Territorial patterns of Dickerson’s collared lizard, Crotaphytus dickersonae
Lieke Faber a, Melissa Plasman b, *, Marie José H. M. Duchateau a
a Department of Biology, Animal Ecology, Utrecht University, Padualaan 8, 3584 CH, Utrecht, The Netherlands
b Laboratorio de Conducta Animal, Departamento de Ecología Evolutiva, Instituto de Ecología, Universidad Nacional Autónoma de México, Circuito Exterior s/n anexo Jardín Botánico exterior, Ciudad Universitaria, 04510 Ciudad de México, Mexico
Current address: Centro Tlaxcala de Biología de la Conducta, Universidad Autónoma de Tlaxcala, carretera Tlaxcala-Puebla Km 1.5, 90062 Tlaxcala, Mexico
*Corresponding author: melissaplasman@hotmail.com (M. Plasman)
Abstract
High quality males generally obtain better territories, resulting in enhanced survival rates and reproduction. In our study we examined which phenotypic traits play a role in obtaining the best territories in males of the highly territorial and sexually dimorphic lizard species Crotaphytus dickersonae. For each lizard, we measured snout-vent length, body mass, head width, hind limb length, and tail length as phenotypic traits that might promote success in competition over territories. In addition, we examined whether the bright blue coloration of the males is related to territory quality. We assessed territory quality by determining the size, the number of overlapping female home ranges, the exclusivity of each territory, and the number of refuges and basking sites. Males with longer tails and wider heads had the best territories with more females and less overlapping male territories. Bluer males tended to have less overlapping male territories and more overlapping female home ranges, suggesting that the blue coloration is related to male quality. Since tail length and head width influence fighting ability in lizards, males with longer tails and wider heads probably obtain the best territories through male-male competition.
Keywords:
Territory quality; Color signal; Spatial behavior; Female home range; Exclusivity; Morphology; Resource-holding potential
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Patrón territorial de la lagartija de collar de Dickerson, Crotaphytus dickersonae
Resumen
Los machos de alta calidad generalmente obtienen mejores territorios, resultando en tasas de sobrevivencia y reproducción altas. Este estudio examina las características fenotípicas importantes para que machos de la altamente territorial y sexualmente dimórfica lagartija de collar, Crotaphytus dickersonae, obtengan los mejores territorios. Como caracteres fenotípicos que promueven el éxito en la competencia sobre los territorios, se midió la longitud hocico cloaca, la masa corporal, el ancho de la cabeza, la longitud de la pata trasera y la longitud de la cola. Adicionalmente, examinamos la relación de la coloración azul brillante de los machos con la calidad del territorio. Estimamos la calidad del territorio de acuerdo con su tamaño, el número de hembras y machos, refugios y sitios de asoleo. Los machos con colas más largas y cabezas más anchas ocuparon los mejores territorios traslapándose con más hembras y menos machos. Los machos más azules tendieron a traslaparse menos con otros machos y más con hembras, sugiriendo que la coloración azul está relacionada con la calidad del macho. La longitud de la cola y el ancho de la cabeza influenciaron positivamente en la habilidad de pelea de las lagartijas que les permite competir por mejores territorios.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras claves:
Calidad de territorio; Señal de calidad; Conducta territorial; Rango hogareño de hembras; Exclusividad; Morfología; Capacidad de retención de los recursos
Introduction
Animal spatial organization ranges from widely overlapping home ranges to clearly defined exclusive territories owned by strong competitors. A home range can be described as the total area in which the animal lives and reproduces, though it may be undefended, whereas an area is a territory only if it is defended (Burt, 1943; Maher & Lott, 1995). Defended areas can vary greatly in their size and characteristics, ranging from small areas within the home range significant to habitation, reproduction, and feeding (e.g., nests or feeding places), to the entire home range area (Maher & Lott, 1995; Stamps, 1977).
Territorial animals enhance their chances of reproduction and survival by gaining access to mates and excluding potential competitors from their territory. In many lizard species, males are highly territorial (Baird, 2013) and defend their entire home range (Stone & Baird, 2002). In lizards, access to possible mates is often considered the primary motive for defending a territory (Baird, 2013). In such cases males may defend territories that include the home ranges of several females. These females typically mate with the current territory holder (Kiester, 1979; Stamps, 1983, 1987; Tokarz, 1998) and territorial males often have higher reproductive success than non-territorial males within the same species (Baird, 2013; Zamudio & Sinervo, 2003). As such, to ensure paternity of the female’s offspring, male lizards will have to exclude potential competitors from their territory. Defense of a territory is costly in terms of both time and energy, and fights can result in injuries (Baird, 2013; Marler & Moore, 1988; Olsson, 1994). Individuals that are stronger, faster and/or healthier have higher resource-holding potential (RHP) and obtain better territories (Andersson, 1994). Often performance traits that determine RHP are related to morphological traits, like size, leg length, head width, etc. (Baird, 2013). To reduce fighting costs, in some species individuals signal their RHP and assess winning chance before engaging in a fight. Signals of high RHP include armaments (Berglund et al., 1996), but can also include ornaments like color (Baird, 2013; Lappin et al., 2006; Whiting et al., 2003).
In this study, we examined the relation between morphological traits and territory quality in Dickerson’s collared lizard, Crotaphytus dickersonae. This is a desert-dwelling lizard, endemic to Mexico, and can only be found in the rocky hills of Isla Tiburón in the Sea of Cortez and in the adjacent mountainous coastline of western Sonora (McGuire, 1996). Males of this species are highly territorial. They emerge from hibernation in March and immediately form territories, as breeding season starts in April (McGuire, 1996), and which they likely maintain during the entire reproductive season (Baird, 2013). This polygynous lizard species displays a strong sexual dimorphism; males are larger, heavier and have wider heads than females (McGuire, 1996; Plasman et al., 2007). Sexual dimorphism in these traits is often explained by sexual selection through male-male competition (Baird, 2013). Crotaphytus dickersonae also displays a strong sexual dimorphism in coloration: Males display a deep cobalt blue color over their entire body while females are a dull brown (McGuire, 1996). This bright coloration over the whole body is exceptional because most animals only display color patches, which in lizards are often only exposed during displays (Andersson, 1994; Whiting et al., 2003). The striking blue coloration of C. dickersonae males makes them highly conspicuous in the open habitat, possibly facilitating assessment of opponents if color functions as an honest signal of RHP. Indeed, an earlier study showed that bluer males had higher running speed and higher bite force and thus likely have higher RHP (Plasman et al., 2015). The exceptional coloration and strong sexual dimorphism of C. dickersonae makes it an excellent model for sexual selection studies. Yet, the species is poorly studied. Here, we examined whether the male’s color and several morphological measures of Dickerson’s collared lizard are related to the quality of the territory obtained by the male.
Materials and methods
We studied adult Crotaphytus dickersonae in the coastal hills close to the village Bahía de Kino in Sonora, Mexico. Our study took place from May 2011 until September 2011. The focus of our study involved 2 sites. The sizes of the study sites were approximately 9.0 and 2.7 hectares. Both study sites were situated at a slope of a hill and consisted of granite rock, with some big rocks and many stones, and with little vegetation (i.e., Jatroph cuneata, Fouquieria splendens, Pachycereus pringlei). We caught 95% of all observed collared lizards, 21 lizards (10 males and 11 females) at study site 1 and 15 lizards (4 males and 11 females) at study site 2. We assumed the lizards of these study sites to be of the same population, given that the study sites were located in proximity (< 400 m) and only separated by a piece of flat desert land without geographic interruptions (i.e., no road, river, building, etc.). Additionally, habitat measures of both study sites were similar, giving us the confidence to combine relevant data regarding lizards at both study sites.
Lizards were caught using a pole and a noose made of fishing line. After capture we took a photo of the lateral sides of each lizard’s body to allow for photo identification. Crotaphytus dickersonae have white dots on their body and the pattern and distribution of these dots is unique for each individual lizard (Faber et al., 2013). To allow identification from a distance, we gave each lizard 4 small dots of paint on the dorsal base of the tail in a unique combination of green, red, and yellow non-toxic acrylic paint. When lizards had shed their skin, and thus the paint, they were caught again, identified by their photos, and repainted.
Phenotypic traits were measured on the event of the lizard’s first capture. We measured snout-vent length (SVL; ± 1 mm) and tail length (from the cloaca to the tip of the tail; ± 1 mm) with a flexible ruler. Body mass was measured with a Pesola scale (± 0.5 g). We measured the length of the left lower hind limb (from the knee joint to the ankle joint) with a caliper (± 1 mm). Head width of each lizard was measured with a caliper (± 1 mm) at the widest point of the head where the lateral extent of the jaw adductor muscles was largest. Body condition index (BCI), often used as an indication of the general physical status of the lizard, was estimated as the standardized residuals of the linear regression of body mass on SVL (Schulte-Hostedde et al., 2005).
We took a measurement of the blue color on both lateral sides of the dorsum of the male lizards with a portable spectrophotometer (MINOLTA CM-2600d, Minolta Co. Ltd., Osaka Japan). Blue chroma was calculated as reflection from 400-480 nm divided by the total reflection measured by the spectrophotometer (360-740 nm). In subsequent analyses we used the average blue chroma of both measurements. Blue chroma was determined for 7 males. For 6 of them we had information on territory variables. For these 7 males, color was measured in the same week and around the same time (16:00 h) after the males had been allowed to sunbathe in an enclosure consisting of a wooden frame covered with mesh (60 × 60 × 60 cm).
One of the male lizards we caught had lost one of its forelimbs and part of his tail. We decided to include this lizard in our study because we considered its short tail would affect his quality similarly as a naturally shorter tail would and his SVL, body mass, length of the hind leg, head width, and blue chroma did not fall outside the range of the measurements in other males.
Stone and Baird (2002) found that male collared lizards defend their entire home range against conspecific males. We therefore treated the home ranges of the males in our study as their territory, as has been done in other studies on collared lizards (Baird et al., 2007; Husak et al., 2008). Territories of males and home ranges of females were determined based on point sightings collected from May 3 until August 23 during daily walks through the study sites in a routine way between 8:00 and 17:30 h. We recorded the exact location of lizards with a Garmin GPSmap 62s (< 3 m accuracy). When a lizard was encountered twice during a day, we only recorded the second sighting when at least 2 hours had passed since the first sighting. We used a minimum of 15 sightings to calculate territory and home range size with the minimum convex polygon technique (Rose, 1982), the only exception to which was a lizard for which we used only 12 sightings to calculate territory size. This lizard had not been seen after July 16 and we assumed that he had died. This was the same lizard that had lost his forelimb and part of its tail. We collected enough sightings to estimate territory size for 6 males and home range size for 10 females.
We determined the home range of females for which we had a minimum of 15 sightings. We recorded 1) the number of overlapping female home ranges for each male territory, and 2) the total number of females that were seen in a male’s territory, including those females for which we did not calculate home range size.
We determined the exclusivity of a territory by 1) the number of overlapping male territories, 2) the percentage of overlap with territories of other males, and 3) the number of other males seen in the territory that were observed fewer than 15 times.
A higher number of refuges and basking sites may indicate higher territory quality. Collared lizards may hide below trees and shrubs from possible predators. Furthermore, the shade of these plants allow for thermoregulation opportunities. Large rocks are the preferred basking sites of males (McGuire, 1996). For each territory, we counted the number of trees and shrubs, and rocks taller than 30 cm to calculate the number of refuges and basking sites per m2, respectively.
We recorded all sightings of potential predators of C. dickersonae for our assessment of territory quality. The most dangerous terrestrial predators of Crotaphytus species are considered to be the roadrunner (Geococcyx californianus, a terrestrial bird) and the coachwhip (Masticophis flagellum, a diurnal snake) (Husak, Macedonia et al., 2006). Crotaphytus species are also predated by flying avian predators, but given that their hunting areas easily encompass the total area of both the study sites, making them an equal threat to all of the lizards in our study, they were not included in our consideration of territory quality. Furthermore, during our study we observed only 1 potential terrestrial predator of C. dickersonae (a rattlesnake species), therefore we excluded predator abundance of the territory quality assessment.
Territory and home range analyses (size and overlap) were performed with GIS and ArcView 3.2a with the Convex Hulls v. 1.24 extension by Jenness Enterprises (Jenness, 2008). Statistical analyses were performed in R, version 3.3.3 (R Core Team, 2017). We used non-parametric tests: Mann Whitney U tests to evaluate differences between males with and without territories, and Spearman rank correlation tests to assess correlations between morphological traits and aspects of their territories.
Results
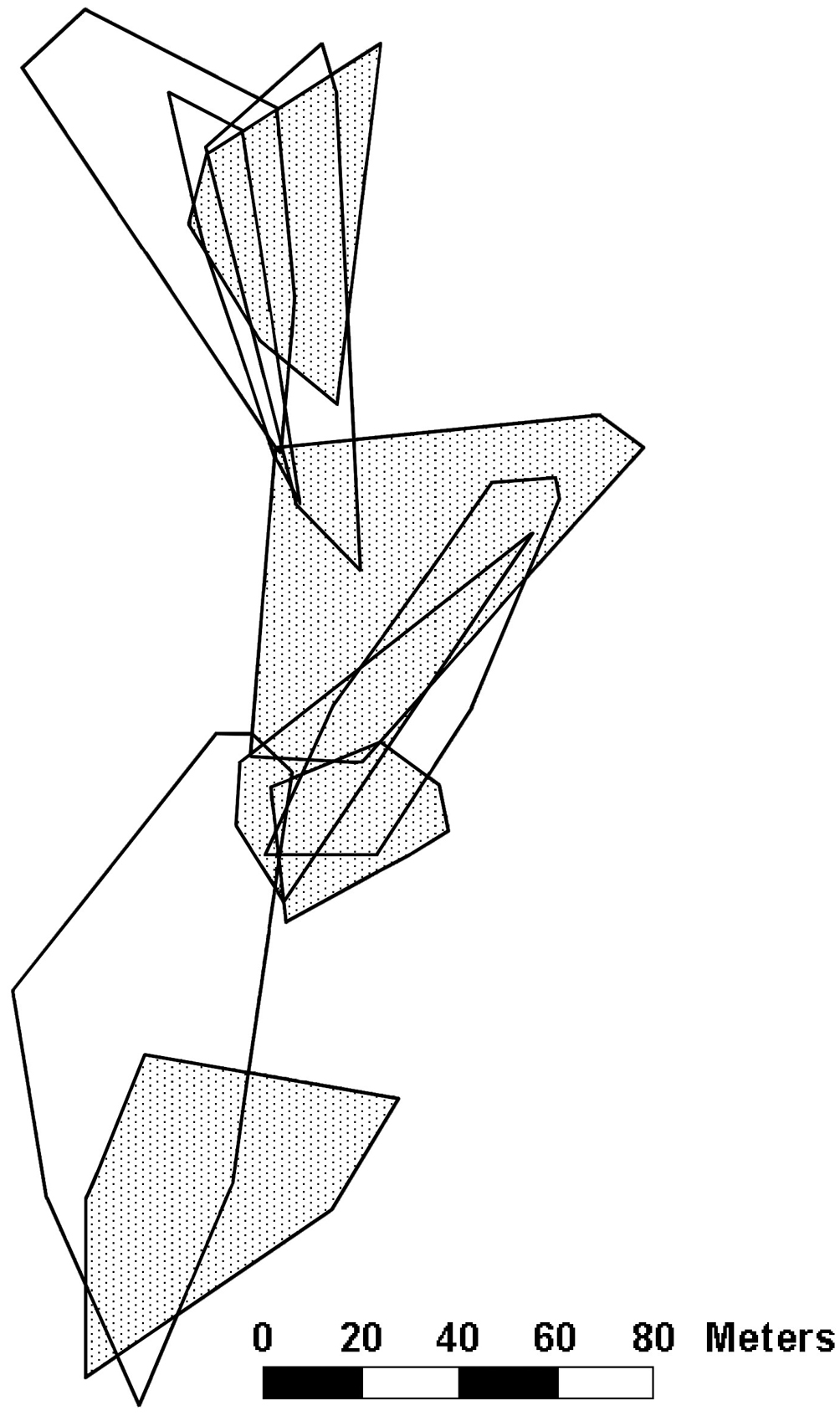
At study site 1, we collected enough sightings to plot the territories of 5 males and the home ranges of 5 females (Fig. 1). We saw 5 additional males (each observed between 1 and 6 times) and 6 additional females (with sightings ranging from 1 to 10).
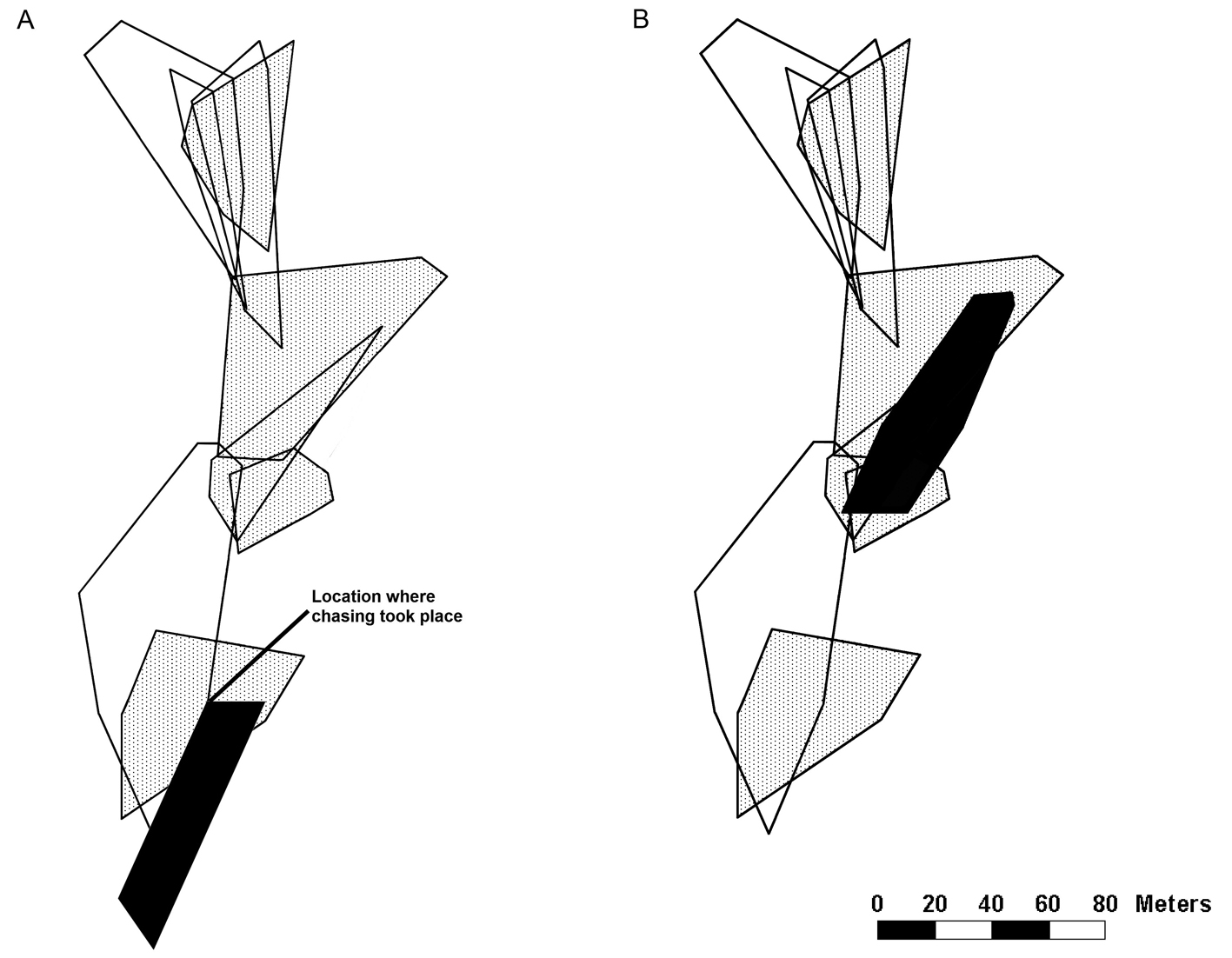
Additionally, we observed a bout of interactions between 2 males at study site 1 on June 4, resulting in the apparent expulsion of one male from its territory. One of the males ran from a distance of > 30 m to another male and chased him away repeatedly. After June 4, the lizard that had been chased away was not seen again at the coordinates at which the chase had taken place, though he did establish a new territory within our study site (Fig. 2). For our calculations of territory quality and territory overlap of this lizard, we only used the sightings that we collected after June 4.
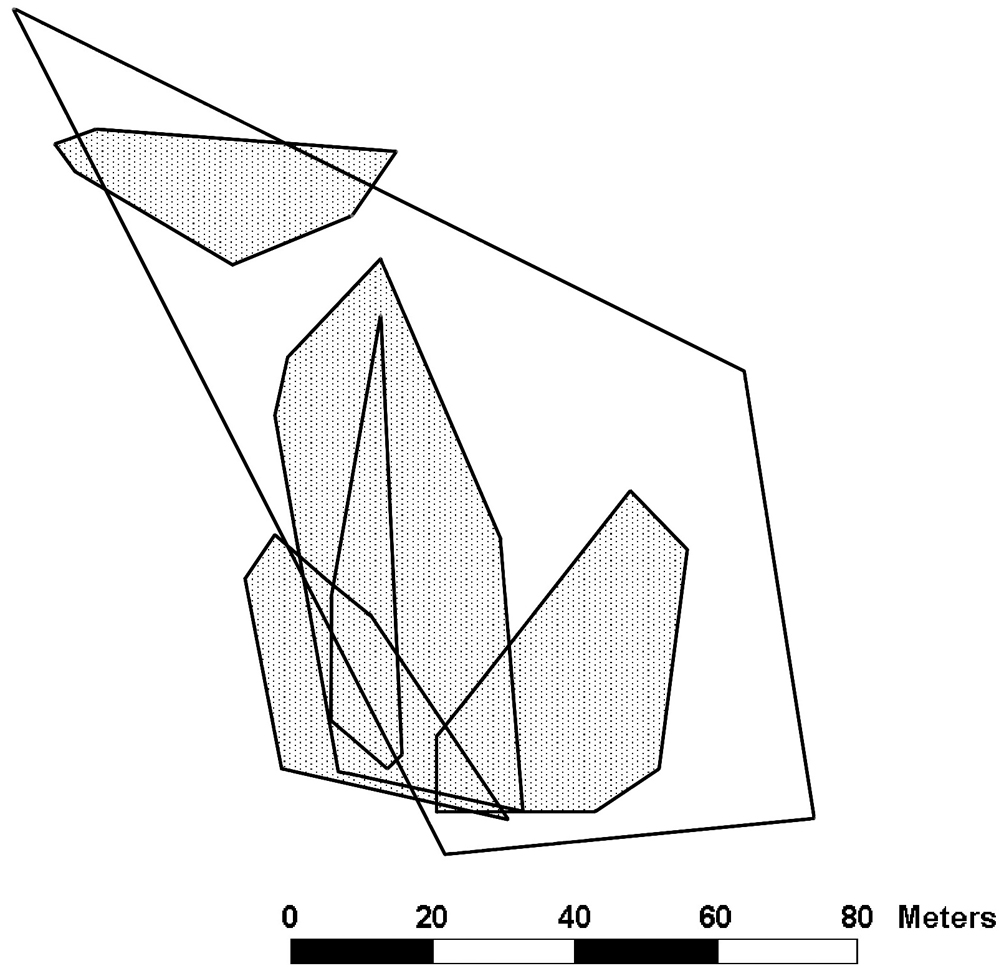
At study site 2 we collected enough sightings to plot the territory of 1 male and home ranges of 5 females (Fig. 3). We saw 3 additional males (each observed between 1 and 3 times) and 6 additional females (each observed between 1 and 6 times).
Phenotypic traits did not differ between territorial males (> 12 sightings, n = 6) and other males (≤ 6 sightings, n = 10; Mann-Whitney U tests: U > 23.00, p > 0.44; Table 1). Males with higher SVL had longer tails (rs = 0.83, p = 0.04), wider heads (rs = 0.90, p = 0.01), and longer legs (rs = 0.99, p = 0.0003). Strangely, SVL did not relate with body mass (rs = 0.60, p = 0.21). Heavier males tended to have longer tails (rs = 0.77, p = 0.07) and wider heads (rs = 0.75, p = 0.08). When corrected for SVL, body mass, tail length, head width, and leg length were uncorrelated (rs < 0.66, p > 0.16). For further analyses we used morphology traits corrected for SVL. Bluer males had wider heads (rs = 0.93, p = 0.01), and tended to be heavier (rs = 0.77, p = 0.07), but blue chroma did not correlate with SVL (rs = 0.71, p = 0.11) or tail length (rs = 0.66, p = 0.16).
Male territory size averaged 2,912.9 m2, ranging from 693.5 m2 to 6,236.0 m2 (n = 6) (Figs. 1, 3). We found no significant correlations between phenotypic traits and the size of the territory (Table 2).
Table 1
Morphological traits of territorial and non-territorial males. Average ± SD are given. Differences were not significant.
|
Males |
||
|
with territory |
without territory |
|
|
SVL (mm) |
84 ± 8 |
84 ± 8 |
|
Weight (g) |
23 ± 8 |
28 ± 11 |
|
Head width (mm) |
20 ± 5 |
22 ± 3 |
|
Tail length (mm) |
187 ± 39 |
197 ± 14 |
|
Hind limb length (mm) |
29 ± 5 |
30 ± 3 |
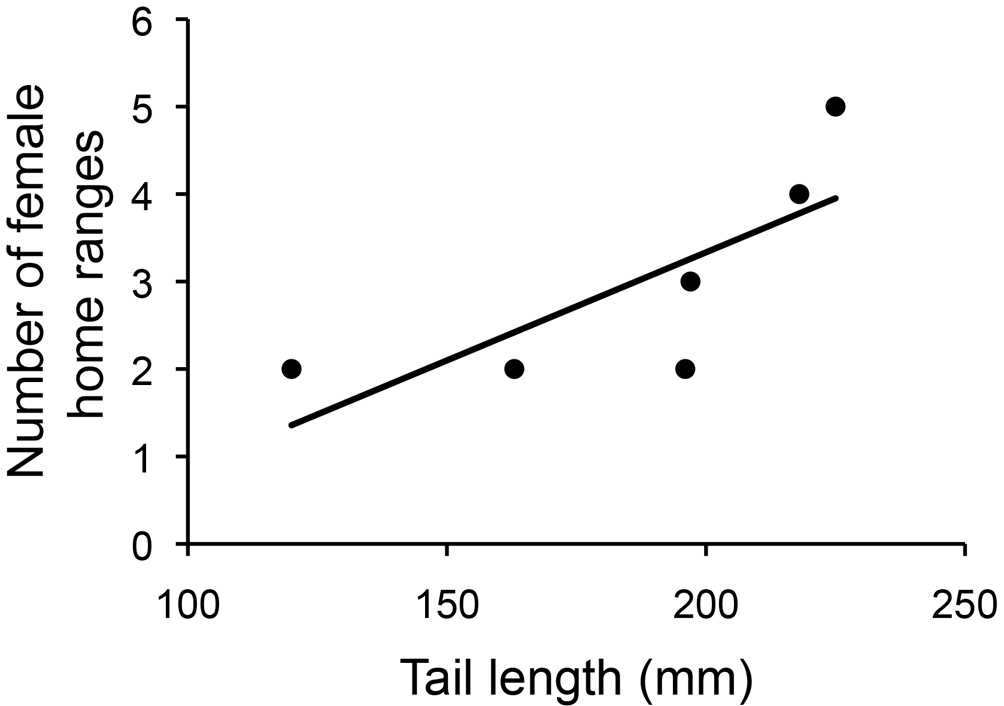
The average number of overlapping female home ranges for each male territory was 3 and ranged from 2 to 5 (Figs. 1, 3). Males with longer tails had more overlapping female home ranges (rs = 0.94, p = 0.005, Fig. 4). Males with longer SVL, wider heads, and higher blue chroma tended to have more overlapping female home ranges (Table 2). However, when correcting for territory size all these correlations became non–significant (rs < 0.26, p > 0.623). There was no correlation between hind limb length and the number of overlapping female home ranges.
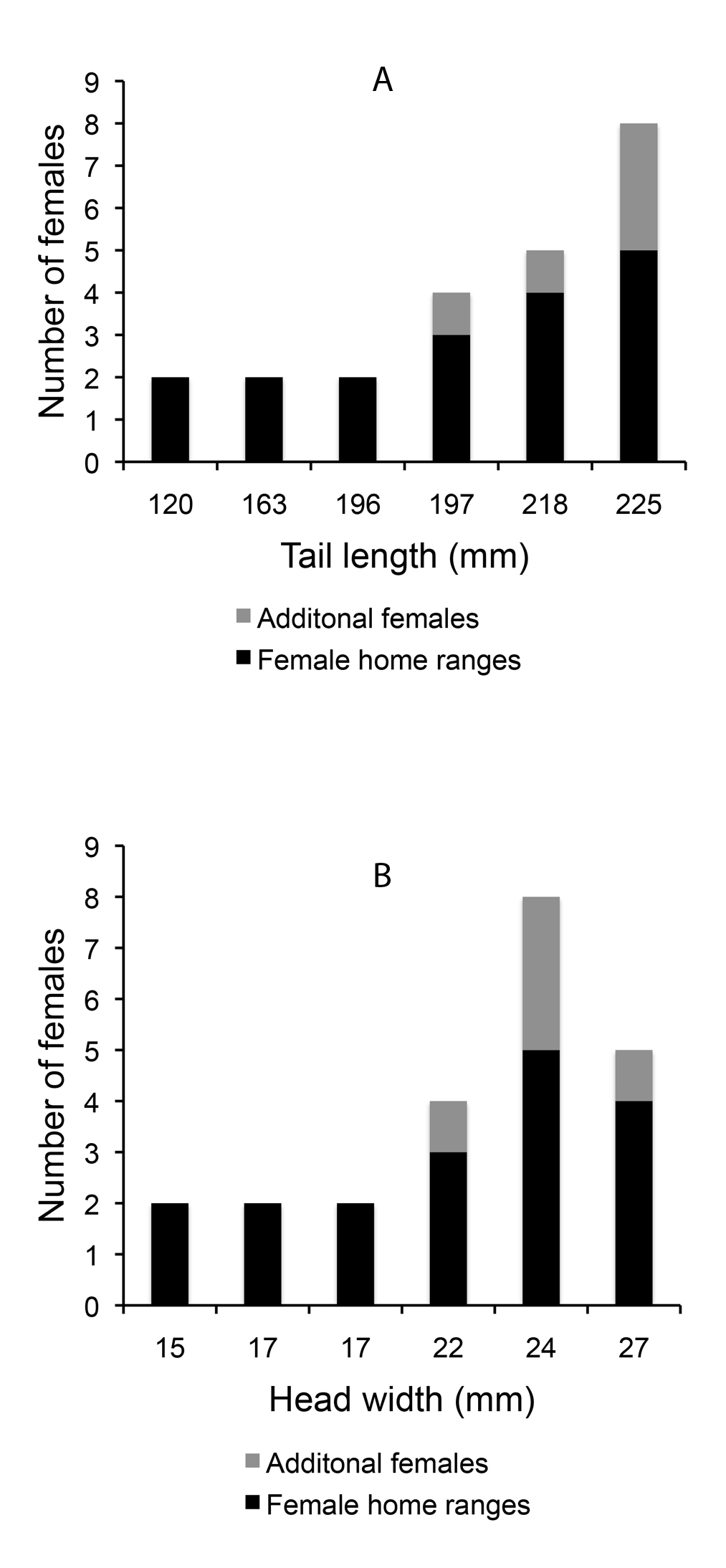
The average of the total number of females observed in a male’s territory (including the females for which we did not calculate home range size) was 3.8 and ranged from 2 to 8. The total number of females observed in a male’s territory was significantly higher for males with longer tails (rs = 0.94, p = 0.005) and tended to be higher for males with longer SVL, wider heads, and higher blue chroma (Table 2). We found no correlation between the total number of females seen in a male’s territory and the hind limb length or body mass of the male. When correcting for territory size, no morphological measure bore statistical relation to the total number of females observed in the territory (p > 0.20). Interestingly, the additional females, with too few sightings to calculate their home range, were only seen in the territories of the males with the widest heads and the longest tails (Fig. 5).
The number of overlapping male territories averaged 1.33 and ranged from 0 to 2. Males with longer tails had less overlapping male territories (rs = -0.93, p = 0.008). There was a trend that males with wider heads and higher blue chroma had less overlapping male territories (Table 2). We found no significant correlation between the SVL and the hind limb length of the male and the number of overlapping male territories (Table 2).
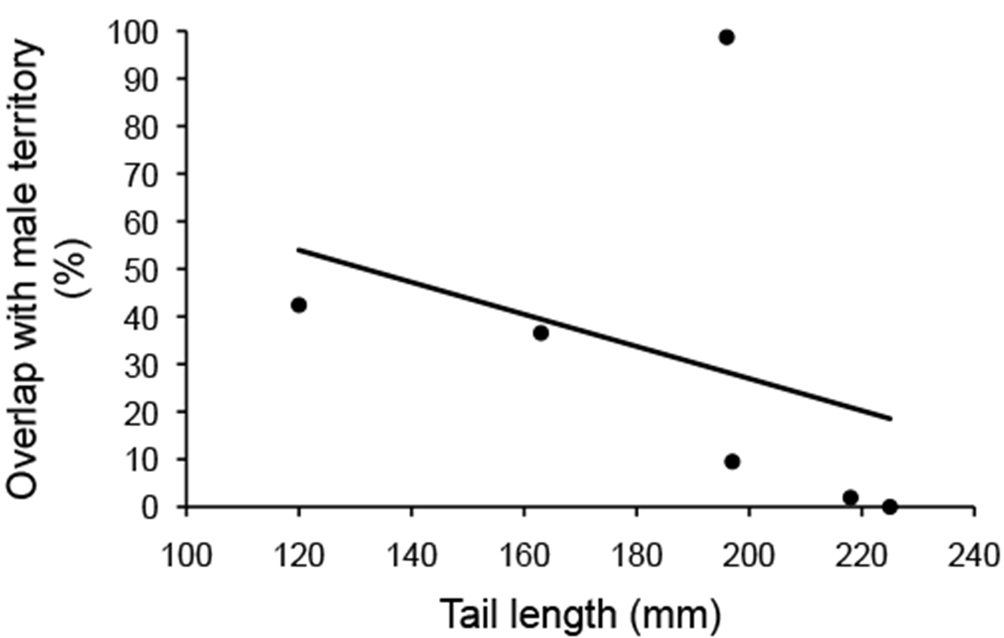
The percentage of overlap with other male territories averaged 31.5% and ranged from 0% to 98.7%. Males with longer tails had significantly less overlap with other male’s territories (Table 2; Fig. 6). There was no significant correlation between the other phenotypic traits and the percentage of overlap with other male territories (Table 2).
Males with longer tails, wider heads, and higher blue chroma had a higher number of additional males (males for which we did not calculate territory size) in their territories (Table 2). Males with higher SVL also tended to have more incidences of additional males in their territories (Table 2). When correcting for territory size, head width and blue chroma still related positively with the number of additional males (Table 2). Interestingly, the number of additional males was correlated with the number of females in the territory (rs = 0.89, p = 0.02).
The number of trees and shrubs in a territory ranged from 150 to 456 and averaged 248. The territories had an average of 0.107 trees and shrubs per m2; this ranged from 0.069 to 0.216. The total number of rocks in a territory ranged from 75 to 526 and averaged 224.5. The number of rocks per m2 averaged 0.080 and ranged from 0.042 to 0.108. When corrected for territory size, the number of refuges or basking sites did not correlate with any of the phenotypic traits evaluated (Table 2). Furthermore, the number of refuges or basking sites did not correlate with the number of females present in the territory, whether considering total number of females, percent of overlap with female home ranges, or additional females (p > 0.11).
Table 2
Spearman correlations between territory aspects and morphological traits of the male lizards. Results are given with and without correcting for territory size. Tail length and head width were corrected for SVL. Body mass and hind limb length were excluded from the table, as they did not correlate with any of the territory aspects. · indicates a trend (0.10 < p > 0.05), * indicates p < 0.05, and ** indicates p < 0.01.
|
Territory quality |
SVL |
Tail length |
Head width |
Blue chroma |
||||
|
rs |
p |
rs |
p |
rs |
p |
rs |
p |
|
|
Territory size: |
0.54 |
0.27 |
0.60 |
0.21 |
0.60 |
0.21 |
0.66 |
0.16 |
|
Female presence: |
||||||||
|
Nr of female’s home ranges overlap |
0.76 |
0.08˙ |
0.94 |
0.01** |
0.76 |
0.08˙ |
0.76 |
0.08˙ |
|
corrected for territory size |
-0.03 |
0.70 |
-0.26 |
0.62 |
-0.26 |
0.62 |
-0.26 |
0.62 |
|
Additional females |
0.83 |
0.14 |
0.93 |
0.01** |
0.74 |
0.09˙ |
0.74 |
0.09˙ |
|
corrected for territory size |
0.94 |
0.36 |
0.76 |
0.08˙ |
0.76 |
0.08˙ |
0.76 |
0.08˙ |
|
Total nr of females |
0.76 |
0.08˙ |
0.94 |
0.01** |
0.76 |
0.08˙ |
0.76 |
0.08˙ |
|
corrected for territory size |
0.60 |
0.40 |
0.49 |
0.33 |
-0.30 |
0.96 |
0.14 |
0.79 |
|
Exclusivity: |
||||||||
|
Nr of male territories |
-0.68 |
0.14 |
-0.93 |
0.01** |
-0.74 |
0.09˙ |
-0.74 |
0.09˙ |
|
corrected for territory size |
-0.60 |
0.21 |
-0.77 |
0.07˙ |
-0.77 |
0.07˙ |
-0.77 |
0.07˙ |
|
% Overlap male territories |
-0.54 |
0.27 |
-0.83 |
0.04* |
-0.83 |
0.21 |
-0.66 |
0.16 |
|
Additional males |
0.77 |
0.07˙ |
0.83 |
0.04* |
0.93 |
0.01** |
0.93 |
0.01** |
|
corrected for territory size |
0.52 |
0.30 |
-0.60 |
0.12 |
0.88 |
0.02* |
0.88 |
0.02* |
|
Habitat: |
||||||||
|
Refuges (per m2) |
-0.49 |
0.33 |
-0.43 |
0.40 |
-0.60 |
0.21 |
-0.54 |
0.27 |
|
Basking sites (per m2) |
0.14 |
0.79 |
0.31 |
0.54 |
-0.20 |
0.70 |
-0.14 |
0.79 |
Discussion
We studied the relation of phenotypic traits with territory quality in Crotaphytus dickersonae. Results show that males with longer tails had higher female presence in their territory and less overlap with other male territories. Additionally, bluer males and males with wider heads tended to have territories with more females and less overlapping male territories, yet these males had more additional males in their territories.
Tail length and head width are morphological features that can be related to RHP. Longer tails increase agility, allowing the animal to run faster without losing stability (Wheatley et al., 2015; Wynn et al., 2015). Tail length is related to sprint speed in lizards. Experimental reductions in tail length decreased sprint speed in some lizard species (Ballinger et al., 1979; Downes & Shine, 2001; Formanowicz et al., 1990), but increased sprint speed in others (Daniels, 1983; Li et al., 2011). Garland (1985) suggested that longer tails would be especially beneficial for lizards that run bipedally. Crotaphytus species are well known for their bipedal gait (McGuire, 1996), and longer tails may allow them to run faster. Lizards that can run faster have larger territories (Peterson & Husak, 2006) and are more likely to achieve dominance (Garland et al., 1990; Robson & Miles, 2000). This suggests that running speed is important in male-male competition. Accordingly, Husak and Fox (2006) found that male C. collaris use near maximum sprint speed when they respond to other males in their territory. In the interactions between males that we observed, a typical pattern of behavior involved one of the males running quickly to the other male, with the latter subsequently running away. We observed several such interactions between 2 males, which even led to territory replacement of one of the males (see results). The lizard that had chased away the other male was the male with the widest head and longest tail of all males included in this study. Hence, faster and stronger males may be able to maintain exclusivity of their territory. These males might also be better able to expand the boundaries of their territory, possibly increasing the number of females in their territory while still being able to keep other males away from those females.
Biting is often used in agonistic encounters (Hall et al., 2010; Lailvaux & Irschick, 2006) and can be considered an important trait determining RHP (Lailvaux & Irschick, 2006). Male lizards that bite harder are more likely to win fights (Husak, Lappin et al., 2006; Huyghe et al., 2005; Lailvaux et al., 2004), have larger territories with more overlap with female home ranges (Lappin & Husak, 2005), and they have a higher reproductive success (Husak et al., 2009; Lappin & Husak, 2005). Head size and shape are strongly correlated with bite force (Anderson et al., 2008). In male lizards, head width is a predictor of bite force; males with wider heads bite harder (Herrel et al., 2006; Husak, Lappin et al., 2006; Lappin & Husak, 2005). The positive relation between head width and territory quality suggests that biting also plays a major role in territory acquisition and defense in C. dickersonae. Head width, however, correlated positively with additional males. Perhaps males are attracted by the higher territory quality of these males, in which more female encounters may be worth the risk of the attack of the strong territory owner.
We found that bluer males tended to have more exclusive territories. The intensity of the blue color relates to running speed and bite force in C. dickersonae and could thus be an honest signal of RHP (Plasman et al., 2015). Morphological features may not be easily distinguished from long distances, whereas bright coloration can be easily detected (Losos, 1985; Whiting et al., 2003), especially in the open habitat of the Sonoran desert. The blue color may facilitate the assessment of the opponent’s RHP over long distances (preliminary opponent assessment), whereas morphological traits may be assessed in a later stage when animals are nearer (secondary opponent assessment; Kokko, 2013). The blue color may thus dissuade other males to invade their territory. Contrarily, however, we observed more additional males in the territories of bluer males. Likewise, more additional males were observed in territories of males with longer tails and wider heads. These additional males may temporarily sneak into these territories with the intention to steal copulations. This strategy is frequently seen in species where part of the population is not able to obtain a territory (Gross, 1996; López-Sepulcre & Kokko, 2005) and has been found to have equal fitness benefits as that of territory owners in the C. collaris (York et al., 2014). The big size of the territories of C. dickersonae prevent males from guarding the entire area simultaneously, leaving parts open to opportunistic males. Further, the number of additional males correlated with female abundance. Interestingly, the strong correlation of number of additional males with blue chroma of the resident male may suggest that the sneaker males perhaps uses the information broadcasted by the territorial male to estimate female presence.
More females were found in the territories of males with longer tails, wider heads and higher blue chroma. Higher female abundance may occur due to high territory quality. Although we did not find correlations with abundance of basking sites and refuges, or the presence of predators, we cannot exclude other territory characteristics that were not measured here (e.g., food abundance). On the other hand, female abundance may be higher, because females prefer the territory owner as mate. Females may be attracted by traits used in male-male competition, as these traits may relate to general condition or quality (Berglund et al., 1996) and provide the female with good genes for her offspring (Hamilton & Zuk, 1982; Zahavi, 1975) or give direct benefits in the form of a high quality territory and protection from other males (Emlen & Oring, 1977). Female choice has rarely been documented in lizards (Olsson & Madsen, 1995; Olsson et al., 2013) and generally the female mates with the territory owner with which her home range overlaps (Kiester, 1979; Stamps, 1983, 1987; Tokarz, 1998). Nevertheless, females may temporarily leave their home range to mate with a high quality male (Calsbeek & Sinervo, 2002; Vitousek et al., 2007). In the collared lizard, C. collaris, almost 40% of the offspring was from a male other than the owner of the territory with which their home range overlapped (Husak et al., 2008) and multiple mating may be common (York & Baird, 2015). However, the lack of difference between offspring fitness from territorial and non-territorial males and the thermoregulation costs associated to fleeing from males, suggests that females may accept copulations to avoid male harassment (York & Baird, 2015). On the other hand, in an earlier study with C. collaris it was found that females discriminated against young males (Baird et al., 1996). Furthermore, we found that only males with long tails and wide heads had additional female visitors, which may suggest female choice. To what extent female choice determines overlap of male territories with female home ranges or additional female visitors remains unclear and is an interesting topic for future studies.
Although we assumed basking sites and refuges to be important factors for survival in C. dickersonae, their quantity did not relate to any male trait or to female presence. Possibly refuges and basking sites are not considered when selecting a territory, due to the high abundance of rocks and bushes in our study site. As such, territory quality was determined primarily by its size and the presence of conspecifics, and obtaining mates is likely the most important motive for territory defense in this species (Baird, 2013).
Our results indicate that, in male Crotaphytus dickersonae, head width (i.e., bite force) and especially tail length (i.e., running speed) are important traits for the intra-sexual contest for territory acquisition and determine access to females for mating. The blue coloration of the males probably signals male dominance status (Plasman et al., 2015) and helps to estimate the opponent’s RHP over long distances and possibly attracts females. The conspicuous sexual signals can thus increase fitness benefits (e.g., repel opponents and attract mates). Yet, the higher number of additional males in territories of bluer males suggests that opponents may exploit the signal when intercepting the mates attracted by the signal (Perril et al., 1978). Further, our results indicate that territories of C. dickersonae males are large compared to those of other animals of similar size (Candolin & Voigt, 2001; McLoughlin & Ferguson, 2000), other lizards (Molnár et al., 2016; Stehle et al., 2017), or even other collared lizard species (Schwartz et al., 2007; although this may depend on population, see McCoy et al., 2003). Due to their size, the number of territories that can be established is probably limited, whereas costs for patrolling and defending this big area are likely high, and low female density may result in low reproductive payoff (Briffa & Sneddon, 2007; Lappin & Husak, 2005). Hence, it could be concluded that C. dickersonae is a species with relatively few territorial males and many floaters (López-Sepulcre & Kokko, 2005). It needs to be determined if these 2 mating strategies form an ESS (evolutionary stable strategy) with equal success or that being a floater is ‘making the best of a bad job’.
Acknowledgments
We thank G. E. Castro and A. B. Fleishman for help with fieldwork, G. W. Heil and A. B. Fleishman for help with GIS ArcView, H. de Vries for help with statistics, V. H. Reynoso for logistical and technical support, and Prescott College for facilitating lodging. We thank R. Torres for lending equipment and also for arranging the capture-release permits for C. dickersonae, which were obtained from the Secretaría del Medio Ambiente y Recursos Naturales with permit number SGPA/DGVS/02084/11. This study was performed according to Mexican regulations of animal welfare.
References
Andersson, M. (1994). Sexual selection. Princeton: Princeton University Press.
Anderson, R. A., McBrayer, L. D., & Herrel, A. (2008). Bite force in vertebrates: opportunities and caveats for use of a nonpareil whole-animal performance measure. Biological Journal of the Linnean Society, 93, 709–720.
Baird, T. A. (2013). Lizards and other reptiles as model systems for the study of contest behaviour. In I. C. W. Hardy, & M. Briffa (Eds.), Animal contests (pp. 258–286). Cambridge: Cambridge University Press.
Baird, T. A., Acree, M. A., & Sloan, C. L. (1996). Age and gender-related differences in the social behavior and mating success of free-living collared lizards, Crotaphytus collaris. Copeia, 1996, 336–347.
Baird, T. A., Hranitz, J. M., Timanus, D. K., & Schwartz, A. M. (2007). Behavioral attributes influence annual mating success more than morphological traits in male collared lizards. Behavioral Ecology, 18, 1146–1154.
Ballinger, R. E., Nietfeldt, J. W., & Krupa, J. J. (1979). An experimental analysis of the role of the tail in attaining high running speed in Cnemidophorus sexlineatus (Reptilia: Squamata: Lacertilia). Herpetologica, 35, 114–116.
Berglund, A., Bisazza, A., & Pilastro, A. (1996). Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biological Journal of the Linnean Society, 58, 385–399.
Briffa, M., & Sneddon, L. U. (2007). Physiological constraints on contest behaviour. Functional Ecology, 21, 627–637.
Burt, W. H. (1943). Territoriality and home range concepts as applied to mammals. Journal of Mammalogy, 24, 346–352.
Calsbeek, R., & Sinervo, B. (2002). Uncoupling direct and indirect components of female choice in the wild. Proceedings of the National Academy of Sciences, 99, 14897–14902.
Candolin, U., & Voigt, H. R. (2001). Correlation between male size and territory quality: consequence of male competition or predation susceptibility? Oikos, 95, 225–230.
Daniels, C. B. (1983). Running: an escape strategy enhanced by autotomy. Herpetologica, 39, 162–165.
Downes, S., & Shine, R. (2001). Why does tail loss increase a lizard’s later vulnerability to snake predators? Ecology, 82, 1293–1303.
Emlen, S. T., & Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science, 197, 215–223.
Faber, L., Plasman, M., & Duchateau, M. J. (2013). The use of photo identification on Dickerson’s collared lizard (Crotaphytus dickersonae). Herpetological Review, 44, 600–603.
Formanowicz, D. R., Jr., Brodie, E. D., Jr., & Bradley, P. J. (1990). Behavioural compensation for tail loss in the ground skink, Scincella lateralis. Animal Behaviour, 40, 782–784.
Garland, T., Jr. (1985). Ontogenetic and individual variation in size, shape and speed in the Australian agamid lizard Amphibolurus nuchalis. Journal of Zoology, 207, 425–439.
Garland, T., Jr., Hankins, E., & Huey, R. B. (1990). Locomotor capacity and social dominance in male lizards. Functional Ecology, 4, 243–250.
Gross, M. R. (1996). Alternative reproductive strategies and tactics: diversity within sexes. Trends in Ecology and Evolution, 11, 92–98.
Hall, M. D., McLaren, L., Brooks, R. C., & Lailvaux, S. P. (2010). Interactions among preformance capacities predict male combat outcomes in the field cricket. Functional Ecology, 24, 159–164.
Hamilton, W. D., & Zuk, M. (1982). Heritable true fitness and bright birds: a role for parasites? Science, 218, 384–387.
Herrel, A., Joachim, R., Vanhooydonck, B., & Irschick, D. J. (2006). Ecological consequences of ontogenetic changes in head shape and bite performance in the Jamaican lizard Anolis lineatopus. Biological Journal of the Linnean Society, 89, 443–454.
Husak, J. F., & Fox, S. F. (2006). Field use of maximal sprint speed by collared lizards (Crotaphytus collaris): compensation and sexual selection. Evolution, 60, 1888–1895.
Husak, J. F., Fox, S. F., & Van Den Bussche, R. A. (2008). Faster male lizards are better defenders not sneakers. Animal Behaviour, 75, 1725–1730.
Husak, J. F., Lappin, A. K., Fox, S. F., & Lemos-Espinal, J. A. (2006). Bite-force performance predicts dominance in male venerable collared lizards (Crotaphytus antiquus). Copeia, 2006, 301–306.
Husak, J. F., Lappin, A. K., & Van Den Bussche, R. A. (2009). The fitness advantage of a high-performance weapon. Biological Journal of the Linnean Society, 96, 840–845.
Husak, J. F., Macedonia, J. M., Fox, S. F., & Sauceda, R. (2006). Predation cost of conspicuous male coloration in collared lizards (Crotaphytus collaris): an experimental test using clay-covered model lizards. Ethology, 112, 572–580.
Huyghe, K., Vanhooydonck, B., Scheers, H., Molina-Borja, M., & Van Damme, R. (2005). Morphology, performance and fighting capacity in male lizards, Gallotia gallotia. Functional Ecology, 19, 800–807.
Jenness, J. (2008). Convex hulls around points (conv_hulls_pts.avx) extension for ArcView 3.x, v. 1.23. Jenness Enterprises. Available at: http://www.jennessent.com/arcview/convex_hulls.htm
Kiester, A. R. (1979). Conspecifics as cues: a mechanism for habitat selection in the Panamanian grass anole (Anolis auratus). Behavioral Ecology and Sociobiology, 5, 323–330.
Kokko, H. (2013). Dyadic contest: modelling fights between two individuals. In I. C. W. Hardy, & M. Briffa (Eds.), Animal contests (pp. 5–32). Cambridge: Cambridge University Press.
Lailvaux, S. P., Herrel, A., VanHooydonck, B., Meyers, J. J., & Irschick, D. J. (2004). Performance capacity, fighting tactics and the evolution of life-stage male morphs in the green anole lizard (Anolis carolinensis). Proceedings of the Royal Society B, 271, 2501–2508.
Lailvaux, S. P., & Irschick, D. J. (2006). A functional perspective on sexual selection: insights and future prospects. Animal Behaviour, 72, 263–273.
Lappin, A. K., Brandt, Y., Husak, J. F., Macedonia, J. M., & Kemp, D. J. (2006). Gaping displays reveal and amplify a mechanically based index of weapon performance. The American Naturalist, 168, 100–113.
Lappin, A. K., & Husak, J. F. (2005). Weapon performance, not size, determines mating success and potential reproductive output in the collared lizard (Crotaphytus collaris). The American Naturalist, 166, 426–436.
Li, C., Lian, X., Bi, J., Fang, H., Maul, T. L., & Jian, Z. (2011). Effects of sand grain size and morphological traits on running speed of toad-headed lizard Phrynocephalus frontalis. Journal of Arid Environments, 75, 1038–1042.
López-Sepulcre, A., & Kokko, H. (2005). Territorial defense, territory size, and population regulation. The American Naturalist, 166, 317–329.
Losos, J. B. (1985). An experimental demonstration of the species-recognition role of anolis dewlap color. Copeia, 1985, 905–910.
Maher, C. R., & Lott, D. F. (1995). Definitions of territoriality used in the study of variation in vertebrate spacing systems. Animal Behaviour, 49, 1581–1597.
Marler, C. A., & Moore, M. C. (1988). Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behavioral Ecology and Sociobiology, 23, 21–26.
McCoy, J. K., Baird, T. A., & Fox, S. F. (2003). Sexual selection, social behavior, and the environmental potential for polygyny. In S. F. Fox, J. K. McCoy, & T. A. Baird (Eds.), Lizard social behavior (pp. 149–171). Baltimore, London: Johns Hopkins University Press.
McGuire, J. A. (1996). Phylogenetic systematics of Crotaphytid lizards (Reptilia: Iguania: Crotaphytidae). Bulletin of Carnegie Museum of Natural History, 32, 1–143.
McLoughlin, P. D., & Ferguson, S. H. (2000). A hierarchical pattern of limiting factors helps explain variation in home range size. Écoscience, 7, 123–130.
Molnár, O., Bajer, K., Szövényi, G., Török, J., & Herczeg, G. (2016). Space use strategies and nuptial color in European green lizards. Herpetologica, 72, 40–46.
Olsson, M. (1994). Nuptial coloration in the sand lizard, Lacerta agilis: an intra-sexually selected cue to lighting ability. Animal Behaviour, 48, 607–613.
Olsson, M., & Madsen, T. (1995). Female choice on male quantitative traits in lizards-why is it so rare? Behavioral Ecology and Sociobiology, 36, 179–184.
Olsson, M., Stuart-Fox, D., & Ballen, C. (2013). Genetics and evolution of colour patterns in reptiles. Seminars in Cell and Developmental Biology, 24, 529–541.
Perrill, S. A., Gerhardt, H. C., & Daniel, R. (1978). Sexual parasitism in green tree frogs (Hyla cinerea). Science, 200, 1179–1180.
Peterson, C. C., & Husak, J. F. (2006). Locomotor performance and sexual selection: individual variation in sprint speed of collared lizards (Crotaphytus collaris). Copeia, 2006, 216–224.
Plasman, M., Duchateau, M. J. H. M., & Macedonia, J. M. (2007). Anti-predation behaviour of Dickerson’s collared lizard, Crotaphytus dickersonae. Animal Biology, 57, 231–246.
Plasman, M., Reynoso, V. H., Nicolás, L., & Torres, R. (2015). Multiple colour traits signal performance and immune response in the Dickerson’s collared lizard Crotaphytus dickersonae. Behavioral Ecology and Sociobiology, 69, 765–775.
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Robson, M. A., & Miles, D. B. (2000). Locomotor performance and dominance in male tree lizards, Urosaurus ornatus. Functional Ecology, 14, 338–344.
Rose, B. (1982). Lizard home ranges: methodology and functions. Journal of Herpetology, 16, 253–269.
Schulte-Hostedde, A. I., Zinner, B., Millar, J. S., & Hickling, G. J. (2005). Restitution of mass-size residuals: validation body condition indices. Ecology, 86, 155–163.
Schwartz, A. M., Baird, T. A., & Timanus, D. K. (2007). Influence of age and prior experience on territorial behavior and the costs of defense in male collared lizards. Ethology, 113, 9–17.
Stamps, J. A. (1977). Social behavior and spacing patterns in lizards. In C. Gans, & D. W. Tinkle (Eds.), Biology of the Reptilia. Vol. 7. Ecology and Behaviour (pp. 265–334). New York: Academic Press.
Stamps, J. A. (1983). Sexual selection, sexual dimorphism, and territoriality. In R. B. Huey, E. R. Pianka, & T. W. Schoener (Eds.), Lizard ecology: studies of a model organism (pp. 169–204). Cambridge, MA: Harvard University Press.
Stamps, J. A. (1987). Conspecifics as cues to territory quality: a preference of juvenile lizards (Anolis aeneus) for previously used territories. The American Naturalist, 129, 629–642.
Stehle, C. M., Battles, A. C., Sparks, M. N., & Johnson, M. A. (2017). Prey availability affects territory size, but non territorial display behavior, in green anole lizards. Acta Oecologia, 84, 41–47.
Stone, P. A., & Baird, T. A. (2002). Estimating lizard home range: the Rose model revisited. Journal of Herpetology, 36, 427–436.
Tokarz, R. R. (1998). Mating pattern in the lizard Anolis sagrei: implications for mate choice and sperm competition. Herpetologica, 54, 388–394.
Vitousek, M. N., Mitchell, M. A., Woakes, A. J., Niemack, M. D., & Wikelski, M. (2007). High costs of female choice in a lekking lizard. Plos One, 2, e567.
Wheatley, R., Angilletta, M. J., Jr., Niehaus, M. C., & Wilson, R. S. (2015). How fast should an animal run when escaping? An optimality model based on the trade-off between speed and accuracy. Integrative and Comparative Biology, 55, 1166–1175.
Whiting, M. J., Nagy, K. A., & Bateman, P. W. (2003). Evolution and maintenance of social status signalling badges: experimental manipulations in lizards. In S. F. Fox, J. K. McCoy, & T. A. Baird (Eds.), Lizard social behavior (pp. 47–82). Baltimore, London: Johns Hopkins University Press.
Wynn, M. L., Clemente, C., Nasir, A. F. A. A., & Wilson, R. S. (2015). Running faster causes disaster: trade-offs between speed and manoeuvrability and motor control when running around corners in northern quolls (Dasyurus hallucatus). Journal of Experimental Biology, 218, 433–439.
York, J. R., & Baird, T. A. (2015). Testing the adaptive significance of sex-specific mating tactics in collared lizards (Crotaphytus collaris). Journal of the Linnean Society, 115, 423–436.
York, J. R., Baird, T. A., & Haynie, M. L. (2014). Unexpected high fitness payoff of subordinate social tactics in male collared lizards. Animal Behaviour, 91, 17–25.
Zahavi, A. (1975). Mate-selection –a selection for a handicap. Journal of Theoretical Biology, 53, 205–214.
Zamudio, K. R., & Sinervo, B. (2003). Ecological and social context for the evolution of alternative mating strategies. In S. F. Fox, J. K. McCoy, & T.A. Baird (Eds.), Lizard social behavior (pp. 83–106). Baltimore, London: John Hopkins University Press.



