niedbała – (1specimen); near Puerto Vallarta, El Edén – Mismaloya, near road jungle, litter, 30 IX 1995, leg. W. Niedbała – (1 sp.); 107 km N of Chamela, litter of Pinus oocarpa? leg. F. Mata – (1 sp.). Veracruz: Cabanas, ex trunk, 11 IX 1983, leg. J. Palacios – (1 specimen); Palma Sola (700 m asl), litter of tropical rainforest, 1 X 1976, leg. P. Lavelle – (1 specimen). Guerrero: Taxco, cave “Aguacachil”, 25 I 1981, leg. J. Palacios col. – (1 specimen); Sotano Tilaco, soil, 21 XII 1980, leg. A. Guzmán – (4 specimens); Puebla, Yohualichán, litter of coffee plantation, 24 VII 1981, leg. J. Palacios – (1 specimen); Oaxaca, El Suspiro, Carr. Tuxtepec-Oaxaca, Santiago Comaltepec (2,000 m asl), 29 III 1988, leg. A. Luis – (1 specimen.); Yucatán, ca 100 km S of Mérida, between Lol-Tun and Labna, tropical forest, 11 IX 2002, leg. W. Niedbała – (12 specimens). New records: Hidalgo: Cañada Otongo, 700 m.a.s.l., Tropical forest, Pinus–Quercus forest, ex litter, 10- X-80, 10-V-80, R. Johansen leg. Zacualtipán, 24-I-81, R. Johansen leg. Guerrero: Gruta de Acuitlapán, ex soil and litter, 24-I-81, J. Palacios leg. Grutas de Juxtlahuaca, Deciduous forest, ex soil, 11-IV-81, J. Palacios leg., 24-VII-82, M. Ojeda leg. Road to Colotlipa riverside ex soil, decaying trunk and litter, 5-VIII-82, M. Ojeda leg. Piedras Negras, ex detritus, 25-VIII-81, J. Palacios leg. Morelos: Derrame del Chichinautzin, ex litter, decaying trunk, 27-XI-76, J. Palacios leg., 7-XI-76, P. Rico leg. Puebla: Villa Juárez, Rancho Grande, ex soil, 28-X-79, J. Palacios leg. Yohualichán, ex litter in coffee plantation, 24-VII-77, J. Palacios leg. San Luis Potosí: Sótano Tlamayo, ex soil, 14-III-82, H. Guzmán leg. Huichihuayán, rainforest, ex soil, 22-XI-75, J. Palacios leg.
Remarks
Oribatid ptyctimous mites are a diverse and abundant group with few specialists around the world. Due to this, many of the descriptions that are available have not addressed in detail many of the morphological characteristics for the recognition of the species. Among them are the leg chaetotaxy, type of infracapitulum, details that we add for the 2 Atropacarus (Hoplophorella) redescribed here. For Atropacarus (Hoplophorella) hamatus we observe some differences from what was originally described. The Mexican specimens have the spoon-shape notogastral setae but they do not present the small spines on the distal portion. However, all other characters described for the species can be seen in the specimens from Quintana Roo, so the differences could be taken as morphological variations.
The ptyctimous mite fauna of the Neotropical Region is characterized by a great richness and diversity, a large number of endemic species and is much richer than the faunas of other regions of the world (Niedbała, 2004). The total number of species known from this region is 305, with a dominant number of Phthiracaroidea (193 species). Records of 60 species, 17 genera from 6 families have been reported for Mexico (Niedbała 2004; Niedbała & Liu 2018). The family Steganacaridae is represented by the highest number (6 genera; 21 spp.). According to Niedbała (2004) Atropacarus (Hoplophorella) species are poorly represented in Mexican territory and are rather common in Central America and the Antilles. The 5 species of Atropacarus (Hoplophorella) previously known from Mexico are recorded in 7 states (Guerrero, Jalisco, Oaxaca, Puebla, Quintana Roo, San Luis Potosí and Yucatán) most of them associated with leaf litter of tropical forest, some living in caves and others under decaying tree trunks (Table 2). A. (H.) cucullatus, A. (H.) hamatus and A. (H.) vitrinus have a semi cosmopolitan distribution, (A. (H.) brachys and A. (H.) brevipilosus) are clearly Neotropical and are distributed in Mesoamerica and the Antilles, and 1 is endemic (Atropacarus (Hoplophorella) plumatus). The record of a new locality for Atropacarus (Hoplophorella) hamatus expands its presence in 7 states of the country. As for the new record of Atropacarus (Hoplophorella) singularis is consistent with the general similarity between the fauna of the Antilles and the lowlands of Mexico, as pointed out by Niedbała (2004) for the general pattern of neotropical ptichoid mites.
Key to the identification of the Atropacarus (Hoplophorella) species from Mexico.
1. Notogaster with a well-developed anterior hood (also named as cowl by Niedbala); notogastral setae short, smooth, leaf shaped; ad2 leaf shaped ………………………. A. (H.) cucullatus (Ewing, 1909)
-. Notogaster without a well developed anterior hood (cowl); notogastral setae various shapes ………………………. 2
2. Notogastral setae setiform, barbed fairly short to long ………………………. 3
-. Notogastral setae not setiform and barbed, various shapes as leaf shaped, clavate, or barbulated ………………………. 4
3. Setae in long, robust, erected covered with spines in distal half. Notogastral setae are rigid, erect, fairly short with barbs at distal half. Sensilli long, with club-like, elongated head ………………………. A. (H.) brachys Niedbała, 2004
-. Setae in longer and thicker than sensilli. Notogastral setae thick with barbs at 1/3 distal end. Sensilli with narrow pedicel and globular head, rounded distally ………………………. A. (H.) singularis (Sellnick, 1959)
4. Notogastral setae setiform, very short; Sensilli rather short, with short pedicel and elongated head; ad3 shortest and spiniform ………………………. A. (H.) brevipilosus Niedbała, 2004
-. Notogastral setae leaf-shaped, or lanceolate ………………………. 5
5 . Rostral setae directed inwards. Notogastral setae foliate fairly short and covered with spines. Sensilli long, narrow, inflated in the middle covered with spines. Setae ad2 foliate, ad3 the shortest ………………………. A. (H.) vitrinus (Berlese, 1913)
-. Rostral setae directed forwards. Notogastral setae spoon-shape short covered with small spines. Sensilli long, narrow, sickle-shaped with narrow pedicel and broad head covered with thin spines. Setae ad2 spoon-shape, ad3 shorter ………………………. A. (H.) hamatus (Ewing, 1909)
Acknowledgements
María Magdalena Vázquez (Universidad de Quintana Roo, Chetumal, Mexico) invited the corresponding author to collect in the Yucatán Peninsula. Jair Páez helped in the digitalization of figures. This project was partially supported by DGAPA (UNAM) to J.G.P.-V. Xavier Gamboa and Acacia López, from UMA “El Tepez”, in Raudales, Quintana Roo, who gave facilities for collecting in their locality. We are also very grateful to the two anonymous reviewers that helped to improve the manuscript.
References
Balogh, J., & Balogh, P. (1992). The oribatid mites genera of the world, Vol. 1. Budapest: Hungarian National Museum Press.
Grandjean, F. (1954). Essai de classification des oribates. Bulletin de la Société Zoologique de France, 78, 421– 446.
Grandjean, F. (1967). Nouvelles observations sur les oribates (5e serie). Acarologia, 9, 242–272.
Niedbała, W. (1986). Système des Phthiracaroidea (Oribatida, Euptyctima). Acarologia, 27, 61–84.
Niedbała, W. (1992). Phthiracaroidea (Acari, Oribatida). Systematic studies. Warszawa, Poland: PWN-Polish Scientific Publishers.
Niedbała, W. (1994). Supplement to the classification of Phthiracaroidea, with redescriptions and descriptions of some species (Acari, Oribatida, Euptyctima). Genus, 5, 1–152.
Niedbała, W. (2004). Ptyctimous mites (Acari: Oribatida) of the Neotropical region. Annales Zoologici, 54, 1–288. https://doi.org/10.24349/acarologia/20174217
Niedbała, W., & Ermilov, S. (2017). New data on ptyctimous mites (Acari: Oribatida) from Mexico and Peru with description of two new species. Systematic & Applied Acarology, 22, 759–765. https://doi.org/10.11158/ssa.22.6.1
Niedbała, W., & Liu, D. (2018). Catalogue of ptyctimous mites (Acari: Oribatida) of the world. Zootaxa, 4393, 1–238. https://doi.org/10.11646/zootaxa.4393.1.1
Norton, R. A., & Behan-Pelletier, V. M. (2009). Oribatida. In G. W. Krantz, & D. E. Walter, (Eds.), A manual of acarology, Chapter 15 (pp. 430–564). Lubbock: Texas Tech University Press.
Ojeda, C. M. (1983). Contribución al conocimiento de los Ptyctimina (Acarida: Oribatei) neotropicales (Tesis). Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad de México.
Palacios Vargas, J. G., & Iglesias, R. (2004). Oribatei (Acari). In J. Llorente-Bousquets, J. J. Morrone, O. Yáñez- Ordóñez, & I. Vargas-Fernández (Eds.), Biodiversidad, taxonomía y biogeografía de artrópodos de México: hacia una síntesis de su conocimiento, Vol. IV (pp. 431–468). Facultad de Ciencias, UNAM, México.
Sanders, F. H., & Norton, R. A. (2004). Anatomy and function of the ptychoid defensive mechanism in the mite Euphthiracarus cooki (Acari: Oribatida). Journal of Morphology, 259, 119-154.
Subías, L. S., Shtanchaeva, U. Y A., & Arillo, A. (2012). Listado de los ácaros oribátidos (Acariformes, Oribatida) de las diferentes regiones biogeográficas del mundo / Checklist of the oribatid mites (Acariformes, Oribatida) of the different world biogeographical regions. Monografías electrónicas Sociedad Entomológica Aragonesa, Vol. 4. http://sea-entomologia.org/Publicaciones/MonografiaElectronica4/ACARI_ORIBATIDA_MESEA4.pdf
Vázquez, M. M., & Prieto-Trueba, D. (2001). Oribatida. In M. M. Vázquez (Ed.), Fauna edáfica de las selvas tropicales de Quintana Roo (pp. 71–84). Universidad de Quintana Roo, México.
Woas, S. (2002). Acari: Oribatida. In J. Adis (Ed.), Amazonian Arachnida and Myriapoda (pp. 21–291). Sofia: Pensoft Publ.