Diana A. Ahuatzin a, Wesley Dáttilo a, Fernando Escobar-Hernández a, Albina Demeza b, Lucrecia Arellano a, *
a Instituto de Ecología, A.C., Red de Ecoetología, Carretera Antigua a Coatepec Núm. 351, 91073 Xalapa, Veracruz, Mexico
b Reserva Ecológica Comunitaria Bajlum Pakal, Ejido Nueva Betania, Carretera Fronteriza Sur, a 55 km de Palenque, 29963 Palenque, Chiapas, Mexico
*Corresponding author: lucrecia.arellano@inecol.mx (L. Arellano)
Received: 1 December 2021; accepted: 28 November 2022
Abstract
Despite the relevance of community ecological reserves, little is known about changes in spatio-temporal biodiversity patterns in these reserves. Here, we analyzed the spatio-temporal dynamics of the dung beetle assemblages in 4 different environments (primary forest, riparian vegetation, secondary forest, and pasture) in the Bajlum Pakal Community Ecological Reserve, in Chiapas, Mexico. We found that the spatial dynamics showed differences among the analyzed environments, with higher diversity in the tropical rainforest and riparian vegetation. The temporal dynamics did not present a relationship with the richness and diversity of species; therefore, spatial dynamics were more important for explaining the diversity of beetles from all environments. When evaluating the spatial turnover, we found that the most important component of β diversity was β2 (between environments), which suggests that the environmental variation provided by the great spatial heterogeneity of the environments modulates the diversity patterns of beetles in this reserve. Conservation strategies must consider changes in the spatio-temporal dynamics that modulate species assemblages to determine their responses to changes in land use. It is important to maintain the existence of community ecological reserves that conserve biodiversity and connectivity in tropical landscapes, as well as the quality of life of its inhabitants.
Keywords: Biodiversity; Chiapas; Rural reserves; Land-use; Seasonality; Tropical rainforest; Species turnover
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Variación espacio-temporal del ensamble de escarabajos coprófagos (Coleoptera: Scarabaeidae) de una reserva ecológica comunitaria del sureste de México
Resumen
A pesar de la importancia de las reservas ecológicas comunitarias, se sabe poco sobre los patrones espacio-temporales de su biodiversidad. Analizamos la dinámica espacio-temporal de los ensambles de escarabajos coprófagos en 4 ambientes (bosque tropical húmedo, vegetación riparia, bosque secundario y pastizal) en la Reserva Ecológica Comunitaria Bajlum Pakal, en Chiapas, México. Encontramos una mayor diversidad en el bosque tropical húmedo y en la vegetación riparia. En contraste, la dinámica temporal no presentó relación con la riqueza y diversidad de especies, por lo que la dinámica espacial fue más importante para explicar la diversidad de escarabajos coprófagos en todos los ambientes. Encontramos que el componente más importante de la diversidad β espacial fue β2 (entre ambientes), lo que sugiere que la heterogeneidad espacial de los ambientes modula los patrones de diversidad de escarabajos en esta reserva. Las estrategias de conservación deben considerar los cambios en la dinámica espacio-temporal de la diversidad ya que modulan la respuesta de los ensambles de especies a los cambios en el uso del suelo. Es importante mantener la existencia de reservas ecológicas comunitarias pues conservan la biodiversidad y la conectividad en los paisajes tropicales, así como la calidad de vida de sus habitantes.
Palabras clave: Biodiversidad; Chiapas; Reservas rurales; Uso del suelo; Estacionalidad; Selva tropical; Recambio de especies
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
In tropical regions, rural communities have established actions to conserve and manage their natural resources, creating ecological community reserves that function as reservoirs of local biodiversity. They include strategies and practices that have been carried out in many regions worldwide since ancient times as part of a vision of rustic use-conservation (Bezaury-Creel & Gutiérrez-Carbonell, 2009; Boege, 2008; Halffter, 2005; Toledo & Barrera-Bassols, 2008). For instance, rural communities can plan the management of their lands, plan their future use, maintain their economic potential, and at the same time promote the conservation of natural resources. Moreover, some activities can be developed in ecological community reserves, such as forest exploitation, UMA (i.e., Units for Conservation, Management, and Sustainable Use of Wildlife), nurseries, ecotourism, coffee plantations, aquaculture, agriculture, and livestock, if the activities are focused on sustainability and trying to minimize the environmental impacts that could be generated. However, converting tropical forest into different land uses generates environmental variations that affect spatial and temporal biodiversity patterns and species distributions (Foley et al., 2005; Tilman, 2017) in different ways (Foley et al., 2005; Gibson et al., 2011; Reiners et al., 1994; Tilman, 2017).
In general, conversion and degradation of forests reduce the probability of population persistence by modifying the capacity of habitats to maintain their biodiversity (Barlow et al., 2007; Fahrig, 1997, 2003; Gibson et al., 2011; Nichols et al., 2007; Regolin et al., 2020). Although there is evidence of the negative impacts of different land uses on biodiversity (McGill, 2015; Newbold et al., 2015), it is necessary to consider the study of temporal changes in species richness and diversity in human-modified systems as seasonal fluctuations in resource availability, which are related to human activities, may also alter species-habitat relationships (Yabuhara et al., 2019; Yeiser et al., 2021). In fact, understanding temporal dynamics is important because it is highly related to species phenological, physiological, and behavioral changes (Leong et al., 2016). Therefore, knowing the magnitude of spatio-temporal variation of species diversity could improve our understanding of the dynamics of species assemblages and their role in maintaining biodiversity.
In ecological community reserves, spatio-temporal patterns of biodiversity could be at risk due to land use changes and other local activities. The use of bioindicator species can help us evaluate the importance and quality of rural nature reserves for the conservation of native flora and fauna (Adrián, 2021; de la Vega et al., 2014; León-Espinosa & Mujica, 2018; Vidaurre et al., 2008). In this sense, dung beetles (Coleoptera: Scarabaeidae) are excellent focal organisms for studying the effects of anthropogenic disturbances on diversity and ecosystem function in tropical regions (Escobar et al., 2008; Favila & Halffter, 1997; Slade et al., 2011). These organisms use decomposing organic matter for food and reproduction and provide various ecosystem services through their removal and burial of matter (e.g., incorporating nutrients into soil, bioturbation, indirect control of flies and livestock parasites, and secondary seed dispersal) (Alvarado et al., 2019; Nichols et al., 2008). Moreover, dung beetles are highly sensitive to environmental disturbances and are used as indicators of natural or anthropogenic environmental disturbances in tropical forests (Filgueiras et al., 2011, 2015; Favila & Halffter, 1997). Studies have shown that vegetation structure, elevation, atmospheric temperature, relative humidity, soil type, and habitat quality can influence the species occurrence and abundance of dung beetles (Joaqui et al., 2021; Pinto-Leite et al., 2018). Moreover, we have evidence that fragmentation and habitat loss are important processes that shape dung beetle diversity patterns and body condition (Escobar et al., 2008; Nichols et al., 2007; Salomão et al., 2018). Such studies have found a general pattern in which there is a high loss of species as habitat disturbance increases (e.g., Damborsky et al., 2015; De Juan & Hewit, 2014; Escobar et al., 2008; Filgueiras et al., 2015; Frank et al., 2017; Gómez-Cifuentes et al., 2017; Howden & Howden, 2001; Keyton et al., 2016; Quintero & Halffter, 2009). In addition, studies evaluating the temporal changes in the richness and diversity of species of dung beetles in tropical environments have found that there is high variation in species composition over time (i.e., species turnover) (Novais et al., 2016). However, this temporal variation tends to be context-dependent, since some studies considered annual changes (Noriega et al., 2021), while others considered the changes between seasons (Costa-Batista et al., 2016; Vernes et al., 2005) or carried out long-term evaluations (Escobar et al., 2008).
Specifically for Mexico, studies dealing with dung beetles in the state of Chiapas have increased considerably in the last 15 years (Rodríguez-López et al., 2019). Most of the studies analyzed the composition and structure of the assemblages in federally protected natural areas and their zones of influence, mainly from the Lacandon rainforest region (Navarrete & Halffter, 2008a; Sánchez-de Jesús et al., 2016; Santos-Heredia et al., 2018), and the Zoque rainforest region (Arellano et al., 2008, 2013; Blas-López & Gómez, 2009; Gómez et al., 2017; Sánchez-Hernández et al., 2018), while others provided new geographic distribution data or descriptions of new species (Chamé-Vázquez & Gómez, 2005; Gómez & Chamé-Vázquez, 2003; Halffter & Halffter, 2009; Morales et al., 2004; Navarrete & Halffter, 2008b; Sánchez-Hernández & Gómez, 2018; Sánchez-Hernández et al., 2017). Nevertheless, there are still extensive areas of the Chiapas territory that are little explored, among which are most of the state-protected natural areas (Rodríguez-López et al., 2019) and community ecological reserves stand out. For these reasons, evaluating spatio-temporal patterns can be decisive for the development of more efficient management and conservation strategies (Correa et al., 2021).
In this study, we analyzed the spatial and temporal changes in the diversity of dung beetle species in 4 different environments (primary forest, secondary forest, riparian forest, and pasture for livestock use) in the Bajlum Pakal Community Ecological Reserve, Chiapas, southeastern Mexico. We postulated that changes in the original vegetation (i.e., humid tropical forest) to other land uses (i.e., secondary vegetation, remnants of disturbed riparian vegetation, and grasslands) would negatively influence the richness and species composition. Specifically, we expected that there would be higher species diversity in more conserved environments (i.e., primary forest) compared to environments with more habitat disturbance (i.e., secondary forest, riparian forest, and pasture). This should occur because primary forest presents a higher habitat quality and resource availability, allowing the coexistence of different trophic guilds characterized by species with high habitat specialization, while in mostly disturbed sites, there is a dominance of generalist and opportunistic beetle species in terms of habitat and resource use (Filgueiras et al., 2015, 2016; Kenyon et al., 2016). Moreover, we expected that changes in species composition would be related to monthly variation in local activities and climatic and environmental conditions throughout a sampling year, with a higher species number in the warmer and rainy months (March-October). This is because dung beetles in the region tend to be more active from the first rains until the end of summer when there is a greater availability of high nutritional quality resources compared to other colder seasons (Estrada et al., 1993; Flota-Bañuelos et al., 2012; Kaur et al., 2021; Morón-Ríos & Morón, 2016).
Materials and methods
This study was carried out in the Bajlum Pakal Community Ecological Reserve (BPCER), which is located 6 km south of the town of Nueva Betania, Municipality of Palenque, Chiapas, Mexico (17°16’48.09” N, 91°39’11.96” W), with an elevational range between 156 and 260 m asl. The predominant climate is hot and humid with rain in the summer accompanied by an annual average temperature of 26 °C and a rainfall of 2,156 mm per year. May is the warmest month (29.7 °C) and January is the coldest (22.4 °C) (García, 1988). The dominant vegetation in the BPCER was high evergreen forest; however, given deforestation and the change in land use over time, there is a mosaic of different plant associations (primary forest, secondary forest, remnants of riparian vegetation, lands under cultivation, and pastures for livestock). Evergreen forest (in different conservation states) occupies approximately 50% of the area surrounding the reserve, and the rest is occupied by cattle ranches, corn, and rural areas dedicated to ecotourism.
Currently in BPCER, evergreen forest and riparian forest have mainly been modified to open roads and rustic stops for ecotourism tours, generally preserving the tropical forest, but changing the understory and the herbs. Moreover, about 33% of the reserve has historically been used for livestock and agriculture, but some of those spaces have been abandoned and now are secondary forest.
The Nueva Betania community was founded in 1969 and is mainly inhabited by indigenous Choles people who have created an alternative tourism center based in Community Based Tourism (Palomino et al., 2007). The REBP maintains the connectivity of the tropical rainforest due to its strategic location. It is a part of one of the most important tourist corridors in Chiapas, connecting with Bonampak, Yaxchilán, and the Lacandon Jungle. The reserve was decreed to conserve the high evergreen forest in the communal lands that have not been used due to their topographic conditions and difficult access, so that the remnants of the original forest are regenerated and for the natural reintroduction of species (CDI, 2010).
Beetle samplings were carried out every month from March 2015 to February 2016. Within the reserve, 4 types of environments and 3 sampling points were used: primary forest (represents the native forest cover), secondary forest (forest in an advanced regeneration stage enriched with fruit trees), remnants of riparian vegetation (cover constituted by arboreal vegetation located on the margins of water courses), and pasture (areas destined for cattle ranching). At each sampling point we established 2 transects with 6 baited fall traps (Halffter & Arellano, 2002), each 500 m apart. We placed the traps 50 m away from each other (Larsen & Forsyth, 2005), and they were alternately baited with 60 g of human excreta, bovine manure, and decomposing fish to record a greater number of copronecrophagous beetles. The use of multiple attractants allowed us to determine the largest number of species and trophic guilds associated with the use of the resource (Cajaiba et al., 2017). We checked the traps after 48 h of exposure and the organisms within them were collected. This methodology was repeated every month for a year. Organisms were identified at the species level, and the material was deposited in the Red de Ecoetología of Instituto de Ecología, A.C. (Mexico). We included all species of recorded copronecrophagous beetles.
We estimated the completeness of the beetle species inventory as the sample coverage for each type of environment. The sample coverage represents the proportion (with respect to all individuals in the community) that constitutes the individuals of the species collected in the sample (Hsieh et al., 2016). Subsequently, we evaluated the diversity of beetles in each environment using the method proposed by Jost (2006), which recognizes “true diversity” through the effective number of species. Specifically, this index measures the diversity that a community composed of i equally common species would have. The effective number of species is calculated using the equation:
where qD is diversity, pi is the proportion of individuals in the total sample that belongs to species i, and q is a constant that determines the influence of common and rare species on true diversity. The exponent q = 0 is completely insensitive to the species abundances, and therefore it is the number of species present, while values of q < 1 overestimate rare species and q = 1 indicates that all species are included with a weight exactly proportional to their abundance in the community. The values of q > 1 consider the most common species (Moreno et al., 2011). All indices were obtained through the “vegan” package (Oksanen et al., 2017) in the R statistical program (R Core Team, 2021).
The graphical representation of the species composition by each environment and the sites (3 sampling units by environment) was carried out using a non-metric multidimensional scaling analysis (NMDS). This type of ordering analysis is one of the most robust and often summarizes more information in fewer axes than other techniques (Legendre & Legendre, 1998). A distance matrix (4 sites [12 rows] × species [37 columns]) was used with the Bray-Curtis dissimilarity index as a measure. This index is a measure of dissimilarity between communities that allows for the evaluation of differences in terms of abundance and species composition. This analysis was carried out with the R program (R Core Team, 2021) using the “vegan” package (“metaMDS” function) (Oksanen et al., 2017). To evaluate whether the groupings observed in the NMDS present differences in community structure, we performed a multivariate analysis of variance with 999 permutations (PERMANOVA) using the “vegan” package (“adonis” function) (Oksanen et al., 2017). Subsequently, PERMDISP analyses were performed to verify species homogeneity and comparability using the “betadisper” function in the R program (R Core Team, 2021).
We evaluated the relationship between species richness (q0) and Shannon diversity (q1) and time (months of the year) using linear mixed effects models (LMMs). In these models, we used observations each month as an independent variable and the species richness and Shannon diversity as dependent variables, with type of environment as a random factor to consider the effects of site. In this way, we were able to assess the temporal variation in species diversity more robustly regardless of the type of environment in which they occur. Due to the nature of the response variables, a Poisson error distribution was considered for species richness, and for the other indices we used a Gaussian error distribution (Guisan et al., 2002). The analyses were performed using the nlme version 3.1-152 (Pinheiro et al., 2022), and lmerTest version 3.1-3 (Kuznetsova et al., 2017) packages. LMMs were performed in R software (R Core Team, 2021).
We evaluated the diversity components to determine the spatial dynamics of the beetle assemblage between environments. To determine the contribution of each sampling point and of the environments to the total diversity (γ), we carried out an additive partition for 3 levels of diversity: average diversity between sampling points (ᾱ), diversity between traps (β1), and diversity between types of environments (β2).
γ = ᾱ+β1+ β2
The observed values were contrasted using a random distribution (n = 999 permutations) generated by null models, where the presence of the species is randomly distributed among the samples using the “adipart” function in the R package “vegan” (Oksanen et al., 2017). The p-value was obtained by comparing the random distribution generated by the null models against the values observed for each of the diversity levels (Crist et al., 2003).
Results
We registered a total of 2,555 individuals belonging to 15 genera and 37 species. Sample coverage indicated that a completeness of almost 99% was obtained for all environments (the riparian forest presented 98% completeness) (Supplementary material: Appendix 1). The most abundant genera were Canthidium (n = 500 individuals, 4 species), Onthophagus (n = 484, 8 species), and Uroxys (n = 412, 3 species). Regarding the species, the most abundant were Copris laeviceps (n = 373 individuals), found mainly in evergreen forest environments and Canthidium pseudopuncticolle (n = 296 individuals) and Onthophagus cyclographus (n = 280 individuals), both mainly associated with disturbed environments (Supplementary material: Appendix 2). There were 6 rare species (< 5 individuals): Anaides laticollis, Anomiopus cirulito, Copris lugubris (evergreen forest), Eurysternus mexicanus and Onthophagus crinitus (secondary forest), and Sisyphus mexicanus (Supplementary material: Appendix 2).
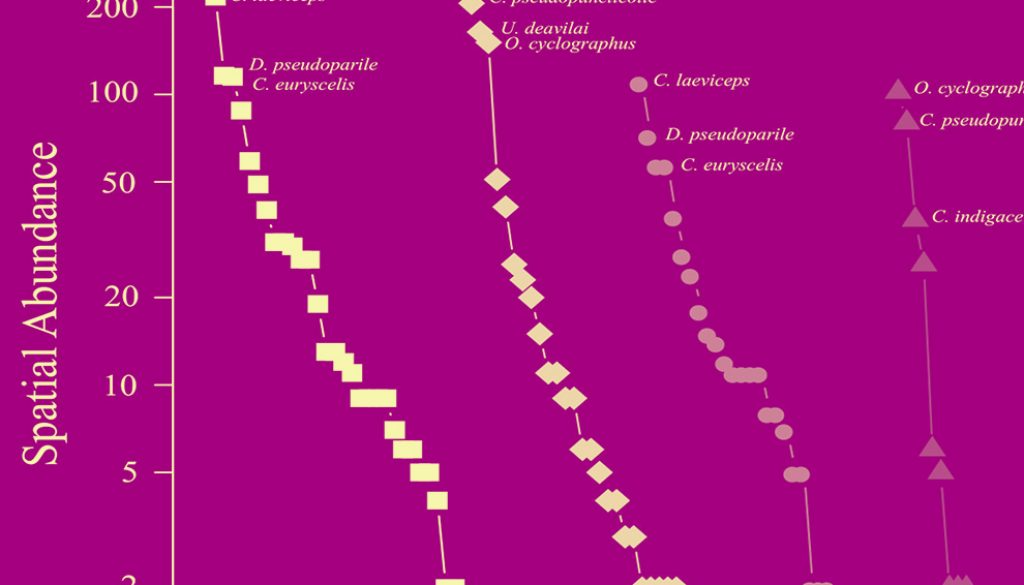
The environments with the highest species richness (q0) were the primary forest (S = 34) and the secondary forest (S = 29). The environments that presented the highest species diversity were the primary forest (q1 = 16.2 species) and riparian vegetation remnants (q1 = 15.2 species). We observed that in the primary forest and remnants of the riparian vegetation, the most abundant species were C. laeviceps, Deltochilum pseudoparile, and Canthon euryscelis. However, even though the most abundant species were similar in the secondary forest and in the pasture, these species change in their hierarchical order (C. pseudopuncticolle and O. cyclographus) according to their abundances (Fig. 1). Canthon indigaceus chiapas was one of the dominant species in the pastures but was not present in the secondary forest. Conversely, Uroxys deavilai was dominant in the secondary forest but not in the pasture.
The months that presented the highest species richness are found in the period from June to September (Fig. 2a), while the highest diversity for most of the environments occurred between June and August (Fig. 2b). The highest abundances were found from January to April. The species that were recorded with the highest abundances in the REBP during all months of the year were Copris laeviceps (n = 373) and Deltochilum pseudoparile (n = 191), which represented mostly conserved areas. Various species had low abundances during the year (fewer than 10 individuals), such as Onthophagus crinitus and Copris luubris, which each only had 1 record in June (Supplementary material: Appendix 3). Other species had high abundances during the cold season, such as C. laeviceps, Bdelyropsis bowditchi, C. pseudoperceptibile, Onthophagus maya, Pseudocanthon perplexus, Uroxys micros, and U. deavilai (Supplementary material: Appendix 3).
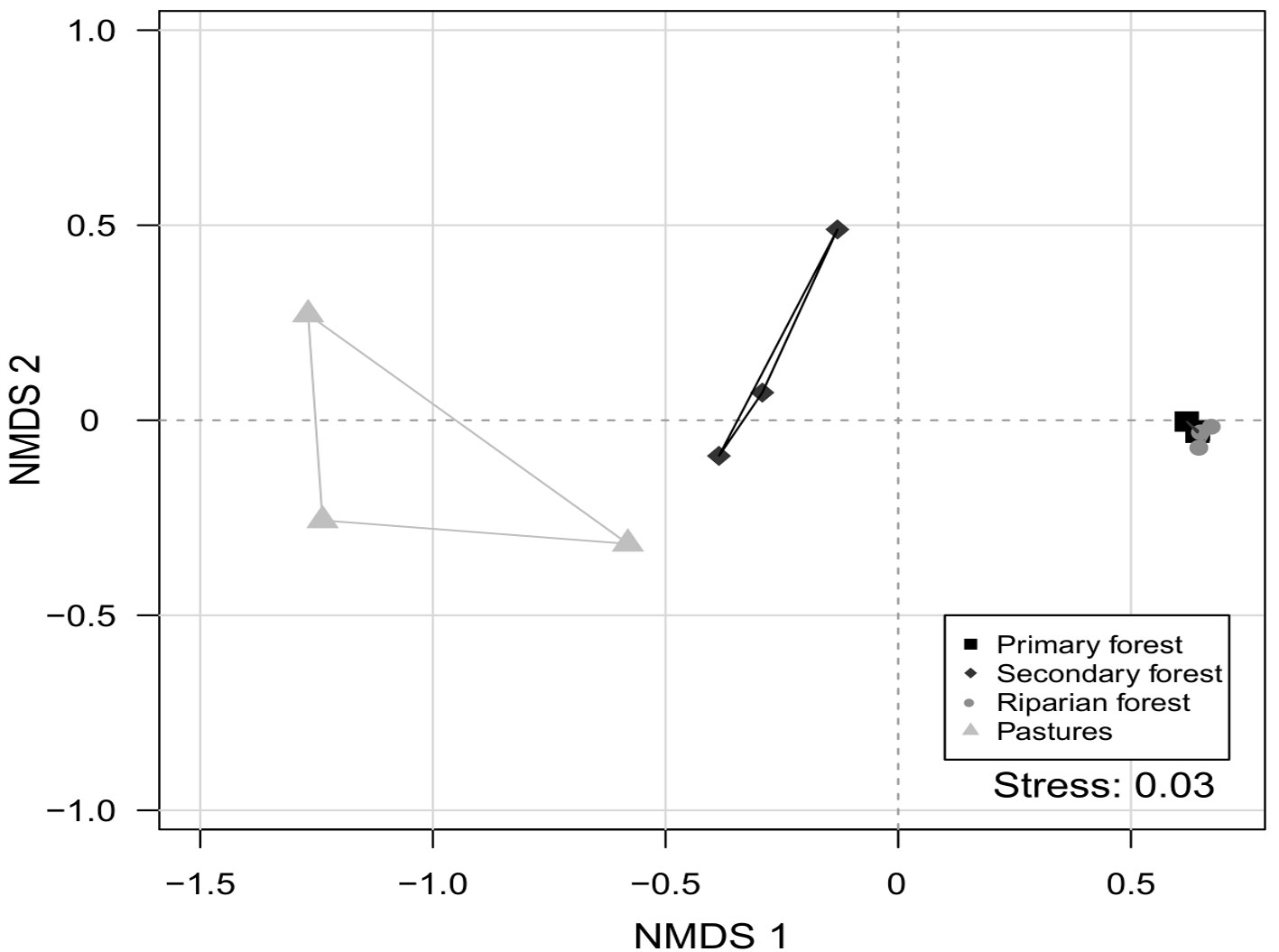
We observed that species composition differed among the 4 studied environments (dimensions = 2, stress value = 0.038; PERMANOVA, p = 0.002) (Fig. 3). This indicates that each environment presents a different species assemblage. In addition, no environment was heterogeneous in species composition (PERMDISP, p = 0.476). This indicates that the difference in species composition is more important than the differences within each environment.
When evaluating the relationship between species richness (q0) and Shannon diversity (q1) with time (months of the year), we found that neither the richness (LMM, χ2 = 0.01, p = 0.90) nor Shannon diversity present differences (LMM, χ2 = 0.12, p = 0.72) changes throughout the year.
Spatial dynamics were more important for explaining species richness and diversity in all environments. We found that the component of dung beetle diversity that explained the highest proportion of gamma diversity (set of total species) was β diversity (55%) (i.e., species turnover between environments). The most important component of β diversity was β2 (between environments) and it did not show differences as expected by chance (p = 0.42), which indicates that the distribution of species is random. The component that had the least importance for gamma diversity was β1 (between traps) as it was even lower than expected by chance (p < 0.05). Land use changes increased the composition dissimilarities between environments, being higher than replacement at the trap level. On the other hand, the diversity ᾱ explained 45% of the gamma diversity and was significantly higher than expected by chance (p < 0.05) (Supplementary material: Appendix 4).

Discussion
Our results indicated that the structure of dung beetle assemblages changed in different environments associated with land use changes in a tropical rainforest in southern Mexico. We found a higher richness and diversity of dung beetle species in more forested environments (i.e., primary forest and riparian forest). When evaluating the effect of the temporal factor, we did not find a relationship between species richness or Shannon diversity and monthly variation. We observed differences in species composition between environments, which could indicate that the type of environment had a greater influence on the patterns of richness and diversity of beetle species. On the other hand, the most important component of β diversity was β2 (between environments), which suggests that the environmental variation given by the high spatial heterogeneity of the different environments modulates the patterns of richness and diversity of beetle species in the Bajlum Pakal Community Ecological Reserve.
Previous research has indicated that geographic location and landscape context appeared to modify dung beetle response under habitat modification and fragmentation scenarios (Nichols et al., 2007). We showed how the spatial factor is important in modulating richness and diversity patterns of dung beetles in a rural reserve. These results are consistent with the general pattern found in some tropical environments, which indicates that species richness and abundance declined with increasing habitat modification (Nichols et al., 2007). The REBP is in an area with some of the greatest biodiversity in America (called Selva Maya), and the area where it is located has few years of occupation and exploitation (a little more than 50 years), which allows it to still preserve an important richness of dung beetles (37 species) despite being in an important tourist corridor. This species richness is slightly greater than the average species richness found in other landscapes with tropical humid forest in Mexico (31 ± 9.85 species): Sian Ka´an, Quintana Roo with 20 species (Morón et al., 1986), Yaxchilán, Chiapas with 25 species (Palacios-Ríos et al., 1990), Boca de Chajul, Chiapas with 27 (Morón et al., 1985) and 49 species (Navarrete & Halffter, 2008a), El Ocote, Chiapas with 29 species (Sánchez-Hernández et al., 2018), Los Tuxtlas, Veracruz with 30 species (Díaz et al., 2010), and Los Chimalapas, Oaxaca with 40 species (Moctezuma, 2019). This highlights the REBP as a great reservoir of local biodiversity in southern Mexico.

In this study we evaluated different measures of taxonomic diversity beyond species richness, including Shannon and Simpson diversities, to evaluate the different responses to land use change. All these biodiversity components are affected negatively by habitat modification, as we found a decline in species richness, diversity, and dominance in more modified environments (pastures). These results are consistent with Nichols et al. (2007), who indicated that some environments such as secondary forests, selectively logged forests, and agroforests supported rich communities with many intact forest species, while cattle pastures and clear-cuts contained fewer species. Furthermore, the spatial dynamics of the dung beetle richness and diversity in the REBP showed differences between the environments, finding the greatest richness and diversity in the primary forest and in the remnants of riparian vegetation, as we expected. It is well-known that habitat conservation status influences the diversity of Scarabaeinae in Neotropical forests, since most of its species have a strong association with humid and shady areas (Halffter, 1991; Favila & Halffter, 1997; Navarrete & Halffter, 2008; Nichols et al., 2007). The most conserved environments such as the tropical rainforest and the remnants of riparian vegetation are characterized by greater forest cover and vegetation complexity (Tews et al., 2004). Increased species richness is often associated with greater habitat complexity, so the microclimatic characteristics of these sites could allow a greater number of species to coexist (Halffter & Arellano, 2002; Navarrete & Halffter, 2008). The sites with evergreen forest (i.e., primary, secondary, and riparian) are preferred by tourism and local inhabitants conserve and protect these areas because they have a higher value than open areas in the reserve.
In addition to the different spatial patterns of richness and diversity of dung beetles, the temporal patterns did not show a relationship with species richness, Shannon diversity, or Simpson diversity during the sampling period, which is contrary to other studies that found changes in the patterns of species richness and diversity over time (Correa et al., 2021; Noriega et al., 2021). We observed the highest abundance from January to April, which coincides with some of the coldest months of the region (Comisión Nacional del Agua, 2019). Species such as Copris laeviceps, Bdelyropsis bowditchi, Canthidium pseudoperceptibile, Onthophagus maya, Pseudocanthon perplexus, Uroxys micros, and U. deavilai (most being nocturnal, according to Demeza’s personal observations) were favored by cold season conditions and because these are the preferred months of tourists, who sometimes defecate in the areas they visit, representing a source of alternative resources for the species.
Changes in temporal patterns have been attributed to various factors, including variation in climatic and environmental conditions and the availability of resources (Ferreira et al., 2019). However, other factors not considered in this study (e.g., different activity patterns of some species, physiological tolerances to certain environmental conditions, and more food availability in months with greater tourist activity) could also influence the presence or absence of species throughout the year in the different study environments (Slade & Roslin, 2016).
Here we showed that the spatial variation in the beetle species had a greater effect due to the change in land use and it is relatively more important than temporal richness and diversity, which suggests that the environmental variation given by the high spatial heterogeneity of the different environments modulates the patterns of richness and diversity of beetle species in the REBP. Land use change involves various processes (i.e., habitat loss and fragmentation) that directly influence the spatial patterns of the diversity of dung beetles (Alonso et al., 2020). We found that the component of beetle diversity that explained the highest proportion of gamma diversity (set of total species) was β diversity, where the most important component of β diversity was β2 (between environments). Accordingly, our results corroborate the idea that species turnover is the main component of spatial β diversity (Ferreira et al., 2019; Soininen et al., 2018). The spatial replacement of species is partially associated with environmental filters generated by the environmental variation of different classes of land use (Filgueiras et al., 2016; Scheffler, 2005). The microclimatic changes (i.e., canopy cover, vegetation structure, pH, environmental humidity, litter volume) in habitat conditions as a consequence of human activity are important factors that modulate the patterns of richness and diversity of beetles that inhabit the soil (Lassau et al., 2005). An example of this are the species with high habitat specialization that were found at more conserved sites of the REBP (i.e., D. pseudoparile), and that have preferences for certain environmental conditions (Campos & Hernández, 2013; Halffter, 1991). Many of these species cannot occupy areas with open vegetation (Almeida et al., 2011; Campos & Hernández, 2013; Spector & Ayzama, 2003), generating changes in the composition of species between environments. In contrast, in mostly disturbed sites such as pastures and secondary forest, we found more species of generalist and opportunistic beetles (Canthidium pseudopuncticolle and Onthophagus cyclographus), which were favored by the environmental conditions that allowed an increase in the abundances of these species (Roslin & Koivunen, 2001). This is a well-known pattern in areas such the REBP where the tropical rain forest dominated and where pastures are historically new environments (little more than 50 years old). However, in some cases the apparent spatial specialization of species may be due to other factors such as low dispersal ability, low availability of mates or resources, restricted distribution in time (certain month or some hours of the day), having a lower fecundity or higher mortality rate (Noriega & Costa, 2011), or simply a sampling bias where commonly used sampling methods may not provide an accurate representation of the dung beetle community (Larsen et al., 2006). Further studies at a larger number of sites are needed to explain the low density of species.
The differences in beetle compositions between the most conserved and the most disturbed environments indicate that the high environmental variation seems to modulate the presence of certain species capable of tolerating the conditions of the different environments. This information allows us to highlight the importance of primary forest sites as reservoirs for a great diversity of species (Korasaki et al., 2013). On the other hand, native species from open areas still represent a low proportion in the pool of species of the REBP since the matrix that surrounds the pastures includes fragments of forest or adjacent living fences, which could favor the presence of edge or generalist species. In addition, in the REBP, the exotic species of beetles such as Digitonthophagus gazella or Euoniticellus intermedius have not yet been found in the open areas (often associated with this type of environment), suggesting that the existing matrix surrounding the REBP does not facilitate their presence. It is important to reflect on what will happen to native beetle species in open areas in response to climate change (the main cause of the reduction of their distribution areas), and in response to livestock practices such as the use of antiparasitics. The decrease in their populations in the medium and long term due to the synergistic effects of these factors could generate a decrease in the ecosystem services they provide (Maldaner et al., 2021).
The diversity of species changes in time and space due to a great variety of factors that involve phenological patterns and life strategies, which in turn are modulated by environmental changes and variation in the availability of food resources (Pinheiro et al., 2002; Wolda, 1988). Thus, conservation strategies must consider the spatio-temporal changes that modulate species assemblages since organisms have different biological responses to land use change (Moreno & Halffter, 2001). Furthermore, changes in the spatio-temporal dynamics are reflected in the biodiversity and functioning of the ecosystem.
Finally, we highlight that our study has been developed in a community ecological reserve that provides several free benefits (scenic and cultural) and well-being for nearby and global populations, but is also a refuge for beetle species and conservation of their biodiversity. Therefore, the conservation of forest fragments and wooded areas, such as secondary vegetation or remnants of riparian vegetation, which are elements of connectivity between tropical forests, requires the active intervention of local communities and the responsible participation of all sectors of society.
Acknowledgments
To Alonso Méndez Vázquez for his help with the fieldwork. We recognize Erick Corro and Pedro Luna for helping obtain diversity parameters. Our sincere gratitude goes out to the Bajlum Pakal Community, especially to Jerónimos Álvaro Arcos, Mateo Álvaro Arcos, Armando Cruz Vázquez, Felipe Álvaro Cruz, and the Méndez Vázquez family for their support and hospitality. LA thanks (20030/10229 INECOL) for financial support.
References
Adrián, A. Q. A (2021). Descripción comunitaria de escarabajos peloteros del Bosque Protector Cerro Blanco, y su relevancia en ciencias aplicadas (Tesis). Facultad de Ciencias Naturales. Universidad de Guayaquil, Ecuador.
Almeida, S., Louzada, J., Sperber, C., & Barlow, J. (2011). Subtle land-use change and tropical biodiversity: dung beetle communities in Cerrado grasslands and exotic pastures. Biotropica, 43, 704–710. https://doi.org/10.1111/j.1744-7429.2011.00751.x
Alonso, C. B. G., Zurita, G. A., & Bellocq, M. I. (2020). Dung beetles response to livestock management in three different regional contexts. Scientific Reports, 10, 1–10. https://doi.org/10.1038/s41598-020-60575-5
Alvarado, F., Dáttilo, W., & Escobar, F. (2019). Linking dung beetle diversity and its ecological function in a gradient of livestock intensification management in the Neotropical region. Applied Soil Ecology, 143, 173–180. https://doi.org/10.1016/j.apsoil.2019.06.016
Arellano, L., León-Cortes, J. L., & Halffter, G. (2008). Response of dung beetle assemblages to landscape structure in remnant natural and modified habitats in southern Mexico. Insect Conservation and Diversity, 1, 253–262. https://doi.org/10.1111/j.1752-4598.2008.00033.x
Barlow, J., Gardner, T. A., Araujo, I. S., Avila-Pires, T. C., Bonaldo, A. B., Costa, J. E. et al. (2007). Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proceedings of the National Academy of Sciences, 104, 18555–18560. https://doi.org/10.1073/pnas.0703333104
Bezaury-Creel, J., & Gutiérrez-Carbonell, D. (2009). Áreas naturales protegidas y desarrollo social en México. In Conabio. Capital natural de México, Vol. II. (pp. 382–431). México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Blas-López, M., & Gómez, B. (2009). Escarabajos (Coleoptera: Scarabaeidae). In A. Riechers, J. E. Morales-Pérez, & G. E. Hernández (Eds.), Laguna Bélgica: patrimonio natural e interpretación ambiental (pp. 75–87). Tuxtla Gutiérrez, Chiapas: Instituto de Historia Natural.
Boege, E. (2008). El patrimonio biocultural de los pueblos indígenas de México: hacia una conservación in situ de la biodiversidad y agrodiversidad en los territorios indígenas. Cd. de México: Instituto Nacional de Antropología e Historia/ Comisión Nacional para el Desarrollo de los Pueblos Indígenas.
Cajaiba, R. L., Périco, E., da Silva, W. B., & Santos, M. (2017). Attractiveness of Scarabaeinae (Coleoptera: Scarabaeidae) to different baits in the Brazilian Amazon region. Revista de Biología Tropical, 65, 917–924. https://doi.org/10.15517/rbt.v65i3.29433
Campos, R. C., & Hernández, M. I. M. (2013). Dung beetle assemblages (Coleoptera, Scarabaeinae) in Atlantic forest fragments in southern Brazil. Revista Brasileira de Entomologia, 57, 47–54. https://doi.org/10.1590/S0085-56262013000100008
Chamé-Vázquez, E. R., & Gómez, B. (2005). Primer registro de Canthon angustatus Harold 1867 en México (Coleoptera: Scarabaeoidea). Acta Zoológica Mexicana (n.s.), 21, 159–160. https://doi.org/10.21829/azm.2005.2131981
Comisión Nacional del Agua. (2019). Coordinación General del Servicio Meteorológico Nacional. Proyecto de Bases de datos Climatológicos. Recuperado el 2 junio, 2021 de: https://smn.conagua.gob.mx/tools/RESOURCES/Mensuales/chis/00007085.TXT
CDI (Comisión Nacional para el Desarrollo de los Pueblos Indígenas, México). (2010). Turismo alternativo en zonas indígenas. 11. Bajlum Pakal. Recuperado el 12 mayo, 2021 de: http://www.cdi.gob.mx/turismo/index.php?option=com_content&view=article&id=56:bajlum-pakal&catid=36:chiapas&Itemid=54
Correa, C., da Silva, P. G., Puker, A., Gil, R. L., & Ferreira, K. R. (2021). Rainfall seasonality drives the spatiotemporal patterns of dung beetles in Amazonian forests in the arc of deforestation. Journal of Insect Conservation, 25, 453–463. https://doi.org/10.1007/s10841-021-00313-y
Costa-Batista, M. C., Lopes, G. D. S., Marques, L. J. P., & Teodoro, A. V. (2016). The dung beetle assemblage (Coleoptera: Scarabaeinae) is differently affected by land use and seasonality in northeastern Brazil. Entomotropica, 31, 95–104.
Crist, T. O., Veech, J. A., Gering, J. C., & Summerville, K. S. (2003). Partitioning species diversity across landscapes and regions: a hierarchical analysis of α, β, and γ diversity. The American Naturalist, 162, 734–743. https://doi.org/10.
1086/378901
Damborsky, M. P., Bohle, M. A., Polesel, M. I., Porcel, E. A., & Fontana, J. L. (2015). Spatial and temporal variation of dung beetle assemblages in a fragmented landscape at Eastern Humid Chaco. Neotropical Entomology, 44, 30–39. https://doi.org/10.1007/s13744-014-0257-2
De Juan, S., & Hewitt, J. (2014). Spatial and temporal variability in species richness in a temperate intertidal community. Ecography, 37, 183–190. https://doi.org/10.1111/j.1600-0587.2013.00048.x
De la Vega, C., Elizalde, H. y González, M. (2014). Escarabajos estercoleros para la ganadería de la Región de Aysén. Boletín INIA N° 295. Coyhaique, Chile: Instituto de Investigaciones Agropecuarias, INIA-Tamel Aike.
Díaz, A., Galante, E., & Favila, M. E. (2010). The effect of the landscape matrix on the distribution of dung and carrion beetles in a fragmented tropical rain forest. Journal of Insect Science, 10, 1–16. https://doi.org/10.1673/031.010.8101
Escobar, F., Halffter, G., Solís, A., Halffter, V., & Navarrete, D. (2008). Temporal shifts in dung beetle community structure within a protected area of tropical wet forest: a 35-year study and its implications for long-term conservation. Journal of Applied Ecology, 45, 1584–1592. https://doi.org/10.1111/j.1365-2664.2008.01551.x
Estrada, A., Halffter, G., Coates-Estrada, R., & Meritt, D. A. (1993). Dung beetles attracted to mammalian herbivore (Alouatta palliata) and omnivore (Nasua narica) dung in the tropical rain forest of Los Tuxtlas, Mexico. Journal of Tropical Ecology, 9, 45–54. https://doi.org/10.1017/S0266467400006933
Fahrig, L. (1997). Relative effects of habitat loss and fragmentation on population extinction. The Journal of Wildlife Management, 61, 603–610. https://doi.org/10.2307/3802168
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34, 487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Favila, M. E., & Halffter, G. (1997). The use of indicator groups for measuring biodiversity as related to community structure and function. Acta Zoológica Mexicana (ns), 72, 1–25. https://doi.org/10.21829/azm.1997.72721734
Ferreira, S. C., Da Silva, P. G., Paladini, A., & Di Mare, R. A. (2019). Climatic variables drive temporal patterns of α and β diversities of dung beetles. Bulletin of Entomological Research, 109, 390–397. https://doi.org/10.1017/S0007485318000676
Filgueiras, B. K., Tabarelli, M., Leal, I. R., Vaz-de-Mello, F. Z., & Iannuzzi, L. (2015). Dung beetle persistence in human-modified landscapes: combining indicator species with anthropogenic land use and fragmentation-related effects. Ecological Indicators, 55, 65–73. https://doi.org/10.1016/j.ecolind.2015.02.032
Filgueiras, B. K., Tabarelli, M., Leal, I. R., Vaz-de-Mello, F. Z., Peres, C. A., & Iannuzzi, L. (2016). Spatial replacement of dung beetles in edge-affected habitats: biotic homogenization or divergence in fragmented tropical forest landscapes? Diversity and Distributions, 22, 400–409. https://doi.org/10.1111/ddi.12410
Filgueiras, B. K., Iannuzzi, L., & Leal, I. R. (2011). Habitat fragmentation alters the structure of dung beetle communities in the Atlantic Forest. Biological Conservation, 144, 362–369. https://doi.org/10.1016/j.biocon.2010.09.013
Flota-Bañuelos, C., López-Collado, J., Vargas-Mendoza, M., Fajersson, P., González-Hernández, H., & Martínez-Morales, I. (2012). Efecto de la ivermectina en la dinámica espacio-temporal de escarabajos estercoleros en Veracruz, México. Tropical and Subtropical Agroecosystems, 15, 227–239.
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R. et al. (2005). Global consequences of land use. Science, 309, 570–574. https://doi.org/10.1126/science.1111772
Frank, K., Hülsmann, M., Assmann, T., Schmitt, T., & Blüthgen, N. (2017). Land use affects dung beetle communities and their ecosystem service in forests and grasslands. Agriculture, Ecosystems and Environment, 243, 114–122. https://doi.org/10.1016/j.agee.2017.04.010
García, E. (1988). Modificaciones al Sistema de Clasificación Climática de Köppen. 5ª Edic. Ciudad de México: Talleres Offset Larios, S.A.
Gibson, L., Lee, T. M., Koh, L. P., Brook, B. W., Gardner, T. A., Barlow, J. et al. (2011). Primary forests are irreplaceable for sustaining tropical biodiversity. Nature, 478, 378–381. https://doi.org/10.1016/j.agee.2017.04.010
Gómez, B., Pozo, C., de la Mora-Estrada, L. F., Domínguez, M. R., Rodríguez-López, M. E., & Ruiz-Montoya, L. (2017). Diversidad de insectos colectados en cuatro localidades de la Reserva de la Biosfera Selva El Ocote. In L. Ruiz- Montoya, G. Álvarez-Gordillo, N. Ramírez-Marcial, & B. Cruz-Salazar (Eds.), Vulnerabilidad social y biológica ante el cambio climático en la Reserva de la Biosfera Selva El Ocote (pp. 171–254). San Cristóbal de Las Casas: El Colegio de la Frontera Sur, Chiapas.
Gómez, B., & Chamé-Vázquez, E. R. (2003). Primeros registros de Goniophileurus femoratus y Sisyphus mexicanus para Chiapas, México (Coleoptera: Scarabaeoidea). Folia Entomológica Mexicana, 42, 103–104.
Gómez-Cifuentes, A., Munevar, A., Gimenez, V., Gatti, M., & Zurita, G. (2017). Influence of land use on the taxonomic and functional diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina. Journal of Insect Conservation, 21, 147–156. https://doi.org/10.1007/s10841-017-9964-4
Guisan, A., Edwards, T. C. Jr., & Hastie, T. (2002). Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecological Modelling, 157, 89–100. https://doi.org/10.1016/S0304-3800(02)00204-1
Halffter, G. (1991). Historical and ecological factors determining the geographical distribution of beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Biogeographia–The Journal of Integrative Biogeography, 15, 11–40. https://doi.org/10.21426/B615110376
Halffter, G. (2005). Towards a culture of biodiversity conservation. Acta Zoológica Mexicana (n.s.), 21, 133–153. https://doi.org/10.21829/azm.2005.2121991
Halffter, G., & Arellano, L. (2002). Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica, 34, 144–154. https://doi.org/10.1111/j.1744-7429.2002.tb00250.x
Halffter, V., & Halffter, G. (2009). Nuevos datos sobre Canthon (Coleoptera: Scarabaeinae) de Chiapas, México. Acta Zoológica Mexicana (n.s.), 25, 397–407. https://doi.org/10.21829/azm.2009.252646
Howden, H., & Howden, A. (2001). Change through time: a third survey of the Scarabaeinae (Coleoptera: Scarabaeidae) at Welder Wildlife Refuge. The Coleopterists Bulletin, 55, 356–362. https://doi.org/10.1649/0010-065X(2001)055[0356:CTTATS]2.0.CO;2
Hsieh, T., Ma, K., & Chao, A. (2016). Package “iNEXT: iNterpolation and EXTrapolation for species diversity”. R package version 2.0. 12 Recuperado el 12 mayo, 2021 de: http://chao.stat.nthu.edu.tw/blog/software-download
Joaqui, T., Cultid-Medina, C. A., Dáttilo, W., & Escobar, F. (2021). Different dung beetle diversity patterns emerge from overlapping biotas in a large mountain range of the Mexican Transition Zone. Journal of Biogeography, 48, 1284–1295. https://doi.org/10.1111/jbi.14075
Jost, L. (2006). Entropy and diversity. Oikos, 113, 363–375. https://doi.org/10.1111/j.2006.0030-1299.14714.x
Kaur, A. P., Holley, J. M., & Andrew, N. R. (2021). Assessing how changes in temporal resource quality influence the reproductive performance of the dung beetle Onthophagus binodis. Ecological Entomology, 46, 1315–1323. https://doi.org/10.1111/een.13078
Kenyon, T. M., Mayfield, M. M., Monteith, G. B., & Menéndez, R. (2016). The effects of land use change on native dung beetle diversity and function in Australia’s Wet Tropics. Austral Ecology, 41, 797–808. https://doi.org/10.1111/aec.12366
Korasaki, V., Braga, R. F., Zanetti, R., Moreira, F. M., Vaz-de-Mello, F. Z., & Louzada, J. (2013). Conservation value of alternative land-use systems for dung beetles in Amazon: valuing traditional farming practices. Biodiversity and Conservation, 22, 1485–1499. https://doi.org/10.1007/s10531-013-0487-3
Kuznetsova, A., Brockhoff, P. B., & Christensen, H. B. (2017). lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software, 82, 1–26. https://doi.org/10.18637/jss.v082.i13
Larsen, T. H., & Forsyth, A. (2005). Trap spacing and transect design for dung beetle biodiversity studies. Biotropica, 37, 322–325. https://doi.org/10.1111/j.1744-7429.2005.00042.x
Larsen, T. H., Lopera, A., & Forsyth, A. (2006). Extreme trophic and habitat specialization by Peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 60, 315–324. https://doi.org/10.1649/0010-065X(2006)60[315:ETAHSB]2.0.CO;2
Lassau, S. A., Hochuli, D. F., Cassis, G., & Reid, C. A. (2005). Effects of habitat complexity on forest beetle diversity: do functional groups respond consistently? Diversity and Distributions, 11, 73–82. https://doi.org/10.1111/j.1366-9516.2005.00124.x
Legendre, L., & Legendre. P. (1998). Numerical ecology. Amsterdam, The Netherlands: Elsevier Science BV.
León-Espinosa, L. F., & Mojica-Soto, R. A. (2018). Géneros de escarabajos coprófagos (Scarabaeidae: Scarabaeinae) de dos zonas de la reserva natural el Caduceo, San Martin Meta, Colombia (Tesis). Departamento de Biología, Universidad Pedagógica Nacional. Bogotá, Colombia.
Leong, M., Ponisio, L. C., Kremen, C., Thorp, R. W., & Roderick, G. K. (2016). Temporal dynamics influenced by global change: bee community phenology in urban, agricultural, and natural landscapes. Global Change Biology, 22, 1046–1053. https://doi.org/10.1111/gcb.13141
Maldaner, M. E., Sobral-Souza, T., Prasniewski, V. M., & Vaz-de-Mello, F. Z. (2021). Effects of climate change on the distribution of key native dung beetles in South American grasslands. Agronomy, 11, id2033. https://doi.org/10.3390/agronomy11102033
McGill, B. (2015). Biodiversity: land use matters. Nature, 520, 38–39. https://doi.org/10.1038/520038a
Moctezuma, P. J. V. (2019). Escarabajos del estiércol (Scarabaeinae) de los Chimalapas, México: análisis taxonómico, ecológico y evolutivo (Tesis doctoral). Instituto de Ecología, A.C. Xalapa, Veracruz, México.
Morales C. J., Ruiz, R., & Delgado, L. (2004). Primer registro de Euoniticellus intermedius (Reiche, 1849) y datos nuevos de distribución de Digitonthophagus (Fabricius, 1787) (Coleoptera: Scarabaeidae) e Hybosorus illigeri Reiche, 1853 (Coleoptera: Hybosoridae) para el estado de Chiapas. Dugesiana, 11, 21–23. https://doi.org/10.32870/dugesiana.v11i2.3803
Moreno, C. E., Barragán, F., Pineda, E., & Pavón, N. P. (2011). Reanálisis de la diversidad alfa: alternativas para interpretar y comparar información sobre comunidades ecológicas. Revista Mexicana de Biodiversidad, 82, 1249–1261. https://doi.org/10.22201/ib.20078706e.2011.4.745
Moreno, C. E., & Halffter, G. (2001). Spatial and temporal analysis of the alpha, beta, and gamma diversities of bats in a fragmented landscape. Biodiversity and Conservation, 10, 367–382. https://doi.org/10.1023/A:1016614510040
Morón, M. A., Camal, J. F., & Canul, O. (1986). Análisis de la entomofauna necrófila del área Norte de la Reserva de la Biosfera «Sian Ka’an», Quintana Roo, México. Folia Entomológica Mexicana, 69, 83–98.
Morón, M. A., Villalobos, F. J., & Deloya, C. (1985). Fauna de coleópteros lamelicornios de Boca del Chajul, Chiapas, México. Folia Entomológica Mexicana, 66, 57–118.
Morón-Ríos, A., & Morón, M. A. (2016). Evaluación de la fauna de Coleoptera Scarabaeoidea en la Reserva de la Biósfera de Calakmul, Campeche, México. Southwestern Entomologist, 41, 469–484. https://doi.org/10.3958/059.041.0217
Navarrete, D., & Halffter, G. (2008a). Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: the effects of anthropogenic changes. Biodiversity and Conservation, 17, 2869–2898. https://doi.org/10.1007/s10531-008-9402-8
Navarrete, D. A., & Halffter, G. (2008b). Nuevos registros de escarabajos copro-necrófagos (Coleoptera: Scarabaeidae: Scarabaeinae) para México y Chiapas. Acta Zoológica Mexicana (n.s.), 24, 247–250. https://doi.org/10.21829/azm.2008.241637
Newbold, T., Hudson, L. N., Hill, S. L., Contu, S., Lysenko, I., Senior, R. A. et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520, 45–50. https://doi.org/10.1038/nature14324
Nichols, E., Larsen, T., Spector, S., Davis, A. L., Escobar, F., Favila, M. et al. (2007). Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biological Conservation, 137, 1–19. https://doi.org/10.1016/j.biocon.2007.01.023
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., Favila, M. E. et al. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation, 141, 1461–1474. https://doi.org/10.1016/j.biocon.2008.04.011
Noriega, J. A., & Acosta, A. (2011). Population size and dispersal of Sulcophanaeus leander (Coleoptera: Scarabaeidae) on riverine beaches in the Amazonian region. Journal of Tropical Ecology, 27, 111–114. https://doi.org/10.1017/S0266467410000581
Noriega, J. A., Santos, A. M., Calatayud, J., Chozas, S., & Hortal, J. (2021). Short-and long-term temporal changes in the assemblage structure of Amazonian dung beetles. Oecologia, 195, 719–736. https://doi.org/10.1007/s00442-020-04831-5
Novais, S., Evangelista, L. A., Reis-Júnior, R., & Neves, F. S. (2016). How does dung beetle (Coleoptera: Scarabaeidae) diversity vary along a rainy season in a tropical dry forest? Journal of Insect Science, 16, id81. https://doi.org/10.1093/jisesa/iew069
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D. et al. (2017). “vegan: Community Ecology Package”. R Package 2.4-3. Recuperado el 12 mayo, 2021 de: https://CRAN.R-project.org/package=vegan
Palacios-Ríos, M., Rico Gray, V., & Fuentes, E. (1990). Inventario preliminar de los Coleoptera Lamellicornia de la zona de Yaxchilan, Chiapas, México. Folia Entomológica Mexicana, 78, 49–60.
Pinheiro, F., Diniz, I. R., Coelho, D., & Bandeira, M. P. S. (2002). Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecology, 27, 132–136. https://doi.org/10.1046/j.1442-9993.2002.01165.x
Pinheiro, J., Bates, D.& R Core Team. 2022. Nlme: Linear and Nonlinear Mixed Effects Models. https://svn.r-project.org/R-packages/trunk/nlme/
Pinto-Leite, C.M., Mariano-Neto, E., & da Rocha, P. L. B. (2018). Biodiversity thresholds in invertebrate communities: the responses of dung beetle subgroups to forest loss. Plos One, 13, e0201368. https://doi.org/10.1371/journal.pone.0201368
Quintero, I., & Halffter, G. (2009). Temporal changes in a community of dung beetles (Insecta: Coleoptera: Scarabaeinae) resulting from the modification and fragmentation of tropical rain forest. Acta Zoológica Mexicana, 25, 625–649. https://doi.org/10.21829/azm.2009.253665
R Core Team. (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
Regolin, A. L., Muylaert, R. L., Crestani, A. C., Dáttilo, W., & Ribeiro, M. C. (2020). Seed dispersal by Neotropical bats in human-disturbed landscapes. Wildlife Research, 48, 1–6. https://doi.org/10.1071/WR19138
Reiners, W. A., Bouwman, A. F., Parsons, W. F. J., & Keller, M. (1994). Tropical rain forest conversion to pasture: changes in vegetation and soil properties. Ecological Applications, 4, 363–377. https://doi.org/10.2307/1941940
Rodríguez-López, M. E., Sánchez-Hernández, G., & Gómez, B. (2019). Escarabajos coprófagos (Coleoptera: Scarabaeidae: Scarabaeinae) en la reserva El Zapotal, Chiapas, México. Revista Peruana de Biología, 26, 339–350. http://dx.doi.org/10.15381/rpb.v26i3.16778
Roslin, T., & Koivunen, A. (2001). Distribution and abundance of dung beetles in fragmented landscapes. Oecologia, 127, 69–77. https://doi.org/10.1007/s004420000565
Salomão, R. P., González-Tokman, D., Dáttilo, W., López-Acosta, J. C., & Favila, M. E. (2018). Landscape structure and composition define the body condition of dung beetles (Coleoptera: Scarabaeinae) in a fragmented tropical rainforest. Ecological Indicators, 88, 144–151. https://doi.org/10.1016/j.ecolind.2018.01.033
Sánchez-de Jesús, H., Arroyo-Rodríguez, V., Andresen E., & Escobar, F. (2016). Forest loss and matrix composition are the major drivers shaping dung beetle assemblages in a fragmented rainforest. Landscape Ecology, 31, 843–854. https://doi.org/10.1007/s10980-015-0293-2
Sánchez-Hernández, G., Gómez, B., Delgado, L., Rodríguez-López, M. E., & Chamé-Vázquez, E. R. (2018). Diversidad de escarabajos copronecrófagos (Coleoptera: Scarabaeidae: Scarabaeinae) en la Reserva de la Biosfera Selva El Ocote, Chiapas, México. Caldasia, 40, 144–160. https://dx.doi.org/10.1544 6/caldasia.v40n1.68602
Sánchez-Hernández, G., Gómez, B., Delgado, L., & Rodríguez-López, M. E. (2017). Primer registro de Onthophagus longimanus Bates, 1887 (Coleoptera: Scarabaeidae: Scarabaeinae) en Chiapas, México. Dugesiana, 24, 57–59. https://doi.org/10.32870/dugesiana.v24i1.6236
Sánchez-Hernández, G., & Gómez, B. (2018). First precise locality data for Onthophagus atriglabrus Howden and Gill and new state record for Onthophagus anewtoni Howden and Génier (Coleoptera: Scarabaeidae: Scarabaeinae) in Mexico. The Coleopterists Bulletin, 72, 873–876. https://doi.org/10.1649/0010-065X-72.4.873
Santos-Heredia, C., Andresen, E., Zárate, D. A., & Escobar, F. (2018). Dung beetle and their ecological functions in three agroforestry systems in the Lacandona rainforest of Mexico. Biodiversity and Conservation, 27, 2379–2394. https://doi.org/10.1007/s10531-018-1542-x
Scheffler, P. Y. (2005). Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. Journal of Tropical Ecology, 21, 9–19. https://doi.org/10.1017/S0266467404001683
Slade, E. M., Mann, D. J., & Lewis, O. T. (2011). Biodiversity and ecosystem function of tropical forest dung beetles under contrasting logging regimes. Biological Conservation, 144, 166–174. https://doi.org/10.1016/j.biocon.2010.08.011
Slade, E. M., & Roslin, T. (2016). Dung beetle species interactions and multifunctionality are affected by an experimentally warmed climate. Oikos, 125, 1607–1616. https://doi.org/10.1111/oik.03207
Soininen, J., Heino, J., & Wang, J. (2018). A meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Global Ecology and Biogeography, 27, 96–109. https://doi.org/10.1111/geb.12660
Spector, S., & Ayzama, S. (2003). Rapid turnover and edge effects in dung beetle assemblages (Scarabaeidae) at a Bolivian neotropical forest-savanna ecotone1. Biotropica, 35, 394–404. https://doi.org/10.1111/j.1744-7429.2003.tb00593.x
Tews, J., Brose, U., Grimm, V., Tielbörger, K., Wichmann, M. C., Schwager, M. et al. (2004). Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography, 31, 79–92. https://doi.org/10.1046/j.0305-0270.2003.00994.x
Tilman, D., Clark, M., Williams, D. R., Kimmel, K., Polasky, S., & Packer, C. (2017). Future threats to biodiversity and pathways to their prevention. Nature, 546, 73–81. https://doi.org/10.1038/nature22900
Toledo, V. M., & Barrera-Bassols, N. (2008). La memoria biocultural: la importancia ecológica de las sabidurías tradicionales. Barcelona: Icaria Editorial.
Vernes, K., Pope, L. C., Hill, C. J., & Bärlocher, F. (2005). Seasonality, dung specificity and competition in dung beetle assemblages in the Australian Wet Tropics, north-eastern Australia. Journal of Tropical Ecology, 21, 1–8. https://doi.org/10.1017/S026646740400224X
Vidaurre, T., González, L., & Ledezma, M. (2008). Escarabajos coprófagos (Scarabaeidae: Scarabaeinae) del palmar de las islas, Santa Cruz-Bolivia. Kempffiana, 4, 3–20.
Wolda, H. (1988). Insect seasonality: Why? Annual Review of Ecology and Systematics, 19, 1–18. https://doi.org/10.1146/annurev.es.19.110188.000245
Yabuhara, Y., Yamaura, Y., Akasaka, T., Yamanaka, S., & Nakamura, F. (2019). Seasonal variation in patch and landscape effects on forest bird communities in a lowland fragmented landscape. Forest Ecology and Management, 454, 117140. https://doi.org/10.1016/j.foreco.2019.01.030
Yeiser, J. M., Chandler, R. B., & Martin, J. A. (2021). Distance-dependent landscape effects in terrestrial systems: a review and a proposed spatio-temporal framework. Current Landscape Ecology Reports, 6, 1–8. https://doi.org/10.1007/s40823-020-00061-w