New host record of Sclerotium rolfsii causing crown and root rot on Pseudogynoxis benthamii
Marta C. Rivera a, b,*, Eduardo R. Wright b, Luciana Silvestro c, Sebastián Stenglein c, d, Adriana Kato a
a Instituto de Floricultura, Instituto Nacional de Tecnología Agropecuaria, De Los Reseros y Nicolás Repetto s/n, Hurlingham, 1686 Buenos Aires, Argentina
b Cátedra de Fitopatología, Facultad de Agronomía, Universidad de Buenos Aires, Av. San Martín 4453, 1417 Buenos Aires, Argentina
c Laboratorio de Biología Funcional y Biotecnología, CICBA, INBIOTEC-CONICET. Av. República de Italia 780, 7300 Azul, Argentina
d Área de Microbiología, Facultad de Agronomía-UNCPBA. Av. República de Italia 780, 7300 Azul, Argentina
*Corresponding author: rivera.marta@inta.gob.ar (M.C. Rivera)
Abstract
Symptoms of a wilt disease were observed on 10 of 40 plants of Pseudogynoxis benthamii grown in Buenos Aires, Argentina, in March 2016. The aim of this study was to identify the causal agent of the disease. Five phenotypically identical fungal isolates were obtained from sclerotia that developed on the roots of wilted plants. One of them was inoculated onto healthy plants, and caused symptoms in 2 weeks. The pathogen was identified as Sclerotium rolfsii, on the basis of morphological characteristics. The nuclear ribosomal DNA internal transcribed spacer region of the isolate was PCR-amplified and sequenced. DNA analysis revealed a 99-100% similarity with S. rolfsii. This is the first report of S. rolfsii causing wilt on P. benthamii and the first pathogen reported on this plant species worldwide.
Keywords:
Wilt; Soilborne fungi; Inoculation; Morphological and molecular characterization
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Registro de Sclerotium rolfsii como causante de pudrición de corona y raíces de Pseudogynoxis benthamii
Resumen
En marzo de 2016, de un total de 40 plantas de Pseudogynoxis benthamii cultivadas en Buenos Aires, Argentina, se observó marchitez en 10 de ellas. El objetivo de este trabajo fue identificar el agente causal de la enfermedad. Se obtuvieron 5 aislados fúngicos de características similares a partir de esclerocios desarrollados sobre las raíces, de los cuales se seleccionó 1, que luego de ser inoculado en plantas sanas, causó síntomas en un período de 2 semanas. El patógeno fue identificado como Sclerotium rolfsii sobre la base de sus características morfológicas. Se amplificó y secuenció la región de la espaciadora interna transcrita de ADN ribosomal del núcleo del aislado. El análisis de ADN mostró 99-100% de similitud para S. rolfsii. Este es el primer reporte de S. rolfsii como agente causal de marchitez en P. benthamii y el primer patógeno citado sobre esta especie en el mundo.
Palabras clave:
Marchitez; Hongo de suelo; Inoculación; Caracterización morfológica y molecular
Pseudogynoxis benthamii Cabrera (= P. cabrerae H.Rob. & Cuatrec.) is an evergreen, suffrutescent, climbing, ornamental Asteraceae native to Argentina, Brazil, and Paraguay. Its stout peduncles hold capitula with bright orange female ray-florets, and reddish hermaphrodite disk florets (Hind, 1992; Robinson & Cuatrecasas, 1977). In March 2016, 10 of 40 plants of P. benthamii in breeding programs in Hurlingham (Buenos Aires, Argentina) suddenly wilted after infrequent hot conditions (Fig. 1a, b). The lower foliage turned yellow and brown, and plants died within a week. White mycelial mats and roundish sclerotia appeared at the basal portion of the stems (Fig. 1c, d), and the lesions spread rapidly to girdle and rot the base of the stems and roots (Fig. 1e). The aim of this study was to identify the causal agent of the disease.
Sclerotia that had developed at the base of 2 wilting plants were gently picked with a tong, disinfected by immersion in a NaOCl: water solution (20% Cl) for 30 s, washed in sterilized distilled water for 1 min, and incubated on potato dextrose agar (PDA, Merck) at 27 ºC. The growing fungal colonies were kept at 8 ºC in darkness, and one of the 5 phenotypically identical isolates that developed from the sclerotia was chosen for further studies.
Vigorous shoots with 2 leaf buds were cut from young non-lignified branches of mother plants of P. benthamii and dipped into a talcum powder mixture containing 250 ppm of indolbutiric acid (Hartman et al., 2011). The cuttings were planted in 25-alveole plastic molded plug trays filled with a mixture of Grow Mix Tabaco S2 (Terrafertil) and expanded perlite (1:1), and placed on a bench in a greenhouse equipped with 80% shadow netting and mist system, at 23-26 ºC. After 15 days, each rooted plantlet was transplanted into a 12 cm diameter plastic pot filled with the same substrate, watered and fertilized routinely for 3 months prior to inoculation.
The fungal isolate was cultivated on PDA at 25 ºC and pieces of 1.7 cm2 were cut from the edge of 5-days colonies with a scalpel. Pathogenicity was tested in a climatic chamber on twelve potted plants inoculated by placing 2 plugs of inoculum on the soil near the stem bases. For the control, 5 plants were treated with sterile PDA plugs. Each plant was enclosed in a transparent polyethylene bag, and incubated at 27 °C for 72 h. The plants remained in the chamber with natural daylight conditions. The test was repeated twice. To satisfy Koch’s postulates, the fungus was re-isolated from symptomatic tissues. Following DNA extraction (Stenglein & Balatti, 2006), a PCR was carried out in an XP thermal cycler (Bioer Technology) to amplify the nuclear ribosomal DNA internal transcribed spacer (ITS) region using primer pairs ITS1 (5´-TCC GTA GGT GAA CCT GCG G-3´) / ITS4 (5´-TCC TCC GCT TAT TGA TAT GC-3´) (White et al. 1990). The sequence fragment was compared with BLASTn (Altschul et al., 1990) to publicly-available sequences deposited in GenBank. The sequence was subsequently submitted to GenBank (accession KY216142).

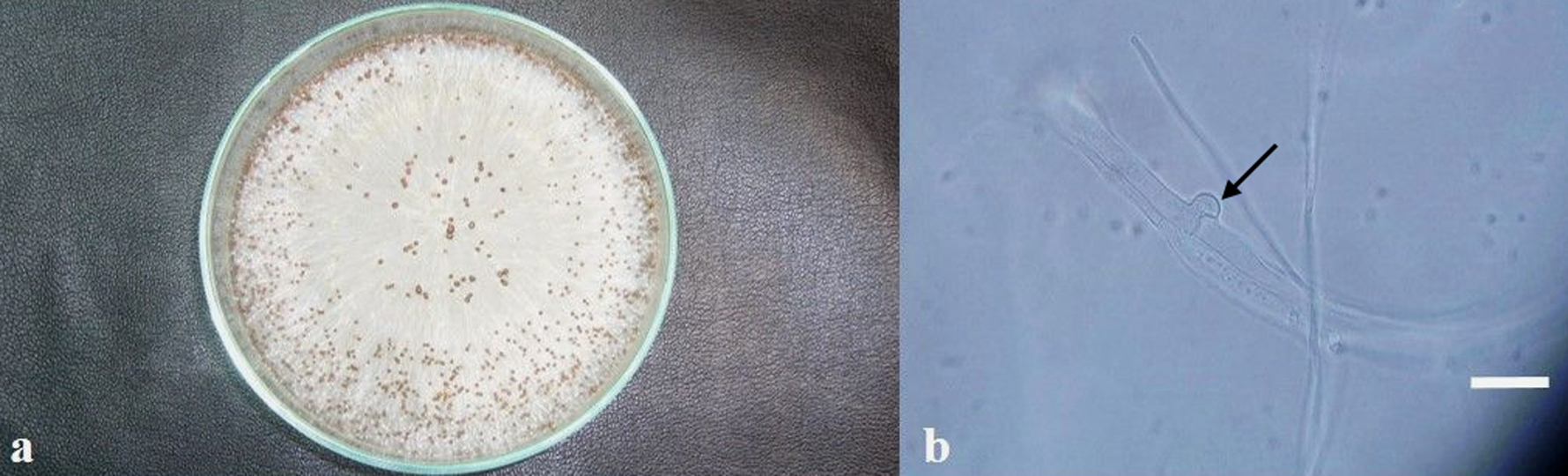
Fungal colonies consistently developed on PDA, and were preliminarily placed in the genus Sclerotium. They grew fast on PDA, covering the whole area of the 10 cm diameter plates in 4 days, and even growing outwards in 7 days, while the aerial mycelium was observed to reach the lid of the plates. The mycelium was white, with strands showing a fan shaped expansion. Almost spherical sclerotia 0.7 to 1.5 mm diameter with a shiny surface developed from the mycelium. They were white at first, and turned tan to brown with age (Fig. 2a). The hyphae formed typical clamp connections (Fig. 2b).
Two days after inoculation, hyphal strands emerged from the plugs and grew towards the substrate and the base of the plants (Fig. 3a), which gradually withered (Fig. 3b). The lower stems appeared surrounded by pathogen’s hyphae (Fig. 3c), and showed water-soaked lesions 5 days from inoculation. All the inoculated plants died within 2 weeks, while the controls remained healthy.
On the basis of colony characteristics, symptoms, and pathogenicity to the host, the isolate was identified as S. rolfsii Sacc. (Mordue, 1974). It was deposited in the fungal collection the Instituto de Floricultura INTA (entry coded INTA-IF-501). The resulting 684 bp of ITS region sequence revealed that it was 99-100% identical to S. rolfsii (ex. HQ420816, KX186998, KU760984).
Sclerotium rolfsii usually infects the lower stem near the soil surface of many crops causing the disease known as Southern blight. Infections are favored by high temperature and moisture (Punja, 1985), which correspond to the environmental conditions before the disease was detected in this case. This is a widely distributed soil-borne species, but P. benthamii is not included in the 2,579 fungus-host records for Sclerotium rolfsii (Farr & Rossman, 2017). The pathogen can overwinter as mycelium in infected plants, plant debris, or as sclerotia. These data should to be taken into account when planning control measures. To the best of our knowledge, this is the first report of S. rolfsii on P. benthamii and the first pathogen anywhere reported on this plant species.

References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Farr, D. F., & Rossman, A. Y. (2017). Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. Retrieved 08 Mar 2018 from: http://nt.ars-grin.gov/fungaldatabases/
Hartmann, H. T., Kester, D. E., Davies Jr., F. T., & Geneve, R. L. (2011). Hartmann and Kester’s plant propagation: principles and practices. Boston: Prentice Hall.
Hind, N. (1992). Pseudogynoxis cabrerae. Compositae. Curtis’s Botanical Magazine, 9, 153–156.
Mordue, J. E. M. (1974). Corticium rolfsii. In Commonwealth Mycological Institute (Ed.), CMI descriptions of pathogenic fungi and bacteria (pp. 410). Kew, Surrey: CMI.
Punja, Z. K. (1985). The biology, ecology and control of Sclerotium rolfsii. Annual Review of Phytopathology, 23, 97–127.
Robinson, H., & Cuatrecasas, J. (1977). Notes on the genus and species limits of Pseudogynoxys (Greenm.) Cabrera (Senecioneae, Asteraceae). Phytologia, 36, 177–192.
Stenglein, S., & Balatti, P. (2006). Genetic diversity of Phaeoisariopsis griseola in Argentina as revealed by pathogenic and molecular markers. Physiological and Molecular Plant Pathology, 68, 158–167.
White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: a guide to methods and applications (pp. 315–322). San Diego, CA: Academic Press.
