Reproductive output in spiny lizards (genus Sceloporus) with different reproductive mode. A comparative approach
Saúl López-Alcaide a, Lorena Y. Cuateta-Bonilla b, Rodrigo Macip-Ríos c, d, *
a Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad, Liga Periférico-Insurgentes Sur, Núm. 4903, Parques del Pedregal, Tlalpan, 14010 Ciudad de México, Mexico
b Escuela de Biología, Benemérita Universidad Autónoma de Puebla, Blvd. Valsequillo y Av. San Claudio, Edificio 112-A, Ciudad, Universitaria, Col. Jardines de San Manuel, 72570 Puebla, Puebla, Mexico
c Escuela Nacional de Estudios Superiores, Universidad Nacional Autónoma de México, Campus Morelia, Antigua Carretera a Pátzcuaro Núm.8701, Col. Ex Hacienda de San José de la Huerta, 58341 Morelia, Michoacán, Mexico
d Laboratorio Nacional de Síntesis Ecológica y Conservación de Recursos Genéticos, Universidad Nacional Autónoma de México, Campus Morelia, Antigua Carretera a Pátzcuaro Núm.8701, Col. Ex Hacienda de San José de la Huerta, 58341 Morelia, Michoacán, Mexico
*Corresponding author: rmacip@enesmorelia.unam.mx (R. Macip-Ríos)
Received: 5 April 2019; accepted: 19 February 2020
Abstract
The analysis of the reproductive output variation within a lineage is fundamental to understand life-history evolution. When different reproductive modes occur within the same lineage, the comparison of the reproductive output of each mode becomes relevant to understand how reproductive output is linked to the reproductive mode, evolutionary history, and environmental factors. In this study, we analyzed the bet-hedging life-history model approach to the reproductive output in the spiny lizards, genus Sceloporus. We conducted a comparative phylogenetic and standard statistical analysis on published and original reproductive output and environmental data from Sceloporine lizards. No statistical differences in the reproductive output variables were detected between oviparous and viviparous species. Clutch/litter size was negatively correlated with average and minimum environmental temperature. Furthermore, body size was positively correlated with clutch/litter size. A high phylogenetic signal was detected for body size, body mass, and clutch/litter size, but not for reproductive effort. All life-history traits analyzed seem to have a deep evolutionary history, however they are still correlated with environmental variables indicating some evidence for bet-hedging strategy. Results also were discussed under the climate change effects.
Keywords: Reproductive effort; Cutch size; Body size; Phylogenetic signal; Bet-hedging
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Inversión reproductora en lagartijas espinosas (género Sceloporus) con diferente modo reproductor. Una aproximación comparativa
Resumen
El análisis de la variación reproductora dentro de un linaje es fundamental para entender la evolución de las historias de vida. Cuando en un mismo linaje existen diferentes modos de reproducción, la comparación de la inversión reproductora entre cada modo reproductor es relevante para entender su relación con la historia evolutiva y factores ambientales. En este estudio se analizó la inversión reproductora en las lagartijas espinosas, género Sceloporus, desde el punto de vista del modelo de historia de vida “apostador a lo seguro”. Se utilizaron datos publicados e inéditos de rasgos de historia de vida y variables ambientales de sceloporinos. Los datos se analizaron por métodos filogenéticos comparados y estándar. No se detectaron diferencias entre los organismos vivíparos y ovíparos en lo referente a la inversión reproductora. El tamaño de la nidada/camada se relacionó de manera negativa con los valores mínimos y promedio de la temperatura ambiente. El tamaño del cuerpo se relacionó positivamente con el tamaño de la nidada/camada. Se detectó un nivel alto de señal filogenética para el tamaño del cuerpo, masa corporal, tamaño de nidada, pero no para el esfuerzo reproductor. Aun cuando existe un efecto profundo de la historia evolutiva, las correlaciones entre las variables ambientales y de historia de vida indican evidencia de una estrategia “apostador a lo seguro”. Los resultados se discutieron en un escenario de cambio climático.
Palabras clave: Esfuerzo reproductor; Tamaño de nidada; Tamaño del cuerpo; Señal filogenética; Apostar a lo seguro
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Among the life-history evolution models, bet-hedging has been proposed as a good explanatory model for those species with low survivorship in early stages, inhabiting seasonally variable environments, and have reduced reproductive effort (Charnov, 2002, 2005; Enium & Fleming, 2004). Life-history evolution is closely linked with the environmental factors met by the organisms during speciation or local adaptation (Roff, 2002; Stearns, 1992), then, the reproductive output will correlate with environmental conditions by moving forward (reduced reproductive output) or reverse (increment in output) to a bet-hedging life history strategy under high or less environmental (i.e., climate) variability, which represents a direct effect of the environment on the fitness of local populations (Breandle et al., 2011; Roff, 1981, 2002; Stearns, 1989, 1992).
Reproductive output components such as clutch/litter size, egg/neonate size, clutch/litter frequency, and reproductive effort (relative clutch/litter mass) can show plasticity (Shine & Brown, 2008; Shine & Schwarzkof, 1992; Smith & Fretwell, 1974; Tinkle, 1969; Tinkle & Hadley, 1975; Tinkle et al., 1970; Vitt & Prince, 1982). Thereof, plasticity responses in life history traits may also occur among populations or lineages according to the environmental factors they faced during its lifetime (Macip-Ríos et al., 2017; Shine & Schwazkopf, 1992), especially if the environment changes rapidly or if it is seasonally variable, with a higher degree of stochasticity. Hence it is important to investigate the life-history variation during climate change to asses population viability of lineages with potentially imperiled lizards (Sinervo et al., 2010).
In lizards and snakes several life history traits could be fixed or be highly variable (Tinkle et al., 1970). The evolution of life-history traits such as body size, reproductive effort, egg/neonate size, and clutch/litter size could be constrained by the reproductive mode (Rodríguez-Romero et al., 2002; Zúñiga-Vega et al., 2016). Understanding how life-history traits evolve and how they are directly related with environmental variables (i.e., climate, food, light, etc.) is crucial to assess how global environmental changes may affect species occurrence in a fast-changing world (Angilletta et al., 2004; Brandt & Navas, 2011).
There is extensive evidence that lizards have the capacity to adapt to variable environmental conditions (Angilletta et al., 2004; Chamaillé-Jammes et al., 2006; Huey et al., 2012; Logan et al., 2014). That is why it is so important to analyze possible complex interactions between life-history traits such as reproductive mode and body size with reproductive output and with historical environmental (climatic) variables (Andrews & Schwarzkopf, 2012; Meiri et al., 2013).
We used as a study system the spiny lizards from the genus Sceloporus (Iguanidae: Phrynosomatidae). These lizards have been recognized as a suitable model because they inhabit several types of environments within an elevation range from sea level to near 4,000 m (Mathies & Andrews, 1995). Spiny or Sceloporine lizards are exclusive from North America and the lineage includes both, oviparous and viviparous species, a trait that evolved at least 6 times in their evolutionary history (Lambert & Wiens, 2013). The lineage is particularly diverse in Mexico and the southwestern US (Flores-Villela & García-Vázquez, 2014). The referred characteristics make this group of lizards an ideal system to test the evolution of the reproductive output and its relationship with climatic variables at a local scale.
Sceloporine lizards are also facing a population decline due to climate change (Sinervo et al., 2010). According to the latest forecasts, the mean temperature would rise 1.5 °C between 2030 and 2052 (IPCC, 2018). This represents a potential threat to biodiversity at the global and local scale (Tewksbury et al., 2008), and particularly for ectotherms such as reptiles. The global climate change will increase the variation in rainfall and climatic stability leading to fast environmental temperature warming and/or atypical dry conditions episodes (Walters et al., 2012). These new environmental conditions will affect lizards (Deutsch et al., 2008; Huey et al., 2012; Scheffers et al., 2013), which are highly sensitive to environmental temperature variation. Most of their basic biological functions (physiological, reproductive, metabolic, and locomotion) occur within a narrow body temperature range (Angilletta, 2009). Frequently, these body temperature ranges could be evolutionarily static (Grigg & Buckley, 2013), which could drive to short-term extinction processes when environmental conditions change rapidly (Sinervo et al., 2010).
The aim of this study was to analyze the potential correlations between reproductive output traits with environmental variables (climate variation) in a lineage of lizards with different reproductive modes. We hypothesize that the reproductive output of viviparous and oviparous species will correlate with environmental conditions by moving forward to a bet-hedging life history strategy (reduced reproductive output) under high climate variability.
Materials and methods
To explore how reproductive output evolved in spiny lizards (Sceloporus), we conducted a comparative study using the reproductive and phylogenetic data available from the genus. We revised the literature for available data on body size (snout-vent length-SVL), clutch/litter size (CS/LS), and reproductive effort (relative clutch mass or litter) (RCM/RLM, Cuellar, 1984); we also added unpublished data (Table 1). We revised Leaché (2010), Wiens and Reeder (1997), and Wiens et al. (2010), for a phylogenetic hypothesis that better fit our reproductive output database and to have at least one representative taxa from the entire Sceloporine lineage within the phylogeny. In other words, we tried to match the OTU’s present in each topology with the reproductive output data base we gathered.
Since we did not gather data for all the species included in the consulted phylogenies (Wiens & Reeder, 1997; Wiens et al., 2010), we decided to generate our own phylogeny that matched our database. We downloaded the sequences used by Leaché (2010) from GenBank (Geer et al., 2010) for the nuclear markers: RAG-1, BDNF, RNA finger print protein 35, and the PNIN gene. We also used the available mtDNA sequences for 12S, NAD1, and NAD4. Sequence alignment was performed using Clustal W (Larkin et al., 2007). The nucleotide data base was run in Modeltest 3.06 (Posada, 2008; Posada & Crandall, 1998) to determine the evolution model used in the phylogenetic inference, then based on Leaché (2010) topology we constructed a template to perform a constrained phylogenetic analysis to conserve Leaché (2010) main topology but setting free the branch calculation for the new topology. We performed a maximum likelihood phylogenetic analysis using RAxLM (Stamatakis, 2014) in a graphical interface called raxmlGUI (Silvestro & Michalak, 2011). Uta stansburiana was used as out-group. We performed 1,000 random bootstrap re-samples to calculate branch support. The phylogenetic tree was visualized and edited in Figtree 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
We used WorlClim data layers to calculate climatic variation per locality (or localities) from where life-history data were originally sampled (Hijmans et al., 2005). To extract climate data, we used a 100 × 100 m2 grid to extract average climatic data. To compare temporal variation between localities, we searched for 2 basic types of climatic variables; temperature, including mean, minimum, and maximum monthly temperature; and precipitation, including total rain per month, monthly mean evapotranspiration, and maximum rain per day. Since our objective was to describe temporal environmental variation as a main assumption for the bet-hedging life-history strategy (Charnov, 2002, 2005), we calculated standard deviations for each variable and a mean of standard deviations for every type of variable, thermal (TV) and rain variation (RV). We also combined both standard deviations of variables in an average of thermal and rain variation, which was called total climate variation (TCV).
The dataset was analyzed in 2 basic ways: 1) by standard parametric statistical analyses, and 2) by comparative phylogenetic methods (Goolsby, 2015). To determine the correlation between body sizes with reproductive output, we conducted a series of linear correlations. We compared life-history traits of oviparous with viviparous species by an Ancova, using the body size as a covariate (Zar, 1999). This procedure did not imply a phylogenetic correction.
Since our data set is hierarchically ordered following the phylogenetic relationships, we proceeded using phylogenetic comparative methods (Harvey & Pagel, 1991). We used Felsenstein phylogenetic independent contrast (PIC) to identify correlations between life-history traits (Felsenstein, 1985, 2008). We ran the PIC tests with Mesquite ver. 3.04 (Maddison & Maddison, 2018) using the PDAP package (Garland et al., 1999; Garland & Ives, 2000).
Table 1
Life-history traits evaluated among the Sceloporus phylogeny. O = Oviparous, V = viviparous. RMC = Reproductive clutch mass, RLM = reproductive litter mass, CS = clutch size, LS = litter size, SVL = snout-vent length (body size).
|
Table 1 Continued |
|||||||
|
Species |
Rep. Mode |
RCM/ RLM |
CS/ LS |
SVL |
Body mass |
Locality |
Source |
|
Species |
Rep. Mode |
RCM/ RLM |
CS/ LS |
SVL |
Body mass |
Locality |
Source |
|
S. ochoterenae |
O |
0.147 |
5 |
46.4 |
3.34 |
Cerro calera chica, Morelos, Mexico |
Bustos-Zagal et al., 2011 |
|
S. jalapae |
O |
0.47 |
5.6 |
46 |
Tehuacán, Puebla, Mexico |
Ramírez-Bautista et al., 2005 |
|
|
S. gadoviae |
O |
0.6 |
3.9 |
50.4 |
6.03 |
Tehuacán, Puebla, Mexico |
Ramírez-Bautista et al., 2005 |
|
S. melanorhinus |
O |
0.65 |
7.7 |
87.9 |
Chamela, Jalisco, Mexico |
Ramírez-Bautista et al., 2006 |
|
|
S. torquatus |
V |
0.3 |
6.5 |
90.6 |
26.17 |
Pedregal de San Ángel, México D.F. |
Feria-Ortiz et al., 2001 |
|
S. jarrovi |
V |
0.468 |
4.9 |
60.9 |
14.57 |
Pinal de Amoles, Querétaro, Mexico |
Ramírez-Bautista et al., 2002 |
|
S. microlepidotus |
V |
0.48 |
5.2 |
48.5 |
Zoquiapan Edo. de Méx., Mexico |
Guillette & Casas-Andreu, 1980 |
|
|
S. duguessi |
V |
0.192 |
4.6 |
61.5 |
Tzintzuntzan, Michoacán, Mexico |
Ramírez-Bautista y Dávila, 2009 |
|
|
S. poinsetti |
V |
0.5 |
6.3 |
85.8 |
30.94 |
Mapimí, Durango, Mexico |
Gadsen et al., 2005 |
|
S. cyanogenys |
V |
0.3 |
13 |
106 |
39 |
Webb, County Texas, USA |
Hunsaker, 1959; Garrick, 1974 |
|
S. serrifer |
V |
0.0548 |
3.9 |
82.2 |
23.47 |
Conkal, Yucatán, Mexico |
López-Alcaide, unpublished data |
|
S. mucronatus |
V |
0.27 |
5.1 |
83.5 |
19.47 |
Zoquiapan, Edo. de Méx., Mexico |
Rodríguez-Romero et al., 2005 |
|
S. macdougalli |
V |
0.116 |
3.7 |
74.8 |
16.03 |
Santa Cruz Bamba, Oaxaca, Mexico |
López-Alcaide, unpublished data |
|
S. mucronatus aureolus |
V |
0.0842 |
6.5 |
71.9 |
16.27 |
Tlaxiaco, Oaxaca, Mexico |
López-Alcaide, unpublished data |
|
S. grammicus |
V |
0.49 |
5.1 |
50.9 |
5.18 |
Pachuca, Hidalgo, Mexico |
Ramírez-Bautista et al., 2009 |
|
S. formosus |
V |
0.433 |
6.6 |
64.8 |
12.62 |
San Pablo Etla, Oaxaca, Mexico |
Ramírez-Bautista y Pavón, 2009 |
|
S. horridus |
O |
0.7 |
15 |
91.4 |
19.46 |
El Rodeo, Morelos, Mexico |
Valdéz-González & Ramírez-Bautista, 2002 |
|
S. spinosus |
O |
0.75 |
19 |
98.6 |
Las Minas, Puebla, Mexico |
Valdéz-González & Ramírez-Bautista, 2002 |
|
|
S. undulatus |
O |
0.43 |
9.1 |
71.9 |
9.51 |
Burlington, New Jersey, USA |
Angilletta et al., 2001 |
|
S. virgatus |
O |
0.289 |
10 |
61.5 |
8.48 |
Chiricahua, Arizona, USA |
Smith et al., 1995 |
|
S. occidentallis |
O |
0.318 |
4.2 |
74.4 |
14.29 |
Lyle, Washington, USA |
Sinervo et al., 1991 |
|
S. scalaris |
O |
0.86 |
9.4 |
52.8 |
5.8 |
Cochise, Arizona USA |
Mathies & Andrews, 1995 |
|
S. aeneus |
O |
0.24 |
3.9 |
47.5 |
4.3 |
Milpa Alta, D.F., Mexico |
Rodriguez-Romero et al., 2002 |
|
S. bicanthalis |
V |
0.52 |
5.9 |
49.2 |
4.18 |
Zoquiapan, Edo. de Méx. Mexico |
Rodriguez-Romero et al., 2002 |
|
S. orcutti |
O |
0.6 |
11 |
92 |
22.63 |
Riverside, California, USA |
Mayhew, 1963 |
|
S. graciosus |
O |
0.196 |
3.7 |
56.4 |
6.8 |
National Park Zion, Utah, USA |
Tinkle et al., 1993 |
|
S. variabilis |
O |
0.212 |
4.6 |
55.4 |
7.19 |
Bastonal, Veracruz, Mexico |
Benabib, 1994 |
|
S. utiformis |
O |
0.6 |
6.9 |
63.8 |
9.18 |
Chamela, Jalisco, Mexico |
Ramírez-Bautista & Gutiérrez-Mayén, 2003 |
|
S. pyrocephalus |
O |
0.78 |
5.8 |
47 |
Tejupilco, Edo. de Méx. Mexico |
Ramírez-Bautista & Olvera-Becerril, 2004 |
|
|
S. bicanthalis_2 |
V |
0.47 |
7.2 |
52.1 |
5.97 |
Nevado de Toluca, Edo. de Méx., Mexico |
Rodríguez-Romero et al., 2004 |
|
S. jarrovi_2 |
V |
0.468 |
7.8 |
72.9 |
Pabellón de Arteaga, Aguscalientes, Mexico |
Ramírez-Bautista et al., 2002 |
|
|
S. mucronatus_2 |
V |
0.37 |
6.5 |
79.4 |
19.47 |
Tecocomulco, Hidalgo, Mexico |
Villagrán et al., 2009 |
|
S. torquatus_2 |
V |
0.34 |
9.7 |
72 |
26.17 |
San Juan Teotihuacán, Edo. de Méx. Mexico |
Guillette & Méndez-de La Cruz, 1993 |
|
S. horridus_2 |
O |
0.0251 |
16 |
94 |
24.49 |
Xalitla, Guerrero, Mexico |
López-Alcaide unpublished data |
|
S. occidentallis_2 |
O |
0.281 |
3.9 |
74.4 |
14.29 |
Terrebone, Oregon, USA |
Sinervo et al., 1991 |
|
S. undulatus garmani |
O |
1 |
6.2 |
64.6 |
5.26 |
Statfford, Kansas, USA |
Ferguson & Snell, 1986 |
|
S. variabilis_2 |
O |
0.203 |
4.3 |
55.4 |
7.04 |
Montepío, Veracruz, Mexico |
Benabib, 1994 |
|
S. spinosus albiventris |
O |
0.0503 |
10 |
74 |
15 |
Chamela, Jalisco, Mexico |
López-Alcaide unpublished data |
A phylogenetic Anova was also used to compare between oviparous and viviparous species (Garland et al., 1993). We ran the phylogenetic Anova in phytools in R (R Development Core Team, 2008; Revell, 2012). To detect the phylogenetic signal on the reproductive output traits evaluated, we used the K statistic also in phytools (Bloomberg et al., 2003; Revell, 2012). K statistic works
as a gauge of phylogenetic signal in a trait that allows the comparison between traits and between trees (Münkemüller et al., 2012). Transformations, parametric assumptions tests, and standard statistical analyses were conducted with JMP ver. 5.0.1 (SAS Institute, 2002).
Results
Data set was composed by 38 operative taxonomic units (OTU’s), including 28 species, approximately 31% of all the species contained in the lineage (Table 1). Some species were over represented by more than 1 population, we included all observations to achieve a larger sample. We gathered information from 21 oviparous and 17 viviparous species. Our sampling allowed to include at least 1 species of each of the major and minor clades of the lineage. The data set comprised a 27° range in latitude from Sceloporus occidentalis from Oregon (44.32° N), to Sceloporus mucronatus from Oaxaca (17.26° N), Mexico. Overall, according to our database and among the Sceloporus lineage, body size (SVL) averages 70 mm (± 16.8, range 106-46), body mass 14.28 g (± 9.04, ranges 3.34-39), clutch size or litter size 7.2 (± 3.6, range 4-19), and relative clutch mass or relative litter mass 0.38 (± 0.21, ranges 86-0.025).
When we compared oviparous with viviparous taxa in a phylogenetic context (Fig. 1), the phylogenetic Anova did not detect significant variation in body size (F = 0.25, p = 0.808), CS/LS (F = 0.36, p = 0.74), and RCM/RLM (F = 0.007, p = 0.95), nevertheless, the phylogenetic signal was statistically detectable for body size (K = 1.11, p = 0.001), body mass (K = 1.42, p = 0.001), and CS/LS (K = 0.6, p = 0.047), but not for RCM/RLM (K = 0.18,
p = 0.99).
Felsenstein independent contrast analysis between climatic variables and life-history traits showed a significant inverse correlation between minimum environmental temperature (T°min) and average environmental temperature (T°env) with CS/LS (r2 = -0.43, F = 7.41, p = 0.01 and r2 = -0.33, F = 4.40, p = 0.04; Table 2). Also, body size and body mass showed direct correlations with CS/SL (r2 = 0.56, F = 16.87, p = 0.00021 and r2 = 0.45, F = 7.61, p = 0.009). No correlations between other life history traits or/with environmental variables were detected (Table 2).
Standard statistical analyses only showed moderate to weak correlations between body size (SVL), CS/LS (r2 = 0.29, p = 0.005) and body mass to CS/LS (r2 = 0.15, p = 0.02). No other correlations were detected, not even with any of the environmental variables (Table 3). We found significant variation in body mass between oviparous and viviparous species (t = 2.49, p = 0.02), but we did not find any variation in body size (t = 0.74, p = 0.46). We ran an Ancova using body mass as a covariable to compare CS/LS and RCM/RLM. Variation was only detected for CS/LS (F = 7.75, p = 0.002) among reproductive mode. According to our results oviparous species produce larger clutches than viviparous ones. Reproductive effort (RCM/RLM) did not show any variation (F = 0.32, p = 0.72) between reproductive mode.
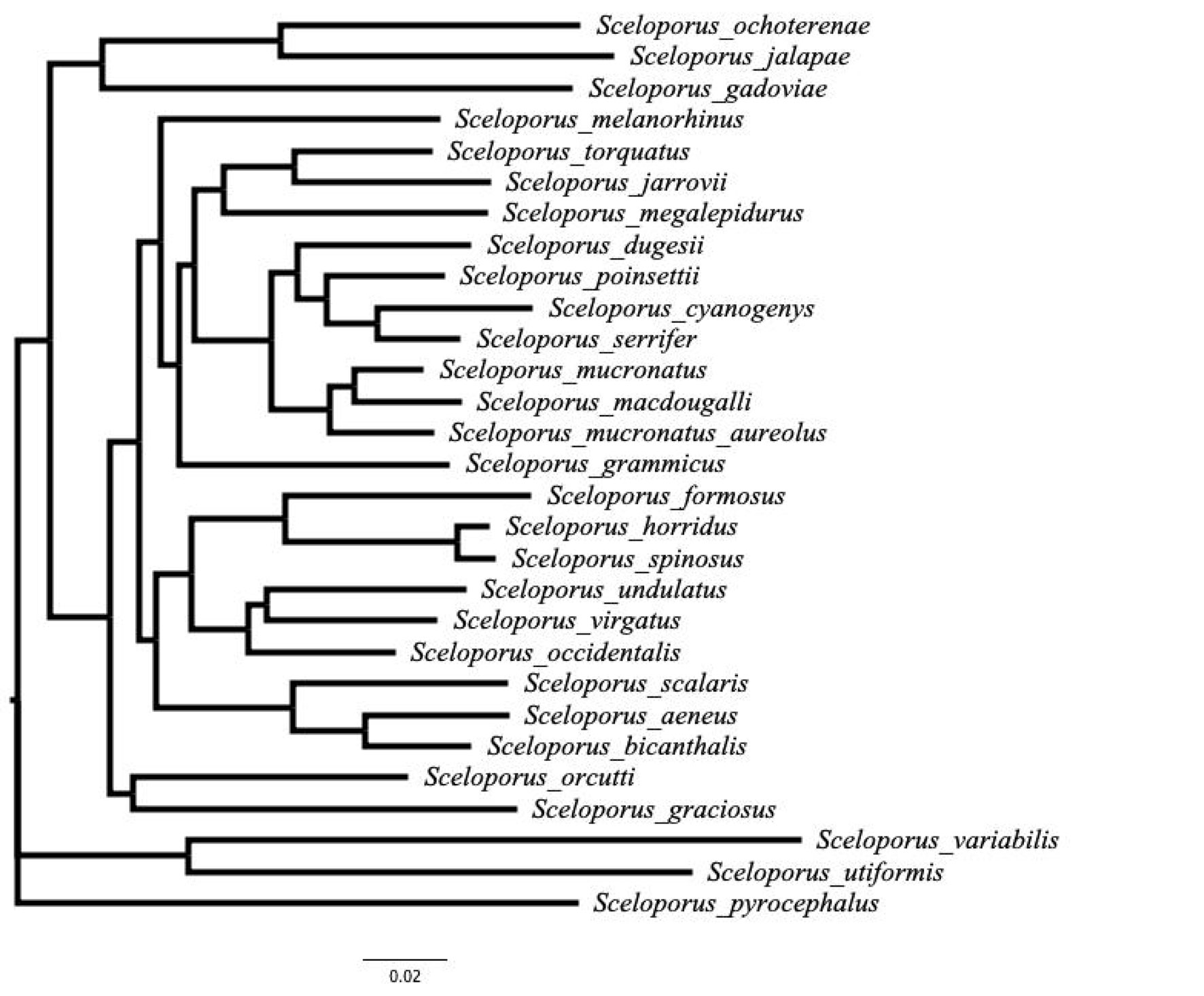
Table 2
Correlations through the origin after phylogenetic independent contrast between life-history traits and climatic variables for the Sceloporine sample. RMC = relative clutch mass, RLM = relative litter mass, CS = clutch size, LS = litter size, body size = snout vent length, T°max = maximum monthly temperature at site, T°min = minimum monthly temperature at site, T°env = mean monthly temperature, Prec(X) = monthly precipitation, SD = standard deviation.
|
Table 2 Continued |
||||||
|
Y |
X |
r2 |
F |
p |
Origin |
Slope |
|
Y |
X |
r2 |
F |
p |
Origin |
Slope |
|
CS/BS |
Body size |
0.56 |
16.87 |
0.00021 |
0.49 |
0.13 |
|
RCM/RLM |
Body size |
0.21 |
1.71 |
0.19 |
-3.07 |
4.78 |
|
CS/BS |
Body mass |
0.45 |
7.61 |
0.009 |
1.26 |
-0.02 |
|
RCM/RLM |
Body mass |
-0.08 |
0.23 |
0.06 |
-0.09 |
-0.31 |
|
CS/BS |
T°max |
-0.21 |
1.68 |
0.20 |
0.41 |
-0.50 |
|
RCM/RLM |
T°max |
-0.16 |
0.09 |
0.32 |
0.53 |
-1.07 |
|
Body size |
T°max |
-0.01 |
0.005 |
0.94 |
0.22 |
-0.12 |
|
Body mass |
T°max |
-0.07 |
0.15 |
0.69 |
0.80 |
-0.15 |
|
CS/BS |
T°min |
-0.43 |
7.41 |
0.01 |
-0.29 |
0.52 |
|
RCM/RLM |
T°min |
-026 |
2.42 |
0.12 |
0.75 |
-0.77 |
|
Body size |
T°min |
-0.04 |
0.06 |
0.80 |
0.11 |
-0.03 |
|
Body mass |
T°min |
-0.11 |
0.30 |
0.58 |
1.67 |
-0.18 |
|
CS/BS |
T°env |
-0.33 |
4.40 |
0.04 |
0.45 |
-0.54 |
|
RCM/RLM |
T°env |
-0.25 |
2.50 |
0.12 |
1.00 |
-1.40 |
|
Body size |
T°env |
-0.13 |
0.61 |
0.43 |
0.34 |
-0.20 |
|
Body mass |
T°env |
-0.17 |
0.90 |
0.34 |
0.23 |
-0.15 |
|
CS/BS |
Prec (X) |
-0.02 |
0.02 |
0.87 |
0.11 |
-0.01 |
|
RCM/RLM |
Prec (X) |
0.05 |
0.09 |
0.76 |
0.39 |
0.004 |
|
Body size |
Prec (X) |
-0.04 |
0.07 |
0.78 |
0.35 |
-0.03 |
|
Body mass |
Prec (X) |
-0.08 |
0.20 |
0.65 |
5.03 |
-0.44 |
Table 3
Correlations between the life-history traits and environmental variables analyzed for the Sceloporine lineage. RMC = Relative clutch mass, RLM = relative litter mass, CS = clutch size, LS = litter size, body size = snout vent length, T°max = maximum monthly temperature at site, T°min = minimum monthly temperature at site, T°env = mean monthly temperature, Prec(X) = monthly precipitation, SD = standard deviation.
|
Y |
X |
r2 |
F |
p |
Origin |
Slope |
|
CS/BS |
Body size |
0.29 |
14.88 |
0.005 |
-2.12 |
0.95 |
|
RCM/RLM |
Body size |
0.03 |
1.2 |
0.28 |
1.32 |
-0.59 |
|
CS/BS |
Body mass |
0.15 |
5.26 |
0.02 |
1.24 |
0.24 |
|
RCM/RLM |
Body mass |
0.044 |
1.31 |
0.26 |
-0.64 |
-0.24 |
|
CS/BS |
T°max |
0.01 |
0.45 |
0.50 |
1.20 |
0.18 |
|
RCM/RLM |
T°max |
0.005 |
0.21 |
0.64 |
-0.34 |
-0.24 |
|
Body size |
T°max |
0.03 |
1.31 |
0.25 |
3.61 |
0.17 |
|
CS/BS |
T°min |
0.002 |
0.07 |
0.78 |
1.95 |
-0.03 |
|
RCM/RLM |
T°min |
0.071 |
-0.28 |
0.37 |
-0.28 |
-0.37 |
|
Body size |
T°min |
0.022 |
0.71 |
0.40 |
4.0 |
0.06 |
|
CS/BS |
T°env |
0.006 |
0.22 |
0.63 |
1.55 |
0.10 |
|
RCM/RLM |
T°env |
0.04 |
1.76 |
0.19 |
0.44 |
-0.54 |
|
Body size |
T°env |
0.004 |
0.14 |
0.70 |
4.06 |
0.04 |
|
CS/BS |
Prec (X) |
0.006 |
0.23 |
0.62 |
1.80 |
0.02 |
|
RCM/RLM |
Prec (X) |
0.06 |
2.59 |
0.11 |
-1.56 |
0.12 |
|
Body size |
Prec (X) |
0.003 |
0.12 |
0.72 |
4.17 |
0.008 |
|
CS/BS |
SD of T°max |
0.0001 |
0.0048 |
0.94 |
1.86 |
0.007 |
|
RCM/RLM |
SD of T°max |
0.0002 |
0.009 |
0.922 |
-1.17 |
0.02 |
|
Body size |
SD of T°max |
0.00006 |
0.002 |
0.96 |
4.20 |
-0.003 |
|
CS/BS |
SD of T°min |
0.01 |
0.37 |
0.54 |
1.83 |
0.062 |
|
RCM/RLM |
SD of T°min |
0.013 |
0.50 |
0.48 |
-1.23 |
0.13 |
|
Body size |
SD of T°min |
0.05 |
2.23 |
0.14 |
4.15 |
0.08 |
|
CS/BS |
SD of T°env |
0.0008 |
0.03 |
0.86 |
1.86 |
0.01 |
|
RCM/RLM |
SD of T°env |
0.0007 |
0.028 |
0.86 |
-1.17 |
0.03 |
|
Body size |
SD of T°env |
0.01 |
0.58 |
0.44 |
4.17 |
0.04 |
|
CS/BS |
SD of Prec (X) |
0.01 |
0.40 |
0.53 |
1.80 |
0.024 |
|
RCM/RLM |
SD of Prec (X) |
0.04 |
1.82 |
0.18 |
-1.42 |
0.09 |
|
Body size |
SD of Prec (X) |
0.02 |
1.00 |
0.32 |
4.14 |
0.02 |
Discussion
As expected, and described in single species studies (e.g., Abell, 1999; Benabib, 1994; Feria et al., 2001; Gadsden et al., 2005; Mathies & Andrews, 1995), clutch/litter size was positively correlated with body size. Longer females produce larger clutches or litters, which under certain environmental conditions is advantageous to increase fitness (Roff, 2002; Stearns, 1992). According to our results, at lower environmental temperatures, females tend to produce smaller clutches/litters, which seem to be a direct response to a critical thermal environment. We did not test the correlation of hatchling size with environmental variables, but the clutch/litter size correlation with body size suggests a trade-off resolution through few, but large offspring in cooler habitats. This response seems logical to provide offspring with more mass and energy in colder environments, however the production of more offspring (with smaller size) in tropical environments (also more thermally stable) could be selected (Mesquita, Costa et al., 2016; Mesquita, Gomes-Faria et al., 2016). Our interpretation agrees with Bergmann’s Rule, which has been tested with several groups of vertebrates such as mammals (Ashton et al., 2000), turtles (Lewis et al., 2018), lizards, and snakes (Angilletta et al., 2004; Ashton & Feldman, 2003).
Our analysis showed that oviparous and viviparous species have equal CS/LS and reproductive effort when the effect of body size is removed, this result agrees with the correlation of body size with CS/LS and indicates that body size has a deep influence in CS/LS and then in life history variation, which also agrees with Mesquita, Costa et al. (2016) who did not find differences in CS/LS among several lineages of lizards with different reproductive mode. Oviparous and viviparous species did not differ in their reproductive output, nevertheless, both reproductive modes have been hypothesized as adaptations to thermal conditions (cold weather hypothesis; Lawing et al., 2016; Martínez-Méndez et al., 2019; Shine, 2004) rather than reproductive output strategies. Results from Zúñiga-Vega et al. (2016) support that the correlation between life history traits with environmental factors in prhynosomoatid lizards, could be related to their evolutionary history under cooling conditions.
Bloomberg et al. (2003) mentioned that the K statistic for the phylogenetic signal could show high values for large sample sizes when concerning body size or body mass. We obtained higher values for both traits. Then, according to this interpretation, reproductive output could potentially be affected by the phylogenetic inertia of body size and its correlation with other reproductive output traits, however, reproductive effort seems not to be affected by the phylogenetic signal, body size or any of the environmental variables tested. It was interesting to discover a correlation of reproductive effort with climatic variation. This also has been reporterd for other groups of lizards (Mesquita, Costa et al., 2016), snakes (Shine & Schwarzkopf, 1992), and turtles (Macip-Ríos et al., 2017). Recently, Mesquita, Gomes-Faria et al. (2016) argued that lizard life history strategies could be classified by clutch size, the age of maturity, and investment per progeny (reproductive effort). Based on these 3 axes, lizards occupy a narrow space in a 3D plot, going from low clutch size, low age at maturity, and middle investment per progeny, to low clutch size, delayed age at maturity, and low reproductive effort. It seems that Sceloporine lizards fit the general pattern. However, our lack of data of several life-history traits to compare with Mesquita, Gomes-Faria et al. (2016) data limits the overall interpretation.
Our results agree with Mesquita, Costa et al. (2016) main conclusions of high phylogenetic signal in RCM/RLM, CS/LS, and body size and correlation between climatic variables with life-history traits. It seems that deep evolutionary history has an important role in life-history variation. Compared with Mesquita, Costa et al. (2016) and other studies of life-history patterns in lizards (Dunham et al., 1988; Mesquita, 2010; Miles & Dunham, 1992; Vitt & Congdon, 1978; Zúñiga-Vega et al., 2016), our results match in a general way what has been described as lizard life history strategy, however it is interesting how the reproductive mode in the life-history traits mentioned above has no influence (except CS/LS). Recently Zúñiga-Vega et al. (2016) also documented the same lack of influence of reproductive mode and suggested how viviparity could constraint the evolution of life-history traits, rather than oviparity. Because our main objective was to test the bet-hedging strategy, we consider that Sceloporus lizards partially followed the bet-hedging predictions, nevertheless, other life-history models should be tested in Sceloporine lizards such as the fast-slow continuum hypothesis as pointed out by Pérez-Mendoza and Zúñiga-Vega (2014).
Since climate change will affect thermal niches, lizard life-history could face severe constraints that could reduce species geographic distributions and the viability of local populations (Gadsden et al., 2018; Martínez-Méndez et al., 2019). Lawing et al. (2016) demonstrated that Sceloporine lizards diversified during the last ice age, and viviparity became an important trait to colonize new environments, therefore during global warming and generalized climate change, the perspectives of survivorship of this group of lizards could be imperiled. Sinervo et al. (2010) demonstrated important population exctinctions on the genus Sceloporus by climate change due to a direct consequence of the restrictions that high environmental temperatures impoose on their activity out of shelters, which reduces their food intake and their reproductive cycles. Moreover, Sceloporine lizards exhibit a conserved physiology relative to reproductive traits (high phylogenetic signal). A good example of this is the narrow temperature range of healthy embryo development, which occurs between 31.3 ± 0.2 and 32.8 ± 0.1 °C (Andrews et al., 1997). In addition, it is also known that most reptiles have limited dispersal abilities and Sceloporine lizards are not the exception. To face a fast-climatic change, spiny lizards may not be able to migrate in the short term to sites with suitable conditions. Consequently, these lizards are among the most vulnerable groups of organisms threatened by climatic change (Araujo et al., 2006; Carvalho et al., 2010; Chen et al., 2011).
Nevertheless, lizards are not inert biological entities unable to respond to environmental variation, it would be reasonable to expect that they may show some type of adjustment of life history traits to thermal conditions when climate changes occur. Environmental temperatures can exert strong selection pressures and some species may exhibit rapid adaptation, either genetically regulated or by phenotypic plasticity (Moritz et al., 2012). Hence, a thermal regime that spiny lizards could experiment could drive changes in their life history traits (if there is enough heritability; Bradshaw & Holzapfel, 2006; Kellermann et al., 2012) and confer resiliency to warmer thermal conditions in a relatively short period of time, and then favor populations to increase their survival probabilities or face local extinctions (Leal & Gunderson, 2012; Logan et al., 2014; Sinervo et al., 2010).
Acknowledgments
This study was partially funded by Conacyt scholarships during graduate studies of SLA and RMR. Rodrigo Macip thanks the Benemérita Universidad Autónoma de Puebla, which supported his position during the final development of this study. Two anonymous reviewers made important comments to improve this manuscript.
References
Abell, J. A. (1999). Variation in clutch size and offspring size relative to environmental conditions in the Lizard Sceloporu virgatus. Journal of Herpetology, 33, 173−180. https://doi.org/10.2307/1565712
Andrews, R. M., Qualls, C. P., & Rose, B. R. (1997). Effects of low temperature on embryonic development of Sceloporus lizards. Copeia, 1997, 827–833.
Andrews, R. M., & Schwarzkopf, L. (2012). Thermal performance of squamate embryos with respect to climate, adult life history, and phylogeny. Biological Journal of the Linnean Society, 106, 851−864. https://doi.org/10.1111/j.1095-8312.2012.01901.x
Angilletta, M. J. (2009). Thermal adaptation: a theorical and empirical synthesis. Oxford: Oxford University Press.
Angilletta, M. J., Niewiarowski, P. H., Durham, A. E., Leaché, A., & Porter, W. P. (2004). Bergmann’s clines in ectotherms: illustrating a life-history perspective with Sceloporine lizards. The American Naturalist, 164, E168−E183. https://doi.org/10.1086/425222
Angilletta, M. J., Sears, M. W., & Winters, R. S. (2001). Seasonal variation in reproductive effort and its consequences for offspring size in the lizard Sceloporus undulatus. Herpetologica, 57, 365−375.
Araujo, M. B., Thuiller, W., & Pearson, R. G. (2006). Climate warming and the decline of amphibians and reptiles in Europe. Journal of Biogeography, 33, 1712–1728. https://doi.org/10.1111/j.1365-2699.2006.01482.x
Ashton, K. G., & Feldman, C. R. (2003). Bergamann’s rule in non-avian reptiles: turtles follow it, lizards and snakes reverse it. Evolution, 57, 1151−1163. https://doi.org/10.1554/0014-3820(2003)057[1151:brinrt]2.0.co;2
Ashton, K. G., Tracy, M. C., & de Querioz, K. (2000). Is Bergmann’s rule valid for mammals? The American Naturalist, 156, 390−415. https://doi.org/10.1086/303400
Benabib, M. (1994). Reproduction and lipid utilization of tropical populations of Sceloporus variabilis. Herpetological Monographs, 8, 160−180. https://doi.org/10.2307/1467079
Bloomberg, S. P., Garland, T., & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57, 717−745. https://doi.org/10.1554/0014-3820(2003)057[0717:tfpsic]2.0.co;2
Bradshaw, W. E., & Holzapfel, C. M. (2006). Evolutionary Response to Rapid Climate Change. Science, 312, 1477−1478. https://doi.org/10.1126/science.1127000
Brandt, R., & Navas, C. A. (2011). Life-history on Tropidurinae lizards: influence on lineage, body size and climate. Plos One, 6, e20040. https://doi.org/10.1371/journal.pone.0020040
Breandle, C., Heyland, A., & Flatt, T. (2011). Integrating mechanistic and evolutionary life history evolution. In T. Flatt, & A. Heyland (Eds.), Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs (pp. 3−10). Oxford: Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199568765.003.0001
Bustos-Zagal, M. G., Méndez-de la Cruz, F. R., Castro-Franco, R., & Villagrán-Santa Cruz, M. (2011). Ciclo reproductor de Sceloporus ochoterenae en el estado de Morelos, México. Revista Mexicana de Biodiversidad, 82, 589−597. https://doi.org/10.22201/ib.20078706e.2011.2.471
Carvalho, S. B., Brito, J. C., Crespo, E. J., & Possingham, H. P. (2010). From climate change predictions to actions – conserving vulnerable animal groups in hotspots at a regional scale. Global Change Biology, 16, 3257–3270. https://doi.org/10.1111/j.1365-2486.2010.02212.x
Chamaillé-Jammes, S., Massot, M., Aragon P., & Clobert, J. (2006). Global warning and positive fitness response in mountain populations of common lizards Lacerta vivipara. Global Change Biology, 12, 292−402. https://doi.org/10.1111/j.1365-2486.2005.01088.x
Charnov, E. L. (2002). Reproductive effort, offspring size, and benefit-cost ratios in the classification of life histories. Evolutionary Ecology Research, 4, 749−758.
Charnov, E. L. (2005). Reproductive effort is inversely proportional to average adult life span. Evolutionary Ecology Research, 7, 1221−1222.
Chen, I. C., Hill, J. K., Ohlemüller, R., Roy, D. B., & Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science, 333, 1024. https://doi.org/10.1126/science.1206432
Cuellar, O. (1984). Reproduction in a parthenogenetic lizars: with a discussion of optimal clutch size and critique of the clutch weigth/body weigth ratio. American Midland Naturalist, 111, 242−258. https://doi.org/10.2307/2425319
Deutsch, C., Tewksbury, J. J., Huey, R. B., Sheldon, K., Ghalambor, C. K., Haak, D. C. et al. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences Biology, 105, 6668−6672. https://doi.org/10.1073/pnas.0709472105
Dunham, A. E., Miles, D. B., & Reznick, D. N. (1988). Life history patterns in squamate reptiles. In C. Gans, & R. B. Huey (Eds.), Biology of the Reptilia. Vol. 16: Ecology B. Defense and life history (pp.441–522). New York: Alan R. Liss.
Enium, S., & Felmin, I. A. (2004). Environmental unpredictability and offspring size: conservative versus diversified bet-hedging. Evolutionary Ecology and Research, 6, 443−455.
Felsenstein, J. (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1−15.
Felsenstein, J. (2008). Comparative methods with sampling error and within-species variation: contrasts revisited and revised. The American Naturalist, 171, 713−725. https://doi.org/10.1086/587525
Ferguson, G. W., & Snell, H. L. (1986). Endogenous control of seasonal change of egg, hatchling, and clutch size of the lizard Sceloporus undulatus garmani. Herpetologica, 42, 185−191.
Feria, O. M., Nieto, M. A., & Salgado, U. I. (2001). Diet and Reproductive Biology of the Viviparous Lizard Sceloporus torquatus torquatus (Squamata: Phrynosomatidae). Journal of Herpetology, 35, 104−112. https://doi.org/10.2307/1566029
Flores-Villela, O., & García-Vázquez, U. O. (2014). Biodiversidad de reptiles en México. Revista Mexicana de Biodiversidad, 85, S467−S475. https://doi.org/10.7550/rmb.43236
Gadsden, H., Rodríguez, R. F., Méndez-de la Cruz, F., & Gil, M. R. (2005). Ciclo reproductor de Sceloporus poinsettii Bair y Girard 1852 (Squamata: Phynosomatidae) en el centro del desierto chihuahuense. Acta Zoológica Mexicana (n.s), 21, 93−107. https://doi.org/10.22201/ib.20078706e.2011.2.471
Gadsden, H., Ruiz, S., Castañeda, G., & Lara-Reséndiz, R. A. (2018). Selected body temperature in Mexican lizard species. Global Journal of Ecology, 3, 1−4. https://doi.org/10.17352/gje.000007
Garland, T., Dickerman, A. W., Janis, C. M., & Jones, J. A. (1993). Phylogenetic analysis of covariance by computer simulation. Systematic Biology, 42, 265−292.
Garland, T., & Ives, A. R. (2000). Using the past to predict the present: Confidence intervals for regression equations in phylogenetic comparative methods. The American Naturalist, 155, 346−364. https://doi.org/10.1086/303327
Garland T., Midford, P. E., & Ives, A. R. (1999). An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral states. American Zoologist, 39, 374−388. https://doi.org/10.1093/icb/39.2.374
Garrick, L. D. (1974). Reproductive influences on behavioral thermoregulation in the lizard, Sceloporus cyanogenys. Physiological Behavior, 12, 85−91. https://doi.org/10.1016/0031-9384(74)90072-9
Geer, L. Y., Marchler-Bauer, A., Geer, R. C., Han, L., He, J., He, S. et al. (2010). The NCBI BioSystems database. Nucleic Acids Research, 38, D492−D496. https://doi.org/10.1093/nar/gkp858
Goolsby, E. W. (2015). Phylogenetic comparative methods for evaluating the evolutionary history of function-valued traits. Systematic Biology, 64, 568−578. https://doi.org/10.1093/sysbio/syv012
Grigg, J. W., & Buckley, L. B. 2013. Conservation of lizard thermal tolerances and body temperatures across evolutionary history and geography. Biology Letters, 9, 20121056. https://doi.org/10.1098/rsbl.2012.1056
Guillette, L. J., & Casas-Andreu, G. (1980). Fall reproductive activity in the high-altitude Mexican lizard, Sceloporus grammicus microlepidotus. Journal of Herpetology, 14, 143−147. https://doi.org/10.2307/1563845
Guillette, L. J., & Méndez-de la Cruz, F. R. (1993). The reproductive cycle of the viviparous Mexican lizard Sceloporus torquatus. Journal of Herpetology, 27, 168−174. https://doi.org/10.2307/1564933
Harvey, P., & Pagel, M. (1991). The comparative method in Evolutionary Biology. Oxford: Oxford University Press.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high-resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965−1978. https://doi.org/10.1002/joc.1276
Huey, R. B., Kearney, M. R., Krockenberger, A., Holtum, J. M., Jess, M., & Williams, S. E. (2012). Predicting organismal vulnerability to climate warming: roles of behavior, physiology, and adaptation. Philosophical Transactions of the Royal Society London B, 367, 1665−1679. https://doi.org/10.1098/rstb.2012.0005
Hunsaker, D. (1959). Birth and utter sizes of the blue spiny lizard Scelporus cyanogenys. Copeia, 1959, 260−261. https://doi.org/10.2307/1440408
IPCC (Intergovernmental Panel on Climate Change). (2018). Summary for Policymakers. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Geneva, Switzerland: World Meteorological Organization.
Kellermann, V., Loeschcke, V., Hoffmann, A. A., Kristensen, T. N., Flojgaard, C., David, J. R. et al. (2012). Phylogenetic constraints in key functional traits behind species’ climate niches: patterns of desiccation and cold resistance across 95 Drosophila species. Evolution, 66, 3377−3389. https://doi.org/10.1111/j.1558-5646.2012.01685.x
Lambert, S. M., & Wiens, J. J. (2013). Evolution of viviparity: a phylogenetic test of the cold-climate hypothesis in phrynosomatid lizards. Evolution, 67, 2614−2630. https://doi.org/10.1111/evo.12130
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H. et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947−2948. https://doi.org/10.1093/bioinformatics/btm404
Lawing, A. M., Polly, P. D., Hews, D. K., & Martins, E. P. (2016). Including fossils in phylogenetic climate reconstructions: A deep time perspective on the climatic niche evolution and diversification of spiny lizards (Sceloporus). The American Naturalist, 188, 133−148. https://doi.org/10.1086/687202
Leaché, D. A. (2010). Species trees for spiny lizards (Genus Sceloporus): Identifying points of concordance and conflict between nuclear and mitochondrial data. Molecular Phylogenetics and Evolution, 54, 162−171. https://doi.org/10.1016/j.ympev.2009.09.006
Leal, M., & Gunderson, A. R. (2012). Rapid change in the thermal tolerance of a tropical lizard. The American Naturalist, 180, 815−22. https://doi.org/10.1086/668077
Lewis, E. L., Iverson, J. B., Smith, G. R., & Reting, J. E. (2018). Body size and growth in the red-eared slider (Trachemys scripta elegans) at the northern edge of its range: Does Bergmann’s rule apply? Herpetological Conservation and Biology, 13, 700−710.
Logan, M. L., Cox, R. M., & Calsbeek, R. (2014). Natural selection on thermal performance in a novel thermal environment. Proceedings of National Academy of Sciences Biology, 111, 14165−14169. https://doi.org/10.1073/pnas.1404885111
Macip-Ríos, R., Ontiveros, R. N., Sánchez-León, A. T., & Casas-Andreu, G. (2017). Evolution of reproductive effort in mud turtles (Kinosternidae): the role of environmental predictability. Evolutionary Ecology and Research, 18, 539−554.
Maddison, W. P., & Maddison, D. R. (2018). Mesquite: a modular system for evolutionary analysis. Version 3.51. http://mesquiteproject.org
Martínez-Méndez, N., Mejía, O., Ortega, J., & Méndez-de la Cruz, F. (2019). Climatic niche evolution in the viviparous Sceloporus torquatus group (Squamata: Phrynosomatidae). PeerJ, 6, e6192. https://doi.org/10.7717/peerj.6192
Mathies, T., & Andrews, R. M. (1995). Thermal and reproductive biology of high and low elevation populations of the lizard Sceloporus scalaris: implications for the evolution of viviparity. Oecologia, 104, 101−111. https://doi.org/10.1007/bf00365568
Mayhew, W. W. (1963). Reproduction of the granite spiny lizard, Sceloporus orcutii. Copeia, 1963, 144−152. https://doi.org/10.2307/1441282
Mesquita, D. O. (2010). Life history patterns in South American tropical lizards. In O. H. Gallegos, F. Méndez-de la Cruz, & J. F. Méndez-Sánchez (Eds.), Reproducción en reptiles: morfología, ecología y evolución (pp. 45−71). Toulca, México: Universidad Autónoma del Estado de México.
Mesquita, D. O., Costa, G. C., Colli, G. R., Costa, T. B., Shepard, D. B., Vitt, L. J. et al. (2016). Life-history patterns of lizards of the world. The American Naturalist, 187, 689−705. https://doi.org/10.1086/686055
Mesquita, D. O., Gomes-Faria, R., Colli, G. R., Vitt, L. J., & Pianka, E. R. (2016). Lizard life-histories strategies. Australian Ecology, 41, 1−5. https://doi.org/10.1111/aec.12276
Meiri, S., Bauer, A. M., Chirio, L., Colli, G. R., Das, I., Doan, T. M. et al. (2013). Are lizards feeling the heat? A tale of ecology and evolution under two temperatures. Global Ecology and Biogeography, 22, 834−845. https://doi.org/10.1111/geb.12053
Miles, D. B., & Dunham, A. E. (1992). Comparative analyses of phylogenetic effects in the life-history patterns of iguanid reptiles. The American Naturalist, 139, 848−869. https://doi.org/10.1086/285361
Moritz, C., Langham, G., Kearney, M., Krockenberger, A., Van DerWal, J., & Williams, S. (2012). Integrating phylogeography and physiology reveals divergence of thermal traits between central and peripheral lineages of tropical rainforest lizards. Philosophical Transactions of the Royal Society London Biology, 367, 1680−1687. https://doi.org/10.1098/rstb.2012.0018
Münkemüller, T., Lavergne, S., Bzeznik, B., Dray, S., Jombart, T., Schiffers, K. et al. (2012). How to measure and test phylogenetic signal. Methods in Ecology and Evolution, 3, 743−756. https://doi.org/10.1111/j.2041-210x.2012.00196.x
Pérez-Mendoza, H., & Zúñiga-Vega, J. J. (2014). A test of the fast-slow continuum model of life-history variation in the lizards Scelporus grammicus. Evolutionary Ecology Research, 16, 235−248.
Posada, D. (2008). jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution, 25, 1253−1256. https://doi.org/10.1093/molbev/msn083
Posada, D., & Crandall, K.A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics, 1, 817−818. https://doi.org/10.1093/bioinformatics/14.9.817
R Development Core Team. (2008): R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available: http://www.R-project.org
Ramírez-Bautitsa, A., & Dávila, U. E. (2009). Reproductive characteristics of a population of Sceloporus dugesii (Squamata: Phrynosomatidae) from Michoacán, México. The Southwestern Naturalist, 54, 400−408. https://doi.org/10.1894/gc-164.1
Ramírez-Bautista, A., & Gutiérrez-Mayén, G. (2003). Reproductive ecology of Sceloporus utiformis (Sauria: Prhynosomatidae) from a tropical dry forest of México. Journal of Herpetology, 37, 1−10. https://doi.org/10.1670/0022-1511(2003)037[0001:reosus]2.0.co;2
Ramírez-Bautista, A., Hernández-Ramos, D., Rojas, A., & Marshall, J. C. (2009). Fat bodies and liver mass cycles in Sceloporus grammicus (Squamata: Phrynosomatidae) from southern Hidalgo. Herpetological Conservation and Biology, 4, 164−170.
Ramírez-Bautista, A., Jiménez, C. E., & Marshall, C. J. (2004): Comparative life history for populations of the Sceloporus grammicus complex (Squamata: Phrynosomatidae). Western North American Naturalist, 64, 175−183.
Ramírez-Bautista, A., Luja, V. H., Balderas-Valdivia, C., & Ortíz-Pulido, R. (2006). Reproductive cycle of male and female spiny lizards, Sceloporus melanorhinus, in a tropical dry forest. The Southwestern Naturalist, 51, 157−162. https://doi.org/10.1894/0038-4909(2006)51[157:rcomaf]2.0.co;2
Ramírez-Bautitsa, A., & Olvera-Becerril, V. (2004): Reproduction in the Boulder spiny lizard, Sceloporus pyrocephalus (Sauria: Phynosomatidae) from a tropical Dry forest of México. Journal of Herpetology, 38, 225−231. https://doi.org/10.1670/200-01a
Ramírez-Bautista, A., Ortiz-Cruz, A. L., Arizmendi, M. C., & Campos, J. (2005). Reproductive characteristics of two syntopic lizard species, Sceloporus gadoviae and Sceloporus jalapae (Squamata: Phrynosomatidae), from Tehuacan Valley, Puebla, Mexico. Western North American Naturalist, 65, 202−209.
Ramírez-Bautista, A., & Pavón, N. P. (2009). Sexual dimorphism and reproductive cycle in the arboreal spiny lizard Sceloporus formosus Weigman (Squamata: Phrynosomatidae) from central Oxaca, Mexico. Revista Chilena de Historia Natural, 82, 553−563. https://doi.org/10.4067/s0716-078×2009000400009
Ramírez-Bautista, A., Ramos-Flores, O., & Sites, J. W. (2002). Reproductive cycle of the spiny lizard Sceloporus jarrovii (Sauriua: Phrynosomatidae) from north-central Mexico. Journal of Herpetology, 36, 225−233. https://doi.org/10.1670/0022-1511(2002)036[0225:rcotsl]2.0.co;2
Revell, L. J. (2012). phytools: A R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217−223. https://doi.org/10.1111/j.2041-210x.2011.00169.x
Rodríguez-Romero, F., Méndez-de la Cruz, F., García-Collazo, R., & Villagrán-Santa Cruz, M. (2002). Comparación del esfuerzo reproductor en dos especies hermanas del género Sceloporus (Sauria: Phrynosomatidae) con diferente modo reproductor. Acta Zoológica Mexicana (n.s), 85, 181−188.
Rodríguez-Romero, F., Méndez. R. F., & López, G. L. (2005). Análisis comparado del esfuerzo reproductor en algunos lacertilios mexicanos de ambiente tropical y templado. Revista de la Sociedad Mexicana de Historia Natural, 2, 168−177.
Rodríguez-Romero, F., Smith, G. R., Cuellar, O., & Méndez-de la Cruz, F. R. (2004). Reproductive traits of a high elevation viviparous lizard Sceloporus bicanthalis (Lacertilia: Phrynosomatidae) from Mexico. Journal of Herpetology, 38, 438–443. https://doi.org/10.1670/7-04n
Roff, D. (1981). Reproductive uncertainty and the evolution of iteroparity: why don’t flatfish put all their eggs in one basket? Canadian Journal of Fish and Aquatic Science, 38, 968−977. https://doi.org/10.1139/f81-130
Roff, D. (2002). Life history evolution. Sunderland, Massachusetts: Sinauer.
SAS Institute. (2002). JMP. Statistical Discovery Software. Ver. 5.01. Cary, North Carolina.
Scheffers, B. R., Brunner, R., Ramírez, S., Shoo, L. P., Diesmos, A., & Williams, S. E. (2013). Thermal buffering of microhabitats is a critical factor mediating warming vulnerability of frogs in the Philippine biodiversity hotspot. Biotropica, 45, 628−635. https://doi.org/10.1111/btp.12042
Shine, R. (2004). Does viviparity evolve in cold climatic reptiles because pregnant females maintain stable (not high) body temperatures? Evolution, 58, 1809−1818. https://doi.org/10.1554/04-123
Shine, R., & Brown, G. P. (2008). Adapting to the unpredictable: reproductive biology of the vertebrates in the Australian wet-dry tropics. Philosophical Transactions of the Royal Society London B Biology, 363, 363−373. https://doi.org/10.1098/rstb.2007.2144
Shine, R., & Schwarzkopf, L. (1992). The evolution of reproductive effort in lizards and snakes. Evolution, 46, 62−75. https://doi.org/10.2307/2409805
Silvestro, D., & Michalak, I. (2011). raxlmGUI: a graphical front-end for RAxLM. Organisms Diversity and Evolution, 12, 335−337. https://doi.org/10.1007/s13127-011-0056-0
Sinervo, B., Hedges, R., & Adolph, S. C. (1991). Decreased sprint speed as a cost of reproduction in the lizard Sceloporus occidentalis: variation among populations. Journal of Experimental Biology, 155, 323−336.
Sinervo, B., Méndez-de la Cruz, F., Miles, D. B., Heulin, B., Bastiaans, E., Villagran-Santa Cruz, M. et al. (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science, 328, 894−899. https://doi.org/10.1126/science.1184695
Smith, C. C., & Fretwell, S. D. (1974). The optimal balance between size and number of offspring. The American Naturalist, 108, 499−506. https://doi.org/10.1086/282929
Smith, R. G., Ballinger, E. R., & Rose. R. B. (1995). Reproduction in Sceloporus virgatus from the Chiricahua mountains of southeastern Arizona with emphasis on annual variation. Herpetologica, 51, 342−349.
Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312−1313. https://doi.org/10.1093/bioinformatics/btu033
Stearns, S. C. (1989). Trade-offs in life history evolution. Functional Ecology, 3, 259−268. https://doi.org/10.2307/2389364
Stearns, S. C. (1992). The evolution of life histories. New York: Oxford University Press.
Tewksbury, J. J., Huey, R. B., & Deustch, C. A. (2008). Putting the heat on tropical animals. Science, 320, 1296−1297. https://doi.org/10.1126/science.1159328
Tinkle, D. W. (1969). The concept of reproductive effort and its relation to the evolution of the life histories of lizards. The American Naturalist, 103, 501−516. https://doi.org/10.1086/282617
Tinkle, D. W., Dungam, A. E., & Congdon, H. D. (1993). Life history and demographic variation in the lizard Sceloporus graciosus: A long-term study. Ecology, 74, 2413−2429. https://doi.org/10.2307/1939592
Tinkle, D. W., & Hadley, N. F. (1975). Lizard reproductive effort: caloric estimates and comments on its evolution. Ecology, 56, 427−434. https://doi.org/10.2307/1934973
Tinkle, D. W., Wilbur, H. M., & Tilley, S. G. (1970). Evolutionary strategies in lizard reproduction. Evolution, 24, 55−74. https://doi.org/10.1111/j.1558-5646.1970.tb01740.x
Valdéz-González, M., & Ramírez-Bautista, A. (2002). Reproductive characteristics of spiny lizard, Sceloporus horridus and Sceloporus spinosus (Squamata: Phrynosomatidae) from México. Journal of Herpetology, 36, 36−43. https://doi.org/10.1670/0022-1511(2002)036[0036:rcotsl]2.0.co;2
Villagrán, M., Hernández, G. O., & Méndez-de la Cruz, F. (2009). Reproductive cycle of the lizard Sceloporus mucronatus with comments on intraspecific geographic variation. Western North American Naturalist, 69, 437−446. https://doi.org/10.3398/064.069.0403
Vitt, L. J., & Congdon, J. D. (1978). Body shape, reproductive effort, and relative clutch mass in lizards: resolution of a paradox. The American Naturalist, 112, 595−608. https://doi.org/10.1086/283300
Vitt, L. J., & Price, H. J. (1982). Ecological and evolutionary determinants of relative clutch mass in lizards. Herpetologica, 38, 237−255.
Walters, R. J., Blanckenhorn, W. U., & Berger, D. (2012). Forecasting extinction risk of ectotherms under climate warming: an evolutionary perspective. Functional Ecology, 26, 1324−1338. https://doi.org/10.1111/j.1365
-2435.2012.02045.x
Wiens, J. J., Kuczynski, C. A., Arif, S., & Reeder, T. W. (2010). Phylogenetic relationships of phrynosomatid lizards based on nuclear and mitochondrial data, and a revised phylogeny for Sceloporus. Molecular Phylogenetics and Evolution, 54, 150−161. https://doi.org/10.1016/j.ympev.2009.09.008
Wiens, J. J., & Reeder, T. W. (1997). Phylogeny of the spiny lizards (Sceloporus) based on molecular and morphological evidence. Herpetological Monographs, 11, 1−101. https://doi.org/10.2307/1467007
Zar, J. (1999). Biostatiscal analysis. 4th Ed. New Jersey: Prentice Hall.
Zúñiga-Vega, J. J., Fuentes, G. J. A., Ossip-Drahos, A. G., & Martins, E. P. (2016). Repeated evolution of viviparity in phrynosomatid lizards constrained interspecific diversification in some life-history traits. Biology Letters, 12, 20160653. https://doi.org/10.1098/rsbl.2016.0653