Judith A. Sánchez-Ledesma a, Gonzalo Guevara-Guerrero b, *, Roberto Garibay-Orijel c, Rodolfo Ángeles-Argáiz c, Verónica Ávila-Rodríguez d, Jesús G. Arreola-Ávila a, Violeta Carrasco-Hernández e, Amparo Borja-de la Rosa e, Fabián González-García a
a Universidad Autónoma Chapingo, Unidad Regional Universitaria de Zonas Áridas, Km. 40, Carretera Gómez Palacio-Chihuahua, 35230 Bermejillo, Durango, Mexico
b Instituto Tecnológico de Cd. Victoria, Av. Portes Gil 1301 Pte., 87010 Cd. Victoria, Tamaulipas, Mexico
c Universidad Nacional Autónoma de México, Instituto de Biología, Circuito exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
d Universidad Juárez del Estado de Durango, Facultad de Ciencias Biológicas, Av. Universidad s/n, Fracc. Philadelphia, 35010 Gómez Palacio, Durango, Mexico
e Universidad Autónoma Chapingo, Km 36.5 Carretera México-Texcoco, 56230 Chapingo, Estado de México, Mexico
*Corresponding author: guevaragg@hotmail.com (G. Guevara-Guerrero)
Received: 15 December 2021; accepted: 4 July 2022
Abstract
Tuber is a genus of ectomycorrhizal fungi with an important diversity of species associated with hosts in Juglandaceae. Tuber caryophilum is proposed as a new species based on ecological, morphological and phylogenetic characters of 2 ribosomal markers (ITS and LSU). This species is characterized by forming ectomycorrhizae on the roots of Carya illinoinensis (nogal pecanero) in the Comarca Lagunera of Coahuila and Chihuahua, Mexico and by exhibiting 18-48 × 10-27 μm echinulate ascospores. Tuber caryophilum belongs to the Rufum clade and is the sister species of Tuber theleascum, a species reported in northern Mexico associated with Quercus canbyi and Q. polymorpha. These 2 truffles belong to a clade from the southern USA and northern Mexico with taxa associated to Quercus and Carya, such as Tuber lyonii.
Keywords: Diversity; Ectomycorrhizal fungi; Hypogeous fungi; Pecan
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Tuber caryophilum, una especie nueva de trufa creciendo en huertos de Carya illinoinensis
Resumen
Tuber es un género de hongos ectomicorrízicos con una importante diversidad de especies asociada a hospederos en Junglandaceae. Tuber caryophilum es propuesta como una especie nueva basada en caracteres ecológicos, morfológicos y filogenéticos de 2 marcadores ribosomales (ITS y LSU). Esta especie se caracteriza por formar ectomicorrizas en las raíces de Carya illinoinensis (nogal pecanero) en la Comarca Lagunera de Coahuila y en Chihuahua, México y por presentar ascosporas equinuladas de 18-48 × 10-27 μm. Tuber caryophilum pertenece al clado Rufum y es la especie hermana de Tuber theleascum, una especie descrita del norte de México asociada con Quercus canbyi y Q. polymorpha. Estas 2 especies de trufas pertenecen a un clado del sur de EUA y norte de México con taxones asociados a Quercus y Carya como Tuber lyonii.
Palabras clave: Diversidad; Hongos ectomicorrizógenos; Hongos hipogeos; Nogal pecanero
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Species of the genus Tuber P. Micheli ex F.H. Wigg belong to the family Tuberaceae (Pezizales) and form ectomycorrhizae with many forest tree species (Bonito et al., 2011; Li et al., 2018). These species are ecologically and economically important (Guevara et al., 2013; Neri-Luna et al., 2012). Approximately 86 species of Tuber are known worldwide (Guevara et al., 2013; Kirk et al., 2008). They grow in mycorrhizal symbiotic association with gymnosperm and angiosperm trees. Thirty-eight species of Tuber have been described in North America (Guevara et al., 2013). The diversity of the genus in Mexico is expected to be high because this region is a center of diversification of Quercus Kappelle, Maarten and Pinus Linneo, 2 of the main ectomycorrhizal hosts of Tuber. Knowledge of Tuber in Mexico has recently expanded. For example, new species belonging to the Maculatum clade, such as Tuber castilloi Guevara, Bonito & Trappe, T. guevarai Bonito & Trappe (Guevara et al., 2013), Tuber mexiusanum Guevara, Bonito & Trappe (Guevara et al., 2013), T. mixtecorum J. García, Ayala Vázquez & de la Fuente (García-Jiménez et al., 2021) and T. theleascum M. Leonardi, A. Paz, G. Guevara & Pacioni (Leonardi et al., 2019) associated with Quercus spp., have been described in northeastern Mexico. Tuber incognitum and T. anniae belonging to the Puberulum clade have also been recorded in association with Quercus spp and Pinus montezumae in central Mexico (Piña-Páez et al., 2018). In association with trees of forest interest in Mexican ecosystems, T. guzmanii, T. separans, and T. pseudoseparans have also been found (Gómez-Reyes et al., 2018; Guevara et al., 2015; Piña-Páez et al., 2018).
Carya illinoinensis K. Koch (pecan) is an agronomically important ectomycorrhizal nut tree in whose plantations in the southern United States truffle species such as Tuber lyonii have been reported (Benucci et al., 2012; Bonito et al., 2011; Rodríguez et al., 2018). Ascomata and ectomycorrhizae of Tuber brennemanii and T. floridanum have also been found in these orchards (Grupe et al., 2018). In Mexico, Tuber diversity has not been explored in pecan plantations, although due to their particular soil and climatic conditions it is to be expected that there are species not yet described. In this paper, Tuber caryophilum sp. nov., a species in the Rufum clade, whose distribution includes Chihuahua and la Comarca Lagunera of Coahuila, Mexico, is described based on morphological, ecological and molecular characters.
Materials and methods
Ascomata were collected from a Carya illinoinensis orchard in the Comarca Lagunera of Coahuila and characterized following the recommendations of Castellano et al. (1989) and Pegler et al. (1993). Duplicates of the specimens were deposited in the José Castillo Tovar herbarium (ITCV) and MEXU. Characters examined included ascoma size and color, asci shape, asci wall thickness, and number of spores per ascus. Sections were cut by hand and then mounted in 5% KOH and Melzer’s reagent for light microscopy. Thirty measurements of different structures such as spores and asci were made in 5% KOH. Microscopic structures were measured and photographed on a Velab VE-B3 microscope and a ZEISS Scope A1 optical stereoscope.
DNA was extracted by the CTAB method and amplified by PCR in 25 µl reactions according to Sambrook et al. (1989). The reactions consisted of 2.5 µl of 10X PCR buffer, 2.0 µl of 2.5 Mm MgCl final concentration, 2.0 µl of dNTPs, 2.0 µl of each primer 10 µM, 1.5 units of Taq polymerase (GoTaq®, Promega, WI), 11.3 µl of MiliQ grade water and 3 µl of DNA. The ribosomal internal transcribed spacer (ITS) region was amplified with the ITS4 and ITS5 oligonucleotides. The PCR program consisted of an initial denaturation at 94 °C for 3 min, followed by 34 cycles of 94 °C, 51 °C and 72 °C for 1 min each and a final extension at 72 °C for 8 min (Taylor et al., 2006). Amplification was carried out on a MiniAmp Plus Thermal Cycler (Applied Biosystems, USA). A section of the large ribosome subunit (LSU) was amplified with the LR0R and LR5 oligonucleotides (Vilgalys & Hester, 1990) and the enzyme Taq & Load (Avantor, PA, USA). The 25 µl reactions consisted of 5 µl of master mix, 0.25 µl of each oligonucleotide 50 µM, water, and 1 µl of DNA. The PCR program began with denaturation at 94 ºC for 4 min, followed by 35 cycles at 94 ºC, 54 ºC and 72 ºC for 1 min each and final extension at 72 ºC for 10 min. PCR products were cleaned with ExoSAP-IT (Thermofisher, USA) with some modifications (Ángeles-Argáiz et al., 2016). DNA and PCR products were reviewed on 0.8% and 1.5% agarose gels with 0.5% TBE buffer. Samples were stained with Gel Red (Bioitium, CA, USA) using a 100 bp molecular weight marker as reference. Gels were photographed on a Multidoc-IT photodocumenter (Analytik Jena Company, CA, USA). ITS PCR products were sequenced in both directions at Macrogen (Rockville, MD, USA), with PCR primers. LSU sequences were obtained at the Biodiversity and Health Sequencing Laboratory of the Institute of Biology, UNAM, using BigDye Terminator 3.1 (Thermofisher), also in both directions.
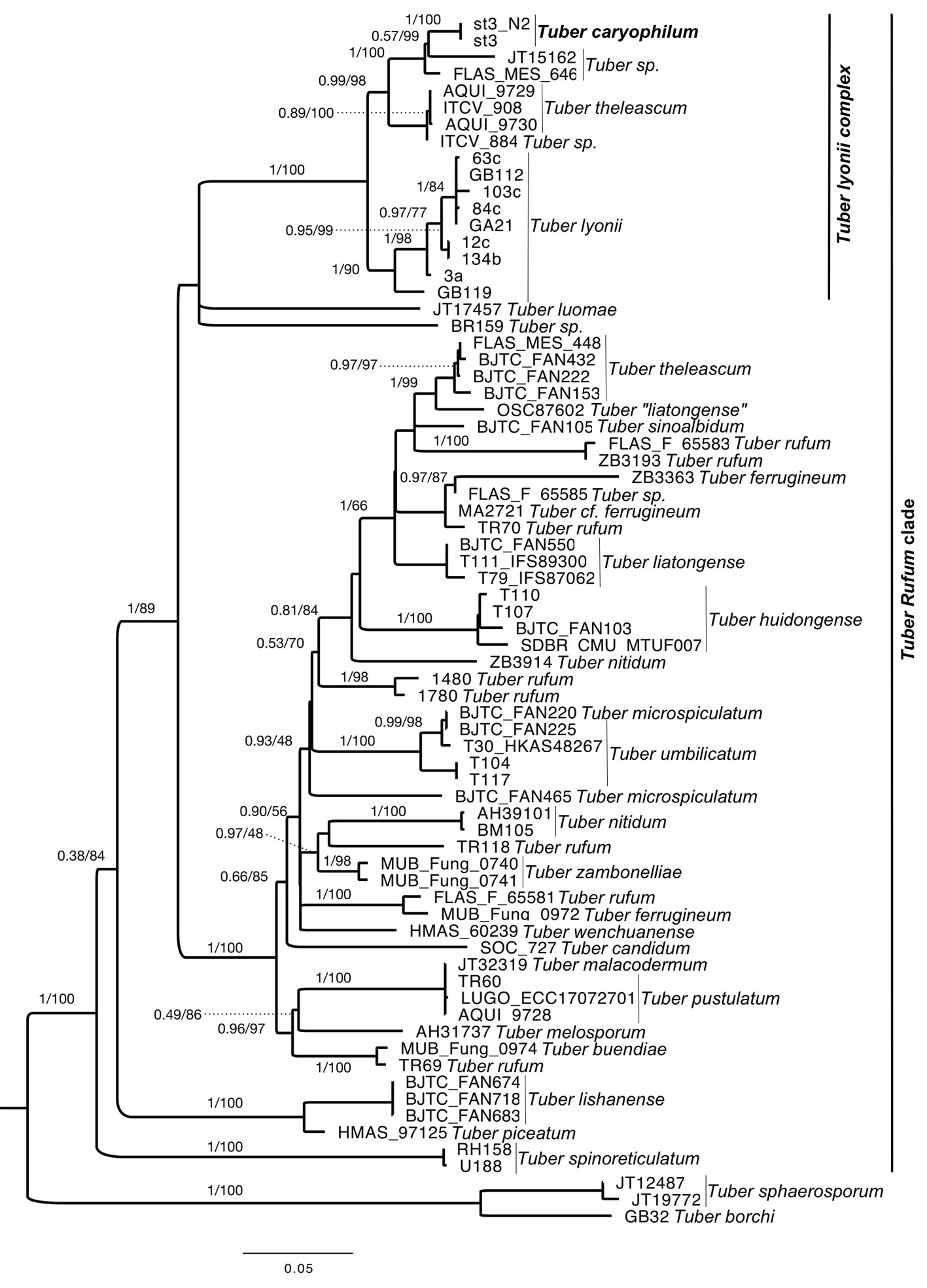
Nucleotide sequences were edited and aligned in Geneious Prime version 2021 with the MUSCLE algorithm (Maddison & Maddison, 2016). Sequences of T. caryophilum voucher materials were deposited in GenBank under accession numbers MZ092919 and OK642388 for ITS, OK642397 and OK642398 for LSU and OK642406 for the ITS of ectomycorrhizae. The alignment for phylogenetic analyses included the sequences generated in this study, those previously included in analyses of the Rufum clade (Eberhart et al., 2020; Leonardi et al., 2019) and sequences of high nucleotide similarity obtained from the GenBank database by means of the BLAST algorithm (Altschul et al., 1990) (Table 1). A total of 72 samples from 26 taxa of the Rufum clade and 1 outgroup were aligned (Fig. 1). The concatenated alignment had 1,076 bp where bases 1-556 corresponded with the ITS and bases 557-1,076 with the LSU. The alignments were reviewed manually excluding ambiguous regions.
Phylogenetic analyses and evolutionary model selection were performed using IQ-TREE (v2.1.4, Minh et al., 2020) from the concatenated and partitioned alignment (IQ-TREE execution line: iqtree -s ../data/concat.fasta -p Partition.nex -m MFP –runs 100 –abayes -B 1000 -T AUTO -ntmax 28). The best evolutionary models were selected with ModelFinder (Kalyaanamoorthy et al., 2017). The best evolutionary model for the ITS marker was TIM2+F+I+G4 and for LSU it was TIM3e+I+G4. The resulting tree is the consensus of 100 replicates of 2 phylogenetic analyses; an ultra-fast Maximum Likelihood analysis (Hoang et al., 2018) with 1,000 bootstrap replicates (MVB), complemented with a Bayesian approximation branch support (BPP) analysis (Anisimova et al., 2011). To show the ectomycorrhizal status and distribution of T. caryophilum, the ITS sequence of the holotype was contrasted by means of nucleotide similarity (% ITS NS) against ectomycorrhizal sequences of Carya illinoinensis obtained from an orchard in Chihuahua, Mexico.
Table 1
Tuber species and DNA sequences used in the phylogenetic analyses. New sequences are in bold.
| Voucher/Isolate | Species | ITS | 28S | Country |
| GB32 | Tuber borchii | FJ809799 | FJ809799 | Italy |
| MUB_Fung-0974 | Tuber buendiae | MT006095 | NG_073829 | Spain |
| SOC_727 | Tuber candidum | AY830856 | – | |
| st3 | Tuber caryophilum | MZ092919 | OK642388 | Mexico |
| st3_N2 | Tuber caryophilum | OK642397 | OK642398 | Mexico |
| MA2721 | Tuber cf. ferrugineum | – | FJ809809 | |
| BJTC_FAN465 | Tuber crassitunicatum | MH115295 | – | |
| MUB_Fung-0972 | Tuber ferrugineum | MN962719 | – | Spain |
| ZB3363 | Tuber ferrugineum | – | MT270600 | Hungary |
| BJTC_FAN103 | Tuber huidongense | MH115294 | MH115301 | China |
| SDBR-CMU-MTUF007 | Tuber huidongense | KT758731 | KU207733 | Thailand |
| T107 | Tuber huidongense | – | GU979099 | China |
| T110 | Tuber huidongense | FJ797882 | GU979093 | China |
| BJTC_FAN550 | Tuber liaotongense | MH115302 | – | China |
| OSC87602 | Tuber liaotongense | – | FJ809813 | China |
| T111_IFS89300 | Tuber liaotongense | GU979037 | – | |
| T79_IFS87062 | Tuber liaotongense | GU979036 | – | |
| BJTC_FAN674 | Tuber lishanense | MH115307 | – | China |
| BJTC_FAN683 | Tuber lishanense | MH115305 | – | China |
| BJTC_FAN718 | Tuber lishanense | NR_160619 | NG_064527 | China |
| JT17457 | Tuber luomae | MH142474 | FJ809812 | USA |
| 103c | Tuber lyonii | GQ379726 | GQ379726 | USA |
| 12c | Tuber lyonii | GQ379723 | GQ379723 | USA |
| 134b | Tuber lyonii | GQ379724 | GQ379724 | USA |
| 3a | Tuber lyonii | GQ379725 | GQ379725 | USA |
| 63c | Tuber lyonii | GQ379722 | GQ379722 | USA |
| 84c | Tuber lyonii | GQ379721 | GQ379721 | USA |
| GA21 | Tuber lyonii | – | JQ925698 | USA |
| GB112 | Tuber lyonii | EU394704 | EU394704 | USA |
| GB119 | Tuber lyonii | FJ748911 | FJ809808 | USA |
| JT32319 | Tuber malacodermum | FJ809889 | JQ925702 | Spain |
| AH31737 | Tuber melosporum | JN392144 | JN392202 | Spain |
| BJTC_FAN220 | Tuber microspiculatum | MH115315 | MH115316 | China |
| AH39101 | Tuber nitidum | JX402092 | JN392331 | |
| BM105 | Tuber nitidum | FJ809885 | FJ809807 | Spain |
| ZB3914 | Tuber nitidum | – | MT270604 | Hungary |
| HMAS_97125 | Tuber piceatum | NR_160620 | NG_064528 | China |
| AQUÍ_9728 | Tuber pustulatum | – | MK211311 | France |
| LUGO_ECC17072701 | Tuber pustulatum | MW376716 | – | Spain |
| Table 1. Continued | ||||
| Voucher/Isolate | Species | ITS | 28S | Country |
| TR60 | Tuber pustulatum | MW077451 | MW076943 | |
| 1480 | Tuber rufum | EF362476 | – | Italy |
| 1780 | Tuber rufum | EF362474 | – | France |
| FLAS_F-65581 | Tuber rufum | MT374048 | – | France |
| FLAS_F-65581 | Tuber rufum | – | MT350486 | France |
| TR118 | Tuber rufum | – | MT270605 | Italy |
| TR69 | Tuber rufum | – | MT270608 | Spain |
| TR70 | Tuber rufum | – | MT270602 | Spain |
| ZB3193 | Tuber rufum | – | MT270603 | Slovakia |
| BJTC_FAN105 | Tuber sinoalbidum | MH115298 | MH115299 | China |
| FLAS_MES-646 | Tuber sp. | MT156470 | – | USA |
| FLAS-F-65585 | Tuber sp. | – | MT350482 | France |
| JT15162 | Tuber sp. | HM485391 | – | USA |
| BR159 | Tuber sp. BR-2020a | – | MW579345 | USA |
| JT12487 | Tuber sphaerosporum | FJ809853 | FJ809853 | USA |
| JT19772 | Tuber sphaerosporum | FJ809854 | FJ809854 | USA |
| RH158 | Tuber spinoreticulatum | GQ221454 | FJ809814 | USA |
| U188 | Tuber spinoreticulatum | FJ809884 | NG_059919 | USA |
| BJTC_FAN153 | Tuber subglobosum | – | MH115322 | China |
| BJTC_FAN222 | Tuber subglobosum | KF002728 | MH115324 | China |
| BJTC_FAN432 | Tuber subglobosum | MH115323 | – | China |
| FLAS_MES-448 | Tuber subglobosum | MT156449 | MT156449 | China |
| AQUI_9729 | Tuber theleascum | MK211283 | MK211312 | Mexico |
| AQUI_9730 | Tuber theleascum | MK211284 | MK211313 | Mexico |
| ITCV_884 | Tuber theleascum | HM485426 | – | Mexico |
| ITCV_908 | Tuber theleascum | NR_164592 | – | Mexico |
| BJTC_FAN225 | Tuber umbilicatum | MH115325 | MH115326 | China |
| T104 | Tuber umbilicatum | FJ797879 | – | |
| T117 | Tuber umbilicatum | FJ797880 | – | |
| T30_HKAS48267 | Tuber umbilicatum | GU979032 | GU979088 | China |
| HMAS_60239 | Tuber wenchuanense | JX267044 | MH115327 | Italy |
| MUB_Fung-0740 | Tuber zambonelliae | MW632952 | – | Spain |
| Mub_Fung-0741 | Tuber zambonelliae | MW632953 | – | Spain |
Results
The consensus tree of Bayesian approximation and Maximum likelihood shows the Rufum clade as monophyletic and with high support (BPP = 1, MV = 100). Within this clade, T. caryophilum is an independent, monophyletic and well-supported clade (BPP = 1, MVB = 100). This species appears as the sister clade of T. theleascum (BPP = 0.99, MVB = 98). In turn, these 2 species are grouped with the T. lyonii complex in a well-supported clade (BPP = 1, MVB = 100) made up of species from the southern USA and northern Mexico. Moreover, we found that the ITS sequence of the T. caryophilum holotype had a 99.3% NS (4 substitutions/549 bp) with mycorrhizal sequences from a pecan orchard in Chihuahua. This indicates that, like T. lyonii, T. caryophilum is an ectomycorrhizal symbiont of C. illinoinensis. Consequently, Tuber caryophilum is designated as a new species supported by ITS and LSU phylogenetic analyses of rnDNA, morphological characters, and ecology.
Description
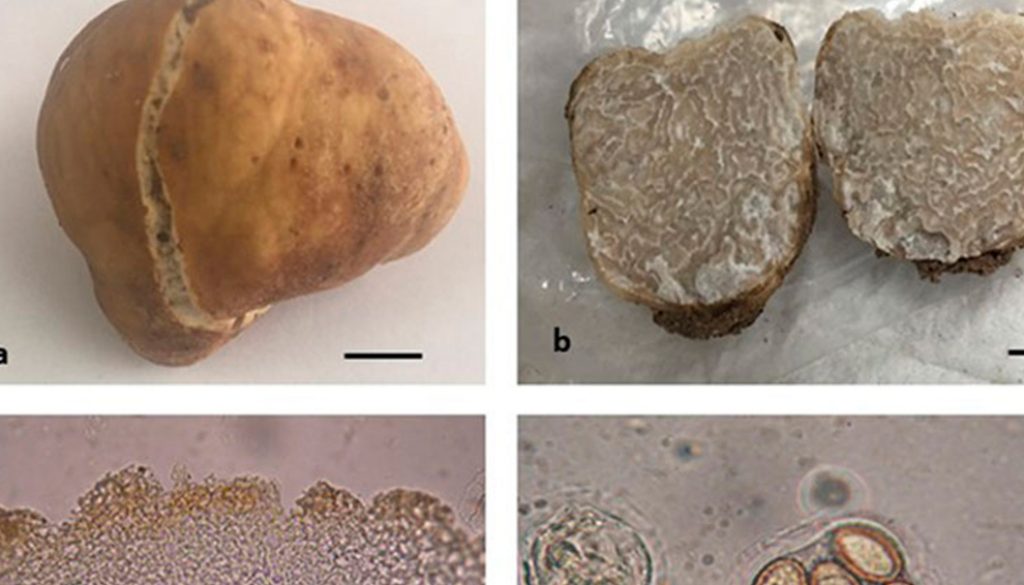
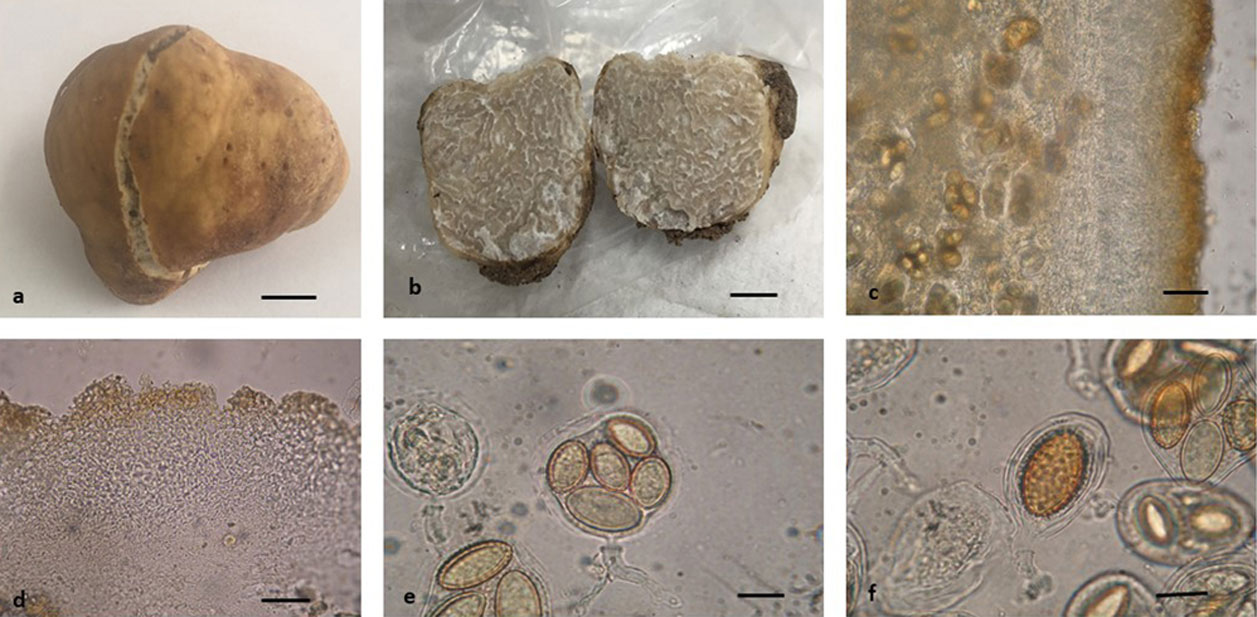
Tuber caryophilum J.A. Sánchez, G. Guevara and R. Garibay-Orijel, sp. nov.
Fig. 2a-f
MycoBank 840581
GenBank MZ092919 (ITS), OK642397 (LSU)
Type. Mexico, Coahuila, Municipality of Viesca, Tierra Blanca Orchard, September 4, 2019, Sánchez st3 (ITCV 1888 “José Castillo Tovar” herbarium).
Diagnosis. Cream peridium with translucent veins towards the epicutis; pseudoparenchymatous epicutis mainly although in some areas it is prosenchymatous, isodiametric hyphae of 3-17 μm; echinulate ascospores of 18-48 × 10-27 μm; it grows in pecan (Carya illinoinensis) orchards.
Ascoma. Subglobose to irregular, 19 × 10 × 18 mm, translucent veins on the light brown to dark brown peridium when dry, with a white to cream furrow and an irregular linear or “V”-shaped margin continuing into the gleba as veins, some areas dark brown with cherry tints and with dark brown to reddish insect caverns; peridium smooth, some areas rough, separable from the gleba, without cystidia. White, cream gleba that is gray to dark when dried, marbled with white to gray, dark brown to reddish brown veins that continue towards the peridium (furrows). Strong, very pleasant and distinctive odor, unrecorded taste.
Peridium. 110-220 μm thick, epicutis 50-75 μm, pseudoparenchymatous in its outermost part, although in some parts it is prosenchymatous with hyphae 3-17 μm wide, versiform to angular or isodiametric, wall 1-4 μm thick, yellow to orange-reddish in KOH; subcutis 70-150 μm wide, pseudoparenchymatous strongly interwoven, hyaline hyphae in KOH, septate 2-4 μm wide. Gleba, intertwined vein hyphae, 2-4 μm at widest part. Asci: 47-105 × 32-50 μm (Q = 1.07-2.63), average 67.1 × 40.6 μm (Q = 1.7) including pedicel, subglobose to broadly ellipsoid, hyaline in KOH, 1-2 μm double wall may have a short to very long pedicel or in some it is absent, 1-5 ascospores per ascus. Ascospores: 18-48 × 10-27 μm (Q = 1-2.40), average 30.1 × 17.2 (Q = 1.76) subglobose to broadly ellipsoid or spindle-shaped excluding ornamentation; echinulate, echinulae mostly free, in some of them a subreticula can be observed, 1-4 μm high. Asci with 1 ascospore 35-48 × 18-27 μm (Q = 1.30-2.33), average 42.1 × 22.4 (Q = 1.91); 2 ascospores 20-38 × 15-21μm (Q = 1-2.4), average 31.3 × 17.7 μm (Q = 1.79); 3 ascospores 18-33 × 14-18 μm (Q = 1.06-2), average 27.1 × 16.6 (Q = 1.63); 4 ascospores 21-33 × 12-18 μm (Q = 1.44-2.13), average 26.4 × 15.1 (Q = 1.76); 5 ascospores 19-33 × 10-17 μm (Q = 1.19-2.36), average 24 × 14.4 (Q = 1.69).
Taxonomic summary
Etymology. Refers to the ectomycorrhizal association between T. caryophilum and Carya illinoinensis.
Distribution and ecology. In northern Mexico in la Comarca Lagunera of Coahuila and Chihuahua, ectomycorrhizal symbiont of Carya illinoinensis. To date it has only been found in pecan orchards, not in natural habitats.
Habitat. Hypogeous, solitary or gregarious under pecan trees (Carya illinoinensis).
Collections examined in Mexico. Coahuila, Municipality of Viesca, Tierra Blanca Orchard, September 4, 2019, Sánchez st3_N2, MEXU 30227; Sánchez st3_N3, ITCV 1890; Sánchez st3_N4, ITCV 1891.
Remarks
Phylogenetic analyses show that Tuber caryophilum belongs to the Rufum clade and, together with T. theleascum (ITS NS = 93.6-93-9%), is related (ITS NS = 90.4-93.1%) to the T. lyonii complex, which is also an ectomycorrhizal species of Carya illinoinensis. Tuber caryophilum differs from T. theleascum because the latter has ascomata without translucent areas on the peridium, a pseudoparenchymatous epicutis with elongated prostrate or intertwined hyphae 4-7 μm wide and smooth, while the former species has translucent areas on the peridium and a pseudoparenchymatous epicutis with isodiametric hyphae 13-17 μm wide. Furthermore, they differ in ascospores size; T. caryophilum has 18-48 × 10-27 μm ascospores, whereas in T. theleascum they are 18-44 × 13-25 μm. Tuber caryophilum is also similar to T. lyonii, an edible truffle species native to the southeastern USA (Bonito et al., 2013; Sharma et al., 2012). However, they differ macro- and microscopically; in T. lyonii, peridium width is larger (300-500 µm), ascospores are ellipsoid 30-37 × 22-24 µm and epicutis width is 20-40 μm, with hyphae 6-10 μm wide (Healy et al., 2016; Sharma et al., 2012). In contrast, in T. caryophilum, the peridium width is 110-220 μm, its ascospores are 18-48 × 10-27 μm and epicutis width is 50-75 µm, with 3-17 μm hyphae. These 3 species share important microscopic features such as the pseudoparenchymatous peridium surface and most of their ascospores are subglobose to ellipsoid. Other common features are that all 3 are closely related in the same clade of the Rufum section and that they develop in pecan orchards (Grupe et al., 2018; Sharma et al., 2012; Trappe et al., 1996).
Other Tuber species associated with pecan plantations have been described but these do not belong to the Rufum section (Table 2). One of these species is Tuber brennemanii, which belongs to the Maculatum clade and therefore differs morphologically and molecularly from species belonging to the Rufum clade. For example, T. brennemanii presents anamorphic ascospores and a periclinal subperidium (Grupe et al., 2018). Likewise, Tuber floridanum, also on the Maculatum clade, has been found in pecan orchards. This species is distinguished by the presence of dermatocystidia and commonly has 2-4 spores and reticulate ornamentation as present in the Maculatum (Grupe et al., 2018). Regarding T. floridanum, it is known to have been unintentionally introduced into southern Brazil on the roots of pecan tree seedlings.
Table 2
Tuber species related to Tuber caryophilum or associated with Carya illinoinensis.
| Species | Peridium Surface | Peridium color and thickness | Epicutis / subcutis and cell size | Ascospores size without spines/alv. | Ascospores shape | Ascopores by asci | Geography and host |
| Tuber caryophilum | Smooth, separable without dermatosictidia | Yellow to reddish orange 110-220μm | Ps. 50-75 µm, 3-17 μm / Pr. 70-150μm | 18-48 × 10-27 μm | Subglobose to broadly ellipsoid | 1-5 | Comarca Lagunera, Mexico. Carya illinoinensis |
| Tuber lyonii | Smooth and slightly pruinose | Yellowish brown 300-500 μm | Ps. 20-40 µm, 6-10 µm | 30-37 × 22-24 µm | Ellipsoid | 1-4 | Northeastern Mexico; Florida, USA. Quercus, C. illinoinensis |
| Tuber theleascum | Smooth | Yellow to reddish brown 160-250 μm | Ps. 45-150 μm, 4-7 μm / Pr. 150 μm | 18-44 × 13-25 μm | Claviform to subglobose | 1-4 | Nuevo Leon, Mexico. Quercus canbyi, Q. polymorpha, Q. laeta, Arbutus |
| Tuber brennemanii | Smooth | Yellow to reddish brown 80-600 µm | Ps. 50-200 µm, 2.5-25 µm | 28-61 × 20-36 µm | Isodiametric globose to subglobose | 1-4 | Nuevo Leon, Mexico; Massachusetts and Georgia, USA. C. illinoinensis, Quercus, and other Fagales |
| Tuber floridanum | Smooth | Reddish brown
300-1120 µm |
Ps. 140-800 µm, 5-35 µm | 36-51 × 26-38 µm | Isodiametric globose to subglobose | 2-4 | Florida, Georgia, and Mississippi, USA. C. illinoinensis and other Fagales |
Ps: Pseudoparenchymatous; Pr: prosenchymatous.
Given the phylogenetic closeness of T. caryophilum to T. lyonii, its discovery in pecan orchards opens the door to its use in northern Mexico. To develop this, it will be necessary to carry out mycorrhization experiments in nurseries and to know its organoleptic properties. This could promote a system of co-cultivation between T. caryophilum and C. illinoinensis as occurs in orchards in southeastern Florida and Europe where various species of truffles are harvested alongside nut production (Bonito et al., 2013; Lefevre et al., 2012; Trappe et al., 1996).
Acknowledgements
To Conacyt for the financial support and the producers of pecan for the access to the Carya illinoinensis orchards. GG, thanks TecNM for research support.
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Anisimova, M., Gil, M., Dufayard, J. F., Dessimoz, C., & Gascuel, O. (2011). Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Systematic Biology, 60, 685–699. https://doi.org/10.1093/sysbio/syr041
Benucci, G. M. N., Bonito, G., Falini, L. B., & Bencivenga, M. (2012). Mycorrhization of Pecan trees (Carya illinoinensis) with commercial truffle species: Tuber aestivum Vittad. and Tuber borchii Vittad. Mycorrhiza, 22, 383–392. https://doi.org/10.1007/s00572-011-0413-z
Benucci, G. M. N., Csorbai, A. G., Falini, L. B., Bencivenga, M., Di Massimo, G., & Donnini, D. (2012). Mycorrhization of Quercus robur L., Quercus cerris L. and Corylus avellana L. seedlings with Tuber macrosporum Vittad. Mycorrhiza, 22, 639–646. https://doi.org/10.1007/s00572-012-0441-3
Bonito, G., Smith, M. E., Nowak, M., Healy, R. A., Guevara, G., Cázares, E. et al. (2013). Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. Plos One, 8, e52765. https://doi.org/10.1371/journal.pone.0052765
Bonito, G., Brenneman, T., & Vilgalys, R. (2011). Ectomycorrhizal fungal diversity in orchards of cultivated pecan (Carya illinoinensis; Juglandaceae). Mycorrhiza, 21, 601–612. https://doi.org/10.1007/s00572-011-0368-0
Castellano, M. A. (1989). Key to spores of the genera of hypogeous fungi of north temperate forests with special reference to animal mycophagy. Eureka, USA: Mad River Press.
Eberhart, J., Trappe, J., Páez, C. P., & Bonito, G. (2020). Tuber luomae, a new spiny-spored truffle species from the Pacific Northwest, USA. Fungal Systematics and Evolution, 6, 299. https://doi.org/10.3114/fuse.2020.06.15
Fan, L., Han, L., Zhang, P. R., & Yan, X. Y. (2016). Molecular analysis of Chinese truffles resembling Tuber californicum in morphology reveals a rich pattern of species diversity with emphasis on four new species. Mycologia, 108, 344–353. https://doi.org/10.3852/14-343
García-Jiménez, J., Ayala-Vásquez, O., Guevara-Guerrero, G., Garza-Ocanas, F., & De La Fuente, J. I. (2021). Tuber mixtecorum (Tuberaceae, Pezizales) a new truffle in the Maculatum clade from Mexico. Phytotaxa, 509, 113-120.
Gómez-Reyes, V. M., Vázquez-Marrufo, G., Ortega Gómez, A. M., & Guevara Guerrero, G. (2018). Ascomicetos hipogeos de la región occidental del Sistema Volcánico Transversal, México. Acta Botanica Mexicana, 125, 37–48. https://doi.org/10.21829/abm125.2018.1327
Grupe, A. C., Sulzbacher, M. A., Grebenc, T., Healy, R., Bonito, G., & Smith, M. E. (2018). Tuber brennemanii and Tuber floridanum: Two new Tuber species are among the most commonly detected ectomycorrhizal taxa within commercial pecan (Carya illinoinensis) orchards. Mycologia, 110, 780–790. https://doi.org/10.1080/00275514.2018.1490121
Guevara, G., Bonito, G., & Cázares, E. (2013). Revisión del género Tuber (Tuberaceae: Pezizales) de México. Revista Mexicana de Biodiversidad, 84, S39–S49. https://doi.org/10.7550/rmb.31981
Guevara, G., Bonito, G., Cázares, E., Rodríguez, J., Vilgalys, R., & Trappe, J. M. (2008). Tuber regimontanum, new species of truffle from Mexico. Revista Mexicana de Micología, 26, 17–20.
Guevara, G., Bonito, G., Trappe, J. M., Cázares, E., Williams, G., Healy, R. A. et al. (2013). New North American truffles (Tuber spp.) and their ectomycorrhizal associations. Mycologia, 105, 194–209. https://doi.org/10.3852/12-087
Guevara-Guerrero, G., Bonito, G., Cázares-González, E., Healy, R., Vilgalys, R., & Trappe, J. (2015). Novel Tuber spp. (Tuberaceae, Pezizales) in the Puberulum Group from Mexico. Ascomycete.org, 7, 367–374. https://doi.org/10.25664/art-0161
Healy, R., Bonito, G. M., & Smith, M. E. (2016). A brief overview of the systematics, taxonomy, and ecology of the Tuber rufum clade. In A. Zambonelli, M. Iotti, & C. Murat (Eds.), True truffle (Tuber spp.) in the World (pp. 125–136). Berlin: Springer. https://doi.org/10.1007/978-3-319-31436-5_8
Hoang, D. T., Chernomor, O., Von Haeseler A., Minh, B. Q., & Vinh, L. S. (2018). UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution, 35, 518–522. https://doi.org/10.1093/molbev/msx281
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K., Von Haeseler, A., & Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589.
Kirk, P. M., Cannon, P. F., Minter, D. W., & Stalpers, J. A. (2008). Ainsworth and Bisby’s dictionary of the fungi. Wallingford: CABI.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
Lancellotti, E., Iotti, M., Zambonelli, A., & Franceschini, A. (2016). The Puberulum group sensu lato (whitish truffles). In A. Zambonelli, M. Iotti, & C. Murat (Eds.), True truffle (Tuber spp.) in the World (pp. 105–124). Berlin: Springer. https://doi.org/10.1007/978-3-319-31436-5_7
Lefevre, C. (2012). Native and cultivated truffles of North America. In A. Zambonelli, & G. Bonito (Eds.), Edible ectomycorrhizal mushrooms (pp. 209–226). Berlin: Springer. https://doi.org/10.1007/978-3-642-33823-6_12
Leonardi, M., Paz-Conde, A., Guevara, G., Salvi, D., & Pacioni, G. (2019). Two new species of Tuber previously reported as Tuber malacodermum. Mycologia, 111, 676–689. https://doi.org/10.1080/00275514.2019.1603777
Li, Q., Yan, L., Ye, L., Zhou, J., Zhang, B., Peng, W. et al. (2018). Chinese black truffle (Tuber indicum) alters the ectomycorrhizosphere and endoectomycosphere microbiome and metabolic profiles of the host tree Quercus aliena. Frontiers Microbiology, 9, 2202. https://doi.org/10.3389/fmicb.2018.02202
Maddison, W. P, & Maddison, D. R. (2016). Mesquite: a modular system for evolutionary analysis (Version 3.10) http://mesquiteproject.org
Marozzi, G., Sánchez, S., Benucci, G. M. N., Bonito, G., Falini, L. B., Albertini, E., & Donnini, D. (2017). Mycorrhization of pecan (Carya illinoinensis) with black truffles: Tuber melanosporum and Tuber brumale. Mycorrhiza, 27, 303–309. https://doi.org/10.1007/s00572-016-0743-y
Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., Von Haeseler, A., & Lanfear, R. (2020). IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37, 1530–1534. https://doi.org/10.1093/molbev/msaa015
Nei, M., & Kumar, S. (2000). Molecular evolution and phylogenetics. Oxford: Oxford University Press.
Neri-Luna, C., & Villarreal-Ruiz, L. (2012). Simbiosis micorrícica: un análisis de su relevante función ecosistémica y en la provisión de servicios ambientales. In M. Huerta, & F. Castro (Comps.), Interacciones ecológicas (pp. 37–61). Guadalajara: Universidad de Guadalajara. https://doi.org/10.1007/s00572-016-0743-y
Pegler, D. N., Spooner, B. M., & Young, T. W. K. (1993). British truffles, a revision of British hypogeous fungi. Kew, UK: Royal Botanic Gardens.
Pina-Paez, C., Bonito, G., Guevara-Guerrero, G., Castellano, M. A., Garibay Orijel, R., Trappe, J. M. et al. (2018). Description and distribution of Tuber incognitum sp. nov. and Tuber anniae in the Transmexican Volcanic Belt, Mycokeys, 41, 17–27. https://doi.org/10.3897/mycokeys.41.28130
Rincón, A., Alvarez, I. F., & Pera, J. (2001). Inoculation of containerized Pinus pinea L. seedlings with seven ectomycorrhizal fungi. Mycorrhiza, 11, 265–271. https://doi.org/10.1007/s005720100127
Rodríguez, R. A., Valdés, M. P., & Ortiz, S. (2018). Características agronómicas y calidad nutricional de los frutos y semillas de zapallo Cucurbita sp. Revista Colombiana de Ciencia Animal Recia, 10, 86–97. https://doi.org/10.24188/recia.v10.n1.2018.636
Sambrook, J., Fritsch, E., & Maniatis, T. (1989). Molecular cloning: a laboratory manual. (No. Ed. 2). Long Island, NY: Cold Spring Harbor Laboratory Press.
Sharma, J., Trela, B., Wang, S., Smith, M., & Bonito, G. (2012). Pecan truffle (Tuber lyonii) in Texas. Pecan South, 2012, 16–24.
Trappe, J. M. (1979). The orders, families, and genera of hypogeous Ascomycotina (truffles and their relatives). Mycotaxon, 9, 297–340.
Trappe, J. M., Jumpponen, A. M. J., & Cazares, E. (1996). NATS truffle and truffle-like fungi. 5. Tuber lyonii (= T. texense), with a key to the spiny-spored Tuber species groups. Mycotaxon, 60, 365–372.
Vilgalys, R., & Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology, 172, 4238–4246.