Oscar A. Flores-Villela a, *, Eric N. Smith b, Luis Canseco-Márquez a,
Jonathan A. Campbell b
a Universidad Nacional Autónoma de México, Facultad de Ciencias, Museo de Zoología, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán 04510 Ciudad de México, Mexico
b University of Texas, Department of Biology, 701 S. Nedderman Drive, Arlington, Texas 76019-0498, USA
*Corresponding author: ofv@unam.mx (O.A. Flores-Villela)
Received: 23 February 2021; accepted: 19 April 2021
http://zoobank.org/urn:lsid:zoobank.org:pub:8050D5B1-99C3-4C7C-AFF0-74511C90DC28
Abstract
A new snake species of the genus Rena is described from northern Jalisco, Mexico. The new species represents an isolated member of the R. dulcis group in the extreme southwest Mesa Central of the country. We redefine the R. dulcis and R. humilis groups within the genus Rena. The status of the other species allocated to these groups is discussed.
Keywords: Blind snakes; Taxonomy; Nomenclature; Squamata; Serpentes
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Una especie nueva de serpiente agujilla de Jalisco, México (Squamata: Leptotyphlopidae)
Resumen
Una especie nueva de serpiente perteneciente al género Rena se describe del norte de Jalisco, México. La nueva especie representa un miembro aislado del grupo de R. dulcis, en el extremo suroeste de la Mesa Central del país. Redefinimos los grupos R. dulcis y R. humilis dentro del género Rena. Se discute el status de otras especies dentro de estos grupos.
Palabras clave: Agujillas; Taxonomía; Nomenclatura; Squamata; Serpientes
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
While surveying amphibians and reptiles and their parasites in northern Jalisco during the summer of 2003, we obtained a specimen of blindsnake east of the town of Bolaños. This specimen belongs to the dulcis group of Leptotyphlops as defined by Klauber (1940), now allocated to the genus Rena (Adalsteinsson et al., 2009). This group is characterized by having a cream-colored ventral surface with scant dark pigmentation, lacking a sharply contrasting white spot on the snout or tail tip, and lacking a pattern of longitudinal lines on the dorsum. Following Klauber (1940), two subgroups usually have been recognized within the genus, the R. dulcis and the R. humilis subgroups, and considered as separated groups by us. For reasons discussed below we refer to the R. dulcis group those species having 10 scale rows around the tail. The only known specimen of the new species was collected 220 km (straight-line) from the closest known locality in San Luis Potosí for any other member of the R. dulcis group. In view of its geographical isolation and distinctive characters, we propose that it be recognized as a new species within the R. dulcis group. The validity of this taxon was tested by Adalsteinsson et al. (2009) using molecular techniques, referred as Rena sp. B.
Materials and methods
Scale nomenclature and the method for determining numbers of middorsal and subcaudal scales follow Klauber (1940), except for nomenclature of the anterior midorsal head scales that we follow Wallach (2003). Counts were done using a dissecting microscope, and measurements were taken with a ruler (to nearest 0.1 mm) or an electronic digital caliper (to nearest 0.01 mm). Values for asymmetric head characters are given in left/right order. Museum specimens examined are listed in the appendix.
The map for the species of the Rena dulcis group was generated with information gathered from the following collections: AMNH, ANSP, AUM, BMNH, BYU, CAS, CM, CNAR, CU, UF (FLMNH), EHT-HMS, FMNH, INHS, KU, LACM, LSU, MCZ, MPM, MSB, MSUM, MVZ, MWSU (Midwestern State University), MZFC, NLU, OMNH, OS, PSM, SDMNH, SM (BCB), SNIB (Sistema Nacional de Información sobre Biodiversidad de México), SRSU, TCWC, TNHC, TTU, UAZ, UCM, UIMNH, UMMZ, UNL (Universidad de Nuevo León), UOMZ, USNM, UTA, UTEP, WTSU (West Texas A&M University), YPM. Localities were obtained directly from museum collections, the HerpNET database (October 2009), from Dixon and Vaughan (2003). Museum acronyms can be found in Sabaj (2016).
Description
The discovery of a snake allied to R. dulcis in northern Jalisco, and the comparison of this snake with other taxa in the Rena dulcis group has led us to conclude that it represents a new species, here named as:
Rena klauberi new species. Fig. 1.
http://zoobank.org/urn:lsid:zoobank.org:act:17770C36-
1C8E-4171-A8D0-4E4682496071
Leptotyphlops sp. B. Adalsteinsson et al. (2009): 7 (Fig. 3), 48 (App. 1).
Rena sp. B. Adalsteinsson et al. (2009): 20, 31 (Fig. 12), 48 (App. 1).
Holotype: MZFC-17047 from Mexico, Jalisco, Río Cartagena, adjacent to the road between Santa María de Los Ángeles and Bolaños, 1,602 m (21º59’0.24” N, 103º20’27.816” W). Original field number JAC 23308, collected by Eric Smith and Jesse Meik, on 10 June 2003.
Diagnosis. A species of the R. dulcis group that differs from R. maxima, in having 10 rows of scales around the tail (vs. 12); from R. bressoni, R. myopica, and R. dissecta in having an undivided anterior supralabial (vs. divided); from R. dulcis in having nine scales on the dorsal surface with dark pigment (vs. seven); from R. humilis in having supraoculars (vs. absent); and by having 10 rows of scales around the tail (12 in R. humilis group members, except for R. h. segrega and R. h. tenuicula that have 10). Rena klauberi differs from all other Mexican Rena, except the holotype of R. d. iversoni, in having a darkly pigmented cloacal plate, while the remainder of the venter is immaculate.
Description of the holotype. Middorsal scales 252 between the rostral scale and the tail tip; 14 rows of smooth, imbricate scales around body, constant between head and level in front of cloaca; 10 rows of scales around midpoint of tail; subcaudals 14, not including tail tip; cloacal plate undivided; rostral scale curving over snout, posterodorsal end rounded; nasal divided horizontally, nostril between upper and lower nasals, located medially between the suture; lower nasals extend to lip; four scales bordering mouth on each side behind rostral (lower nasal, anterior supralabial, ocular, and posterior supralabial); anterior supralabials 1/1, undivided; posterior supralabials 1/1, higher than wide, barely touching corner of mouth; parietals large, three times higher than wide, contacting posterior supralabials; supraoculars 1/1, equal in size to the frontal; occipitals 1/1, subequal to parietals, undivided; temporals 1/1, preventing contact between occipitals and posterior supralabials; postfrontal slightly wider than frontal, and separating supraoculars; interparietal slightly wider than postfrontal and interoccipital (Fig. 2); mental scale about three and a half times wider than long; infralabials 4/4, first pair separated medially by postmental, second pair larger than first and third and separated from each other by three median chin shields, fourth pair slender; body cylindrical from head to tail, with ultimate scale terminating in a well-defined spine.
Coloration. After preservation, nasal openings surrounded by pale coloration; central area of rostral with a pale transverse marking; eyes visible through skin (Figs. 1, 2); seven dorsal scale rows uniformly dark brown; adjacent scale row slightly paler (total nine dark rows), pattern extending along entire length of body and tail (Fig. 1); venter pinkish on anterior half of body, grading to cream on posterior half; cloacal plate covered with dark pigmentation (Fig. 3); midventral row of subcaudal scales cream; paraventral subcaudal rows brown near cloaca and cream color distally. In life, dorsal coloration similar to that in preservative.
Measurements. Total length 265 mm; tail length 14.4 mm; relative tail length (or tail/LOA) 5.4%; horizontal diameter of body at head 3.8 mm, diameter at midbody 4.7 mm, diameter of body at base of tail 4.4 mm; diameter of head at interocular level 3.4 mm; relative rostral width 1.4; relative body proportion (or length/width ratio) 56.4; tail length/tail width ratio 3.27; rostral width/head width ratio 0.71.

Taxonomic summary
Distribution. Rena klauberi is known only from the type-locality (Fig. 4) in dry scrub forest. The holotype was found crawling just after sunset on a dirt path near the outer periphery of a riparian zone consisting of grasses, sedges, and scattered oak trees (Fig. 5). It is likely that this species may also occur in nearby southern Zacatecas, which possesses similar habitat. Several reptile species, apparently endemic to the area, have been reported for both southern Zacatecas and northern Jalisco (Ponce-Campos et al., 2001).
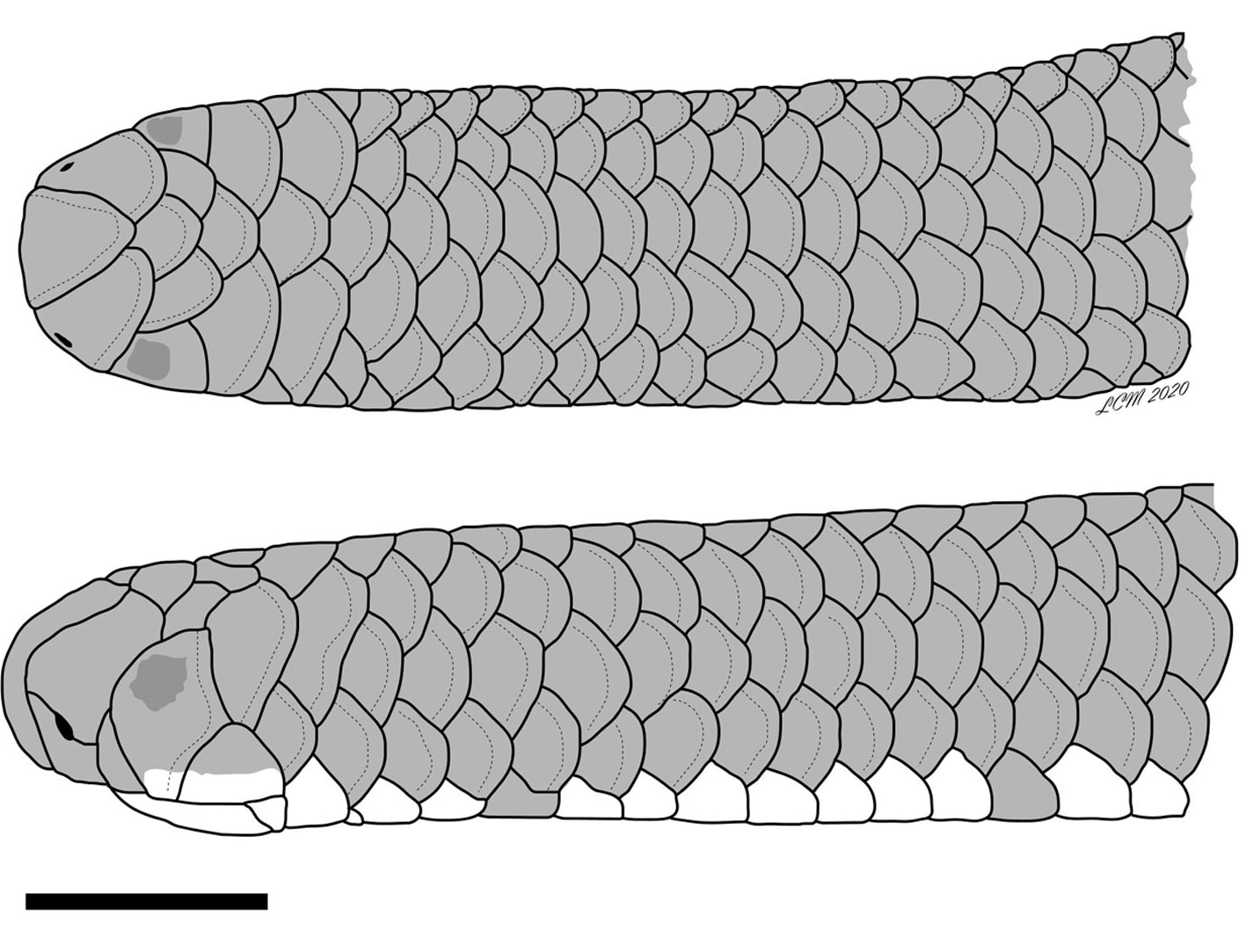

Etymology. This species is named in honor of the late Laurence Monroe Klauber, in recognition of his valuable contributions to North American herpetology, and especially to our knowledge of the genus Leptotyphlops (= Rena) from North America.
Remarks
Based on morphology, R. klauberi appears to be related to R. dulcis. Both R. klauberi and R. dulcis can be distinguished from all other species in the R. dulcis group by having undivided anterior supralabials (typical condition in R. dulcis), 10 scale rows around the tail, and supraocular scales. Adalsteinsson et al. (2009), used DNA to show a close phylogenetic relationship between R. dulcis and R. dissecta (here considered synonymous with R. dulcis), to the exclusion of R. klauberi. Using mitochondrial cytochrome b sequences used by Adalsteinsson et al. (2009), deposited in Genbank, we calculated raw genetic distances among the species they examined. The central Texas R. dulcis and southeastern Arizona R. dissecta show only a 5% sequence difference, a very small distance for samples coming from populations more than 630 km apart, regarded here as within species variation. Between R. dissecta or R. dulcis and R. klauberi there is an 11% sequence divergence, which we attribute to a species level difference. Species from the R. dulcis group differed from those within the R. humilis group by between 14-15%, genetic distance. Additionally, Adalsteinsson et al. (2009) tree (their figure 3) supports the monophyly of the R. humilis and R. dulcis groups.
The taxonomy of the blindsnakes allied to the genus Rena, was reviewed by Adalsteinsson et al. (2009), Dixon and Vaughan (2003), Hahn (1979a, 1979b, 1980a), Klauber (1940) and Smith and Chiszar (1993). These authors reached different conclusions on the status of the taxa associated with the genus and the species that comprise it. Currently, the genus Rena is composed of 10 to 11 species (Table 1), 2 of them with 2 (R. dulcis) and 4 (R. humilis) subspecies respectively (Uetz et al., 2022; Wallach et al., 2014).
For a long time, the species within this genus were placed in the widespread genus Leptotyphlops, until Adalsteisson et al. (2009) found that this genus was composed of 2 clades, one of them containing New World species, restoring the name Rena (Baird & Girard, 1853) for this group of taxa. Nevertheless, the taxonomy of the genus Rena is still controversial and a thorough taxonomic revision is urgently needed. Currently, the genus Rena is distributed in North America from central and southern Mexico to southwestern United States (see below).
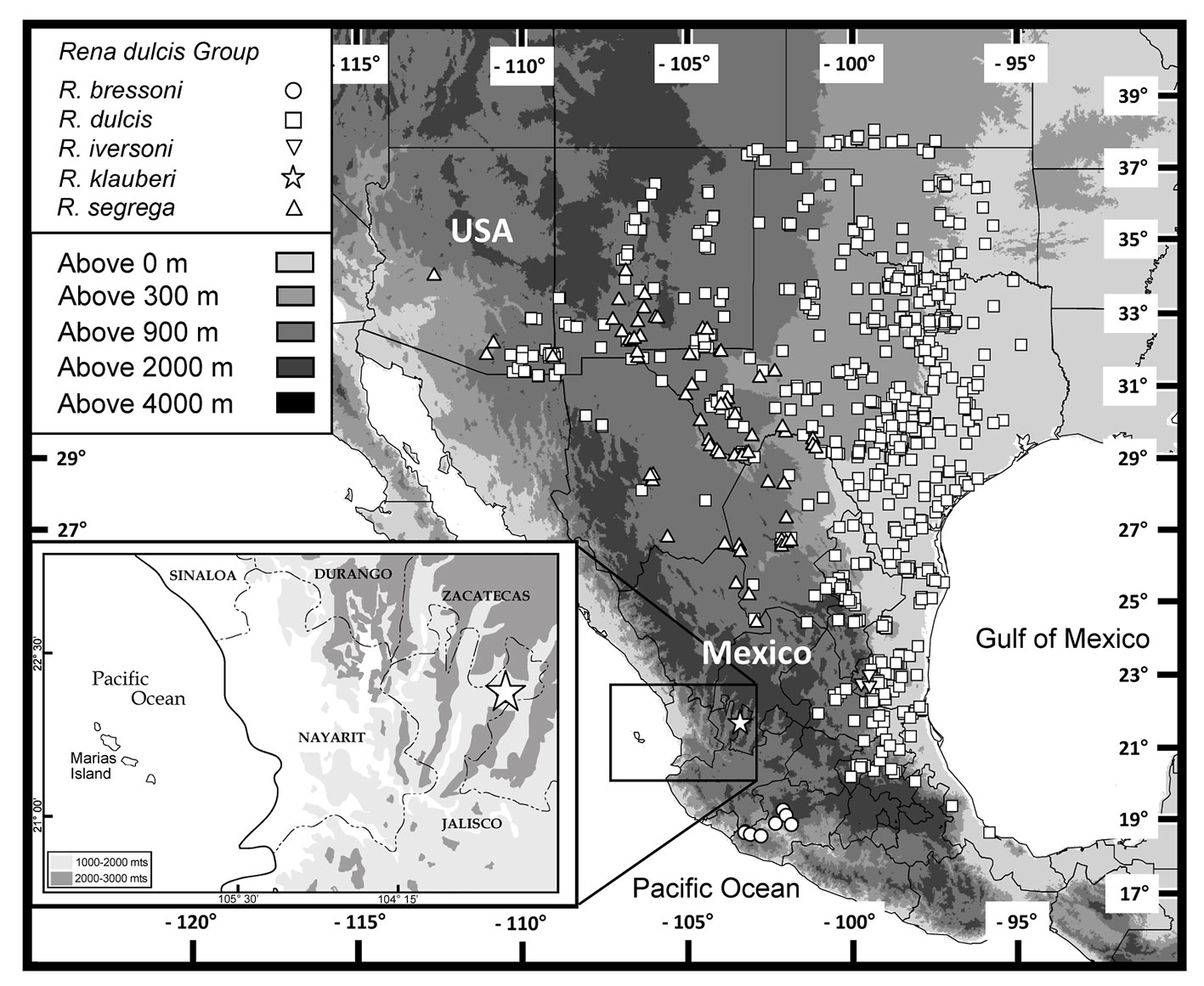
To clarify the status of the taxa allocated to the genus Rena, we reviewed characters that have been used, mainly by Klauber (1940). Klauber (1940) recognized the dulcis and humilis subgroups, which are currently placed in the genus Rena, recognizing four characters differentiating both subgroups (considered separate groups by us); number of middorsal scales (236-246 dulcis; 254-275 humilis), division of the anterior supralabials (undivided in R. dulcis group), total length (R. dulcis under 300 mm; R. humilis over 300 mm), and the presence of supraocular scales in the R. dulcis group, lacking in R. humilis. All of these characters, however, show considerable variation within each group and even within recognized species (Table 2). Therefore, we redefine these groups with a more consistent character, the number of scales around the tail, the R. dulcis group having 10 and the R. humilis group having 12 scales around the tail (Table 2). We adopt this new diagnostic character, the number of scales around the tail, as more important than others in defining these groups in the past (e.g., number of supraoculars); this character defines and coincides with the relationships recovered and depicted by Adalsteinsson et al. (2009). This lepidotic count is consistent within each known species and apportions the taxa to the east and west along the geographically important Cochise Filter Barrier. The Continental Divide has been important in isolating the biota of the Sonoran and Chihuahuan deserts (Castoe et al., 2007; Morafka, 1977; Myers et al., 2017; Provost et al., 2018, and references cited therein), and the formation of this barrier appears to be responsible for separating the snakes of the R. dulcis-humilis groups, partitioning them into 2 distinct taxonomic units. Rena humilis with 12 or more scales around tail, includes species occurring west of the Cochise Filter Barrier: and the R. dulcis group is characterized by 10 scales around tail and occurs east of the Cochise Filter Barrier.

Table 1
Species currently recognized by the two main sources of information about snakes species in the world. Species are sorted by group based on the results of this publication, see text for more details.
|
Species |
The Reptile Data Base |
Wallach et al. (2014) |
|
R. dulcis group |
||
|
R. dulcis (2 ssp.) |
Valid |
Valid |
|
R. dissecta |
Valid |
Valid |
|
R. iversoni |
Valid |
Valid |
|
R. myopica |
Valid |
Valid |
|
R. bressoni |
Valid |
Valid |
|
R. unguirostris |
Valid |
As Siagonodon unguirostris |
|
R. humilis group |
||
|
R. boetgeri |
Valid |
Synonym of R. humilis |
|
R. dugesii |
Valid |
Valid |
|
R. humilis (4 ssp.) |
Valid |
Valid |
|
R. segrega |
Valid |
Valid |
|
R. maxima |
Valid |
Valid |
Below we discuss the status and validity of some taxa in the R. dulcis-humilis groups, particularly as impacted by our newly rearranged groups, and the new species described here. The R. humilis group is presented first for ease of discussion.
Rena dugesii (Bocourt, 1881). Lemos-Espinal et al. (2004) elevated R. h. dugesii to full species status, comparing this taxon to R. h. segrega, in Chihuahua. We agree with Lemos-Espinal and Smith (2007) in restricting the distribution of R. dugesii to the Pacific slopes from Sonora to Colima, considering a Guanajuato record erroneous. That record was based on a specimen sent to the U.S. National Museum by Dugès. There is another specimen at the Dugès Museum in Guanajuato (MADUG-HE 90) from Colima; Dugès did not record this species from Guanajuato (Dugès, 1890, 1895), so it is unlikely that it occurs there. The closest record of this species to Guanajuato is that of Peterson et al. (1995) from Chapala, Jalisco.
Rena humilis tenuicula (Garman, 1883 [1884]). Stenostoma tenuiculum was described from a single specimen from San Luis Potosí. It was diagnosed by having 10 scales around the tail and less than 250 middorsal scales (Klauber, 1940). Brown and Brown (1967) reported a second specimen (BCB 11560) from Llera, Tamaulipas. This second specimen belongs to a population existing in geographical proximity to localities where R. iversoni (Smith, van Breukelen, Auth & Chiszar, 1998) has been collected. Smith et al. (1998) made this specimen (BCB 11560) part of the type series of R. iversoni, arguing that BCB 11560 has divided anterior supralabials, a low number of middorsals, and that the only other known specimen, the holotype of R. humilis tenuicula, comes from the “semiarid plateau in San Luis Potosí”. Klauber (1940) commented that it was not clear whether the type-locality “San Luis Potosí” refers to the state or the city, and as Brown and Brown (1967) recognized, the type-locality may be situated 60-200 miles (96-320 km) from Llera, Tamaulipas, the general area occupied by R. iversoni. It is worth noting that the holotype of R. tenuicula lacks divided anterior supralabials, but has 10 scales around the tail. Based on our examination of the holotype of R. h. tenuicula (Stenostoma tenuiculum, MCZ 4519), and the considerable variation in middorsal scale counts, supraoculars and anterior supralabials exhibited by R. dulcis (Table 2), we consider R. tenuicula as belonging to the R. dulcis group. If proven to be a valid taxon, a matter that needs more investigation; its relationships to other members of the R. dulcis group, should be considered. For the present we place it in the synonym of R. dulcis.
Klauber (1940), thought R. tenuicula might be related to R. dugesii, pending the revision of R. dugesii and confirmation of the number of scales around tail. Uetz et al. (2022) and Wallach et al. (2014) considered R. tenuicula as a synonym of R. dugesii. Our examination of the type of Catodon dugesii (Bocourt, 1881) revealed that it has 12 scale rows around the tail. Therefore, considering this taxon as a synonym of R. dugesii by Uetz et al. (2022) and Wallach et al. (2014) is unjustified.
Rena humilis chihuahuensis (Tanner, 1985). This taxon was synonymized by Lemos-Espinal and Smith (2007) based on variation of middorsal scale counts provided by Klauber (1940) for populations of R. humilis segrega from Arizona (254-280). We agree with these authors in relegating this taxon to R. segrega (see R. h. segrega account below). Therefore it should not be considered as a valid subspecies of R. humilis, as indicated by Uetz et al. (2022).
Rena humilis segrega (Klauber, 1939). This taxon is characterized by having 10 rows of scales around the tail, as in other members of the R. dulcis group, but it lacks supraocular scales, similar to members of the R. humilis group. Nevertheless, its distribution falls mostly to the east of the Cochise Filter Barrier and has undivided supralabials, similar to R. dulcis and R. klauberi of the R. dulcis group. Rena segrega is a distinct species from R. humilis and unique within the R. dulcis group, therefore we recognize it as a valid taxon and part of the R. dulcis group.
Rena dulcis (Baird & Girard, 1853). This species differs from other members in this group, except R. klauberi, by having supraoculars and undivided anterior supralabials (Dixon & Vaughan, 2003). Klauber (1940) recognized 3 subspecies: R. d. dulcis, R. d. myopica and R. d. dissecta. This arrangement was followed by Hahn (1979a, 1980b), McDiarmid et al. (1999) and Smith et al. (1998). Smith et al. (1998) described an additional subspecies, R. d. iversoni (see below). Dixon and Vaughan (2003) elevated to species rank the subspecies of R. dulcis recognized by Klauber (1940), and recognized the taxon R. d. iversoni as a subspecies of R. myopica, on the basis of middorsal scale counts. Dixon and Vaughan (2003) also recognized R. rubella as a subspecies of R. dulcis (see below) and assigned the name to populations from southwestern Texas and adjacent Mexico. Dixon and Vaughan (2003) also recognized an unnamed population of Rena dulcis ssp. from Seminole, Oklahoma, USA, which needs further confirmation.
Table 2
Selected characters used to diagnose species discussed in this paper. Data modified from Klauber (1940), Dixon and Vaughan (2003), and specimens examined (Appendix). Taxa we recognize appear in bold type. Typical condition in number of scales, excluding number of middorsals, appears first and is defined as the condition present in the largest number of individuals examined or reported in the references.
|
Species |
Middorsals |
Scales around body |
Scales around tail |
Supraoculars |
Anterior supralabials |
Parietal-posterior supralabial contact |
Number of pigmented dorsals (adding half scales) |
Dorsal color (as reported for live or freshly preserved) |
Maximum size |
|
Rena dulcis group |
|||||||||
|
R. dulcis |
192-257 |
14 |
10 |
1/1 1/0 0/0 |
1/1 2/2 1/2 2/1 0/1 0/0 |
Yes |
7 (5-8) |
Pinkish, light to medium, and dark brown to black |
272 |
|
R. d. rubella |
222-257 |
14 |
10 |
1/1 |
1/1 |
Yes |
7 (6-7) |
Pale brown |
191 |
|
R. dissecta |
213-255 |
14 |
10 |
1/1 |
2/2 1/2 |
Yes |
7 |
Pinkish |
272 |
|
R. iversoni |
202-226 |
14 |
10 |
0/0 1/0 1/1 |
2/2 |
Yes |
7 (5-8) |
Medium to dark brown |
196 |
|
R. myopica |
192-236 |
14 |
10 |
1/1 |
2/2 1/2 1/1 |
Yes |
7 |
Dark brown to black |
227 |
|
R. bressoni |
227-246 |
14 |
10 |
1/1 |
2/2 1/2 |
No |
7 |
Pale brown |
265 |
|
R. klauberi |
252 |
14 |
10 |
1/1 |
1/1 |
Yes |
9 |
Dark brown |
265 |
|
R. segrega |
253-287 |
14 |
10 |
0/0 0/1 |
1/1 |
Yes |
7 |
Pale to dark brown, grey |
319 |
|
Rena humilis group |
|||||||||
|
R. boettgeri |
244-269 |
14 |
12 |
0/0 |
1/1 |
Yes |
5 |
Pale to dark brown |
253 |
|
R. dugesii |
231-259 |
14 |
12 |
0/0 |
1/1 |
Yes |
7 (7-9) |
Medium brown |
187 |
|
R. h. cahuilae |
280-305 |
14 |
12 |
0/0 |
1/1 |
Yes |
7 (5-7) |
Almost white (pink), brown to orange |
389 |
|
R. h. humilis |
253-291 |
14 |
12 |
0/0 |
1/1 |
Yes |
7 (7-9) |
Medium to dark brown |
315 |
|
R. h. utahensis |
289-308 |
14 |
12 |
0/0 |
1/1 |
Yes |
7 |
Pale gray-brown |
322 |
|
R. h. chihuahuensis |
253-257 |
14 |
10 |
0/0 |
1/1 |
Yes |
7 |
Pigmented |
133 |
|
R. humilis tenuicula |
244 |
14 |
10 |
0/0 |
1/1 |
Yes |
7 (6-7) |
Pale gray-brown |
117 |
|
R. maxima |
216-235 |
14 |
12 |
1/1 |
1/1 |
Yes |
7 |
Dark brown to orange |
300 |
Rena dulcis rubella (Garman, 1883 [1884]). Stejneger (1891) synonymized R. rubella with R. dulcis, demonstrating that the original description of Garman (1883 [1884]), based on a single specimen, had numerous errors. Garman reported erroneously 15 dorsal scale rows in R. dulcis, instead of 14 (as in other members of the genus Rena and most Leptotyphlopids); the rostral separating the nasals as a diagnostic character (as all Leptotyphlopids); 5 infralabials instead of 4 (as in all members of the genus Rena); and the anterior parietal (= parietal) being the only scale contacting the posterior labial, implying that in R. dulcis the posterior parietal (= occipital) also contacts the posterior labial (Stejneger, 1891). The latter condition, as mentioned by Stejneger, is only found on the left side of the holotype of R. rubella, and is variable in R. dulcis, contact only by the parietal being the prevalent condition. Klauber (1940) concurred with Stejneger (1891) and maintained R. rubella in the synonymy of R. dulcis, adding that Garman misinterpreted the original description of Baird and Girard (1853), and pointing out that the type-locality of R. rubella is within the geographical distribution of R. dulcis. This taxon was resurrected as a subspecies of R. dulcis by Dixon and Vaughan (2003). In spite of broad overlap in ranges of variation, the number of middorsal scale rows was one of the criteria used by Dixon and Vaughan (2003: 14, 22: key) to distinguish 3 subspecies of R. dulcis: R. d. dulcis (210-246), R. d. rubella (222-257), and an unnamed subspecies from Oklahoma (202-228). The other diagnostic character was dorsal coloration; “pinkish” for R. d. dulcis and “light to medium brown” for R. d. rubella. No dorsal color pattern was described for the unnamed subspecies. Because these 2 taxa show considerable overlap in middorsal counts, their distributional ranges are parapatric as delimited by Dixon and Vaughan (2003), and their middorsal scale counts decrease in a north to south clinal pattern, we find no valid argument to recognize any subspecies within R. dulcis. Current examination of the holotype of R. rubella (MZC 4584) does not provide evidence of its original color (see Table 2) and it seems necessary to have other evidence, besides scale variation and dorsal coloration, to arrive at a taxonomic decision involving this name. Our examination of R. dulcis from throughout its range in Texas reveals considerable variation in dorsal coloration, from pink to medium brown to black.
Additionally, Dixon and Vaughan (2003) reported a population of Rena from Querétaro and Hidalgo (their Population 5a, central Mexico) which they associated with R. dulcis. We have examined additional material from central Mexico, and from a locality in northeastern Zacatecas. The Zacatecas locality is geographically intermediate between the central Mexico population and those in Tamaulipas and Nuevo León. The Zacatecas and central Mexico specimens coincide in middorsal scale counts with those to the north. We consider R. rubella as a junior synonym of R. dulcis and populations in central Mexico (Querétaro, Hidalgo, and Zacatecas) to represent R. dulcis populations.
Rena myopica (Garman, 1883 [1884]). This species was distinguished from other species in the R. dulcis group (including specimens previously assigned to R. dissecta) by possessing the following combination of characters (Dixon & Vaughan, 2003; Klauber, 1940): divided anterior supralabials; no postocular; parietals contacting posterior supralabials; and 192-255 middorsals. We have examined specimens in which the postoculars and the anterior supralabials are variable; the middorsal scale count falls within the range of R. dulcis, the lower count may be just part of a north-south cline found by Dixon and Vaughan (2003). Therefore, we do not consider this taxon valid, and it should be considered a junior synonym of R. dulcis. Dixon and Vaughan (2003) recognized 2 subspecies, R. m. myopica and R. m. iversoni (status of this taxon discussed below). Wallach et al. (2014), on the basis of Pinto (2010) unpublished dissertation, recognized this taxon as valid as well as Uetz et al. (2022).
Rena dissecta (Cope, 1896). This taxon was distinguished from other members of the R. dulcis subgroup by the following combination of characters: divided anterior supralabials, no postocular, parietals contacting posterior supralabials, middorsal scale counts, and dorsal coloration (pinkish, orange, or pale to dark brown Dixon and Vaughan [2003]). Klauber (1940) stated that R. d. dissecta can be consistently differentiated from R. d. myopica by number of middorsal scales, but Dixon and Vaughan (2003) showed that middorsal scale counts overlap in these taxa. Klauber (1940) considered 2 additional characters to further differentiate these taxa: in R. dissecta the fifth dorsal scale is wider than the fourth (equal width in R. myopica) and the occipital scales are divided on at least one side of the head in many individuals of R. dissecta (59% of the specimens he examined vs. always complete in R. myopica). Dixon and Vaughan (2003) stated that such characters are highly variable and should not be used in diagnoses, and we agree after examination of additional specimens. In 2 specimens of R. myopica that we examined, the fifth dorsal scale is wider than the fourth and the occipitals are single. As currently recognized, R. dissecta occurs in the southwestern United States (Arizona, New Mexico, Oklahoma, and Texas) and northern Mexico (Chihuahua and Coahuila), it seems to be mostly allopatric from R. dulcis and R. myopica, with only limited distributional overlap (see map in Hahn [1979a] and Dixon and Vaughan [2003]). We do not agree with Dixon and Vaughan (2003) in recognizing R. dissecta as a separate species from R. myopica. These authors based the recognition of these as 2 separate taxa primarily on their supposed differences in dorsal color, pinkish in R. dissecta and brown to black in R. myopica. Klauber (1940) reports specimens of R. dissecta ranging from pale to medium brown. We have examined additional specimens of R. dissecta not seen by Dixon and Vaughan (2003), and we observed grey (e.g., UTA R-54613) and brown (UTA R-45091) dorsal colors. We have also observed pinkish (UTA R-3149) and medium brown (UTA R-54555) specimens of R. myopica from Nuevo León and Tamaulipas, respectively. The number of middorsal scales was also stated by Dixon and Vaughan (2003) as significant in differentiating these taxa. Examination of additional material considerably expands the lower limit in the range of these scales for R. dissecta, from 220 to 213, providing considerable overlap between R. dissecta and R. myopica. Based on the lack of differences between the 2 taxa, we consider R. dissecta a junior synonym of R. dulcis (Table 2).
Rena iversoni (Smith, van Breukelen, Auth & Chiszar, 1998). This taxon is of special interest because, unlike all other populations formerly in the R. dulcis group, it lacks supraoculars in most known specimens (10 out of 13). Smith et al. (1998) described this taxon as a subspecies of R. dulcis, based on it having 10 scales around the tail. We agree that the affinities of this taxon are with the R. dulcis group. The absence of supraoculars in R. iversoni may be the result of fusion of the ocular and supraocular scales. We consider the diagnostic features of this taxon are variable, although they may have systematic value. The matter needs to be resolved using molecular techniques. Wallach et al. (2014) on the basis of Pinto (2010) unpublished dissertation recognized this taxon as valid, as well as Uetz et al. (2022). We provisionally accept the status as valid species, pending more evidence.
In summary, we recognize 9 species in the Rena dulcis-humilis groups, including R. bressoni and R. maxima (Table 3). We do not recognize any subspecies for R. dulcis. Within R. humilis we recognize tentatively 2 subspecies, both with 12 scales around the tail and lacking supraoculars (Tables 2, 3). The R. dulcis and R. humilis groups are mainly separated from each other, in the northern part of their distribution, by the Cochise Filter Barrier between the Sonoran and Chihuahuan deserts.
Table 3
Taxonomic changes proposed in this paper.
|
Species previously recognized |
Species recognized in this work |
|
R. dulcis group |
|
|
R. d. dulcis |
R. dulcis |
|
R. d. rubella |
R. dulcis |
|
R. dissecta |
R. dulcis |
|
R. h. tenuicula |
R. dulcis |
|
R. myopica |
R. dulcis |
|
R. iversoni |
R. iversoni |
|
R. klauberi |
R. klauberi |
|
R. bressoni |
R. bressoni |
|
R. h. chihuahuensis |
R. segrega |
|
R. segrega |
R. segrega |
|
R. humilis group |
|
|
R. boettgeri |
R. boettgeri |
|
R. dugesii |
R. dugesii |
|
R. h. humilis |
R. humilis |
|
R. h. utahensis |
R. h. utahensis, not treated here |
|
R. maxima |
R. maxima |
Acknowledgements
We thank J. Meik, J. Malone, T. Devitt, and P. Ponce-Campos for field assistance, J. Rosado and V. Wallach, Museum of Comparative Zoology, for providing us with information related to the type of L. rubellum and photographs of the specimen. We thank for the loan of comparative material or providing specimen information: T. Crumpton of Baylor University, Mayborn Museum Complex (Strecker Museum); G. Schneider of the University of Michigan Museum of Zoology; D. R. Frost of the American Museum of Natural History; S. Rogers of the Carnegie Museum; J. T. Giermakowski of the Museum of Southern Biology; V. H. Reynoso of the Instituto de Biología, UNAM; R. McDiarmid and S. Gotte of the Smithsonian Institution; R. G. Webb and C. Lieb from University of Texas, El Paso; N. Vidal from MNHN-Paris; G. Magaña-Cota from the Museo Alfredo Dugès. We thank F. Wallach and J. Boundy for sending relevant information. For preparing specimen loans we thank E. Pérez Ramos, MZFC, and C. Franklin, UTA. J. Meik and R. Pinto made extensive valuable suggestions. J. A. Hernández Gómez helped with the figures. This paper is based in part upon work supported by the National Science Foundation under grant No. DEB-0613802 to J. A. Campbell; ENS participation was possible by NSF grant DEB-0416160 and a grant from Bioclon, Mexico. Necessary permits were issued by Semarnap to OFV. The final revision of this paper was done while OFV was on sabbatical at UTA supported by the Conacyt and DGAPA, UNAM, Mexico.
Appendix. Specimens examined.
Rena bressoni: Mexico, Michoacán: MZFC-12356–12357: Coalcomán, 2 km SE of Coalcomán; MZFC-12358: Aguililla, 5 km W of Aguililla; MZFC-12390: Coalcomán, 4 km N Puerto de la Zarzamora, 1.5 km NW of C. El Laurel.
Rena dissecta: USA, Arizona: UTA R-26544: Cochise, Portal road, 0.5 km E Portal; UTA R-50598: Cochise, Forest Service barracks; Texas: UTA R-33758: Jeff Davis, 3.0 mi E of end of FM 1832 on FM 1832; UTA R-54613: Terrel.
Rena dulcis: USA, Texas: UTA R-32303: Bosque, 2.5 mi S of jct W FM 927 and Texas 144, W of 144 on AW Vickrey property; UTA R-1596: Cameron, South most Palm Grove, E Brownsville; UTA R-9166-9170: Cooke, 15.8 mi S Gainesville on FM 51; UTA R-36890: Crockett, 8 mi SE of Barnhart; UTA R-57102-103: Crockett, Dirth road to the south of Hwy 67 runs paralell to train tracks; UTA R-896-898: Dallas, Oak Cliff; UTA R-1444: Dallas, Hwy 67 at Hampton Road; UTA R-10273: Dallas, Dallas, Dallas Zoo; UTA R-9171: Denton, 19.4 mi S Gainesville on FM 51; UTA R-16799-800: Denton, N of Aubrey, Main Street ca. 2 km N jct Blackjack Road; UTA R-15680: Denton, unknown locality; UTA R-38466: Eastland, 1.5 mi N Cisco on 380; UTA R-15681–15682: Frio, 9.6 km NE Dilley near jct Leona and Frio Rivers; UTA R-16200: Frio, Panther Hollow Ranch, ca. 11 km NW Dilley; UTA R-10503: Garza, NE Post; UTA R-30067: Goliad, Goliad, grounds of courthouse; UTA R-26552: Henderson, no other data; UTA R-398-399: Hidalgo, E McAllen; UTA R-33378: Jeff Davis, 3.0 mi E of end of FM 1832 on FM 1832; UTA R-32815: Jim Hogg; S of Hebronville on FM 1017, 8.4 mi from jct FM 1017 and FM 285; UTA R-16415-420: Johnson, Johnson Ranch, 21.7 km W Rio Vista; UTA R-38402: Kendall, on 743, 5 mi N of Sisterdale; UTA R-11123-24, 12649-651: Llano, 12.8 km S Cherokee on St Hwy 16, Houston Ranch; UTA R-11075: McMullen, 11.1 km S Tilden on Texas Hwy 16 at creek; UTA R-15176: Montague, Sundance Ranch, S side Sandy Creek, 7.7 km S, 4.2 km E jct US Hwy 287 and FM 1125; UTA R-9172: Palo Pinto, Birdwell Ranch, 1.5 mi S Palo Pinto on FM 4; UTA R-1234: Parker, 3 mi E Cresson; UTA R-1440: Parker, 4.8 km NE of Aledo Mary’s Creek área; UTA R-5043: Parker, FM rd 2376 2 mi S Aledo, behind graveyard; UTA R-9173-74: Parker, 2.5 mi S Aledo, on road off of FM 2376; UTA R-26545-547: Parker, ca. 3 air km NW Wheatland, along Bear Creek; UTA R-7890-91: San Saba, 2.9 mi S, 1.0 mi E Bend; UTA R-55708-709: Shackelford, Hwy 351 S junction with Hwy 180; UTA R-599: Tarrant, Arlington (College Farm); UTA R-600: Tarrant, Arlington (4 blks N of Turnpike on Fielder Road); UTA R-863: Tarrant, Arlington; UTA R-18688: Tarrant, Arlington, UTA campus; UTA R-25669: Tarrant, Arlington, UTA Central Services Building, 1225 West Mitchell; UTA R-25712: Tarrant, Arlington, 1426 S West Street; UTA R-31418: Tarrant, University of Texas at Arlington campus, crossing path near Student Union Building; UTA R-32380: Tarrant, Arlington, in house near UTA campus; UTA R-38873: Tarrant, Arlington, UT Arlington campus, Lipscomb Hall; UTA R-55402: Tarrant, Arlington Veteran´s Park; UTA R-19327: Tarrant, Benbrook-Aledo Road; UTA R-19328: Tarrant, ca. 1 km NW jct Loop 820 and Rufe Snow; UTA R-18272: Tarrant, Euless, 402 Huntington Drive; UTA R-28691: Tarrant, Euless, 607 Bent Tree Court; UTA R-5696: Tarrant, Ft. Worth, 800 block of Sylvania street near Trinity River; UTA R-14727-729: Tarrant; Fort Worth, 600 Congress; UTA R-40737: Tarrant, Fort Worth; UTA R-1233: Tarrant, Lake Worth; UTA R-1930: Tarrant, N. Richland Hill; UTA R-1585-86: Tarrant, Trinity River and Tex 360; UTA R-9175-77: Tarrant, 2.9 km S Jct FM 1886 on White Settlement Road; UTA R-394, 1232, 45050: Travis, Austin; UTA R-1235: Val Verde, Devil’s River, 23.5 mi N Comstock; UTA R-28907: Val Verde, 8.7 km N Baker’s Crossing on St Hwy 163; UTA R-1599: Williamson, Georgetown Country Club; UTA R-8633-37, 8650-52: Wise, 4.0 mi NNW Decatur; UTA R-10010-12: Wise, LBJ National Grassland; UTA R-14726, 15356-57: Wise, 8.0 Km N Decatur, L.B. Johnson National Grassland; UTA R-26549: Wise, 0.3 air km SW Flat Rock Cemetery; UTA R-31419: Wise, 1.6-2.0 air mi S Flat Rock Cemetary, 0.4 air mi W old Decatur Road; UTA R-31420-423: Wise, 3.0 mi N Runaway Bay, Sid Richardson Scout Ranch; UTA R-32459-462: Wise, Sid Richardson Scout Ranch; UTA R-10008: Zapata, 3.7 mi NE jct. US 83 on FM 3169; Mexico, Hidalgo: MZFC-8022: Metztitlán, Metztitlán; Querétaro: MZFC-8482: Arroyo Seco, N Concá-Santa María River Bridge; MZFC-6257–6258: Mesa de León; MZFC-6259: Rancho Nuevo; MZFC-6260: Nopalera Boye; CM-90296: San Juan Del Río, 2.2 mi S.; Zacatecas: CM-59982: 16 mi NE Concepción Del Oro turnoff on Mex Hwy 54.
Rena dugesii: Mexico, Colima: MNHN-RA-0.1651.
Rena dulcis iversoni: Mexico, Tamaulipas: BU-MMC 11522: 20.9 km NE Ignacio Zaragoza, Río Guayalejo (formerly SM 11522); BU-MMC 11560: 1.6 km N Llera; BU-MMC 15094-15096 1.6 km E Llera (all paratypes, all formerly BCB collection).
Rena humilis tenuicula: Mexico, San Luis Potosí: MCZ-R 4519: San Luis Potosí (Holotype).
Rena maxima: Mexico, Guerrero: MZFC-03826: Ixcateopan de Cuauhtémoc, Ixcateopan; Morelos: MZFC-01931: Mazatepec, Mazatepec; Puebla: MZFC-04710: Zapotitlán de las Salinas, Zapotitlán de las Salinas; UTA R-12227: 9 km SSW Zapotitlan Salinás; UTA R-12229: 5.6 km SSW Zapotitlán Salinas; UTA R-14533: vicinity of Zapotitlán Salinas.
Rena myopica: Mexico, Hidalgo: MZFC-7793: 2 km W Arroyo Blanco; Nuevo León: UTA R-3149: Monterrey; Tamaulipas: UTA R-54555: Carretera Estación Calles-Hacienda Acuña, 583 m, 23.18565 N 98.49051 W.
Rena rubella: Mexico, Coahuila: MCZ-R 4584: San Pedro (Holotype).
Rena segrega: USA, Texas: UTA R-17767: Brewster, W side Black Hill, ca. 15 km E, ca. 2 km N jct Dove Mountain Road, and US Hwy 385; UTA R-33761: Brewster, 100 yds. W of Big Bend National Park entrance on TX 118; UTA R-2881: Hudspeth, Eagle Mtns., 7 mi S Hot Wells, UTA R-33759: Presidio, 19.4 mi W of Lajitas on FM 170; UTA R-33760: Presidio, 26.7 mi W of Lajitas on FM 170; UTA R-54619: Terrel, TX 349, 4.2 miles N of Dryden; UTA R-45091: Ward, On Hwy 329, ca. 4.8 km E Grandfalls.
References
Adalsteinsson, S. A., Branch, W. R., Trape, S., Vitt, L. J., & Hedges, S. B. (2009). Molecular phylogeny, classification, and biogeography of snakes of the Family Leptotyphlopidae (Reptilia, Squamata). Zootaxa, 2244, 1–50. https://doi.org/10.11646/zootaxa.2244.1.1
Baird, S. F., & Girard, C. (1853). Catalogue of North American reptiles in the Museum of the Smithsonian Institution. Part I – Serpents. Washington D.C., Government Printing Office. https://doi.org/10.5962/bhl.title.5513
Brown, B. C., & Brown, L. M. (1967). Notable records of Tamaulipan snakes. Texas Journal of Science, 19, 323–326.
Castoe, T. A., Spencer, C. L., & Parkinson, C. L. (2007). Phylogeographic structure and historical demography of the western diamondback rattlesnake (Crotalus atrox): A perspective on North American desert biogeography. Molecular Phylogenetics and Evolution, 42, 193–212. https://doi.org/ 10.1016/j.ympev.2006.07.002
Dixon, J. R., & Vaughan, R. K. (2003). The status of Mexican and southern United Sates blind snakes allied with Leptotyphlops dulcis (Serpentes: Leptotyphlopidae). Texas Journal of Science, 55, 3–24.
Dugès, A. (1890). Fauna del estado de Guajuato. In A. L. Velasco (Ed.), Geografía y estadística del estado de Guajuato. Geografía y estadística de la República Mexica, Vol. 5 (pp. 287–295). Ciudad de México: Oficina Tipográfica de la Secretaría de Fomento.
Dugès, A. (1895). Fauna del estado de Guajuato. In Memoria sobre la administración pública del estado de Guajuato presentada al congreso del mismo por el C. Gobernador constitucional Lic. Joaquín Obregón González”, el 1 de abril de 1895. Morelia: Imprenta y litografía de la Escuela IM Porfirio Díaz.
Garman, S. (1883). The reptiles and batrachians of North American reptiles, part 1, Ophidia. Memoirs of the Museum of Comparative Zoology Harvard, 8, 1–185. https://doi.org/10.5962/bhl.title.50435
Hahn, D. E. (1979a). Leptotyphlops dulcis (Baird & Girard) Texas blind snake. Catalogue of American Amphibians and Reptiles, 231, 1–2.
Hahn, D. E. (1979b). Leptotyphlops humilis (Baird & Girard) Western blind snake. Catalogue of American Amphibians and Reptiles, 232, 1–4.
Hahn, D. E. (1980a). Leptotyphlops maximus Loveridge. Catalogue of American Amphibians and Reptiles, 244, 1.
Hahn, D. E. (1980b). Liste der rezenten Amphibien und Reptilien Anomalepididae, Leptotyphlopidae, Typhlopidae. Das Tierreich, eine zusammenstellung und kennzeichnung der reszenten tierformen. Berlin: Walter de Gruyter.
HerpNET database. Accessed 24 October 2009. http://www.herpnet.org
Klauber, L. M. (1940). The worm snakes of the genus Leptotyphlops in the United States and Northern Mexico. Transactions of the San Diego Society of Natural History, 9, 87–162.
Lemos-Espinal, J. A., & Smith, H. M. (2007). Anfibios y reptiles del estado de Chihuahua, México. México D.F.: UNAM/ Conabio.
Lemos-Espinal, J. A., Smith, H. M. Chiszar, D., & Woolrich-Piña, G. (2004). 2003. Snakes from Chihuahua and adjacent states of Mexico. Bulletin of the Chicago Herpetological Society, 39, 206–213.
McDiarmid, R. W., Campbell, J. A., & Touré, T. A. (1999). Snake species of the world, a taxonomic and geographic reference, Vol. 1. Washington, DC: The Herpetologists League.
Morafka, D. J. (1977). A biogeographical analysis of the Chihuahuan Desert through its herpetofauna. Dordrecht: Springer. https://doi.org/10.1007/978-94-010-1318-5
Myers, E. A., Burgoon, J. L., Ray, J. M., Martínez-Gómez, J. E., Matías-Ferrer, N, Mulcahy, D. G. et al. (2017). Coalescent species tree inference of Coluber and Masticophis. Copeia, 105, 640‒648. https://doi.org/10.1643/CH-16-552
Peterson, H. W., Smith, H. M., & Chiszar, D. (1995). Some noteworthy amphibians and reptiles from the region of Chapala, Jalisco, Mexico. Bulletin of the Chicago Herpetological Society, 30, 90–91.
Pinto, R. R. (2010). Revisão Sistemática da Subtribo Reni (Serpentes: Leptotyphlopidae) (PhD. Thesis). Universidade Federal do Rio de Janeiro, Brazil.
Ponce-Campos, P., Huerta-Ortega, S. M., Nogueira-Gonzalez, C., & Smith, H. M. (2001). Natural history notes on the Southern Plateau night lizard, Xantusia sanchezi. Bulletin of the Maryland Herpetological Society, 37, 18–21.
Provost, K. L., Mauck III, W. M., & Smith, B. T. (2018). Genomic divergence in allopatric Northern Cardinals of the North American warm deserts is linked to behavioral differentiation. Ecology and Evolution, 8, 12456–12478. https://doi.org/10.1002/ece3.4596
Sabaj, M. H. (2016). Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Version 6.5 (16 August 2016). American Society of Ichthyologists and Herpetologists, Washington, DC. Electronically accessible at http://www.asih.org/
Smith, H. M., & Chiszar, D. (1993). Apparent intergradation in Texas between the subspecies of the Texas blind snake (Leptotyphlops dulcis). Bulletin of the Maryland Herpetological Society, 29, 143–155.
Smith, H. M., van Breukelen, F., Auth, D. L., & Chiszar, D. (1998). A subspecies of the Texas blind snake (Leptotyphlops dulcis) without supraoculars. The Southwestern Naturalist, 43, 437–440.
Stejneger, L. (1891). Notes on some North American snakes. Proceedings of the United States National Museum, 14, 501–505. https://doi.org/10.5479/si.00963801.876.501
Uetz, P., Freed, P., & Hošek, J. (Eds.). (2022). The Reptile Database. Accessed January, 10, 2022. http://www.reptile-database.org
Wallach, V. (2003). Scolecophidia miscellanea. Hamadryad, 27, 227–245.
Wallach, V., Williams, K. L., & Boundy, J. (2014). Snakes of the world, a catalogue of living and extinct species. Boca Raton, Florida: CRC Press. https://doi.org/10.1086/679952




