The additive effects of pollinators and herbivores on the vine Bomarea salsilla (Alstroemeriaceae), remain spatially consistent in a fragmented forest
Carlos E. Valdivia a, *, Javier A. Simonetti b
a Laboratorio de Vida Silvestre, Departamento de Ciencias Biológicas y Biodiversidad, Universidad de Los Lagos, Casilla 933, 5311157 Osorno, Chile
b Laboratorio de Conservación Biológica, Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Casilla 653, 7800003 Santiago, Chile
*Corresponding author: carlos.valdivia@ulagos.cl (Carlos E. Valdivia)
Abstract
Modifications in plant-mutualistic and plant-antagonistic interactions driven by habitat fragmentation may have far reaching consequences by affecting plant reproductive success and their microevolutionary dynamics. Mutualists (e.g., pollinators) and antagonists (e.g., herbivores) can exert non-additive effects on plant fitness, which is interpreted as evidence of a pathway for correlated evolution on mutualist- and antagonist-linked traits, respectively. We suggest that a decrease in pollination and herbivory due to habitat fragmentation and proximity to edges may lead plants to face non-correlated fitness effects (i.e., additivity) exerted by pollinators and herbivores. We assessed the effects of pollinators and herbivores on Bomarea salsilla seed set by separately and simultaneously excluding pollinators and herbivores in a fully factorial design. The exclusions were performed in the core and edge of a continuous forest, and in the core and edge of forest fragments. At all sites studied, pollinators, but not herbivores, affected plant fitness, exerting non-correlated fitness effects. Consequently, forest fragmentation and the creation of edge habitats seemed not to affect the pollinator- and herbivore-mediated selection pressures on B. salsilla.
Keywords:
Hummingbirds; Nectarivory; Sephanoides sephaniodes; Temperate forests; Chile
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Los efectos aditivos de polinizadores y herbívoros sobre la enredadera Bomarea salsilla (Alstroemeriaceae) permanecen espacialmente consistentes en un bosque fragmentado
Resumen
Las modificaciones en las interacciones planta-mutualista y planta-antagonista dadas por la fragmentación del hábitat pueden tener consecuencias de largo alcance al afectar el éxito reproductivo de la planta y su dinámica microevolutiva. Los mutualistas (e.g., polinizadores) y antagonistas (e.g., herbívoros) pueden ejercer efectos no aditivos sobre la adecuación de la planta, lo que se interpreta como evidencia de una vía para la evolución correlacionada en rasgos ligados a mutualistas y antagonistas, respectivamente. Sugerimos que una disminución en la polinización y herbivoría debido a la fragmentación del hábitat y la proximidad a los bordes, puede llevar a las plantas a enfrentar efectos no correlacionados en la adecuación (i.e., efectos aditivos) ejercidas por polinizadores y herbívoros. Evaluamos los efectos de los polinizadores y herbívoros en la producción de semillas de Bomarea salsilla excluyendo polinizadores y herbívoros, simultánea y separadamente, en un diseño completamente factorial. Las exclusiones se realizaron en el centro y en el borde de un bosque continuo, y en centro y borde de fragmentos de bosques. En todos los sitios estudiados, los polinizadores, aunque no los herbívoros, afectaron a la planta ejerciendo efectos no correlacionados en la adecuación. En consecuencia, la fragmentación del bosque y la creación de hábitats de borde no afectaron las presiones selectivas ejercidas por polinizadores y herbívoros sobre B. salsilla.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Colibríes; Nectarivoría; Sephanoides sephaniodes; Bosques templados; Chile
Introduction
The extent to which plant-animal interactions are modified by habitat fragmentation is of paramount importance in conservation biology. The reduction in habitat size, increase in isolation, and proximity to edge habitats may reduce the diversity and abundance of interacting species (Aguilar et al., 2006; Steffan-Dewenter et al., 2006; Valiente-Banuet et al., 2015). A reduction in animal abundances may lead to contrasting effects on their plant counterparts depending on whether they are mutualists (e.g., pollinators) or antagonists (e.g., herbivores). Thus, coupled to a decrease in diversity and abundance of pollinators there is usually a decrease in the frequency of pollinator visits and, consequently, a decrease in the reproductive success of plants (Aguilar et al., 2006). When herbivores are also scarce in fragments and edges, however, some sort of compensation might arise in the reproductive success of plants depending on the strength of modifications of mutualisms and antagonisms (i.e., the symmetry of relationships) (Groom, 2001; Kolb, 2008).
Modifications in plant-mutualistic and plant-antagonistic interactions given by habitat fragmentation may have farther reaching consequences than those usually claimed in ecological terms by affecting, for instance, the microevolutionary dynamics of plant-animal relationships (Fontúrbel & Murúa, 2014; Jacquemyn et al., 2012; Valdivia, 2011). Plants may face numerous selective pressures exerted by pollinators and herbivores, which may influence their ecological and evolutionary responses (Herrera & Pellmyr, 2002; Medel & Nattero, 2009). Herbivores may modify plant survivorship and reproductive success because their action may have a significant impact on plants by directly reducing seed production. Furthermore, herbivores may indirectly reduce seed production by modifying floral attractiveness to pollinators (Herrera, 2000; Herrera et al., 2002; Strauss & Zangerl, 2002). Despite the pivotal role played by herbivores in the evolution of pollination-related traits in plants, assessments taking into consideration the selection pressures jointly exerted by herbivores and pollinators have seldom been made (Strauss & Whitall, 2006).
Mutualistic and antagonistic animals may exert non-additive effects on plant fitness, which suggests a pathway for correlated evolution on mutualism- and antagonism-related traits (Gómez, 2005; Herrera, 2000; Herrera et al., 2002; Valdivia & Niemeyer, 2005). Nevertheless, plants may undergo a reduction in the strength of interactions with both types of animals, which in turn may promote an evolutionary pathway in which traits linked to them run along dissimilar lanes (i.e., non-correlated evolution). This fact may occur naturally (Abdala-Roberts et al., 2009; Shahbazi et al., 2017; Valdivia & Niemeyer, 2007) or due to human activities such as habitat fragmentation. Unfortunately, no previous work has stressed this latter possibility.
In central Chile, forest fragmentation and proximity to edges has negatively affected pollination and herbivory on Bomarea salsilla, although only pollinators are proven to exert a significant impact on plant fitness (Valdivia et al., 2011). Nevertheless, the ecological and putative microevolutionary consequences of such reductions for B. salsilla depict a challenge that remains to be clarified. Given that forest fragmentation and proximity to edges negatively affects pollination and herbivory of B. salsilla (Valdivia et al., 2011), we hypothesized that pollinators and herbivores exert non-additive effects on the reproductive success of plants from the core of continuous forest, but not on those plants from the edge of continuous forest or from the edge and core of forest fragments. The aim of this work is to evaluate the combined effects of pollinators and herbivores on B. salsilla seed production, by modifying plant-pollinator and plant-herbivore interactions.
Materials and methods
Fieldwork was conducted in Maulino forest in central Chile, from September 2006 to January 2007, during the austral spring-summer season (35º59’ S, 72º41’ W). The vegetation is a coastal Mediterranean caducifolious forest strata of Nothofagus glauca and Persea lingue, which is highly fragmented due to forestry and agriculture (Luebert & Pliscoff, 2006). Specifically, the study was performed in Los Queules National Reserve and 2 neighboring forest fragments (Bustamante et al., 2005). Los Queules is a protected area of 145 ha embedded in a large tract of 600 ha of continuous forest. Forest fragments, ranging from 1 to 6 ha, are patches surrounded by commercial plantations of Pinus radiata. Here, 4 sites were defined taking into account the spatial arrangement of plant populations: 1 core and 1 edge of continuous forest, both sites placed in Los Queules National Reserve, and 1 core and 1 edge of forest fragments, both sites placed in 2 different fragments neighboring Los Queules National Reserve. While cores were defined as sites placed ≥ 50 m inside the border of each site, edges were defined as sites placed ≤ 10 m inside the forest margin. Distance between sites ranges from 0.5 up to 2.5 km. In the present study, only 1 site per category was included because no other site harboring a Bomarea salsilla L (Herb.) population was found.
Bomarea salsilla (Alstroemeriaceae) is a small-sized climbing perennial vine inhabiting the sclerophyllous and temperate forests of Chile from 33° S to 40° S. It flowers from ca. November to January bearing protandrous bell-shaped red flowers (Fig. 1A). Its breeding system and effectiveness of pollinators is unknown. Nevertheless, 2 pollinator-exclusion experiments performed in the study site, devised to test for autogamy and agamospermy, demonstrated that B. salsilla is a totally pollinator-dependent plant for seed set (i.e., a fully xenogamous plant) (Valdivia et al., 2011). Floral visitors, which exhibited a putative pollinating behavior because they touched the reproductive structures of flowers while feeding, were hummingbirds, Sephanoides sephaniodes (Trochilidae); bumblebees and bees, Bombus dahlbomii, B. terrestris, and Manuelia gayatina (Apidae); butterflies, Mathania leucothea (Pieridae); and flies, Acrophthalmyda paulseni (Bombyliidae) (Valdivia et al., 2011). The mean frequency of visits to flowers (± 1SE) was determined to be 0.16 ± 0.02 visits per flower in 10 minutes at the core of continuous forest, 0.09 ± 0.01 at the edge of continuous forest, 0.11 ± 0.01 at the core of forest fragment, and 0.06 ± 0.01 at the edge of forest fragment (Valdivia et al., 2011). Unfortunately, there are no data about the specific frequency of visits of each floral visitor to B. salsilla flowers at each site studied (Valdivia et al., 2011).
The leaves of B. salsilla, as in all other members of the Alstroemeriaceae family, are resupinate and eaten by unidentified insect larvae and mollusks (Fig. 1B) (Valdivia et al., 2011). The mean herbivory index (± 1SE), following Dirzo and Domínguez (1995), was determined to be 0.43 ± 0.10 for plants at the core of continuous forest, 0.03 ± 0.01 at the edge of continuous forest, 0.03 ± 0.03 at the core of forest fragment, and 0.10 ± 0.03 at the edge of forest fragment. Therefore, herbivores removed less than 6% of foliar surface at all sites studied (Valdivia et al., 2011).
In order to determine a possible pathway for correlated evolution on pollinator- and herbivore-linked traits, during the austral spring-summer season, from September 2006 to January 2007, a field experiment following a 2 × 2 factorial design by excluding separately and simultaneously pollinators and herbivores was conducted. Although efficient in simulating extreme trait variation, the experimental design also seems to limit the level of realism. In fact, pollinator exclusions mimicked a situation whereby plants exhibited a suite of traits which reduced the optimal relationship between plants and pollinators, thereby leading to a putative decreased fitness of plants. By contrast, herbivore exclusions mimicked a situation in which plants presented a suite of traits that allowed them to show resistance to herbivores, which in turn should produced an increased fitness of plants. Therefore, this artificial array allows the dissection of the isolated effects of herbivores and pollinators, as well as the combined action of both (Herrera et al., 2002). Thereafter, the detection of non-additive effects of herbivores and pollinators can be interpreted as the evidence of a pathway for a putative correlated evolution on traits related to them (Herrera et al., 2002). However, because we used the amount of seeds as a measure of fitness, the detection of additive or non-additive effects must be interpreted with caution (Herrera et al., 2002). This occurs because non-additivity of the combined effects of pollinators and herbivores could be expressed in seed quality, in terms of germination capacity, or even during seedling recruitment (Herrera et al., 2002).
Herbivores were excluded by monthly spraying plants with the biocide Fastac, thus ensuring that neither insect larvae nor mollusks fed upon plants. Additionally, control plants (i.e., exposed to herbivores) were equally sprayed but only with water. Pollinators were excluded by enclosing inflorescences at the floral-bud stage with a tulle-mesh bag, but allowing the access of herbivores to leaves. Both treatment levels were factorially combined leading to the following 4 combinations: 1) plants exposed to herbivores and pollinators, 2) plants only exposed to pollinators, 3) plants only exposed to herbivores, and 4) plants simultaneously excluded from both herbivores and pollinators. For each combination, 30 plants were selected and monitored from September 2006 to February 2007 at each study site (n = 4 sites). When the reproductive season was over, developing fruits were enclosed in a tulle-mesh bag for collecting all seeds produced by each plant at the end of the reproductive season after experiencing the 4 treatments aforementioned.
Fitness consequences for B. salsilla plants of pollinators, herbivores, and their interaction were assessed by fitting a linear mixed model to seed production. Sites, pollinators, herbivores, and all two- and three-way interactions were included in the model as fixed effects. Computations were carried out with Statistica software package v. 10.0 (StatSoft Inc., Tulsa, Oklahoma, USA). Mann-Whitney tests were further applied for pairwise comparisons between groups with significant p-values obtained after Bonferroni corrections for each site.
Results
In plants experimentally excluded to pollinators no seed was produced; by contrast, in plants experimentally excluded to herbivores seed production was not significantly different from those plants exposed to herbivores (Fig. 2). Furthermore, a significant effect was detected when the identity of site was incorporated into the model, therefore demonstrating a site effect on the pollinator-mediated seed production (Table 1). Thus, with respect to the core of the continuous forest, seed set was 35.6% to 40.1% lower in the edge of forest fragment, 11.2% to 19.8% lower in the core of forest fragment, and 0.6% to 12.1% higher in the edge of forest fragment (Fig. 2). Consequently, pollinators and sites, but not herbivores, had a significant effect on the seed set of B. salsilla by exerting additive effects (i.e., non-correlated fitness effects) on seed production (Table 1).
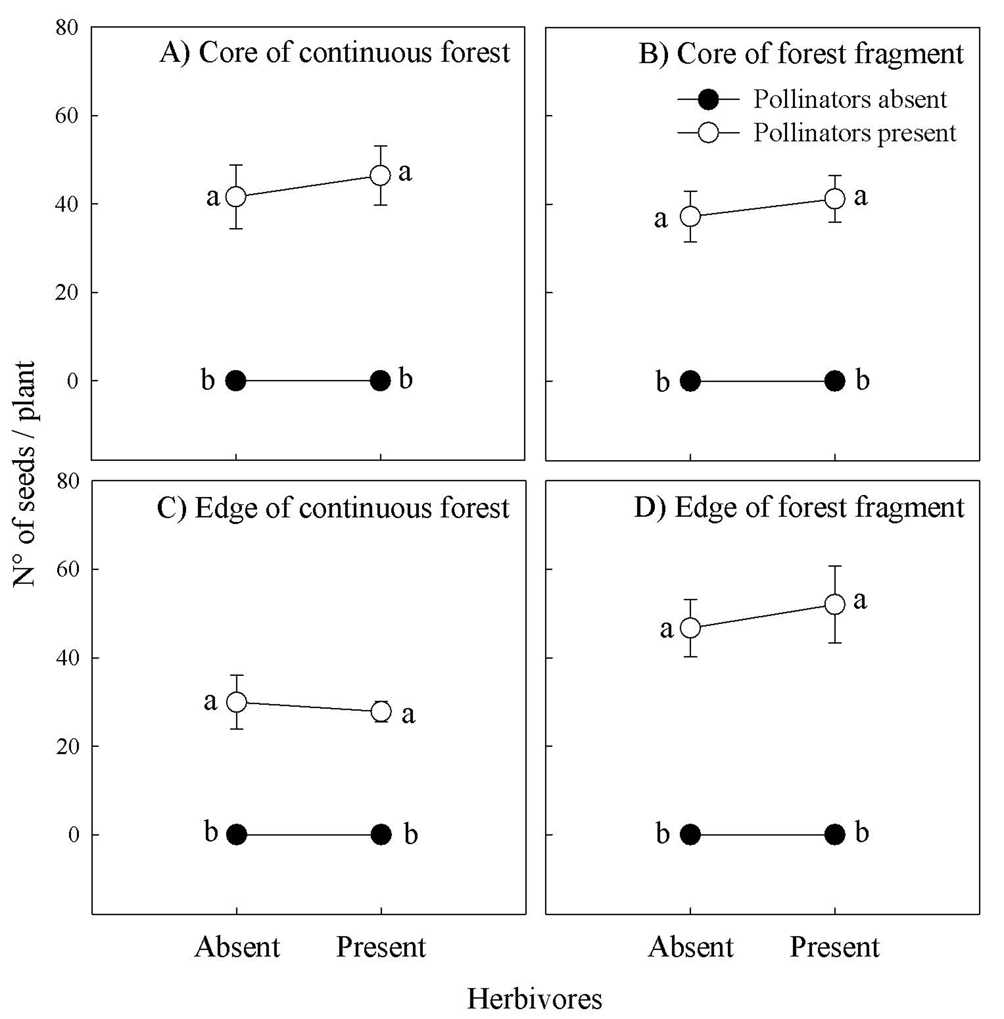
Table 1
Summary of a mixed-model Anova testing for the effects of site (core of continuous forest, edge of continuous forest, core of forest fragment, and edge of forest fragment), pollinators (present or excluded), and herbivores (present or excluded) on the total number of seeds produced by Bomarea salsilla in the fragmented Maulino forest.
|
Source of variation |
df |
MS |
F |
p |
|
Site |
3 |
2,274.7 |
3.808 |
0.010 |
|
Pollinators |
1 |
195,455.4 |
271.186 |
< 0.001 |
|
Herbivores |
1 |
270.0 |
0.375 |
0.542 |
|
Site × pollinators |
3 |
2,274.7 |
3.808 |
0.010 |
|
Site × herbivores |
3 |
89.1 |
0.149 |
0.930 |
|
Pollinators × herbivores |
1 |
270.0 |
0.375 |
0.542 |
|
Site × pollinators × herbivores |
3 |
89.1 |
0.149 |
0.930 |
Pollinators and herbivores had additive effects on B. salsilla fitness at the core of continuous forest as well as at the disturbed sites (i.e., edges and fragments), thus suggesting that pollinator- and herbivore-linked traits currently run along dissimilar lanes (Herrera et al., 2002). This also suggests that there was not a negative effect of forest fragmentation and the increase in edge habitats on the possibility of uncoupling reproductive and vegetative traits through pollinator- and herbivore-mediated fitness effects. This fact contrasts with our original expectations, which were mostly based upon the previously reported negative effects of forest fragmentation on the reproductive success of B. salsilla (Valdivia et al., 2011). However, because the habitats were not replicated, the null effects here observed must be interpreted with caution.
The lack of detection of non-additive effects of pollinators and herbivores on plant fitness probably occurred because seed quantity was not an appropriate measure of fitness (Herrera et al., 2002). Alternatively, non-additive effects of pollinators and herbivores was not observed because it never existed, which seems to be the most plausible explanation. In fact, the absence of non-additive effects probably arose from the evolutionary trajectory of plant-herbivore relationships. Plants currently unaffected by herbivores most likely lose their additive genetic variance conferring susceptibility to the action of herbivores throughout evolutionary time (i.e., an herbivory-related intrinsic factor) due to herbivore-mediated positive selection pressures on resistance. Such plants, therefore, may currently “escape” from the negative impact of herbivory on fitness by avoiding being preyed upon by herbivores. In fact, at the core of continuous forest, herbivores accounted for < 6% of foliar surface removal, thus rendering unlikely the possibility of fitness reduction (Valdivia et al., 2011). For instance, Mothershead and Marquis (2000) found that herbivores accounted for 6.5% of leaf area loss in Oenothera macrocarpa, which translated into a narrow decrease in seed production. In fact, only when herbivory was experimentally increased up to 33.4% of foliar surface loss did plants experience a significant reduction in seed production (Mothershead & Marquis, 2000). Moreover, Núñez-Farfán and Dirzo (1994) in spite of finding a significant herbivore-mediated phenotypic selection pressure on herbivory resistance in the alien plant Datura stramonium from central Mexico, the heritability exhibited by this trait was very small and not significantly different from zero. Taken together, both examples point to consider that a loss in proclivity of plants to be preyed upon by herbivores and a loss of additive genetic variance accounting for such a proclivity is not an uncommon situation.
Contrary to herbivores, pollinators were proven to exert significant effects on B. salsilla fitness in terms of seed production, which also varied spatially. Therefore, it is expected that numerous traits of flowers and inflorescences could be directly selected by them, but not equally among sites. Traits under selection by pollinators could be, for instance, inflorescence architecture, number of flowers per inflorescence, and flower size (Medel & Nattero, 2009; Valdivia to be published). Nevertheless, it is worth noting that pollinator-mediated selection on floral traits may only occur if populations are limited by seed production (Herrera et al., 2002).
The present report failed to disclose non-additive effects on pollinator- and herbivore-linked traits in B. salsilla, and a lack thereof in disturbed habitats due to herbivory-related intrinsic factors. Nowadays, however, many plants grow in highly fragmented habitats and are likely to face a disruption in mutualist- and antagonist-related selection pressures (Fontúrbel & Murúa, 2014; Valiente-Banuet et al., 2015). For this reason, the incorporation of the more progressive and sophisticated frameworks given by evolutionary ecology into conservation biology is of a mandatory importance to understand how plants cope with one of the most pervasive and negative threats to their persistence.
Acknowledgements
We thank to Brenda Valdivia, Carlos O. Valdivia, Florencia Prats, Sandra Valdivia, Fernando Campos, Luciano Silva, Patricio Molina, and Sergio Hernández for their valuable support during fieldwork. The Chilean Forestry Service (CONAF) and Forestal Masisa partially supported fieldwork. This work was funded by Beca de Apoyo a la Realización de Tesis Doctoral, Conicyt 23070138.
References
Abdala-Roberts, L., Parra-Tabla, V., Salinas-Peba, L., & Herrera, C. M. (2009). Noncorrelated effects of seed predation and pollination on the perennial herb Ruellia nudiflora remain spatially consistent. Biological Journal of the Linnean Society, 96, 800–807.
Aguilar, R., Ashworth, L., Galetto, L., & Aizen, M. A. (2006). Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters, 9, 968–980.
Bustamante, R. O., Simonetti, J. A., Grez, A. A., & San Martín, J. (2005). Fragmentación y dinámica de regeneración del bosque Maulino: diagnóstico actual y perspectivas futuras. In C. S. Smith-Ramírez, J. J. Armesto, & C. Valdovinos (Eds.), Historia, biodiversidad y ecología de los bosques costeros de Chile (pp. 555–564). Santiago: Editorial Universitaria.
Dirzo, R., & Domínguez, C. (1995). Plant-herbivore interactions in Mesoamerican tropical dry forests. In S. Bullock, S. Mooney, & E. Medina (Eds.), Seasonally dry tropical forests (pp. 304–345). Cambridge: Cambridge University Press.
Fontúrbel, F. E., & Murúa, M. M. (2014). Microevolutionary effects of habitat fragmentation on plant-animal interactions. Advances in Ecology, 2014, 1–7.
Gómez, J. M. (2005). Non-additive effects of herbivores and pollinators on Erysimum mediohispanicum (Cruciferae) fitness. Oecologia, 143, 412–418.
Groom, M. J. (2001). Consequences of subpopulation isolation for pollination, herbivory, and population growth in Clarkia concinna concinna (Onagraceae). Biological Conservation, 100, 55–63.
Herrera, C. M. (2000). Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology, 81, 2170–2176.
Herrera, C. M., Medrano, M., Rey, P. J., Sánchez-Lafuente, A. M., García, M. B., Guitián, J. et al. (2002). Interactions of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits. Proceedings of the National Academy of Science, 99, 16823–16828.
Herrera, C. M., & Pellmyr, O. (2002). Plant–Animal Interactions. An evolutionary approach. Oxford: Blackwell Publishing.
Jacquemyn, H., de Meester, L., Jongejans, E., & Honnay, O. (2012). Evolutionary changes in plant reproductive traits following habitat fragmentation and their consequences for population fitness. Journal of Ecology, 100, 76-87.
Kolb, A. (2008) Habitat fragmentation reduces plant fitness by disturbing pollination and modifying response to herbivory. Biological Conservation, 141, 2540–2549.
Luebert, F., & Pliscoff, P. (2006) Sinopsis bioclimática y vegetacional de Chile. Santiago: Editorial Universitaria.
Medel, R., & Nattero, J. (2009) Selección mediada por polinizadores sobre el fenotipo floral. In R. Medel, M. A. Aizen, & R. Zamora (Eds.), Ecología y evolución de interacciones planta-animal (pp. 77–94). Santiago: Editorial Universitaria.
Mothershead, K., & Marquis, R. J. (2000). Fitness impacts of herbivory through indirect effects on plant-pollinator interactions in Oenothera macrocarpa. Ecology, 81, 30–40.
Núñez-Farfán, J., & Dirzo, R. (1994). Evolutionary ecology of Datura stramonium L. in central Mexico: natural selection for resistance to herbivorous insects. Evolution, 48, 423–436.
Shahbazi, A., Matinkhah, S., Khajeali, J., Bashari, H., & Esfahani, A. T. (2017) The effects of pollinators and seed predators (Bruchidius koenigi Schilsky) on the breeding biology of Hedysarum criniferum Boiss. Plant Species Biology, 32, 36–44.
Steffan-Dewenter, I., Klein, A. M., Gaebele, V., Alfert, T., & Tscharntke, T. (2006). Bee diversity and plant-pollinator interactions in fragmented landscapes. In N. M. Waser, & J. Ollerton (Eds.), Plant-pollinator interactions, from specialization to generalization (pp. 387–407). Chicago: The University of Chicago Press.
Strauss, S. Y., & Whitall, J. B. (2006). Non-pollinator agents of selection on floral traits. In L. D. Harder, & S. C. H. Barrett (Eds.), Ecology and evolution of flowers (pp. 120–138). Oxford: Oxford University Press.
Strauss, S. Y., & Zangerl, A. R. (2002). Plant-insect interactions in terrestrial ecosystems. In C. M. Herrera, & P. Pellmyr (Eds.), Plant-animal interactions, an evolutionary approach (pp. 77–106). Oxford: Blackwell Science.
Valdivia, C. E. (2011) Microevolución y conservación. In J. A. Simonetti, & R. Dirzo (Eds.), Conservación biológica: perspectivas desde América Latina (pp. 127–141). Santiago: Editorial Universitaria.
Valdivia, C. E., & Niemeyer, H. M. (2005). Reduced maternal fecundity of the high-Andean perennial herb Alstroemeria umbellata (Alstroemeriaceae) by aphid herbivory. New Zealand Journal of Ecology, 29, 321–324.
Valdivia, C. E., & Niemeyer, H. M. (2007). Noncorrelated evolution between herbivore- and pollinator-linked features in Aristolochia chilensis (Aristolochiaceae). Biological Journal of the Linnean Society, 91, 239–245.
Valdivia, C. E., Simonetti, J. A., & Bahamóndez, A. (2011). Negative effects of forest fragmentation and proximity to edges on pollination and herbivory of Bomarea salsilla (Alstroemeriaceae). Plant Ecology and Evolution, 144, 281–287.
Valiente-Banuet, A., Aizen, M. A., Alcántara, J. M., Arroyo, J., Cocucci, A., Galetti, M. et al. (2015). Beyond species loss: the extinction of ecological interactions in a changing world. Functional Ecology, 29, 299–307.



