Luis G. Herrera-Bojórquez a, Ricardo González-Muñoz b, Lorena V. León-Deniz a, *, Gregory Arjona-Torres c, Raúl Sima-Álvarez c
a Universidad Autónoma de Yucatán, Facultad de Medicina Veterinaria y Zootecnia, Departamento de Biología Marina, Km. 15.5 carretera a Xmatkuil, Apartado postal 116, 97315 Mérida, Yucatán, Mexico
b Laboratorio de Biología de Cnidarios, Instituto de Investigaciones Marinas y Costeras, Consejo Nacional de Investigaciones Científicas y Técnicas, Facultad de Ciencias Naturales y Exactas, Universidad Nacional de Mar de Plata, Rodríguez Peña 4046, 7600 Mar del Plata, Argentina
c Laboratorio de Patología Acuática, Centro de Investigación y Estudios Avanzados, Instituto Politécnico Nacional, Unidad Mérida, Km. 6 antigua carretera a Progreso, Apartado postal 73, Cordemex, 97310 Mérida, Yucatán, Mexico
*Corresponding author: lorena.leon@correo.uady.mx (L.V. León-Deniz)
Received: 10 September 2019; accepted: 25 May 2020
Abstract
Sea anemones (order Actiniaria) are among the benthic marine invertebrate groups that commonly inhabit a wide range of coastal environments, including seagrass meadows, rocky bottoms, coral reefs, sandy patches, mangroves, and artificial substrates. However, they are typically overlooked in most faunal studies or biodiversity assessments in shallow water environments along the coastline of the Mexican Atlantic, particularly in Yucatán. Although some sea anemones species have been reported from coral reef localities in the region, no previous studies have been made to inventory the actinofauna in localities of the coast of Yucatán. In this study, we document 8 species of sea anemones from 5 coastal localities in Yucatán, Mexico, and provide short descriptions and images of the external and internal anatomy, and cnidom. Five of these species have been previously reported from coral reefs in Yucatán, whereas 3 of them are reported for the first time in the region. The aim of this contribution is to inventory the most common species of sea anemones in Yucatán and provide an aid to distinguish them to facilitate identification and encourage biological and ecological research requiring species-level resolution.
Keywords: Cnidaria; Benthic fauna; Invertebrates; Gulf of Mexico; Western Atlantic
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Anémonas (Anthozoa: Actiniaria) de la costa de Yucatán, México
Resumen
Las anémonas (orden Actiniaria) se encuentran entre los grupos de invertebrados marinos bentónicos que habitan comúnmente en un amplio rango de ambientes costeros, incluyendo pastos marinos, fondos rocosos, arrecifes de coral, parches arenosos, manglares y sustratos artificiales. Sin embargo, son típicamente pasadas por alto en la mayoría de los estudios faunísticos o evaluaciones de la biodiversidad de ambientes marinos de aguas someras a lo largo de la línea de costa del Atlántico mexicano, particularmente en Yucatán. Aunque algunas especies de anémonas han sido reportadas en la región en localidades de arrecifes de coral, no se han realizado estudios previos para inventariar la actinofauna en localidades de la costa de Yucatán. En este estudio, documentamos 8 especies de anémonas en 5 localidades costeras de Yucatán, México y proveemos descripciones cortas e imágenes de la anatomía externa e interna, y del cnidoma, de cada una de las especies. Cinco de estas especies han sido reportadas previamente en ambientes arrecifales de Yucatán, pero 3 especies son reportadas por primera vez en la región. El objetivo de esta contribución es el inventariar a las especies de anémonas más comunes en Yucatán y proveer un apoyo para distinguirlas y facilitar su identificación, fomentando la investigación biológica y ecológica que requiera de resolución a nivel específico.
Palabras clave: Cnidaria; Fauna bentónica; Invertebrados; Golfo de México; Atlántico Occidental
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
The coastline of Yucatán, in the southeastern part of Mexico, is composed of a variety of ecosystems such as coastal lagoons, coastal seas, mangroves, seagrass meadows, and sandy intertidal patches (Herrera-Silveira et al., 2013), where thousands of species of marine fauna live. Several groups of invertebrates such as mollusks, crustaceans, nematodes, and annelids are among the most studied benthic fauna in the region (Pech et al., 2007; Pech & Ardisson, 2010), with a main interest in those animals with economic importance at the local and regional level, such as shrimps (May-Kú & Ordóñez-López, 2006; May-Kú et al., 2014; Wakida-Kusunoki et al., 2016), lobsters (Ríos-Lara et al., 2010), or sea cucumbers (Hernández-Flores et al., 2015, 2017), among others. In addition, some inventories of these groups have been carried out as valuable steps to study their diversity and their ecological role in the ecosystems (Kuk-Dzul et al., 2019; Palomino-Álvarez et al., 2019; Pech & Ardisson, 2010). However, other invertebrate groups, such as echinoderms, sponges, tunicates, and cnidarians, have been less studied or even overlooked in most faunal studies or biodiversity assessments of the shallow water environments along the coast of Yucatán (Pech & Ardisson, 2010).
Sea anemones (Cnidaria: Anthozoa: Actiniaria) are among the benthic marine cnidarian groups that are commonly found in a wide range of coastal environments, including seagrass meadows, rocky bottoms, coral reefs, sandy patches, mangroves, and artificial substrates (González-Muñoz et al., 2016). They are recognized as valuable elements of the benthic fauna due to their role in the bidirectional exchange of energy between the benthic-pelagic coupling, as well as their propensity to establish close mutualistic relationships with other invertebrates (Daly et al., 2008). Some species of sea anemones have been reported in the coral reef formations off the coast of Yucatán, such as Bajo de Diez, Madagascar, Serpientes, and Alacranes reefs (González-Muñoz et al., 2013), but no previous studies have been conducted to document the species inhabiting other coastal environments along the shoreline.
In this study, we document 8 species of sea anemones from 5 coastal localities along the coast of Yucatán: Chelem, Yucalpetén, Telchac, Chabihau, and Dzilam de Bravo, and provide diagnoses and images of the external and internal anatomy and types of cnidae for 7 of them. Five species, Actinostella flosculifera (Le Sueur, 1817), Bunodosoma granuliferum (Le Sueur, 1817), Anemonia sargassensis Hargitt, 1908, Exaiptasia diaphana (Rapp, 1829), and Calliactis tricolor (Le Sueur, 1817), have been previously reported from the coral reef areas of the region, but Bunodosoma cavernatum (Bosc, 1802), Anthopleura dalyae González-Muñoz, Garese and Acuña, 2018, and Anthopleura krebsi (Duchassaing & Michelotti, 1860) are recorded for the first time in Yucatán.
Materials and methods
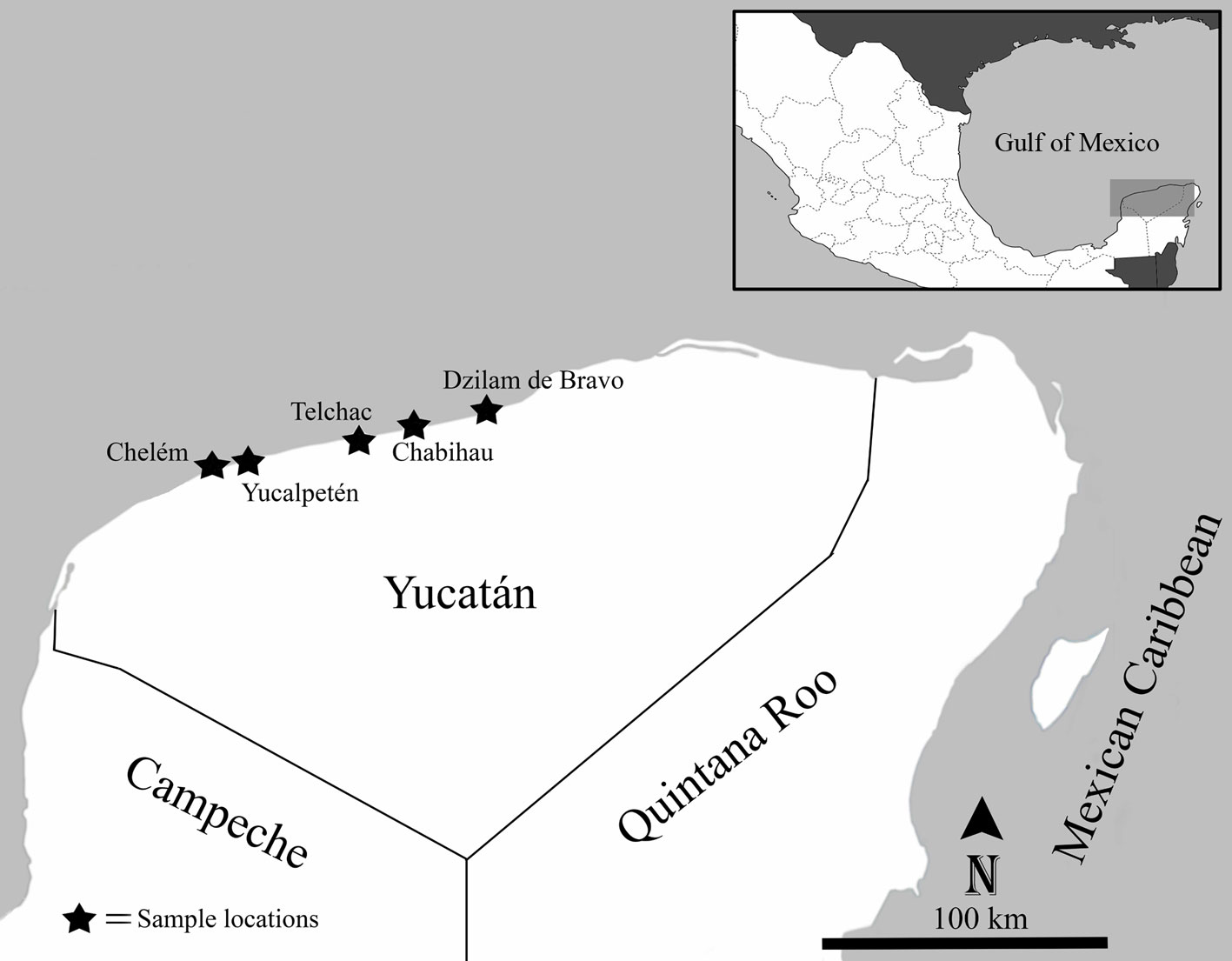
Specimens of sea anemones were collected from 5 beach locations along the Yucatán coastline, Mexico: Chelem (21°16’ N, 89°43’ W), Yucalpetén (21°15’ N, 89°39’ W), Telchac Puerto (21°16’ N, 89°13’ W), Chabihau (21°18’ N, 89°09’ W), and Dzilam de Bravo (21°20’ N, 88°24’ W) (Fig. 1). Specimens were collected by hand and snorkeling from 0-2 m depth, using a hammer and a chisel. Collected specimens were transferred to the laboratory and maintained in an aquarium to photograph their color while alive. Specimens were relaxed in 5% MgCl2 seawater solution and fixed in 10% seawater formalin. Measurements of specimens (i.e., column, oral, and pedal disc diameters, maximum length and width of the body, and number of tentacles) were obtained from fixed specimens. Histological sections 5-10 µm thick were made from 1-2 specimens from each species (except for C. tricolor due to poor preservation), and stained with hematoxylin and eosin (Estrada-Flores et al., 1982). Squash preparations of small amounts of tissue from tentacles, actinopharynx, mesenterial filaments, column, marginal projections, and acontia (if present) were made from at least 1 specimen from each species to observe their cnidae types. Cnidae were examined using a Nikon Eclipse E200 light microscope, and cnidae terminology follows Östman (2000). Specimen identifications are based on Carlgren (1949, 1952), Carlgren and Hedgpeth (1952), González-Muñoz et al. (2012, 2013, 2019), and Daly and den Hartog (2004). We followed the taxonomic classification implemented in Carlgren (1949) with modifications from Rodríguez et al. (2014). Taxa were organized according to their suborder and family and listed in alphabetical order. Voucher specimens of each species were deposited in the zoological collection (YUC-CC) of the Departamento de Zoología, of the Universidad Autónoma de Yucatán (UADY). This study was conducted under SEMARNAT collecting permit SGPA/DGSV/03407/16.
Results
Class Anthozoa
Subclass Hexacorallia
Order Actiniaria Hertwig, 1882
Suborder Enthemonae Rodríguez & Daly, 2014 in Rodríguez et al. (2014)
Superfamily Actinioidea Rafinesque, 1815
Family Actiniidae Rafinesque, 1815
Actinostella flosculifera (Le Sueur, 1817)
(Fig. 2A-P)
Actinia flosculifera Le Sueur (1817)
Metridium praetextum: Couthouy in Dana, 1846
Actinostella Formosa [sic]: Duchassaing, 1850
Oulactis flosculifera: Milne-Edwards, 1857
Oulactis conquilega: Duchassaing & Michelotti, 1860
Oulactis Flosculifera [sic]: Duchassaing, 1870
Evactis flosculifera: Andres, 1883
Oulactis foliosa: Andres, 1883
Oulactis fasciculata: McMurrich, 1889a
Asteractis n. sp.: Duerden, 1897
Asteractis expansa: Duerden in McMurrich, 1898
Cradactis fasciculata: Haddon, 1898
Asteractis flosculifera: Verrill, 1899
Actinactis flosculifera: Verrill, 1900
Actinostella flosculifera: McMurrich, 1905
Actinostella conchilega: McMurrich, 1905
Phyllactis flosculifera: Stephenson, 1922
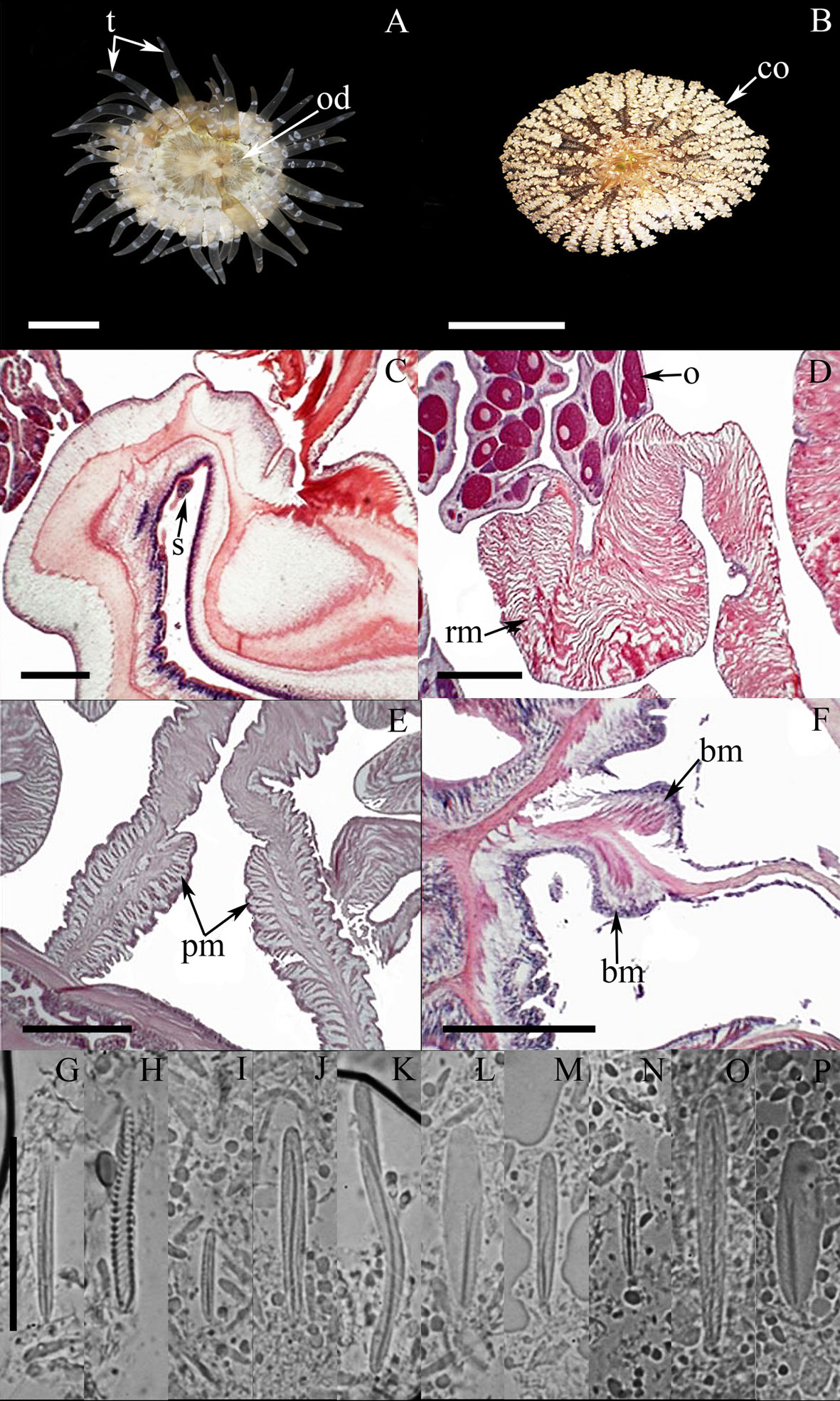
External anatomy. Oral disc flat, 21-31 mm in diameter, beige to dark-brown or light gray, sometimes with white spots; mouth whitish or beige (Fig. 2A). Margin with a collar (also called marginal ruff) formed by 48 rows of small frond-like fused papillae, located in the distal end of the column and surrounding the oral disc (Fig. 2B); collar green to brown or beige, sometimes with dark-red or pink spots. Tentacles smooth, conical, tapering distally, moderately long, contractile, hexamerously arranged in 4 cycles (between 44-48 in number), with the internal cycles longer than outer ones, white, translucent, and with small circular spots on its oral face (Fig. 2A). Column cylindrical, smooth, 20-27 mm in height and 21-34 mm in diameter, with longitudinal rows of small verrucae distally. Pedal disc well-developed, circular. Pedal disc and column beige to light-yellow, translucent when fully expanded.
Internal anatomy. Mesenteries hexamerously arranged in 3 cycles (24 pairs in the specimen examined), first and second cycles perfect, third imperfect; same number of mesenteries distally and proximally. Gametogenic tissue (i.e., oocytes) observed the strongest mesenteries of the first and second cycle, except the directives. Two pairs of directive mesenteries each attached to a well-developed siphonoglyph (Fig. 2C). Retractor muscles strong and restricted (Fig. 2D); parietobasilar muscles well-developed with a free mesogleal pennon (Fig. 2E). Basilar muscles well-developed (Fig. 2F). Marginal sphincter circumscribed. Longitudinal muscles of tentacles ectodermal. Zooxanthellate.
Cnidom. Basitrichs, microbasic b– and p-mastigophores, and spirocysts (Fig. 2G-P). For further information on the size-ranges of cnidae, see González-Muñoz et al. (2012).
Taxonomic summary
Material examined: 26 specimens (YUC-CC-250-9055-19056, 19104-19111, 19232-19246).
Distribution. This species is mainly distributed in the Western Atlantic, from Bermuda to Brazil, and along the Gulf of Mexico and Caribbean Sea (González-Muñoz et al., 2012), but has also been reported from the Canary Islands (Ocaña & den Hartog, 2002) and the Gulf of Guinea (Wirtz, 2003). Actinostella flosculifera has been previously recorded in the rocky intertidal shore and coral reefs from Veracruz (Vassallo et al., 2014; De la Cruz-Francisco & González-Muñoz, 2019), the Campeche Bank (González-Muñoz et al., 2013) and the Mexican Caribbean (González-Muñoz et al., 2012). Here we document for the first time its presence at the coastal localities of Chelem, Yucalpetén, Telchac, Chabihau, and Dzilam de Bravo.
Remarks
The taxonomic characteristics observed in the specimens examined of A. flosculifera agree well with those reported for the species (González-Muñoz et al., 2012; Schlenz & Belém, 1992). In addition, we note the presence of microbasic b-mastigophores (Fig. 2O) in the mesenteric filaments (as is usually observed in other Actiniidae) as well as large curved basitrichs in the actinopharynx (Fig. 2K), that were not previously reported for this species.
Anemonia sargassensis Hargitt, 1908
(Fig. 3A-S)
Anemonia sargassensis Hargitt, 1908
Anemonia antilliensis: Pax, 1924
Anemonia sargassiensis [sic]: Carlgren, 1949
Anemonia melanaster: den Hartog, 1995
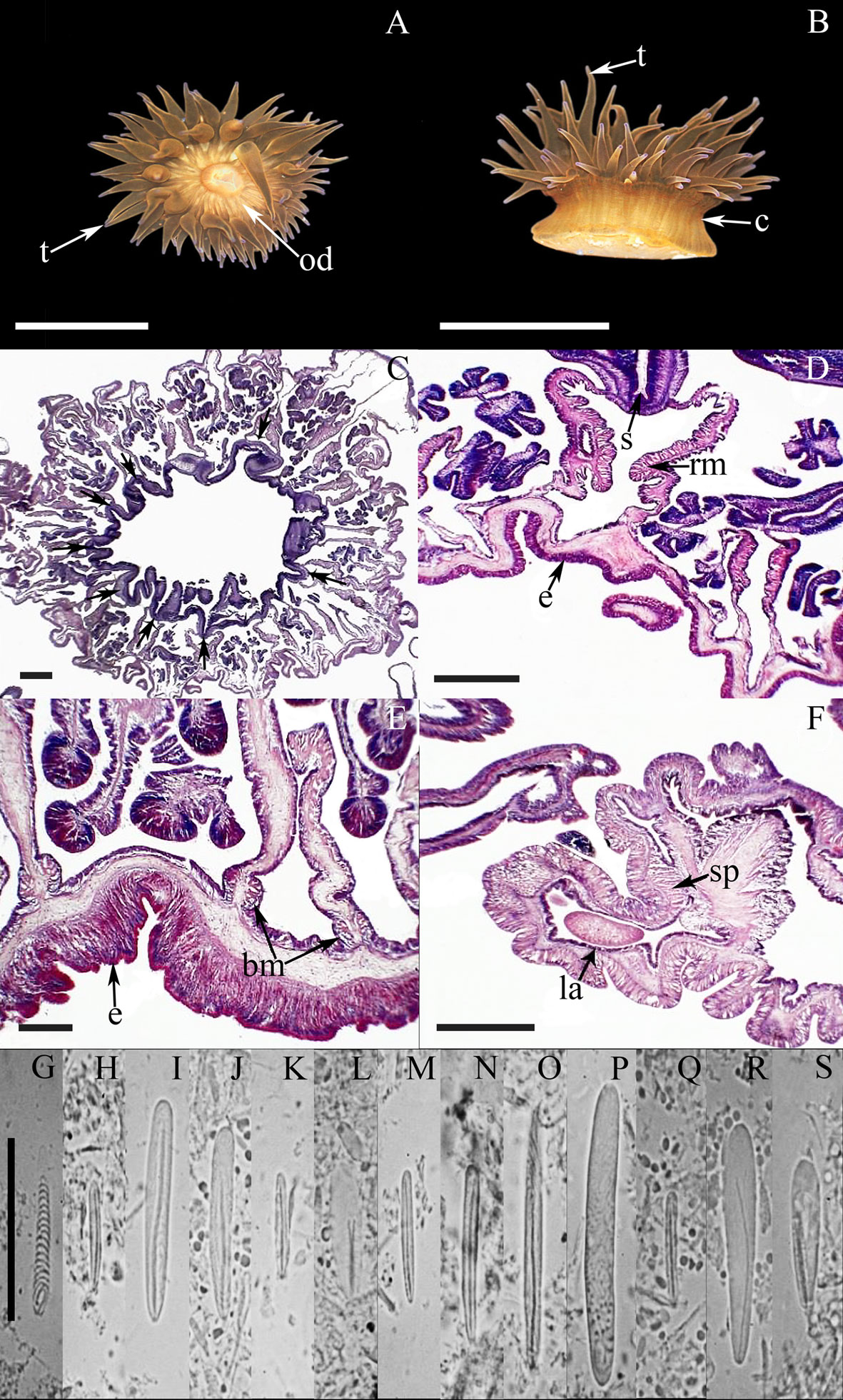
External anatomy. Oral disc flat, wide, 6-14 mm in diameter in specimens examined, dark-orange, dark-red, light-yellow, dark-brown or beige, with white or yellowish endocoelic radial stripes from the base of the tentacles to the mouth (Fig. 3A); mouth yellowish, red, brown or purple. A ring of soft, yellowish or whitish marginal projections with acrorhagi (containing holotrichs and basitrichs) surrounding the oral disc. A shallow fosse is present between the oral disc and the margin of the column. Tentacles smooth, thin, slender, tapering distally, contractile, irregularly arranged in 4-5 cycles (47-111 in number), inner cycles longer than outer ones, dark-red to light-yellow or dark-brown, sometimes with the tips with purple or pink flashes. Column cylindrical, short, cup-shaped, smooth, 3-6 mm in height and 5-11 mm in diameter, bright-red, light-yellow or dark-brown (Fig. 3B). Pedal disc well-developed, circular, wider than column, yellowish to beige.
Internal anatomy. Mesenteries irregularly arranged in 4 cycles (45-52 pairs). Directive mesenteries absent; 5-8 siphonoglyphs observed, each one attached to a regular pair of mesenteries (Fig. 3C). Gametogenic tissue not observed; some larvae observed in the coelenteron. Retractor muscles diffuse to restricted (Fig. 3D); parietobasilar muscles well-developed with a short mesogleal lamella. Basilar muscles well-developed (Fig. 3E). Marginal sphincter muscle endodermal, weak and diffuse (Fig. 3F). Longitudinal muscles of tentacles ectodermal. Zooxanthellate.
Cnidom. Basitrichs, microbasic b– and p-mastigophores, holotrichs, and spirocysts (Fig. 3G-S). For further information on the size-ranges of cnidae see González-Muñoz et al. (2013).
Taxonomic summary
Material examined: 30 specimens (YUC-CC-250-9061-19068, 19112-19121, 19220-19231).
Distribution. Anemonia sargassensis is distributed in the Western Atlantic, from the northern coast of United States to Brazil, and along the Gulf of Mexico and Caribbean Sea (González-Muñoz et al., 2012). This species has been documented in the Mexican Atlantic from coral reefs of Veracruz (De la Cruz-Francisco & González-Muñoz, 2019), the Campeche Bank (González-Muñoz et al., 2013), and the Mexican Caribbean (González-Muñoz, Simões et al., 2015), but this is its first report from Yucatán, at the localities of Chelem and Chabihau.
Remarks
All the taxonomic characteristics observed in A. sargassensis fit well with those previously described, including the presence of more than 2 siphonoglyphs and the absence of directive mesenteries (González-Muñoz et al., 2013). The irregularities of the hexameral pattern are due to asexual reproduction by longitudinal fission, which are commonly reported for this species (Carlgren & Hedgpeth, 1952; González-Muñoz et al., 2013).
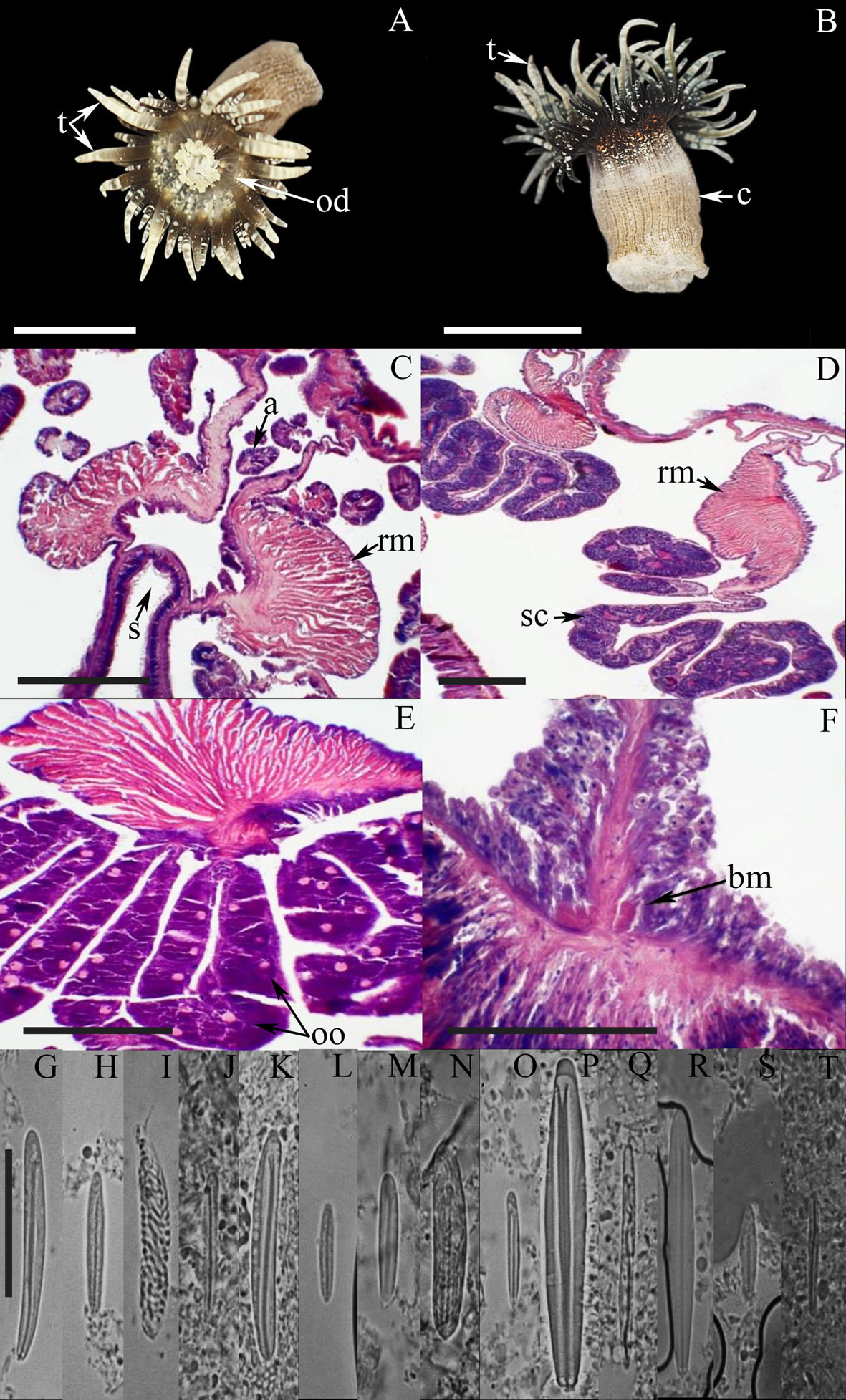
Anthopleura dalyae González-Muñoz, Garese & Acuña, 2018
(Fig. 4A-S)
non Anthopleura varioarmata: Watzl, 1922
? Anthopleura vario-armata [sic]: Carlgren, 1952
Anthopleura varioarmata: Belém & Monteiro, 1981
Anthopleura variarmata [sic]: Zamponi et al., 1998
Anthopleura texaensis: González-Muñoz, Tello-Musi & Simões, 2015
External anatomy. Oral disc smooth and wide, 4-6 mm in diameter, olive-green, light-green to dark-green, or yellowish with white radial stripes (Fig. 4A); mouth dark-green or dark-brown. A ring of rounded marginal projections with acrorhagi in the fosse (containing holotrichs and basitrichs) surrounding the oral disc. A deep fosse is present between the oral disc and the margin of the column. Tentacles smooth, slender, conical, relatively short, tapering distally, contractile, irregularly arranged in 4-5 cycles (41-76 in number), inner cycles longer than outer ones, gray, light-yellow, olive-green to dark-green, with white spots on the oral side and along their entire length (Fig. 4A). Column cylindrical, stout, 6-17 mm in height and 5-12 mm in width, with longitudinal rows of verrucae from margin to limbus but more pronounced distally, olive-green to dark-green, yellowish, pale-orange or whitish (Fig. 4B-C). Pedal disc well-developed, slightly wider than column, beige, and yellowish to white.
Internal anatomy. Mesenteries irregularly arranged in 3-4 cycles (19-31 pairs): first, second, and some of the third cycle perfect, others imperfect; same number of mesenteries distally and proximally. Gametogenic tissue not observed. 2 pairs of directive mesenteries each attached to a well-developed siphonoglyph (Fig. 4D). Retractor muscles strong and restricted; parietobasilar muscles well-developed with a short mesogleal pennon (Fig. 4D). Basilar muscles well-developed (Fig. 4E). Marginal sphincter muscle ectodermal, strong and circumscribed (Fig. 4F). Longitudinal muscles of tentacles ectodermic.
Cnidom. Basitrichs, microbasic b– and p-mastigophores, holotrichs, and spirocysts (Fig. 4G-R). For further information on cnidae size-ranges see González-Muñoz et al. (2019).
Taxonomic summary
Material examined: 18 specimens (YUC-CC-250-19069-19071, 19137-19140, 19247-19257).
Distribution. This species has been reported in Brazil and from the coast of Veracruz, Mexico (Belém & Monteiro, 1981; González-Muñoz et al., 2019), but this is the first record of A. dalyae from Yucatán, at the locality of Chabihau.
Remarks
Taxonomic characteristics observed fit well with those previously described for A. dalyae (González-Muñoz et al., 2019) including the number of tentacles and restricted rather than diffused retractor muscles as in the species Anthopleura texaensis (Carlgren & Hedgpeth, 1952) (Daly & den Hartog, 2004). In addition, we add the presence of microbasic b-mastigophores in the actinopharynx (Fig. 4K) to the cnidom of this species.
Anthopleura krebsi (Duchassaing & Michelotti, 1860)
(Fig. 5A-U)
Anthopleura Krebsi [sic] Duchassaing & Michelotti, 1860
Anthopleura Krebsii [sic]: Duchassaing & Michelotti, 1864
Bunodes Krebsi [sic]: Duerden, 1897
Bunodactis stelloides carneola: Verrill, 1907
Anthopleura varioarmata: Watzl, 1922
Anthopleura kerbsi: Bigger, 1980
Anthopleura carneola: McCommas, 1983
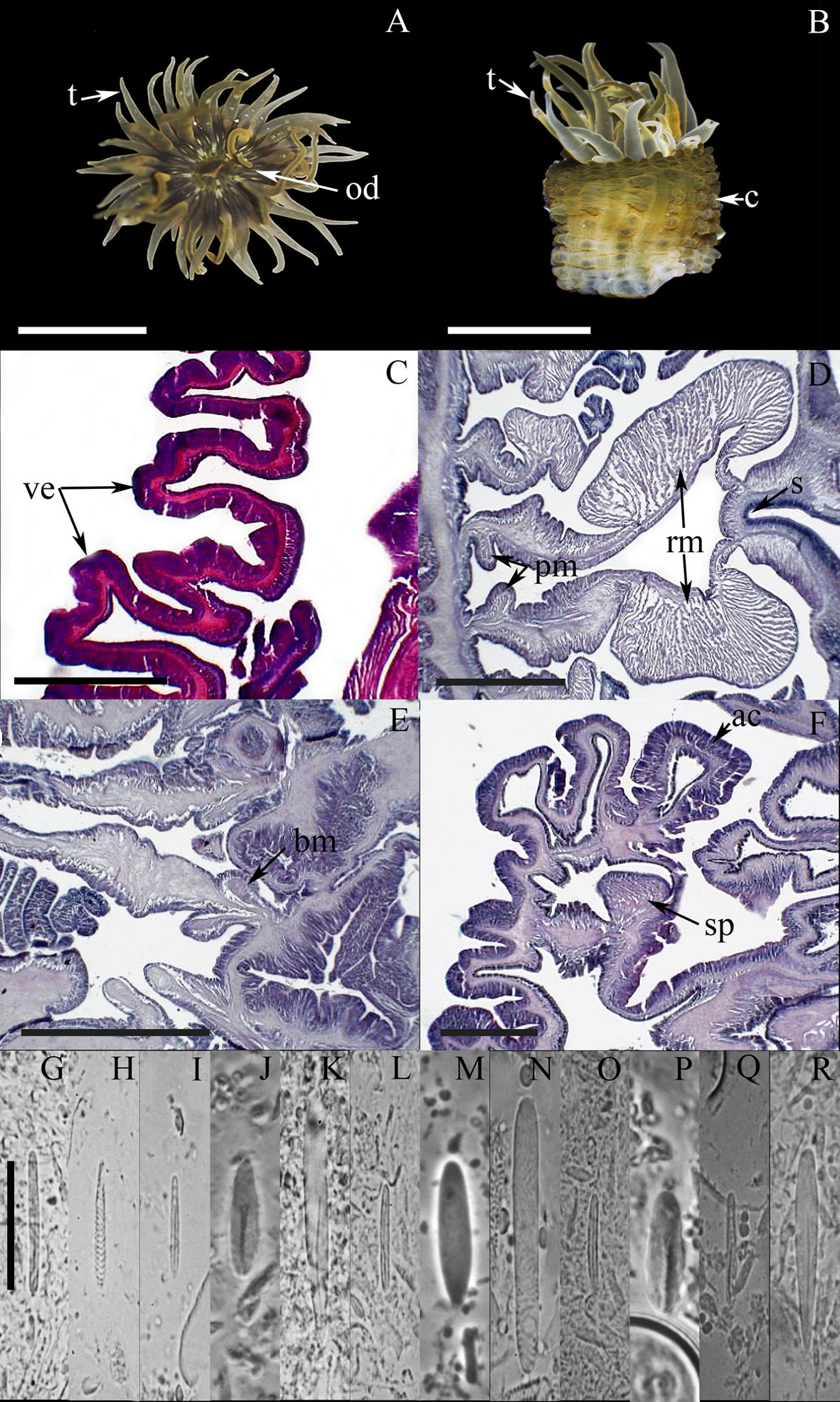
External anatomy. Oral disc smooth, narrow, 5-7 mm in diameter, beige to dark-brown, dark-gray, or olive-green, sometimes with white flashes; mouth whitish or beige (Fig. 5A). A ring of rounded endocoelic marginal projections with acrorhagi in the fosse (containing holotrichs and basitrichs) surrounding the oral disc. A deep fosse is present between the oral disc and the margin of the column. Tentacles smooth, slender, conical, relatively short, tapering distally, contractile, irregularly arranged in 3-4 cycles (19-45 in number), inner cycles longer than outer ones, gray, yellowish to white color, translucent, with oral white spots along their entire length (Fig. 5A-B). Column cylindrical, sometimes trumped-shape, 3-10 mm in height and 3-7 mm in diameter, with longitudinal rows of verrucae from margin to limbus but more pronounced distally, pale-pink with a distinctive bright-red to bright-pink dot on each verrucae (Fig. 5B). Pedal disc well-developed, slightly wider than column, pale-pink to beige.
Internal anatomy. Mesenteries irregularly arranged in 2-3 cycles: first and part of the second cycle perfect, others imperfect. Gametogenic tissue not observed. Directive mesenteries and siphonoglyphs variable in number, 1-4 in specimens examined. Retractor muscles diffuse to restricted (Fig. 5C-D); parietobasilar muscles well-developed with a short mesogleal lamella (Fig. 5C-D). Basilar muscles well-developed (Fig. 5E). Marginal sphincter muscle strong and circumscribed (Fig. 5F). Longitudinal muscles of tentacles ectodermal. Zooxanthellate.
Cnidom. Basitrichs, microbasic b– and p-mastigophores, holotrichs, and spirocysts (Fig. 5G-T). For further information on cnidae size-ranges see Daly and den Hartog (2004).
Taxonomic summary
Material examined: 27 specimens (YUC-CC-250-19072-19082, 19127-19136, 19258-19263).
Distribution. Anthopleura krebsi is distributed in the Western Atlantic, from the Gulf of Mexico and the Caribbean Sea to Brazil (González-Muñoz et al., 2012). In Mexico this species has been previously reported from Veracruz (De la Cruz-Francisco & González-Muñoz, 2019; González-Muñoz, Tello-Musi et al., 2015), and the Mexican Caribbean (INE, 1998), but this is its first record from Yucatán, at the locality of Chabihau.
Remarks
The taxonomic characteristics agree well with those reported for A. krebsi, including the pattern of coloration of column and verrucae, number of tentacles, and cnidom (Belém & Pinto, 1990; Daly & den Hartog, 2004). In addition, we report the presence of microbasic b-mastigophores in the actinopharynx (Fig. 5I); however, we do not observe the small microbasic b-mastigophores in the mesenteric filaments as documented by Daly and den Hartog (2004).
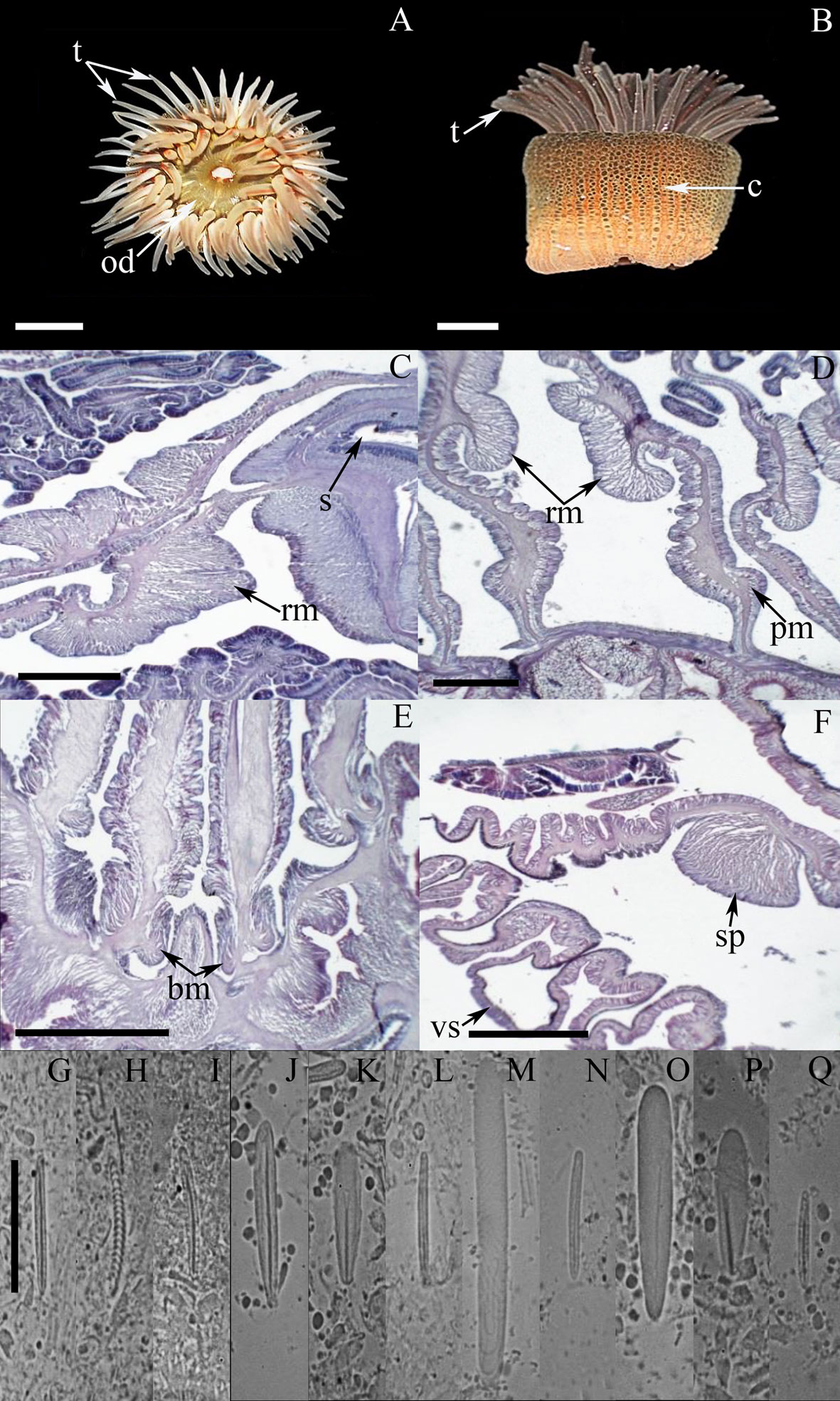
Bunodosoma cavernatum (Bosc, 1802)
(Fig. 6A-Q)
Actinia cavernata Bosc, 1802
Actinia (Monostephanus) cavernata: Brandt, 1835
Urticina cavernata: Duchassaing, 1850
Bunodes cavernata: Verrill, 1864
Phymactis cavernata: Andres, 1883
Bunodosoma cavernata: Verrill, 1899
Anthopleura cavernata: Cary, 1906
Bunodosma [sic] cavernata: Daly, 2003
External anatomy. Oral disc flat, wide and smooth, 8-28 mm in diameter, olive-green, beige or pale-red, with whitish or yellowish radial stripes from tentacles to mid oral disc on endocoelic spaces of the first and second cycles; mouth reddish (Fig. 6A). A ring of rounded marginal projections with acrorhagi (containing holotrichs and basitrichs) surrounds the oral disc. A deep fosse is present between the oral disc and the margin of the column. Tentacles smooth, conical, tapering distally, moderately long, contractile, irregularly arranged in 5 cycles (71-98 in number), internal cycles longer than outer ones, grey, greyish-red or beige, with a red stripe in the aboral side on the tentacles of the inner cycles (never observed in the last cycle of tentacles) (Fig. 6A, B). Column cylindrical, 16-44 mm in length and 14-31 mm in width, densely covered by rounded vesicles arranged in longitudinal rows alternating between large and small vesicles, which are beige or greyish with dark-brown or black in the center each vesicle (Fig. 6B).
Internal anatomy. Mesenteries hexamerously arranged in 4 cycles (48 pairs): first, second, and part of the third cycle perfect, others imperfect; same number of mesenteries distally and proximally. Gametogenic tissue (i.e., spermatic cysts) observed in the strongest mesenteries of the first, second, and third cycle (except the directives). 2 pairs of directive mesenteries each attached to a well-developed siphonoglyph (Fig. 6C). Retractor muscles strong and restricted; parietobasilar muscles well-developed with a free mesogleal pennon (Fig. 6D). Basilar muscles well-developed (Fig. 6E). Marginal sphincter muscle endodermic, strong and circumscribed (Fig. 6F). Longitudinal muscles of tentacles ectodermal. Zooxanthellate.
Cnidom. Basitrichs, microbasic b– and p-mastigophores, holotrichs, and spirocysts (Fig. 6G-Q). For further information on the size-ranges of cnidae, see González-Muñoz et al. (2013).
Taxonomic summary
Material examined: 9 specimens (YUC-CC-250-19188 -19205).
Distribution. Bunodosoma cavernatum is distributed in the Western Atlantic, from the northern coast of United States to the Gulf of Mexico and Caribbean Sea (González-Muñoz et al., 2012, 2013, 2016), and in the Caroline Islands, Micronesia (Bosc, 1802). This species has been reported in coral reefs of Veracruz (De la Cruz-Francisco & González-Muñoz, 2019; González-Muñoz et al., 2013), but this is its first report from Yucatán, at the localities of Chabihau and Dzilam de Bravo.
Remarks
All taxonomic features agree well with those previously reported for B. cavernatum, except for the presence of 2 cnidae size categories of basitrichs in the column, while only one category is clearly distinguishable in the specimens examined. In addition, we found 2 size categories of basitrichs in the actinopharynx (Fig. 6I-J), which were not observed by González-Muñoz et al. (2013).

Bunodosoma granuliferum (Le Sueur, 1817)
(Fig. 7A-R)
Actinia granulifera Le Sueur, 1817
Urticina Lessoni [sic]: Duchassaing, 1850
Oulactis granulifera: Milne-Edwards, 1857
Urticina granulifera: Duchassaing & Michelotti, 1860
Cereus Lessoni [sic]: Duchassaing & Michelotti, 1860
Anthopleura granulifera: Duchassaing & Michelotti, 1864
Anthopleura Granulifera [sic]: Duchassaing, 1870
Aulactinia granulifera: Andres, 1883
Bunodes taeniatus: McMurrich, 1889b
Bunodes granulifera: Duerden, 1897
Bunodosoma granulifera: Verrill, 1899
Bunodosoma granuliferum: Pax, 1910
Phymactis granulifera: Stephenson, 1922
External anatomy. Oral disc flat, wide and smooth, 13-43 mm in diameter, variable in coloration, from white with yellow or greenish to dark-brown, sometimes with whitish of yellowish radial stripes marking the endocoelic spaces; mouth dark-brown, reddish, orange or yellow (Fig. 7A). A ring of rounded marginal projections with acrorhagi (containing holotrichs and basitrichs) surrounds the oral disc. A deep fosse is present between the oral disc and the margin of the column. Tentacles smooth, conical, tapering distally, moderately long, contractile, mostly hexamerously arranged in 5 cycles (83-101 in number), internal cycles longer than outer ones, olive-green, gray, white and translucent, with oral white spots and purple flashes at the tips (Fig. 7A-B). Column cylindrical, 12-24 mm in length and 19-33 mm in width in specimens examined, densely covered by rows of rounded vesicles arranged in 24 alternating dark and light longitudinal bands, which are orange, green, gray or light-pink (Fig. 7B-C). Pedal disc well-developed, cylindrical, orange, olive-green or gray.
Internal anatomy. Mesenteries hexamerously arranged in 4 cycles (48 pairs): first, second, and part of the third cycle perfect, others imperfect; same number of mesenteries distally and proximally. Gametogenic tissue (i.e., spermatic cysts) observed in the strongest mesenteries of the last 2 cycles. 2 pairs of directive mesenteries each one attached to a well-developed siphonoglyph. Retractor muscles strong and restricted; parietobasilar muscles well-developed with a free mesogleal pennon (Fig. 7D). Basilar muscles well-developed (Fig. 7E). Marginal sphincter muscle endodermic, strong and circumscribed (Fig. 7F). Longitudinal muscles of tentacles ectodermal. Zooxanthellate.
Cnidom. Basitrichs, microbasic b– and p-mastigophores, holotrichs, and spirocysts (Fig. 7G-R). For further information on the size-ranges of cnidae see González-Muñoz et al. (2012).
Taxonomic summary
Material examined: 15 specimens (YUC-CC-250-19052, 19122-19126, 19179-19187).
Distribution. This species is distributed in the Western Atlantic, from Bermuda to Brazil, and along the Gulf of Mexico and Caribbean Sea (González-Muñoz et al., 2012). In Mexico, B. granuliferum has been reported from Veracruz (De la Cruz-Francisco & González-Muñoz, 2019; González-Muñoz, Tello-Musi et al., 2015; Vassallo et al., 2014), the Campeche Bank (González-Muñoz et al., 2013), and the Mexican Caribbean (González-Muñoz et al., 2012), but this is its first report from Yucatán, at the localities of Chelem and Chabihau.
Remarks
All the taxonomic characteristics of the specimens examined of B. granuliferum agree well with those reported for the species, including the pattern of column coloration and the number of tentacles, except that we observe only 1 size category of basitrichs in the actinopharynx and column, instead of 2 as reported by González-Muñoz et al. (2012). In addition, we added the presence of microbasic b-mastigophores in the actinopharynx and filaments (Fig. 7K), as well as small basitrichs in filaments (Fig. 7R), to the cnidom of the species.
Superfamily Metridioidea Carlgren, 1893
Family Aiptasiidae Carlgren, 1924
Exaiptasia diaphana (Rapp, 1829)
(Fig. 8A-T)
Actinia diaphana Rapp, 1829
Cribina diaphana: Deshayes & Milne-Edwards, 1840
Actinia elongata Delle-Chiaje, 1841
Adamsia diaphana: Milne-Edwards, 1857
Dysactis pallida: Agassiz in Verrill, 1864
Bartholomea tagetes: Duchassaing & Michelotti, 1864
Bartholomea inula: Duchassaing & Michelotti, 1864
Dysactis mimosa: Duchassaing & Michelotti, 1864
Dysactis minuta: Verrill, 1867
Paranthea minuta: Verrill, 1868
Paranthea pallida: Verrill, 1868
Disactis mimosa [sic]: Duchassaing, 1870
Aiptasia saxicola: Andres, 1881
Aiptasia diaphana: Andres, 1883
Aiptasia Agassizii [sic]: Andres, 1883
Aiptasia inula: Andres, 1883
Aiptasia minuta: Andres, 1883
Aiptasia mimosa: Andres, 1883
Aiptasia tagetes: Andres, 1883
Aiptasia pallida: McMurrich, 1887
Aiptasia leiodactyla: Pax, 1910
Aiptasioides pallida: Stephenson, 1918
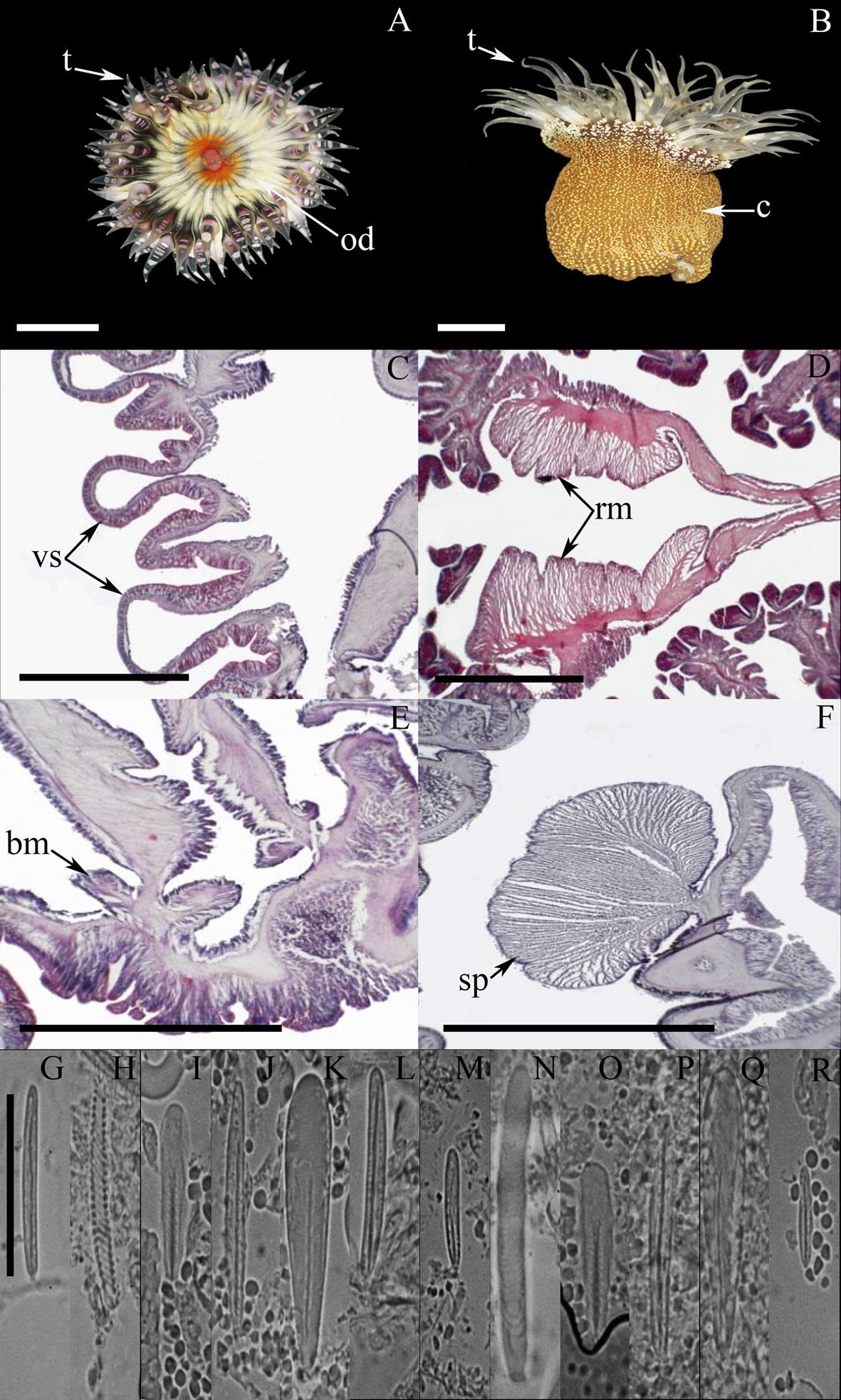
Aiptasiomorpha diaphana: Stephenson, 1920
Aiptasiomorpha leiodactyla: Stephenson, 1920
Aiptasia insignis: Carlgren, 1941
Aiptasia pulchella: Carlgren, 1943
Aiptasia californica: Carlgren, 1952
Aiptasiomorpha minuta: Uchida & Soyama, 2001
Aipstasia pulchella [sic]: Reimer et al., 2007
Exaiptasia pallida: Grajales & Rodríguez, 2014
External anatomy. Oral disc smooth, 1-18 mm in diameter, olive-green to beige, more or less translucent with whitish or yellowish scattered dots; mouth slit-like, white, yellowish or beige (Fig. 8A). Column cylindrical, trumped-shaped when fully expanded, smooth, 2-19 mm in height and 1-20 mm in diameter, divided in capitulum and scapus: capitulum short, beige to dark-brown with whitish, yellowish, or pale-orange spots; scapus long, beige, translucent, with the mesenterial insertions visible (Fig. 8B). 2-3 rings of cinclides around the mid part of scapus. Tentacles smooth, slender, relatively short, irregularly arranged in 3-5 cycles (23–113 in number), inner cycles longer than outer ones, olive-green, yellowish, whitish, dark-brown, beige, or black (Fig. 8A-B). Pedal disc well-developed, beige, translucent. White acontia (with microbasic p-mastigophores and basitrichs).
Internal anatomy. Mesenteries irregularly arranged in 2-4 cycles (about 12-48 pairs in specimens examined): first cycle perfect, others imperfect. Gametogenic tissue (oocysts and spermatic cysts) was observed in the strongest mesenteries of the first, second, and third cycle, except the directives (Fig. 8D-E). 2 pairs of directive mesenteries each one attached to a well-developed siphonoglyph (Fig. 8C). Retractor muscles diffuse to restricted (Fig. 8E); parietobasilar muscles poorly-developed. Basilar muscles well-developed (Fig. 8F). Marginal sphincter muscles mesogleal, short and diffuse. Longitudinal muscles of tentacles ectodermal. Zooxanthellate.
Cnidom. Basitrichs, microbasic b-mastigophores and p-amastigophores, and spirocysts (Fig. 8G-T). For further information on cnidae size-ranges see Carlgren (1952), González-Muñoz et al. (2012), and Grajales and Rodríguez (2014).
Taxonomic summary
Material examined: 27 specimens (YUC-CC-250-19057-19060, 19095-19103, 19206-19219).
Distribution. This species is distributed mainly in the Western Atlantic, from the northeast coast of the United States to Brazil, along the Gulf of Mexico and Caribbean Sea, but it has also been reported from the east and west coasts of the Pacific, Japan, Hawaii, and the islands of Santa Helena (Grajales & Rodríguez, 2014). Exaiptasia diaphana has been previously reported from Veracruz (González-Muñoz, Tello-Musi et al., 2015), the Campeche Bank (González-Muñoz et al., 2013), and the Mexican Caribbean (González-Muñoz et al., 2012), but this is its first report from the coast of Yucatán, at the localities of Chabihau, Yucalpetén, Telchac, and Chelem.
Remarks
All taxonomic characteristics of the examined specimens of E. diaphana are consistent with those reported for the species (González-Muñoz et al., 2012; Grajales & Rodríguez, 2014), including the pattern of cinclides in the column, the pattern of coloration, and cnidae. In addition, we note that the cnidocysts of the column identified as basitrich by González-Muñoz et al. (2012: fig. 9M) corresponds to a microbasic b-mastigophore (Fig. 8N).
Family Hormathiidae Carlgren, 1932
Calliactis tricolor (Le Sueur, 1817)
(Fig. 9A-B)
Actinia tricolor: Le Sueur, 1817
Actinia bicolor: Le Sueur, 1817
Cereus bicolor: Milne-Edwards, 1857
Adamsia tricolor: Milne-Edwards, 1857
Adamsia Egletes [sic]: Duchassaing & Michelotti, 1864
Adamsia egletes: Duchassaing & Michelotti, 1866
Calliactis bicolor: Verrill, 1869
Adamsia sol: McMurrich, 1893
Adamsia bicolor: Andres, 1883
Adamsia tricolor: Andres, 1883
Calliactis tricolor: Haddon, 1898
External anatomy. Oral disc smooth, wide, 5-30 mm in diameter in specimens examined, white to gray with purple flashes; mouth slit-like in shape, with bright-yellow or bright-orange lips, and with a purple or bright-pink ring around the mouth (Fig. 9A). Tentacles smooth, conical, relatively short, contractile, tapering distally, hexamerously arranged in 4-6 cycles (48-192 in number), translucent, beige, with oral white spots along their entire length, sometimes with pink rings distally (Fig. 9A-B). Column cylindrical or trumped-shaped, divided in capitulum and scapus: capitulum very short, smooth, beige to dark-brown; scapus stout, wrinkled in appearance, pale-red to dark-red, beige to dark-brown, or pale-orange. One or 2 rings of dark-red or brown cinclides present in the proximal part of the column, near to the limbus (Fig. 9B). Pedal disc well-developed, wider than column, beige to dark-brown, translucent with the mesenterial insertions and acontia visible.
Internal anatomy. See González-Muñoz et al. (2013).
Cnidom. See González-Muñoz et al. (2013).

Taxonomic summary
Material examined: 5 specimens (YUC-CC-250-
19090-19094).
Distribution. Calliactis tricolor is distributed in the Western Atlantic, from North Carolina to the north coast of Brazil, and along the Gulf of Mexico and Caribbean Sea (Tello-Musi et al., 2018). This species has been previously reported in the Mexican Atlantic, from Veracruz (Tello-Musi et al., 2018), and the Campeche Bank (González-Muñoz et al., 2013), but this is the first report from Yucatán, at the locality of Dzilam de Bravo.
Remarks
All the taxonomic characteristics of the external anatomy of the specimens examined agree well with those reported for the species (González-Muñoz et al., 2013), including the pattern of coloration of the column, cinclides and acontia, and the number of tentacles.
Discussion
Among the species found in the sampled locations, A. flosculifera and E. diaphana are the most widely distributed, being both found in almost all the places visited. Moreover, the nearest previous record of these 2 species in the region, as well as for C. tricolor, is in Madagascar reef (González-Muñoz et al., 2013), located about 20 km from Chelem beach. Actinostella flosculifera is often found in habitats associated with shallow seagrasses and sandy bottoms, and E. diaphana is frequently found attached to small rocks and submerged wood, or even on sponges, between patches of sand and seagrass (González-Muñoz et al., 2012), which are common environments in much of the Yucatán coastal area (Herrera-Silveira et al., 2013). The species C. tricolor is usually found attached to gastropod mollusk shells occupied by hermit crabs (Tello-Musi et al., 2018). However, this species was found attached to buried hard substrate in the intertidal zone of Dzilam de Bravo.
The species A. dalyae, A. krebsi, A. sargassensis, B. cavernatum, and B. granuliferum were found attached to rocks or submerged remains of old constructions that serve these animals as hard substrates. This artificial substrate, such as bricks or concrete remains of the breakwater constructions, as well as the rubble of old summer houses that were affected by the loss of beaches and hurricane events, facilitate the settlement of the sea anemones and other benthic invertebrates in the area. The nearest report of A. sargassensis and B. granuliferum is from Alacranes reef, located approximately 130 km north from the coast of Yucatán (González-Muñoz et al., 2013). The species A. dalyae, A. krebsi, and B. cavernatum were previously recorded in Mexico at the Veracruz Reef System (González-Muñoz, Tello-Musi et al., 2015), but these are their first records in Yucatán.
González-Muñoz et al. (2013) documented the presence of 13 species of sea anemones in coral reefs of Yucatán, 5 of which are also reported here as inhabitants in the coastal area. The other 8 species are documented so far only in coral reef environments in the region (Table 1). The new records of A. dalyae, A. krebsi, and B. cavernatum increase to 16 the number of known species of sea anemones in the region. These species do not represent all species found in Yucatán, but they are those for which there are no unresolved taxonomic problems, and that are more abundant and conspicuous. In addition, we observed and collected some individuals identified so far as Diadumene sp., but their complete taxonomic identification is still in progress.
Table 1
List of sea anemones species reported from the coast and coral reefs of Yucatán. Citation: 1) González-Muñoz et al. (2013). The symbol “*” indicates a new record for the locality, and “†” indicates new records for Yucatán.
|
Species |
Chelém |
Yucalpetén |
Telchac |
Chabihau |
Dzilam de Bravo |
Bajo de Diez |
Madagascar |
Serpientes |
Alacranes |
|
|
1 |
Actinostella flosculifera (Le Sueur, 1817) |
* |
* |
* |
* |
* |
1 |
1 |
||
|
2 |
Anemonia sargassensis Hargitt, 1908 |
* |
* |
1 |
||||||
|
3 |
Anthopleura dalyae González-Muñoz, Garese & Acuña, 2018 † |
* |
||||||||
|
4 |
Anthopleura krebsi (Duchassaing & Michelotti, 1860) † |
* |
||||||||
|
5 |
Anthopleura pallida Duchassaing & Michelotti, 1864 |
1 |
||||||||
|
6 |
Bartholomea annulata (Le Sueur, 1817) |
1 |
1 |
1 |
1 |
|||||
|
7 |
Bunodeopsis antilliensis Duerden, 1897 |
1 |
||||||||
|
8 |
Bunodosoma cavernatum (Bosc, 1802) † |
* |
* |
|||||||
|
9 |
Bunodosoma granuliferum (Le Sueur, 1817) |
* |
* |
1 |
||||||
|
10 |
Calliactis tricolor (Le Sueur, 1817) |
* |
1 |
1 |
||||||
|
11 |
Condylactis gigantea (Weinland, 1860) |
1 |
1 |
1 |
||||||
|
12 |
Exaiptasia diaphana (Rapp, 1829) |
* |
* |
* |
* |
1 |
1 |
1 |
||
|
13 |
Laviactis lucida (Duchassaing & Michelotti, 1860) |
1 |
||||||||
|
14 |
Lebrunia neglecta (Duchassaing & Michelotti, 1860) |
1 |
||||||||
|
15 |
Phymanthus crucifer (Le Sueur, 1817) |
1 |
1 |
|||||||
|
16 |
Stichodactyla helianthus (Ellis, 1768) |
1 |
The documentation of the sea anemones species in the coastal areas of Yucatán is an important step to inventory the actinofauna in Mexico, as well as to study the biological and ecological role of these animals in coastal ecosystems.
Acknowledgements
Thanks to Leopoldina Aguirre Macedo, laboratory of Aquatic Pathology of the Center for Research and Advanced Studies of the IPN, unit Mérida. We also thank Candita, M. Euán Canul, and Magnolia del C. Tzec Gamboa for technical assistance.
References
Belém, M. J. C., & Monteiro, D. C. (1981). Fauna de cnidários do Rio de Janeiro. III. Anthopleura varioarmata Watzl, 1922 (Actiniaria, Endomyaria), uma nova ocurrência de actiniidae. Seminários de Biologia Marinha, Academia Brasileira de Ciências, Rio de Janeiro, 193–203.
Belém, M. J. C., & Pinto, S. M. (1990). Morphological and microanatomical study of Anthopleura krebsi Duchassaing & Michelotti, 1860 (Cnidaria, Anthozoa, Actiniidae), a new record in Brazil. Anais da Academia Brasileira de Ciências (Rio de Janeiro), 62, 183–192.
Bosc, L. A. G. (1802). Historie Naturalle des Vers. Paris: Chez Deterville.
Carlgren, O. (1949). A survey of the Ptychodactiaria, Corallimorpharia and Actiniaria (Cnidaria: Anthozoa). Kunglia Svenska Veteskaps Akademiens Handlingar, 1, 1–121.
Carlgren, O. (1952). Actiniaria from North America. Arkiv für Zoology, 3, 373–390.
Carlgren, O., & Hedgpeth, J. W. (1952). Actiniaria, Zoantharia and Ceriantharia from shallow water in the northwestern Gulf of Mexico. Publications of the Institute of Marine Science, University of Texas, 2, 143–172.
Daly, M., & den Hartog, J. C. (2004). Taxonomy, circumscription, and usage in Anthopleura (Cnidaria: Anthozoa: Actiniaria) from the Gulf of Mexico and the Caribbean. Bulletin of Marine Science, 74, 401–421.
Daly, M., Chaudhuri, A., Gusmão, L., & Rodríguez, E. (2008). Phylogenetic relationships among sea anemones (Cnidaria: Anthozoa: Actiniaria). Molecular Phylogenetics and Evolution, 48, 292–301. https://doi.org/10.1016/j.ympev.2008.02.022
De la Cruz-Francisco, V., & González-Muñoz, R. (2019). Las anémonas marinas de los sistemas arrecifales de Veracruz. En A. Granados-Barba, L. Ortiz-Lozano, C. González-Gandara y D. Salas-Monreal (Eds.), Estudios científicos en el Corredor Arrecifal del Suroeste del Golfo de México (pp.103–116). Campeche: Universidad Autónoma de Campeche (en prensa). https://doi.org/10.26359/epomex0319
Estrada-Flores, E., Peralta, L., & Rivas, P. (1982). Manual de técnicas histológicas. México D.F.: AGT Press.
González-Muñoz, R., Garese, A., & Acuña, F. H. (2019). Anthopleura dalyae sp. nov. (Cnidaria: Actiniaria), a new species of sea anemone from the southern Gulf of Mexico. Marine Biodiversity, 49, 683–690. https://doi.org/10.1007/s12526-018-0844-2
González-Muñoz, R., Simões, N., Guerra-Castro, E., Hernández-Ortiz, C., Carrasquel, G., Méndez, E. et al. (2016). Sea anemones (Cnidaria: Actiniaria, Corallimorpharia, Ceriantharia, Zoanthidea) from marine shallow.water enviroments in Venezuela: new records and an updated inventory. Marine Biodiversity Records, 9, 18. https://doi.org/10.1186/s41200-016-0016-7
González-Muñoz, R., Simões, N., Sánchez-Rodríguez, J., Rodríguez, E., & Segura, L. (2012). First inventory of sea anemones (Cnidaria: Actiniaria) of the Mexican Caribbean. Zootaxa, 3556, 1–38. http://dx.doi.org/10.11646/zootaxa.3556.1.1
González-Muñoz, R., Simões, N., Tello-Musi, J. L., & Rodríguez, E. (2013). Sea anemones (Cnidaria, Anthozoa, Actiniaria) from coral reefs in the southern Gulf of Mexico. ZooKeys, 341, 77–106. http://doi.org/10.3897/zookeys.341.5816
González-Muñoz, R., Simões, N., Tello-Musi, J. L., Sánchez-Rodríguez, J., & Rodríguez, E. (2015). New records of sea anemones (Cnidaria, Anthozoa, Actiniaria) in the Mexican Caribbean. Marine Biodiversity Records, 8, e100. https://doi.org/10.1017/S1755267215000767
González-Muñoz, R., Tello-Musi, J. L., & Simões, N. (2015). Las anémonas del Sistema Arrecifal Veracruzano. En A. Granados-Barba, L. Ortíz-Lozano, D. Salas-Monreal y C. González-Gándara (Eds.), Aportes al conocimiento del Sistema Arrecifal Veracruzano: hacia el Corredor Arrecifal del Suroeste del Golfo de México (pp. 101–118). Campeche: Universidad Autónoma de Campeche.
Grajales, A., & Rodríguez, E. (2014). Morphological revision of the genus Aiptasia and the family Aiptasiidae (Cnidaria, Actiniaria, Metridiodea). Zootaxa, 3826, 55–100. http://dx.doi.org/10.11646/zootaxa.3826.1.2
Hernández-Flores, A., Condal, A., Poot-Salazar, A., & Espinoza-Méndez, J. C. (2015). Geostatistical analyses and spatial modeling of population density for the sea cucumbers Isostichopus badionotus and Holothuria floridana on the Yucatán Peninsula, Mexico. Fisheries Research, 172, 114–124. https://doi.org/10.1016/j.fishres.2015.07.005
Hernández-Flores, A., Cuevas-Jiménez, A., Poot-Salazar, A., Condal, A., & Espinoza-Méndez, J. C. (2017). Bioeconomic modeling for a small-scale sea cucumber fishery in Yucatán, Mexico. Plos One, 13, e0190857. https://doi.org/10.1371/journal.pone,0190857
Herrera-Silveira, J. A., Comin, F. A., & Capurro-Filograsso, L. (2013). Landscape, land-use, and management in the Coastal Zone of Yucatán Peninsula. In J. W. Day, & A. Yáñez-Arancibia (Eds.), Gulf of Mexico: origin, waters, and biota. Volume 4, Ecosystem-based management (pp. 225–242). Harte Research Institute for Gulf of Mexico Studies Series. Corpus Christi: Texas A&M University Press.
INE (Instituto Nacional de Ecología). (1998). Programa de manejo Parque Marino Nacional Costa Occidental de Isla Mujeres, Punta Cancún y Punta Nizuc. Ciudad de México: Instituto Nacional de Ecología.
Kuk-Dzul, J. G., Solís-Marín, F. A., Herrera-Dorantes, M. T., & Ardisson, P. L. (2019). Brittle stars (Echinodermata: Ophiuroidea) of coastal lagoons from the northern Yucatán Peninsula, México. Revista Mexicana de Biodiversidad, 90, e9026982. https://doi.org/10.22201/ib.20078706e.2019.90.2698
May-Kú, M. A., Criales, M. M., Montero-Muñoz, J. L., & Ardisson, P. L. (2014). Differential use of Thalassia testudinum habitats by sympatric penaeids in a nursery ground of the southern Gulf of Mexico. Journal of Crustacean Biology, 34, 144–156. https://doi.org/10.1163/1937240X-00002214
May-Kú, M. A., & Ordóñez-López, U. (2006). Spatial patterns of density and size structure of penaeid shrimps Farfantepanaeus brasiliensis and Farfantepanaeus notialis in a hypersaline lagoon in the Yucatán Peninsula, México. Bulletin of Marine Science, 79, 259–271.
Ocaña, O., & den Hartog, J. C. (2002). A catalogue of actiniaria and corallimorpharia from the Canary Islands and from Madeira. Arquipélago. Life and Marine Sciences, 19A, 33–54. http://hdl.handle.net/10400.3/165
Östman, C. (2000). A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Scientia Marina, 64, 31–46. https://doi.org/10.3989/scimar.2000.64s131
Palomino-Álvarez, L. A., Moreira-Rocha, R., & Simões, N. (2019). Checklist of ascidians (Chordata, Tunicata) from the southern Gulf of Mexico. Zookeys, 832, 1–33. https://doi.org/10.3897/zookeys.832.31712
Pech, D., & Ardisson, P. L. (2010) Diversidad en el bentos marino-costero. En R. Durán y M. Méndez (Eds.), Biodiversidad y desarrollo humano en Yucatán. Mérida: CICY/ PPD-FMAM/ CONABIO/ SEDUMA.
Pech, D., Ardisson, P. L., & Hernández-Guevara, N. A. (2007). Benthic community response to habitat variation: A case of study from a natural protected area, the Celestún coastal lagoon. Continental Shelf Research, 27, 2523–2533. https://doi.org/10.1016/j.csr.2007.06.017
Ríos-Lara, G. V., Zetina-Moguel, C. E., Sánchez-Molina, I., Peniche-Ayora, J. I., Medina-González, R., Espinoza-Méndez, J. C. et al. (2010). Caracterización del hábitat de juveniles de langosta Panulirus argus en la costa central (Dzilam de Bravo) del estado de Yucatán, México. Proceedings of the 63rd Gulf and Caribbean Fisheries Institute, 462–470.
Rodríguez, E., Barbeitos, M. S., Brugler, M. R., Crowley, L. M., Grajales, A., Gusmão, L. et al. (2014). Hidden among sea anemones: the first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. Plos One, 9, e96998. https://doi.org/10.1371/journal.pone. 0096998
Schlenz, E., & Belem, M. (1992). Phyllactis correae n. sp. (Cnidaria, Actiniaria, Actiniidae) from Atol Das Rocas, Brazil, with notes on Phyllactis flosculifera (Le Sueur, 1817). Boletim da Universidade de São Paulo, 12, 91–117.
Tello-Musi, J. L., González-Muñoz, R., Acuña, F. H., & Simões, N. (2018). First record of Calliactis tricolor (Le Sueur, 1817) (Cnidaria, Actiniaria, Hormathiidae) in the Veracruz reef system, southwestern Gulf of Mexico. Check List, 14, 619–631. https://doi.org/10.15560/14.4.619
Vassallo, A., Dávila, Y., Luviano, N., Amozurrutia, S. D., Vital, X. G., Conejeros, C. A. et al. (2014). Inventory of invertebrates from the rocky intertidal shore at Montepio, Veracruz, Mexico. Revista Mexicana de Biodiversidad, 85, 349–362. https://doi.org/10.7550/rmb.42628
Wakida-Kusunoki, A., Rojas-González, R. I., Toro-Ramírez, A., Medina-Quijano, H. A., Cruz-Sánchez, J. L., Santana-Moreno, L. D. et al. (2016). Caracterización de la pesca de camarón en la zona costera de Campeche y Yucatán. Ciencia Pesquera, 24, 3–13.
Wirtz, P. (2003). New records of marine invertebrates from São Tomé Island (Gulf of Guinea). Journal of the Marine Biological Association of the United Kingdom, 83, 735–736. https://doi.org/10.1017/S0025315403007720h