Temporal dynamics of detected transgenes in maize landraces in their center of origin
Beatriz Rendón-Aguilar *, Luis Alberto Bernal-Ramírez, David Bravo-Avilez, Martha Graciela Rocha-Munive
Departamento de Biología, Universidad Autónoma Metropolitana-Iztapalapa, Avenida San Rafael Atlixco Núm. 186, Colonia Vicentina, 09360 Ciudad de México, Mexico
*Corresponding author: bra@xanum.uam.mx (B. Rendón-Aguilar)
Abstract
The first report of transgenic maize in Mexico was published in 2001 for Oaxaca, Mexico. Subsequent studies were controversial, because most of the maize samples analyzed from different years and localities, were negative. The purpose of this paper is to publish records of the presence of transgenic maize in Priority Terrestrial Regions in Oaxaca, and to contribute to the understanding of the movement of transgenic maize through seed management practices. During 2008-2018, 66 municipalities and 148 localities, belonging to 3 PTRs were monitored. A total of 1,210 farmers were surveyed on practices associated with maize management, and 1,412 maize samples were obtained and analyzed using molecular methods. Adventitious presence of transgenic sequences was detected in 1.69% of the samples. The positive samples came from municipalities belonging to Sierra Juárez, Los Loxicha region, and Santa María Huatulco. Farmers’ responses indicated that there is a high movement of seed among localities, but also from other areas of the country. Transgenes appear and disappear, indicating a highly dynamic spatio-temporal replacement over time, mainly due to introduction of foreign material. This aspect must be taken into account to regulate introduction of improved maize to traditional communities.
Keywords:
Zea mays; Diversity; Priority Terrestrial Region; Real-time PCR; GMO detection
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Dinámica temporal de transgenes detectados en variedades nativas de maíz en su centro de origen
Resumen
El primer reporte sobre maíz transgénico presente en las variedades nativas en su centro de origen se publicó en 2001 para Oaxaca, México. Las investigaciones posteriores realizadas en el mismo estado fueron controvertidas: la mayoría de las muestras de maíz analizadas en el periodo de 2001 a 2004 fueron negativas y solo unas pocas muestras resultaron positivas para la presencia de elementos transgénicos en diferentes localidades y años. El propósito de este artículo es documentar la presencia de maíz transgénico en las regiones terrestres prioritarias (RTP) de Oaxaca y contribuir a la comprensión del movimiento del maíz transgénico a través de las prácticas de manejo de semillas. Durante el período 2008-2018, se monitorearon 66 municipios y 148 localidades pertenecientes a 3 RTP. Se encuestó a un total de 1,210 agricultores sobre las prácticas de manejo del maíz y se obtuvieron 1,412 muestras de maíz nativo que se analizaron mediante métodos moleculares. La presencia adventicia de secuencias transgénicas se detectó en 1.69% de las muestras. Las muestras positivas provinieron de municipios de la sierra de Juárez, Los Loxicha (sierra Sur) y Santa María Huatulco en la costa del Pacífico. Las respuestas de los agricultores indicaron que hay un gran movimiento de semillas entre las localidades, pero también se introducen semillas de otras áreas del país. Los datos muestran episodios de aparición y desaparición de los transgenes, lo que indica un reemplazo espacio-temporal altamente dinámico a lo largo del tiempo, causado principalmente por la introducción de material externo. Este último aspecto debe ser tomado en cuenta por las autoridades para la regulación de la introducción de semillas mejoradas a las comunidades tradicionales donde las variedades locales son la base de su subsistencia.
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Zea mays; Diversidad; Regiones Terrestres Prioritarias; PCR tiempo real; Detección de OGM
Introduction
Mexico is the center of origin and domestication of maize. In particular, Oaxaca, in the southeast of the country contains an important proportion of the morphological and genetic diversity of the species. The state contains 54.8% of the 64 agronomic races reported for the country (Aragón-Cuevas et al., 2002, 2005). The maintenance of this diversity is very important in the indigenous and mestizo communities, as more than 90% of the area destined to maize cultivation is planted with some of the landraces managed in traditional farming systems (Aragón-Cuevas et al, 2011). There, farmers produce maize for different uses and choose agronomic, consumption, and management characteristics through local selection. Variations in cultural preferences, as well as micro-environmental conditions, have generated the different races currently present in the “milpas” (Bellon & Risopoulos, 2001; Longmire & Moldashev, 1999).
Besides local selection, it is also common for farmers to buy and/or exchange new varieties, native either to the same or to other geographic areas, or even hybrid lines, in order to test and experiment with them. Crossing between foreign germplasm and local varieties is a common process known as “acriollamiento”, and represents the foundation for new local germplasm.
The perception and degree of acceptance of improved varieties, pure lines, and hybrids by local farmers is very diverse and complex, and although it seems to be related to ethnic identity, the proximity of the communities to the centers of commercialization or the destination of the production, it is a multifactorial phenomenon that must be analyzed. In some cases, farmers have even replaced their local varieties, although this does not imply their total displacement within the community (Dyer & Taylor, 2008). Genetically modified (GM) seeds became part of the complexity in the decision-making of the farmers regarding the germplasm that they decide to plant in their fields, because GM maize can enter into the seed lots without farmers’ awareness; they are not labeled, up to the present they have been incorporated into the fields accidentally, and their adventitious presence in the commercial houses that distribute improved seed, as well as in the governmental programs, is suspected (Piñeyro-Nelson et al., 2009).
Cultivation of GM maize has raised concerns in the country because of its open-pollinated system in which gene flow can occur in closely related fields, as well as the traditional agricultural practices (e.g., introduction of seeds from distant localities, seed exchange within the community, seed replacement). These characteristics seemed to have facilitated the unintended or accidental and even illegal entry of transgenes into traditional cultivars (Bellon & Berthaud, 2004; Ellstrand, 2001; NACEC, 2004; Quist & Chapela, 2001; Warwick et al., 2007) and the ecological and evolutionary implications of this introduction in traditional agroecosystems have been poorly studied (Van Heerwaarden et al., 2012).
Mexico has a privileged and fragile position, because it is the center of origin of maize, but it is also a commercial neighbor of the main producer of GM maize, the USA, and imports large quantities of the grain to meet its internal demands. Although Mexico is the fourth largest maize producer in the world, it is also a major consumer, making it one of the main importers worldwide. It should be noted that Mexico produces mainly white maize, which covers practically the entire demand for this variety. However, production of yellow maize exhibits a deficit, and national demand of this variety to elaborate products such as feed, fructose, starches, and snacks, and to distribute to rural areas, is covered by yellow maize imported from USA, mainly GM maize; consequently, the import requirements are higher than 5 million tons per year (SIAP, 2012).
At the end of the 1990’s, a sample of local varieties of maize was collected in the area of Ixtlán de Juárez, in the northern highlands of Oaxaca, and transgenic maize was detected for the first time in Mexico (Quist & Chapela, 2001). After harsh controversies, due to insufficient scientific evidence, the journal Nature concluded that “the evidence available is not sufficient to justify the publication of the original paper”, afterwards several attempts to understand the presence and fate of those transgenic sequences were carried out (Table 1).
These studies (Landavazo-Gamboa et al., 2006; Ortiz-García et al., 2005; Piñeyro-Nelson et al., 2009), focused on the northern highlands of Oaxaca (where the first evidence was obtained), and collected samples during the period 2000 to 2004, in additional localities near the initial focal area, and some outside the region (Fig. 1). Genetically modified organism (GMO) detection was performed using different methodologies: immunoassay (ELISA) to detect the presence of recombinant proteins and DNA-based methods (end-point and real-time PCR), as well as southern hybridization tests to determine the presence of the 35S promoter from cauliflower mosaic virus (CaMV P35s) and the NOS terminator from Agrobacterium tumefaciens (any of these 2 elements was inserted in all events of GM maize commercialized before 2004). In all cases, except for Ortiz-García et al. (2005), the presence of transgenic maize at different frequencies was detected (Table 1). In addition to the presence of the transgenes, some studies provided statistical models based on pollen and seed flow, which should be considered to estimate the flow of transgenes as well as their frequency and permanence within populations (Piñeyro-Nelson et al., 2009; Snow, 2009 and references therein; Van Heerwaarden et al., 2012).
The objective of the present study was to broaden the transgene monitoring in maize landraces for the subsequent years, from 2008 to 2018, and in geographic regions important for the conservation of the biodiversity in Oaxaca, Mexico, as well as to investigate if transgenes are still present after almost 20 years of their first report from Oaxaca. This work contributes to the understanding of the movement of the transgenes through the practices of traditional management and preservation of seeds.
Table 1
Methodological aspects of previous monitoring studies in Oaxaca, Mexico.
|
Table 1 Continued |
||||||
|
Region |
Sampling year |
Sample size |
Type of maize |
Molecular analysis |
Positive samples |
Reference |
|
Region |
Sampling year |
Sample size |
Type of maize |
Molecular analysis |
Positive samples |
Reference |
|
Localities: 2 Municipalities:1 Ixtlán de Juarez Regions: 1 (North Oaxaca) |
1999 |
4 cobs 1 sample from DICONSA 20 seeds from a seed bank |
Named landrace, but does not explain further Blue Peruvian native collected in 1971 |
End point PCR p35s and Nos terminator |
4/6 cobs and Diconsa sample |
Quist and Chapela, 2001* |
|
Localities: 18 Municipalities:1 Ixtlán de Juarez Regions: 1 (North Oaxaca) |
2003-2004 |
2003 43 plots, 164 plants 50,126 seeds 2004 81 plots, 706 plants, and 51,810 seeds |
Native landraces |
End point PCR p35s and Nos terminator Analysed by two certified laboratories |
All negative |
Ortiz-Garcia et al., 2005 |
|
Localities: 44 Districts:8 (Ixtlán de Juarez, Tlacolula, Ocotlán, Ejutla de Crespo, Etla, Pochutla, Juquila, Jamiltepec) Regions: 3 (Sierra Norte, Valles Centrales, Costa) |
? |
309 plots 10-20 cobs/plot 400 seeds/plot |
Native landraces |
End point PCR p35s Bioessay for phosphinotricine resistance PAT enzyme activity |
23 plants from 5 plots in Sierra Norte |
Landavazo et al., 2006 |
|
Nation wide 49 localities 14 states |
2002 |
419 seed lots from 286 households Two rows/ear Leaf tissue of 20 randomly- chosen individuals per ear was pooled |
Native landraces |
ELISA immunoassay CP4/EPSPS (RoundUp Ready maize) and Cry1Ab/Ac (Bt maize). |
6 samples CP4/EPSPS positive (1.8%) 10 samples Cry1Ab/Ac positive (3.1%) |
Dyer et al., 2009 |
|
2001 States:2 (Puebla and Oaxaca) Localities: 23 Regions 3 (Puebla, southest Oaxaca and North Oaxaca) 2002 State 1 Localities: 9 Municipalities: 4 (Ixtlán de Juárez, Santa Catarina Ixtepeji, Tlalixtac de Cabrera y Capulalpan) 2004 Two localities previously positive (Santiago Xiacui and Santa María Jaltianguis) |
2001, 2002, 2004 |
2001 1 household/ locality 1-5/ears/ household 68 seeds/ laboratory of analysis 2002 9 ears/ plot 117 plots 682 ears 2004 30 plots/ locality 300 leaves randomly selected/ plot 9000 leaves/ locality |
Native landraces Hibrid from DICONSA distributor |
Analyzed in three laboratories End point PCR p35s and Nos terminator Southern blot |
2001 3 localities 2002 All negative 2004 11/60 plots |
Piñeyro-Nelson et al., 2009 |
*Nevertheless Nature magazine in an editorial note on April 2002 declares: “Nature has concluded that the evidence available is not sufficient to justify the publication of the original paper”.
Materials and methods
Seeds were collected from 2008 to 2018 in different municipalities of the state of Oaxaca (Fig. 1, Table 2). Localities were selected within geographical areas that are important for the conservation of biodiversity, which by their particular physical and biotic characteristics are classified as Priority Terrestrial Regions (PTRs) (Arriaga et al., 2000). Three important PTRs were chosen: PTR 129 (Sierra Sur and Costa de Oaxaca), PTR 130 (Sierra Norte and Sierra Mixe), and PTR 132 (Zoque-La Sepultura). These PTRs include different ethnic groups: Zapotecs, Chinantecs, Mixtecs, Mixes, Nahuas, Mazatecs, and Zoques.
Seventy-four municipalities were visited, and 1-2 localities with at least 300 inhabitants were randomly selected in each one (Ventura-Aquino et al., 2008). Eight were sampled more than once (Huatulco, 3 times).
At each site, 10 to 30 farmers were randomly selected; 1,000 seeds from at least 100 different maternal plants were obtained in order to maximize the probability of detecting transgenes at low frequencies (Cleveland et al., 2005; Ortiz-García et al., 2006). In cases where maize was already in storage, producers were asked for a random sample of 500 g. The collected material corresponds mainly to landraces, but some hybrid varieties that are planted locally were also collected.
All samples were accompanied by a brief structured survey (Alexiades, 1996) that included questions regarding the origin, management, and selection of the seed, as well as the names and uses of the varieties sown by each producer. The complete dataset corresponds to 1,412 collected samples belonging to 1,210 farmers, as several producers sow more than one traditional variety. Samples collected in the first year of the study were classified at the agronomic race level (Rendón-Aguilar et al., 2015) (identification performed by Dr. Rafael Ortega-Paczka).
One-thousand two-hundred and sixty eight samples were conserved in paper bags and sent to the GMO Analysis Laboratory of the National Institute of Ecology (accredited at that time under the norm NMX-17025 equivalent to the international standard ISO-17025), where they were processed and analyzed.
Table 2
Temporal and spatial origin of the samples.
|
Project |
Year |
Region |
Municipalities |
Localities |
Farmers |
Samples |
Positive samples |
|
|
Total |
||||||||
|
Repeated |
New |
|||||||
|
JZ003 (CONABIO) |
2008 |
129 |
5 5 |
0 |
13 |
136 |
170 |
13 |
|
INE-UAM |
2010 |
129, 130 y 132 |
50 47 |
3 |
89 |
731 |
817 |
7 |
|
INE-UAM |
2011 |
129 |
3 3 |
3 |
22 |
37 |
38 |
1 |
|
INE-UAM |
2012 |
129 |
1 1 |
1 |
6 |
76 farmers, 5 stores |
94 |
|
|
CORENA-UAM |
2018 |
130 |
15 14 |
1 |
18 |
230 |
293 |
3 |
|
TOTAL |
2008-2018 |
3 regions |
74 66 |
8 |
148 |
1,210 |
1,412 |
24 |
Conabio: Comision Nacional para el Conocimiento y Uso de la Biodiversidad, INE: Instituto Nacional de Ecología (currently Instituto Nacional de Ecología y Cambio Climático, UAM: Universidad Autónoma Metropolitana, CORENA: Comisión de Recursos Naturales, CdMx.
The remaining 144 samples (collected in 2018) were processed and analyzed in the Laboratory for Molecular Diagnostics of the Natural Resources Commission in México City. This laboratory belongs to the Mexican National Network of GMO detection laboratories.
The samples were ground in a Retsch mill model Grindomix 6M 200 as well as industrial blenders and thoroughly homogenized. Samples were analyzed either individually or in groups of 3 to 7 samples, which were referenced as single samples or composite samples, respectively. The composite samples were formed by mixing 2 g of flour from each of the single samples and carefully homogenizing manually. The DNA extraction was done in duplicates of 2 g of flour. For each of the 1,412 samples, a backup flour sub-sample was stored at 4 ºC in a 15 mL polypropylene tube.
The DNA was extracted and purified using the Genetic ID Fast ID kit, according to the manufacturer’s instructions or following the DNA extraction and purification protocol based on CTAB (Alejos-Velazquez et al., 2014). The DNA was quantified by UV spectroscopy and diluted to a final concentration of 50 ng/µL.
Samples taken from 2008 to 2012 were analyzed for the presence of the endogenous maize gene zein to verify the correct DNA amplification and corroborate the absence of DNA inhibitors in the reaction. The 35S promoter (P35s) and the NOS (Tnos) terminator were then amplified by endpoint PCR using the primers listed in Table 3. The PCR conditions were as follows: 1X PCR Buffer (Qiagen), 2.7 mM MgCl2, 0.1 mM dNTPs, 0.4 mM each Q1X Solution (Qiagen) and HotStartTaq DNA Polymerase 1.25 U (Qiagen), 150 ng of DNA template and molecular biology grade water to form a final reaction volume of 25 µL.
The conditions of the thermal cycler were: activation at 95 °C for 15 min; followed by 37 cycles consisting of denaturation of double-stranded DNA at 95 °C for 20 s, alignment of the primers, and extension at 55 °C for 55 s; and a final extension of 72 °C for 10 min.
Certified reference material was used for the controls, the positive control was the ERM-BF416 event MON863 at 9.9% and the negative control was the ERM-BF416 MON863 0% (Fluka / BioChemika). The amplification products were separated by electrophoresis on agarose gels at a concentration of 2% at a constant voltage of 100 V in 1X TAE buffer and then stained with ethidium bromide. A 100 bp molecular weight marker (Invitrogen) was used as a reference to determine the size of the amplified fragments. The gels were evaluated by visual inspection, for the presence or absence of the expected DNA fragments (123 bp for P35s and 118 bp for Tnos). All PCR methods were validated through collaborative trials in the National Network of GMO detection (Pérez-Urquiza et al., 2013).
The composite samples for 2008-2012 and all the samples collected in 2018 were analyzed by real-time PCR to detect and/or quantify the presence of the P35s promoter and Tnos terminator, using the high mobility group (HMG) as endogenous gene. The PCR conditions were as follows: TaqMan Universal PCR Master Mix (Applied Biosystems), 1X, 150 nM each, 50 nM probe, 200 ng of DNA template and molecular biology grade water for a reaction volume of 25 µL.
Table 3
DNA sequences of primers and probes.
|
Gene |
Primer/ probe |
Sequence |
amplicon size(pb) |
Reference |
|
zein |
ZEIN3 ZEIN4 |
5´-AGTGCGACCCATATTCCAG-3´ 5´-GACATTGTGGCATCATCATTT-3´ |
277 |
Studer et al., 1998 |
|
high-mobility group (HMG) |
MaiJ-F2 mhmg-Rev Mhmg-probe |
5´-TTGGACTAGAAATCTCGTGCTGA-3´ 5´-GCTACATAGGGAGCCTTGTCCT-3´ 5´-FAM-CAATCCACACAAACGCACGCGTA-3´ |
79bp |
Paterno et al., 2009 |
|
TNOS |
HA-nos118f HA-nos118r |
5´-GCATGACGTTATTTATGAGATGGG-3´ 5´-GACACCGCGCGCGATAATTTATCC-3´ |
118 |
Lipp et al., 2001 |
|
P35s |
p35S-cf3 p35S-cr4 |
5´-CCACGTCTTCAAAGCAAGTGG-3´ 5´TCCTCTCCAAATGAAATGAACTTCC-3´ |
123 |
Lipp et al., 2001 |
|
P35s |
sF sR 35s-probe |
5´-CGTCTTCAAAGCAAGTGGATTG-3´ 5´-TCTTGCGAAGGATAGTGGGATT-3´ 5´-FAM-TCTCCACTGACGTAAGGGATGACGCA- MGB-3´ |
79 |
Feinberg et al., 2005 |
The reaction was run in an ABI 7500 real-time PCR system, the reaction conditions being as follows: activation 95 °C for 10 min; followed by 40 cycles consisting of DNA denaturation of double stranded 95 °C for 15 s, alignment of the primers, and extension at 60 °C for 60 s. The standard curve was prepared by serial dilutions of standard ERM-BF416 MON863 9.9% (Fluka / BioChemika), and concentrations used were: 9.9%, 5%, 1%, 0.1%, and 0.01%, run in triplicate. The amplification curves were analyzed using the 7500 real-time PCR System Detection Software (SDS). The concentrations of the positive samples were obtained using the standard curve.
When composite samples were positive for the presence of a band and/or an amplification curve, each individual sample of that mix was re-analyzed. DNA extraction was done in duplicate using the same method previously described. In this second step of the analysis, the same methodology was used for the amplification of a P35s promoter region by real-time PCR. The duplicates of the extraction were also analyzed in duplicate by real-time PCR, so 4 results were obtained for each sample.
Those localities where genetic constructs were observed during the first year of sampling, the monitoring was repeated for the subsequent years (Table 2).
In 2011 and 2018, result certificates were delivered to the producers who provided their seeds. Together with the delivery of results, talks were held to explain the nature of GMOs, the potential adverse effects, and the practices necessary to preserve their GMO-free seed stock, with emphasis on avoiding the planting of seeds of unknown origin. In both moments, this action generated subsequent requests from producers to continue the analysis of their seeds, so sampling and analysis of the seeds were repeated (Table 2).
Results
The 1,412 samples obtained during the sampling period (2008-2018), belong to at least 79 traditional landraces plus some improved or hybrid varieties (Rendón-Aguilar et al., 2011, 2015, 2018). Starting from a minimum of 1,000 seeds per sample, it is considered that at least 1,412,000 seeds were analyzed.
The elevational interval of the sampled localities was 0 to 2,531 m asl, covering tropical dry forest, subdeciduous forest, montane cloud forest, pine-oak forest, oak forest, and pine forest (Luna-José & Rendón-Aguilar, 2008; Rendón-Aguilar et al., 2018; Ventura-Aquino et al., 2008). Most of the positive samples from the southern highlands were collected below 1,600 m asl (only samples from San Andrés Paxtlán were collected above 2,300 m asl. In the northern highlands, positive samples came from plots located above 1,900 m asl.
The material collected in 2008 that could be identified as an agronomic race corresponded to 7 of the 10 races reported in 2007 (Rendón-Aguilar et al., 2015). Material collected in 2018 corresponds to 10 agronomic races, from which only 3 were repeated, so 17 different agronomic races are still sown in these regions of Oaxaca, which represent almost half of the total diversity of agronomic races of maize described for Oaxaca.
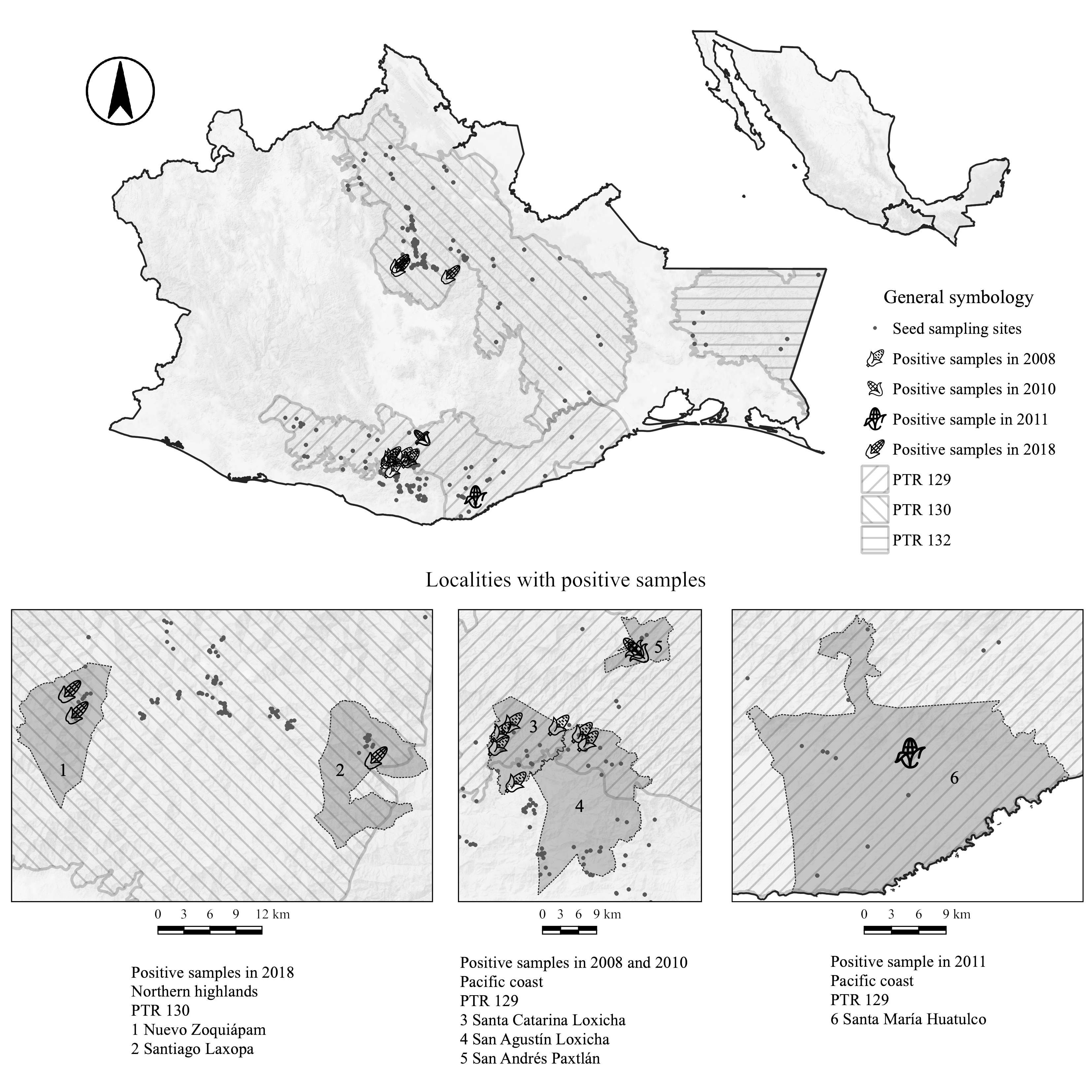
From the 1,412 samples analyzed between 2008 and 2018, 24 (1.69%) were positive for the presence of transgenes at low levels (in most cases, below the limit of quantification). All positive samples belong to Los Loxicha region (Sierra Sur), Santa María Huatulco (Pacific coast), and Sierra Juárez. From the 74 municipalities sampled, 7 (9.45%) exhibited at least one sample with presence of transgenes.
Presence of transgenes fluctuated through the years. In 2008, GM maize were detected in 2 municipalities of Los Loxicha (San Agustín Loxicha and Santa Catarina Loxicha), but in 2010 they were not recorded; other municipalities that were sampled in 2008 were negative for the presence of GM maize, in 2011 some samples were positive (Santa María Huatulco and San Sebastián Coatlán). Some municipalities exhibited transgenes in one year, but some difficulties limited their resampling (San Andrés Paxtlán, San Gabriel Mixtepec, San Sebastián Coatlán). Huatulco exhibited a different pattern, in the first year of sampling no positives were found, one positive record appeared in the second year, and in the final monitoring no positives were found. All localities where samples were taken are separated by at least 8 km.
Sierra Juárez of Oaxaca exhibited a similar pattern. Some municipalities were reported with presence of transgenes during the first decade after the first evidence of their presence was reported (Ixtlán de Juárez, San Juan Chicomezúchil, San Miguel Amatlán) (Table 1, Fig. 1); even more, some of them were sampled more than once, and all cases exhibited positive samples (Ixtlán de Juárez, Santiago Comaltepec, Santa María Jaltianguis, and Santiago Xiacui). Nevertheless, samples analyzed in 2018 were negative. The opposite was found for Nuevo Zoquiapam and Santiago Laxopa, where past monitoring gave negative results, and in 2018 a few samples show the presence of GM maize (Table 2).
In the case of those positive samples whose agronomic race was identified in 2008, it is important to mention that they belonged to 4 races: Olotillo, Tuxpeño, Tepecintle, and Mushito. The first 3 races stand out as the most abundant in the collected material, and those with relatively greater presence of GM maize sequences. It is also remarkable the presence of a positive event in 1 of the 4 Mushito samples, which is considered representative of the highlands of Oaxaca (Conabio, 2012).
From the 1,412 surveys analyzed, a high percentage of producers (71%) select and save the seed from the previous cycle. However, there are farmers who, in addition to keeping the seed, also exchange it with relatives or neighbors of the community (16%), and some even buy seeds from other municipalities of Oaxaca (13%), such as Cuicatlán, Huatulco, Juchitán, Matías Romero, Miahuatlán, Puerto Escondido, Pochutla, Tehuantepec, Tuxtepec, Valle Nacional, Ixtlán, or Oaxaca City, depending on where the locality is situated.
When the seed comes from outside, it usually corresponds to hybrid seeds that are distributed in the agrochemical stores. In 2012 and 2018, we detected that some seed lots, belonging to hybrid lines, came from the state of Sinaloa, located in northern Mexico, in which the experimental release of GM maize was approved from 2009 to 2012 (Table 3).
Another way of obtaining seeds is through agricultural programs and field support promoted by state and municipal governments. In these cases, farmers received improved seed, which is mainly used to make tortillas, but some producers mentioned that if they did not have a good harvest, then they chose to sow it.
Discussion
In the first report of transgene presence in Mexican maize landraces, Quist and Chapela (2001) sampled a few cobs from only 2 localities in the Sierra Norte of Oaxaca, and it was not possible to reach a conclusion on the dispersion of transgenes at that time. Further monitoring efforts carried out in this maize center of origin during the following years were scarce and were focused mainly in the same region and localities. The results presented here respond to different needs: first to expand the scope to other localities at the Sierra Juárez, besides those previously sampled, as well as the Sierra Sur of the state, which had not been sampled before; second, to resample previously reported localities with presence of transgenes; and third, to focus on areas with high levels of bio-cultural diversity.
Like previous studies, most of the material corresponds to common native landraces, although this is the first time where the landrace name is given. Besides common landraces, we also collected rare ecotypes such as “mushito” in the municipality of San Agustín Loxicha, or “ishi”, “mok”, and “bolita”, in the region of the Chimalapas. The distinction among different landraces, the agronomic race to which they belong, and their particular management and movement can be very useful to understand the dynamics of transgene dispersion, and its accumulation in the landraces. Additional knowledge of the geographic distribution and phenology of each landrace can help understand how transgenes move in space and time. For example, some agronomic races, such as Nal-tel and Conejo, include many landraces, which have short production cycles (3-4 months), and particular ecological requirements, as they are only distributed at low elevations (below 1,200 m asl) in the state of Oaxaca. In contrast, agronomic races such as Tepecintle, Olotillo, Tuxpeño, and Mushito include many landraces with longer lifecycles (up to 6 months), and the possibility of overlapping phenology is high. Coincidentally, the Olotillo and Tuxpeño agronomic races have the largest distribution range within the studied region, which might favor the mobility of transgenes by pollen flow (Rendón-Aguilar et al., 2015).
The data on traditional management, as well as the mechanisms for seed acquisition, reinforce the high seed mobility pattern previously reported (Bellon & Berthaud, 2004; Bellon & Brush, 1994; Louette & Smale, 2000; Louette et al., 1997; Perales et al., 2003; Piñeyro-Nelson et al., 2009). Natural curiosity of farmers to try new materials has been part of the traditional seed movement and, in consequence, of the historical enrichment of native maize landraces. Regarding the movement of transgenes, this traditional management might be a disadvantage, because even when people sow seed mainly from the previous harvest, the frequent practice of introducing seeds from outside to try new variants, to increase yield, or to cover other necessities (e.g., forage for cattle), increase the possibility of inadvertently introducing transgenes. Results obtained in the present study support this pattern of transgene introduction. Additionally, government seed distribution policies via municipalities or peasant organizations are another source of introduction of foreign material. In any of these situations, further movement and diffusion of GM maize cannot be determined accurately, because its movement inside each community by pollen flow relies on the phenology of local varieties, so it is possible that the transgenes remain there at very low frequencies.
In addition, the common practice of choosing only a few grains from the most representative ears seems to be an efficient way of purging populations of transgenes that must be genetically linked to other phenotypic characteristics of their parental lines (Mercer & Wainwright, 2008). Natural selection then can act against these phenotypes and foreign genes, because the local varieties are well adapted to the specific climatic conditions. Nevertheless, it is necessary in the future to test the selection pressures of the inserted characters, such as herbicide and insect resistance traits.
Detection of transgenes can be affected by many of the farmers’ decisions on management of seeds (Agapito-Tenfen & Wickson, 2018): a) the number of samples can change from one year to another, for example, in San Agustín Loxicha it was not possible to obtain the same number of samples in 2010 as in 2008, because some farmers decided not to sow maize that year, or b) the local variety is changed from one year to another. In fact, some landraces “suddenly disappeared” from one year to the next one. This is the case of “piñero”, a local variety collected in 2008, but not found in subsequent years. So, it is not ruled out that transgenes might be present and not found in the samples collected, or even that they might have disappeared in some landraces. Nevertheless, some local varieties are a real personal creation of one or 2 peasants inside a community. In this case, if transgenic material has mixed with a local variety, this could be a potential source of transgenic material at least inside the community, as it was detected in Santiago Laxopa in 2018. People feel a lot of attachment with their maize seed, and therefore it is difficult to persuade them to eliminate it.
In the last 18 years, laboratory detection methods have improved enormously, current methodologies are standardized and homogenized worldwide, and online global databases are already available (see for example: Bonfini et al., 2012). At the national level, collaborative trials have allowed the standardization of the optimal conditions for screening tests and their adoption for the specific needs of the country (Pérez-Urquiza et al., 2013). In the future, new methodologies such as digital PCR and next generation sequencing will also be considered for more precision in detection methodologies (Félix-Urquídez et al., 2016; Gutiérrez-Angoa et al., 2015; Pérez-Urquiza & Acatzi-Silva, 2014). It will be possible in the near future to detect the precise insertion sites in the landrace genome as well as to evaluate quantitatively the extent of introgression from hybrids and GM maize. Future monitoring efforts should include these new methodologies.
Acknowledgements
To the hundreds of producers of the 66 municipalities visited, this work could not have been carried out without their support, patience, and confidence. Thanks for keeping traditional knowledge alive, despite the dizzying changes of the world. To the National Commission for Knowledge and Use of Biodiversity (Conabio) for financing the project FZ003: “Diversidad y distribución altitudinal de maíces nativos en la región de los Loxicha, Sierra Madre del Sur, Oaxaca”, where the first samples analyzed to detect GM maize came from; to the National Center for Environmental Research and Training of the National Institute of Ecology (CENICA-INE) for economic support through the project “Monitoreo de la Presencia Accidental o no Intencional de Maíz Genéticamente Modificado en Áreas de Alta Diversidad Genética, en Estados Prioritarios”; to the Natural Resources Commission of Mexico City (CORENA-CdMx) for economic support through the project “Análisis de la presencia de transgenes en maíces nativos de la Sierra Norte de Oaxaca, monitoreo 2017-2018”. To the Laboratory of Molecular Biology of the Universidad Autónoma Metropolitana-Iztapalapa, for the support in the analysis of samples. To María del Consuelo Aragón-Martínez, José Francisco Ávila-Castañeda, Guadalupe Carrillo-Galván, Mireya Hernández-Hernández, Azucena de Lourdes Luna-José, José Miguel Sánchez-García, Anaitzi Rivero-Villar, Asmaveth Solís, Verónica Aguilar-Rojas, Yanin Islas-Barrios, Gabriela Contreras-Bernal, Alejandro López-Arriaga, and Eric Vides-Borrell, for the fieldwork. To Dr. Rafael Ortega-Paczka, for the identification of the agronomic landraces in the years 2007, 2008, and 2018. To Gilberto Hernández-Cárdenas, for the support in the sampling design. To José Miguel Castillo-Minjarez, Berenice Zúñiga-Bustos, Jaime Aportela-Cortés, Rocío Fernández-Suárez, María Teresa Maldonado-Calderón, Erika Lagunes-Fortiz, Sandra Pérez-Briseño, Mayela Flores-Romero, Consuelo Aragón Martínez, Verónica Patiño López, Antonio Ávila, Aldo Valera, and Inocencio Piña for technical support during the processing and analysis of the samples. To Alejandra Serrato-Díaz for all logistic support, advice, and guidance to technicians.
References
Agapito-Tenfen, S. Z., & Wickson, F. (2018). Challenges for transgene detection in landraces and wild relatives: learning from 15 years of debate over GM maize in Mexico. Biodiversity and Conservation, 27, 539–566. https://doi.org/10.1007/s10531-017-1471-0
Alejos-Velázquez, L. P., Aragón-Martínez, C., & Cornejo-Romero, A. (2014). Extracción y purificación de ADN. In A. Cornejo, B. Rendón, A. Serrato, & M. Rocha (Comps.), Herramientas moleculares aplicadas en ecología: aspectos teóricos y prácticos (pp. 1–25). México D.F.: Instituto Nacional de Ecología/ Universidad Autónoma Metropolitana.
Alexiades, N. M. (1996). Collecting ethnobotanical data: an introduction to basic concepts and techniques. In N. M. Alexiades (Ed.), Selected guidelines for ethnobotanical research: a field manual (pp. 53–94). New York: Scientific Publications Department, The New York Botanical Garden.
Aragón-Cuevas, F., Castro-García, F. H., Cabrera-Toledo, J. M., & Osorio-Alcalá, L. (2011). Bancos comunitarios de semillas para conservar in situ la diversidad vegetal. Publicación especial Núm. 9. Oaxaca: Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Centro de Investigación Regional Pacífico Sur, Campo Experimental Valles Centrales de Oaxaca.
Aragón-Cuevas, F., Castro, F. H., Paredes, E., Dillánes, R., Hernández, J. M., Taba S. et al. (2002). Conservación in situ y mejoramiento participativo de la milpa en Oaxaca. In J. L. Chávez-Servia, L. M. Arias-Reyes, D. I. Jarvis, J. Tuxill, D. Lope-Alzina, & C. Eyzaguirre (Eds.), Manejo de la diversidad cultivada en los agroecosistemas tradicionales. Resúmenes del simposio, 13-16 de Febrero del 2002, Mérida, México. Roma: IPGRI.
Aragón-Cuevas, F., Taba, S., Castro-García, F. H., Hernández-Casillas, J. M., Cabrera-Toledo, J. M., Alcalá, L. O. et al. (2005). In situ conservation and use of local maize races in Oaxaca, Mexico: a participatory and decentralized approach. Proceedings of a workshop held at CIMMYT, April 7-10, 2003. In Latin American maize germplasm conservation: regeneration, in situ conservation, core subsets, and prebreeding. Proceedings of a workshop held at Texcoco, Estado de México: CIMMYT.
Arriaga L., Espinoza J. M., Aguilar C., Martínez E., Gómez, L., & Loa, E. (2000). Regiones terrestres prioritarias de México. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. https://doi.org/10.5962/bhl.title.118644
Bellon, M. R., & Berthaud, J. (2004). Transgenic maize and the evolution of landrace diversity in Mexico. The importance of farmers’ behavior. Plant Physiology, 134, 883–888. https://doi.org/10.1104/pp.103.038331
Bellon, M. R., & Brush, S. B. (1994). Keepers of maize in Chiapas, Mexico. Economic Botany, 48, 196–209. https://doi.org/10.1007/bf02908218
Bellon, M. R., & Risopoulos, J. (2001). Small-scale farmers expand the benefits of improved maize germplasm: A case study from Chiapas, Mexico. World Development, 29, 799–811. https://doi.org/10.1016/s0305-750x(01)00013-4
Bonfini, L., van den Bulcke, M. H., Mazzara, M., Ben, E., & Partak, A. (2012). GMOMETHODS: The European Union database of reference methods for GMO analysis. Journal of AOAC International, 95, 1713–1719. https://doi.org/10.5740/jaoacint.12-050
Cleveland, D. A., Soleri, D., Aragón-Cuevas, F., Crossa, J., & Gepts, P. (2005). Detecting (trans) gene flow to landraces in centers of crop origin: lessons from the case of maize in Mexico. Environmental Biosafety Research, 4, 197–208. https://doi.org/10.1051/ebr:2006006
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2012). Razas de maíz de México. Mushito. México D.F., México. Retrieved on February 05 2019, from: https://www.biodiversidad.gob.mx/usos/maices/grupos/mushito.html
Dyer, G. A., Serratos-Hernández, J. A., Perales H. R., Gepts, P., Piñeyro-Nelson, A. et al. (2009). Dispersal of transgenes through maize seed systems in Mexico. Plos One, 4, e5734. https://doi.org/10.1371/journal.pone.0005734
Dyer, G. A., & Taylor, J. E. (2008). A crop population perspective on maize seed systems in Mexico. Proceedings of the National Academy of Sciences, 105, 470–475. https://doi.org/10.1073/pnas.0706321105
Ellstrand, N. C. (2001). When transgenes wander, should we worry? Plant Physiology, 125, 1543–1545. https://doi.org/10.1104/pp.125.4.1543
Feinberg, M., Fernandez, S., Cassard, S., Charles-Delobel, C., & Bertheau, Y. (2005). Quantitation of 35S promoter in maize DNA extracts from genetically modified organisms using real-time polymerase chain reaction, part 2: interlaboratory study. Journal of AOAC International, 88, 558–573.
Félix-Urquídez, D., Pérez-Urquiza, M., Valdez-Torres, J. B., León-Félix, J., García-Estrada, R., & Abraham, A. S. (2016). Development, optimization, and evaluation of a duplex droplet digital PCR assay to quantify the t-nos/hmg copy number ratio in genetically modified maize. Analytical Chemistry, 88, 812–819. https://doi.org/10.1021/acs.analchem.5b03238
Gutiérrez-Angoa, L. E., Castillo-Durán, L. C., Gómez-Casielo, B. E., & Acatzi-Silva, A. I. (2015). Quantification of genetically modified maize with qPCR and dPCR techniques. Agrociencia, 49, 373–394.
Landavazo-Gamboa, D. A., Calvillo-Alba, G. K., Espinosa-Huerta, E., González Morelos, L., Aragón-Cuevas, F., & Torres-Pacheco, I. (2006). Caracterización molecular y biológica de genes recombinantes en maíz criollo de Oaxaca. Agricultura Técnica en México, 32, 267–279.
Lipp, M., Bluth, A., Eyquem, F., Kruse, L., Schimmel, H., Van den Eede, G. et al. (2001). Validation of a method based on polymerase chain reaction for the detection of genetically modified organisms in various processed foodstuffs. European Food Research and Technology, 212, 497–504. https://doi.org/10.1007/s002170000274
Longmire, J., & Moldashev, A. (1999). Farmer management of maize diversity in the central valleys of Oaxaca, Mexico. CIMMYT Economics Working Paper 99-09. Mexico D.F.: CIMMYT.
Louette, D., Charrier, A., & Berthaud, J. (1997). In situ conservation of maize in Mexico: genetic diversity and maize seed management in a traditional community. Economic Botany, 51, 20–38. https://doi.org/10.1007/bf02910401
Louette, D., & Smale, M. (2000). Farmers’ seed selection practices and traditional maize varieties in Cuzalapa, Mexico. Euphytica, 113, 25–41.
Luna-José, A. L., & Rendón-Aguilar, B. (2008). Recursos vegetales útiles en diez comunidades de la Sierra Madre del Sur, Oaxaca, México. Polibotánica, 26, 193–242. Recuperado el 12 de junio, 2019 de: <http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-27682008000200011&lng=es&nrm=iso>
Mercer, K. L., & Wainwright J. D. (2008). Gene flow from transgenic maize to landraces in Mexico: an analysis. Agriculture, Ecosystems & Environment, 123, 109–115. https://doi.org/10.1016/j.agee.2007.05.007
NACEC (North American Commission for Environmental Cooperation). (2004). Maize and biodiversity: the effects of transgenic maize in Mexico. Recuperado el 05 de febrero, 2019 de: http://www.cec.org/sites/default/files/related_documents/maize_biodiversity/4000_Issue_summary-e.pdf
Ortíz-García, S., Ezcurra, E., Schoel, B., & Acevedo, F. (2006). Reply to Cleveland et al.’s “Detecting (trans) gene flow to landraces in centers of crop origin: lessons from the case of maize in Mexico”. Environmental Biosafety Research, 4, 209–215. https://doi.org/10.1051/ebr:2006007
Ortíz-García, S., Ezcurra, E., Schoel, B., Acevedo, F., Soberón, J., & Snow, A. (2005). Absence of detectable transgenes in local landraces of maize in Oaxaca, Mexico (2003-2004). Proceedings of the National Academy of Sciences, 102, 338–343. https://doi.org/10.1073/pnas.0503356102
Paterno, A., Marchesi, U., Gatto, F., Verginelli, D., Quarchioni, C., Fusco, C. et al. (2009). Finding the joker among the maize endogenous reference genes for genetically modified organism (GMO) detection. Journal of Agricultural and Food Chemistry, 57, 11086–11091. https://doi.org/10.1021/jf902560x
Perales, H., Brush, S. B., & Qualset, C. O. (2003). Dynamic management of maize landraces in central Mexico. Economic Botany, 57, 21–34. https://doi.org/10.1663/0013-0001(2003)057[0021:dmomli]2.0.co;2
Pérez-Urquiza, M., & Acatiz-Silva, A. I. (2014). Copy number ratios determined by two digital polymerase chain reaction systems in genetically modified grains. Metrologia, 51, 61–66. https://doi.org/10.1088/0026-1394/51/1/61
Pérez-Urquiza, M., J., Rivera-Mellado, E. A., & Matus-Cundapi (2013). Manual de protocolos de medición de organismos genéticamente modificados. Querétaro: Centro Nacional de Metrología.
Piñeyro-Nelson, A., Van Heerwaarden, J., Perales, H. R., Serratos- Hernández, J. A., Rangel, A., Hufford, M. B. et al. (2009). Transgenes in Mexican maize: molecular evidence and methodological considerations for GMO detection in landrace populations. Molecular Ecology, 18, 750–761. https://doi.org/10.1111/j.1365-294x.2008.03993.x
Quist, D., & Chapela, I. H. (2001). Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico. Nature, 414, 541–543. https://doi.org/10.1038/35107068
Rendón-Aguilar, B., Aguilar-Rojas, V., Aragón- Martínez, M. C., Ávila-Castañeda, J. F., Bernal-Ramírez, L. A., Bravo-Avilez, D. et al. (2015). Diversidad de maíz en la Sierra Sur de Oaxaca, México: conocimiento y manejo tradicional. Polibotánica, 39, 151–174. https://doi.org/10.18387/polibotanica.39.9
Rendón-Aguilar, B., Rocha-Munive, M. G., Sánchez-García, J. M., Aragón-Martínez, M. C., Bernal-Ramírez, L. A., Bravo-Avilez, D. et al. (2018). Análisis de la presencia de transgenes en maíces nativos de la Sierra Norte de Oaxaca, monitoreo 2017-2018. Informe Final. Ciudad de México: Universidad Autónoma Metropolitana-Iztapalapa/ Comisión de Recursos Naturales, Secretaría del Medio Ambiente.
Rendón-Aguilar, B., Rocha-Munive, M.G., Oreo-Arnaiz, A., Aguilar-Rojas, V., Aragón- Martínez, M. C., Ávila-Castañeda, J. F. et al. (2011). Monitoreo de la presencia accidental o no intencional de maíz genéticamente modificado en áreas de alta diversidad genética, en el estado de Oaxaca. Informe final. México D.F.: Universidad Autónoma Metropolitana-Iztapalapa/ Instsituto Nacional de Ecología.
SIAP (Servicio de Información Agroalimentaria y Pesquera). (2012). Situación actual y perspectivas del maíz en México 1996-2012. Recuperado el 05 de febrero, 2019 de: http://www.campomexicano.gob.mx/portal_siap/Integracion/EstadisticaDerivada/ComercioExterior/Estudios/Perspectivas/maiz96-12.pdf
Snow, A. (2009). Unwanted transgenes re-discovered in Oaxacan maize. Molecular Ecology, 18, 569–571. https://doi.org/10.1111/j.1365-294x.2008.04063.x
Studer, E., Rhyner, C., Luthy, J., & Hubner, P. (1998). Quantitative competitive PCR for the detection of genetically modified soybean and maize. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung a-Food Research and Technology, 207, 207–213. https://doi.org/10.1007/s002170050320
Van Heerwaarden, J., Ortega-del Vecchyo, D., Alvarez-Buylla, E., & Bellon, M. R. (2012). New genes in traditional seed systems: diffusion, detectability and persistence of transgenes in a maize metapopulation. Plos One, 7, e46123. https://doi.org/10.1371/journal.pone.0046123
Ventura-Aquino, Y., Rendón, B., Rebollar, S., & Hernández, G. (2008). Use and conservation of forest resources in the municipality of San Agustin Loxicha, Sierra Madre del Sur, Oaxaca, Mexico. Agroforestry Systems, 73, 167–180. https://doi.org/10.1007/s10457-008-9107-8
Warwick, S. I., Légère, A., Simard, M. J., & James, T. (2007). Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Molecular Ecology, 17, 1387–95. https://doi.org/10.1111/j.1365-294x.2007.03567.x