Nathália U. Ferretti-Cisneros a, Alexandre G. S. Silva-Filho b, Felipe Wartchow a, *
a Universidade Federal da Paraíba, Campus I, Departamento de Sistemática e Ecologia, Jardim Universitário s/n, CEP 58051-9000, João Pessoa, Paraíba, Brazil
b Universidade Federal do Rio Grande do Norte, Programa de Pós-Graduação em Sistemática e Evolução, Campus Universitário, Lagoa Nova, CEP 59078-970, Natal, Rio Grande do Norte, Brazil
*Corresponding author: fwartchow@yahoo.com.br (F. Wartchow)
Received: 10 November 2020; accepted: 8 July 2021
Abstract
A novel Leucocoprinus species was collected from the semi-arid region of Brazil in the Caatinga domain. Leucocoprinus rhodolepis is described based on morphological data. It is well characterized by its white pileus with distinct orange-pink pileal squamules at the center, ellipsoid basidiospores measuring 6.9-7.8 × 4.9-5.9 μm lacking an apical germ pore, and clavate to broadly clavate rarely ventricose-capitate cheilocystidia. Detailed macro- and micro-morphological descriptions and illustrations are provided, as well as a comparison with related species and a brief discussion about its conservation status.
Keywords: Agaricales; Agaricomycetes; Biodiversity; Neotropic; Taxonomy
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Leucocoprinus rhodolepis (Agaricaceae: Basidiomycota), una especie nueva de la región semiárida brasileña
Resumen
Una especie nueva de Leucocoprinus se recolectó en la región semiárida de Brasil, en el dominio de la Caatinga. Leucocoprinus rhodolepis se describe con datos morfológicos. Se caracteriza por el píleo blanco con escamas en distintos matices de color rosa anaranjado en el centro, basidiosporas elipsoides sin poro germinal 6.9-7.8 × 4.9-5.9 μm, queilocistidios claviformes o anchamente claviformes, rara vez ventricosos o capitados. Se proporcionan descripciones e ilustraciones macro y micromorfológicas detalladas, así como una comparación con especies relacionadas y una breve discusión sobre su estado de conservación.
Palabras clave: Agaricales; Agaricomycetes; Biodiversidad; Neotrópico; Taxonomía
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Leucocoprinus Pat., 1888 (Agaricaceae) is characterized by its small or sometimes medium-sized basidiomes with a delicate and thin context, membranous pileus generally possessing a plicate-striate margin, pale hymenophore, free lamellae, and central stipe (Singer, 1986; Vellinga, 2004a). Microscopically, it can be recognized by metachromatic and dextrinoid basidiospores with or without evident germ pore, and the presence of pseudoparaphyses (Justo et al., 2020, 2021; Kumar & Manimohan, 2004; Singer, 1986). Leucocoprinus species are saprobic, growing solitarily or gregariously on soil, mostly in the tropics (Guzmán & Guzmán-Dávalos, 1992; Singer, 1986; Vellinga, 2001).
Phylogenetic studies of the Agaricaceae indicate that Leucocoprinus and Leucoagaricus Locq. ex Singer, 1948 are intermixed within a single clade, but without bootstrap support, and this large genus can be recognized or divided into smaller groups (Vellinga, 2004a: 373). Subsequent studies showed that Leucoagaricus/ Leucocoprinus clade is monophyletic only in the rpb2 phylogeny, and both genera can be split in other phylogenies according to morphology (Vellinga et al., 2011: 498). These works also revealed Micropsalliota Höhn., 1914 and Allopsalliota Nauta & Bas, 1999 clustered in a Leucocoprinus/Leucoagaricus clade close to reddening members like Leucoagaricus americanus (Peck) Vellinga, 2000.
Leucocoprinus has a pantropical distribution comprising approximately 50 species worldwide (He et al., 2019; Wijayawardene et al., 2020). In Brazil, at least 14 taxa have been reported from several states belonging, up to now, to Atlantic, Amazon, and Caatinga biomes (Albuquerque & Victoria, 2012; Albuquerque et al., 2006; Capelari & Gimenes, 2004; Ferreira & Cortez, 2012; Nascimento & Alves, 2014; Wartchow et al., 2008). In relation to the northeast region, knowledge is limited to 3 species, L. birnbaumii (Corda) Singer, 1962, L. cretaceus (Bull.) Locq., 1945, and L. fragilissimus (Ravenel ex Berk. & M.A. Curtis) Pat., 1900, reported from Atlantic forest and Caatinga of Ceará and Pernambuco, revealing a low number of taxa (Nascimento & Alves, 2014; Wartchow, 2018; Wartchow et al., 2008).
The ‘Cariri Paraibano’ region constitutes an area of the Caatinga biome (IBGE, 2004) and has a very high dry climate, with an annual rainfall average of only 900-2,500 mm (Nascimento & Alves, 2008). This microregion of the state of Paraíba, located on a “dry diagonal”, where the aridity degree bioclimate can vary between 0.14 (arid climate) for the Eastern Cariri and 0.22 (semi-arid climate) for Western Cariri (Nascimento & Alves, 2008). The region suffers from a high level of desertification, currently intensified by deforestation due to implantation of agriculture and livestock (Sousa et al., 2008). These anthropic pressures in general result in soil depletion, biological diversity loss, and species extinction (Alves, 2009), since the Caatinga biome houses many endemic species (Leal et al., 2003). Despite many problems related to loss of biodiversity in this region, floristic studies reported approximately 400 plant species belonging to 90 families from ‘Cariri Paraibano’ showing a satisfactory richness in some well-preserved areas (Alves, 2009; Barbosa et al., 2007).
During studies of fungal diversity of Paraíba in Northeast Brazil, an interesting species of Leucocoprinus sensu stricto (i.e., species bearing sulcate-plicated pileus and presence of pseudoparaphyses, Singer 1986) with colors not yet observed for this group and basidiospores lacking a germ pore was collected in the ‘Cariri Paraibano’ region. Based on morphological analysis, Leucocoprinus rhodolepis is proposed as a new species, improving the knowledge of Northeastern Brazilian mycota.
Materials and methods
The new species was collected at the ‘Reserva Particular do Patrimônio Natural (RPPN) Fazenda Almas’, in the municipality of São José dos Cordeiros, Paraíba State (7°28’45” S, 36°54’18” W). With an area of 5,502.92 hectare, this reserve belongs to the Caatinga biome (IBGE, 2004), and is composed of a vegetational type called arborous-arbustive Caatinga (Velloso et al., 2002), and a semi-arid hot climate with annual precipitation of 500 to 800 mm and temperatures varying between 26°C and 30°C (Barbosa et al., 2015). It is located in the Western Cariri and corresponds to the largest private reserve in the Caatinga biome of this state (Lima & Barbosa, 2014). For the study, usual methodologies for agaricoid fungi analysis were used (Singer, 1986) and color codes follow Online Auction Color (2004). The statistics of the basidiospores were based on 40 measurements, Q is the quotient between the length and width, and Qm is the median value of Q. The holotype is deposited at JPB (Thiers, 2021, continuously updated).
Description
Leucocoprinus rhodolepis Ferretti-Cisn. & Wartchow, sp. nov. (Fig. 1-3)
Mycobank: MB 838894
Diagnosis. Differs from other Leucocoprinus species by a combination of the following characters: pinkish tints of the pileal squamules, basidiospores measuring 6.9-7.8 × 4.9-5.9 μm, lacking a germ pore, and cheilocystidia measuring 15.5-27.5 × 7.1-11.2 μm, clavate to broadly clavate, rarely ventricose-capitate.
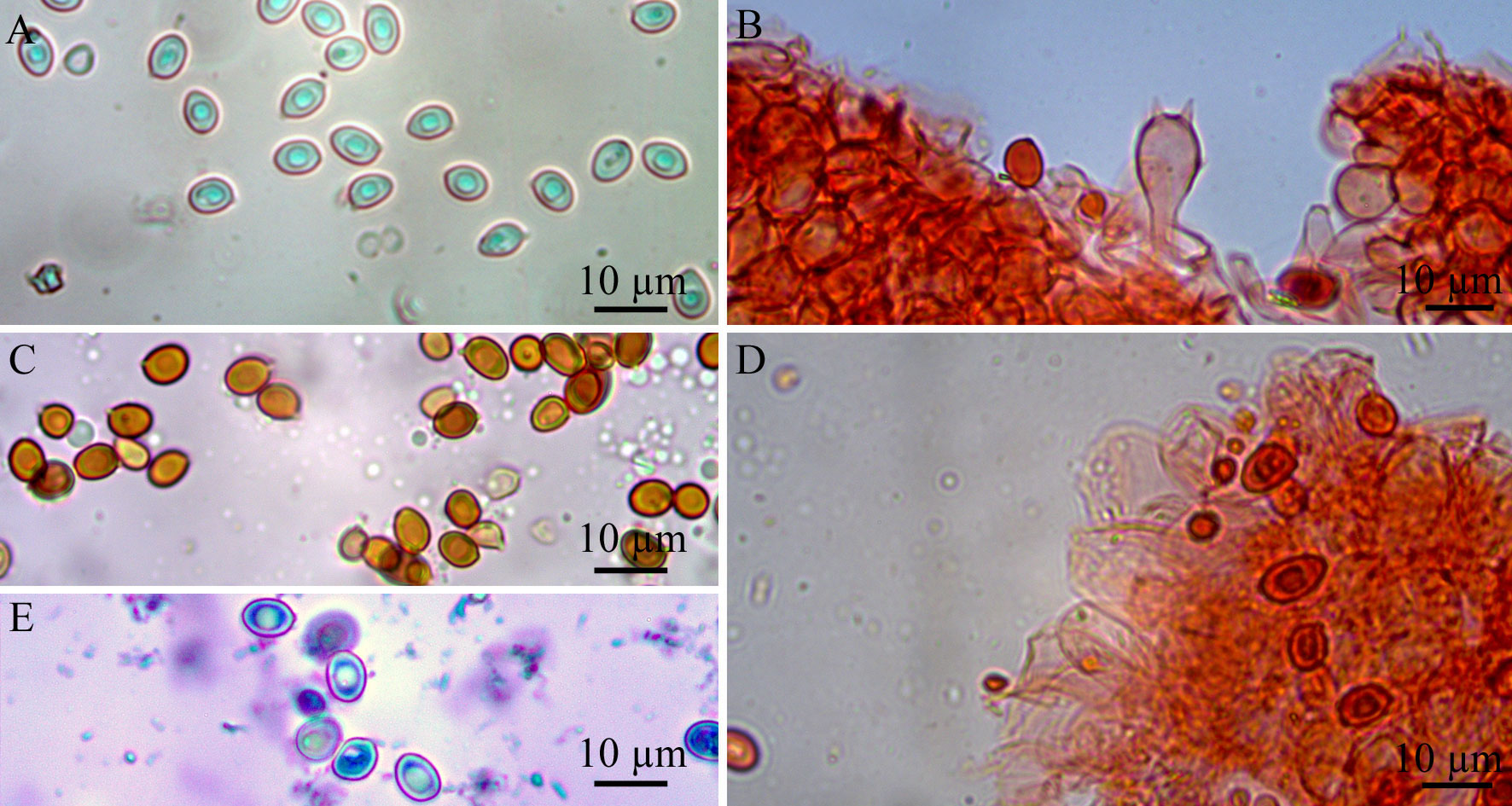
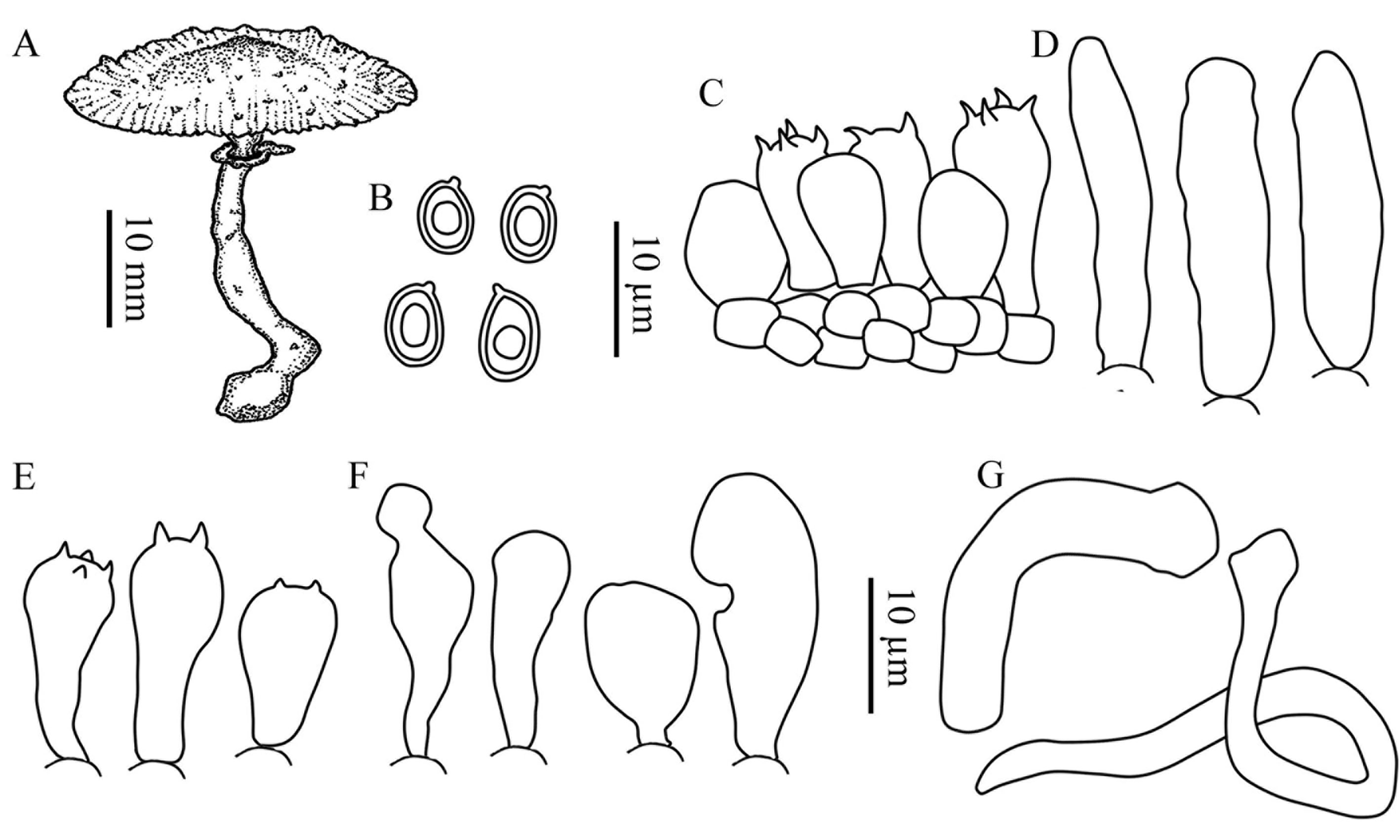
Basidiomata lepiotoid (Largent, 1986). Pileus up to 36 mm, plano-convex umbonate, surface squamulose; grayish-pinkish-orange (OAC 613) at center, disrupting into pinkish orange (OAC 617), then light pink (OAC 620), small squamules more scarce towards margin, on a white (OAC 909) ground; margin very thin, acute, finely plicate-striate, straight to slightly decurved (Fig 1A); context very thin, submembranous, white, unchanging. Lamellae free, crowded to subcrowded, white (OAC 909), edge mostly even, sometimes finely serrulate, concolorous with the side; lamellulae frequent, with 3 lengths (Fig. 1B). Stipe 50 × 4 mm, central, cylindrical to compressed, slightly curved at the base, white (OAC 909) to very pale grayish green (OAC 893); surface smooth, glabrous; base bulbous, ranging to 11 mm diam.; annulus attached on the upper part, funnel shaped, small, white (OAC 909), striate at the inferior part of the annulus (Fig. 1B). Spore print not obtained. Basidiospores 6.9-7.8 (-8.8) × 4.9-5.9 μm, av. 7.7 × 5.1 μm, Q = 1.32-1.59 (-1.79), Qm = 1.47, ellipsoid, rarely oblong, lacking a germ pore, slightly thick-walled, hyaline and colorless in 3% KOH (Fig. 2A), dextrinoid (Fig. 2C), metachromatic in cresyl blue (Fig. 2E) mounts; hilar appendix subapical (Figs. 2A, 3A). Basidia 17.6-20.6 × 7.8-9.8 (-10.8) μm, clavate, 2-4-spored, hyaline, sterigmata 1-3 μm long, surrounded by abundant sphaeropedunculate to balloon-shape pseudoparaphyses (Figs. 2B, 3C, 3E). Pleurocystidia absent. Lamellar edge sterile, composed of numerous cheilocystidia, 15.5-27.5 × 7.1-11.2 μm, clavate to broadly clavate, rarely ventricose-capitate, hyaline, thin-walled (Figs. 2D, 3F). Lamellar trama regular, composed by cylindrical hyphae, 4.1-5.6 μm in diam., thin-walled, colorless. Pileus covering a cutis composed of loosely arranged to pileus surface under microscope elements, mostly cylindrical, 62.5-93.7 × 11.2-22.5 μm, thin walled, pale colorless (Fig. 3D), more abundant at disc. Oleiferous (thrombopleurous) hyphae infrequent to rare, present in lamellar trama and pileus, measuring 4.1-13.3 μm wide (Fig. 3G). Clamp connections absent from all tissues examined.
Taxonomic summary
Holotype: Brazil. Paraíba state, São José dos Cordeiros, RPPN Fazenda Almas, solitary on soil among litter, 26 Feb 2019, leg. A.G.S. Silva-Filho 08-2019 (JPB 64603).
Etymology: from Greek (Borror, 1960) rhodo (= rose-colored), and lepis (= squamules/scales). Due the pinkish squamules on pileus centre.
Habitat and distribution: solitary on soil surrounded by vegetation of Caatinga, only known from the type locality.

Conservation status: the species is only known from the type locality. This place has been the focus of previous floristic studies (Agra et al., 2007; Barbosa et al., 2007) and is considered a somewhat well-preserved area. In addition, it is difficult to define the population of L. rhodolepis due to the extremely dry weather and non-seasonal rains in the region. Thus, we consider the taxon as belonging to the category of ‘Data Deficient’ (DD), and more information needs to be gathered before evaluation against IUCN criteria (IUCN, 2019).
Remarks
Leucocoprinus rhodolepis can be easily recognized in the field by its small basidiomata with white pileus covered by orange-pink squamules on the center and basidiospores without a germ pore. These features are infrequent among Leucocoprinus sensu stricto (Singer 1986). The Neotropical species Lepiota roseolamellata Dennis, 1952 was described as having a plicate-striate surface with white then pink pileus and basidiospores lacking a germ pore, which might be a member of Leucocoprinus. The species can be separated by its smaller pileus 10 mm in diam., white then pink lamellae, more fragile annulus, narrower basidiospores (5.5-7 × 2.5-3 μm), and habit growing on rotten logs (Dennis, 1952). Later, Pegler (1983) revised this species and observed more features such as the absence of clamp connections and a pseudoparenchymatous subhymenial layer. This species has certain similarities with the entity described here, such as the presence of a small annulus and the size and form of the basidiospores, but some differences like the glabrous pileus, pink lamellae, and the smaller pileus distinguish L. roseolamellata from L. rhodolepis (Pegler, 1983).
A species recently mentioned as having pinkish gray hues around the pileus center is L. antillarum Justo, Bizzi & Angelini, 2021 from the Dominican Republic. However, the scales are more scattered and brownish gray, brown-ocher or whitish, the basidiospores are smaller (5-7 × 3.5-5 μm) and amygdaliform, and the cheilocystidia are larger (35-92 × 9-27 μm; Justo et al., 2021).
The species L. rubrosquamosus (Rick) Raithelh., 1991 should also be noted. This species was described under Lepiota (Pers.) Gray, 1821 by Rick (1920) as having small stature, fire-red squamules, whitish margin, small annulus, and small basidiospores (4.5 × 3 μm). Later, Singer (1951) recombined the species to Leucoagaricus rubrosquamosus (Rick) Singer, 1951 (1949). Subsequent study of the type revealed that exsiccata, found in Farlow Herbarium (FH) was deposited under the name ‘Lepiota rubrofibrillosa’ bears reticulate basidiospore walls (Singer, 1953) characteristic of the genus Rugosospora Heinem., 1973 (Heinemann, 1973). Finally, Raithelhuber (2004) concluded that Singer reviewed a different sample and proposed the name Leucocoprinus rubrosquamosus based on material deposited in the herbarium PACA. Thus, this species differs from L. rhodolepis at least in the reddish squamules, and proportionally longer basidiospores (7.8-10 × 4.2-4 μm) although there is no mention of the presence or absence of a germ pore (Raithelhuber, 2004).
Microscopically L. rhodolepis produces basidiospores without a germ pore, a feature also observed in another 16 species: Leucocoprinus antillharum Justo, Bizzi & Angelini, 2021, L. aureofloccosus (Henn.) Bon, 1981, L. cygneus (J.E. Lange) Bon, 1978, L. dominguensis Justo, Bizzi, Angelini & Vizzini, 2020, L. flavescens (Morgan) H.V. Sm., 1981, L. fuligineopunctatus Justo, Bizzi & Angelini, 2021, L. heinemannii Migl., 1987, L. lanzonii Bon, Migl. & Brunori, 1989, L. medioflavus (Boud.) Bon, 1976, L. microlepis Justo, Bizzi & Angelini, 2021, L. parvipileus Justo, Bizzi, Angelini & Vizzini, 2020, L. pepinosporus Heinem., 1977, L. scissus Justo, Bizzi & Angelini, 2021, L. straminellus (Bagl.) Narducci & Caroti, 1995, L. submontagnei Heinem., 1977, and L. tephrolepis Justo, Bizzi, Angelini & Vizzini, 2020, (Table 1) (Birkebak, 2010; Candusso & Lanzoni, 1990; Heinemann, 1977a, b; Justo et al., 2020, 2021; Smith, 1981).
Among the taxa cited, only L. cf. medioflavus was reported from Brazil, occurring in the Atlantic Forest of Rio Grande do Sul (Rother & Silveira, 2008, 2009). However, this material requires a more detailed analysis before confirming its specific status.
Leucocoprinus pepinosporus, L. cygneus, L. submontagnei, L. lanzonii, and L. scissus readily differ in the pileus bearing brownish coloration. Additionally, they present other morphological differences: L. pepinosporus has pinkish tints on the stipe, larger basidiospores (11.2-13.5 × 7.4-8.1 μm), and larger cheilocystidia (45-50 × 14-20 μm, Heinemann, 1977a; 1977b); L. cygneus has grayish granulous flocks on the pileus center and smaller basidiospores (6-7 × 3.5-4.5 μm; Vellinga, 2001); L. submontagnei has dark brown squamules at the center, a more plicate striated pileus, and amygdaliform basidiospores (6.5-8.1 × 4.2-5.1 μm; Heinemann, 1977a; 1977b); L. lanzonii was referred to as having brownish orange tints and also differs in its smaller basidiospores (5-6.5 × 3.5-4.5 μm) and the slightly larger clavate cylindrical cheilocystidia ranging to 60 × 11 μm (Candusso & Lanzoni, 1990); and L. scissus, although described with basidiospores similar in size (6-8 × 4-6 μm), differs in the brown ocher scales on its pileus, and longer cheilocystidia (20-52 × 6-14 μm; Justo et al., 2021).
The other 4 species bearing basidiospores without a germ pore have a yellowish pileus and smaller basidiospores: L. aurefloccosus (5-6.5 × 3.5-4.5 μm; Candusso & Lanzoni, 1990); L. flavescens (4.4-5.9 × 3.6-4.8 μm; Birkebak, 2010); L. straminellus (5-6 × 4-5 μm; Candusso & Lanzoni, 1990; Campi et al., 2015); and L. medioflavus (5.5-6.5 x 3.5-4.2 μm; Candusso & Lanzoni, 1990). Finally, L. heinemannii and other recently described species (L. dominguensis, L. fuligineopunctatus, L. microlepis, L. parvipileus, and L. tephrolepis) differ in the black, purplish black, gray-ash squamules on the pileus center, and the slightly longer cheilocystidia ranging to 46 μm long (Candusso & Lanzoni, 1990; Justo et al., 2020, 2021; Mohr & Ludwig, 2004).
Thus, we consider that our sample collection presents unique features compared to other Leucocoprinus taxa. It corresponds to a sample in perfect condition when gathered, allowing a full and detailed examination of its macro- and microscopical features, which we considered distinct enough to propose it as a new taxon.
Implication for conservation
The northeastern semi-aridity is caused by natural phenomena, but has been intensified by anthropic actions (Sousa et al., 2008). Desertification brings a scarcity of natural resources, which results in greater social, economic, and environmental vulnerabilities, which are reinforced by the absence of consistent public policies (Sousa et al., 2008). Among the factors that contribute to the processes of degradation of the Cariri’s vegetation are the supply of wood for various economic activities in the state of Paraíba, and extensive and semi-extensive local livestock farming. Thus, the sustainability of the Caatinga biome must be accompanied by a policy to combat the degradation of vegetation cover, as pointed out by Alves (2009).
Table 1
Comparison between Leucocoprinus rhodolepis and related species.
| Species | Pileus color | Umbo color | Squamules | Basidiospores (μm) | Cheilocystidia (μm) | Reference |
| Leucocoprinus antillarum | white | brown-ocher, ocher-yellowish | brownish gray, brown-ocher or whitish | 5.5-7 × 3.5-5 | 35-92 × 9-27 | Justo et al. (2021) |
| L. aureofloccosus | sulfur yellow | sulfur yellow | golden floccose | 5-7 × 2.5-3 | not mentioned | Candusso & Lanzoni (1990) |
| L. cygneus | white slightly achraceous | white slightly achraceous | greyish granulose | 6-7 × 3.5-4.5 | Vellinga (2001) | |
| L. dominguensis | white | gray-black | dark-gray to black | 4.5-6 × 3- 4 | 23-46 × 5.5-12.5 | Justo et al. (2020) |
| L. flavescens | sulfur yellow to white tinted with sulfur yellow to naphthalene yellow | barium yellow to amber yellow or faintly tinted brown | not mentioned | 4.4-5.9 × 3.6-4.8 | 11-24.2 × 5.5-13.2 | Birkebak (2010) |
| L. fuligineopunctatus | white | dark brown, brown-black, brown sooty | dark brown, brown-sooty | 5.5-7.5 × 3.5-4.5 (-5) | 20-40 × 6-19.5 | Justo et al. (2021) |
| L. heinemannii | white | black | grey-violet | 6-7.5 × 3.3-4.2 | 24-32 × 7-10 | Candusso & Lanzoni (1990) |
| L. medioflavus | white | light brown | pale yellow to brownish yellow | 5-6.5 × 3-4 | 19-42 × 9-16 | Candusso & Lanzoni (1990) |
| L. microlepis | white | brown-black | brownish gray | 5-7 × 3.5-5 | 16-57 × 8-20 | Justo et al. (2021) |
| L. lanzonii | milky white | brownish | brownish | 5-6.5 × 3.5-4.5 | 30 × 11 | Candusso & Lanzoni (1990) |
| L. parvipileus | white | gray-blackish | gray | 5-7 × 3-4.5 | 22-41 × 7.5-15 | Justo et al. (2020) |
| L. pepinosporus | white | grayish brown | brownish | 11.2-13.5 × 7.4-8.1 | 45-50 × 14-20 (28) | Heinemann (1977a; 1977b) |
| L. rhodolepis sp. nov. | white | grayish-pinkish-orange | pinkish orange to light pink | 6.9-7.8 × 4.9-5.9 | 15.5-27.5 × 7.1-11.2 | This study |
| Lepiota roseoalba | orange-pink to seashell pink | strawberry pink | not mentioned | 6.5-10 × 4-5 | 34-43 × 4.5-11 | Pegler & Rayner (1969) |
| Lepiota roseolamellata | white to flesh-pink | pinkish | not mentioned | 5.5-7 × 2.5-3 | 16-25 × 12 | Dennis (1952) |
| L. scissus | white | brownish ocher | brownish ocher | 6-8 × 4-6 | 20-52 × 6-14 | Justo et al. (2021) |
| L. straminellus | white to yellowish white | pale yellow | white to light yellow | 5-7.5 × 3-4.5 | 15-40 × 6-12 | Kumar & Manimohan (2009), Campi et al. (2015) |
| L. submontagnei | creamy white | brown | brown | 5.5-6.6 × 3.3-4.4 | 25.3-66 × 10-22 | Heinemann (1977a; 1977b) |
| L. tephrolepis | white | gray | gray-ash | 5.5-7 × 3-4 | 23-46 × 55-12.5 | Justo et al. (2020) |
According to Vellinga (2004b), deforestation in areas, particularly in the tropics, may extinguish many habitats for lepiotaceous species. She argued that the conservation of existing diversity would need public policies, supported by new ecological research, more taxonomic studies, and recording projects. In the last decades we observed an increase of recording and describing species of Agaricaceae in Brazil; however, some areas are still underexplored (Nascimento & Alves, 2014; Wartchow et al., 2008). The discovery of this new species in a region that suffers severe aridity shows the importance of inventories of agaricoid fungi in the Brazilian semi-arid region.
Acknowledgements
The authors thank Dr. Rivete S. Lima, and Mr. Wendell Lima Ferreira de Sousa (Laboratório de Anatomia Vegetal-UFPB) for help in microscopic photography; “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for the PhD scholarships awarded to Alexandre G. S. Silva-Filho. FW thanks “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) for funding the projects “Programa de Pesquisa em Biodiversidade” (PPBio-CNPq/MCT Proc. 60/2009) and “Fungos agaricoides em áreas de Mata Atlântica e Caatinga no Estado da Paraíba” (Edital Universal Proc. 420.448/2016-0) and providing a “Produtividade em Pesquisa” (Proc. 307947/2017-3) grant.
References
Agra, M. F., Baracho, G. S., Basílio, I. J. D., Nurit, K., Coelho, V. C., & Barbosa, D. A. (2007). Sinopse da flora medicinal do Cariri Paraibano. Oecologia Brasiliensis, 11, 323–330.
Albuquerque, M. P., & Victoria, F. C. (2012). Leucocoprinus fluminensis (Agaricaceae Basidiomycota), a new species from soutwest Brazilian Rain Forest. Neotropical Biology and Conservation, 7, 158–161. https://doi.org/10.4013/nbc.2012.73.02
Albuquerque, M. P., Victoria, F. C., & Pereira, A. B. (2006). Ecologia e Distribuição do gênero Leucocoprinus Pat. no Rio Grande do Sul, Brasil. Acta Biologica Leopoldensia, 28, 11–16.
Alves, J. J. A. (2009). Caatinga do Cariri Paraibano. Geonomos, 17, 19–25.
Barbosa, M. R. V., Lima, I. B., Lima, J. R., Cunha, J. P., Agra, M. F., & Thomas, W. W. (2007). Vegetação e Flora no Cariri Paraibano. Oecologia Brasiliensis, 11, 313–322.
Barbosa, M. R. V, Pareyn, F. C. G, & Lima, J. R. (2015). Plano de Manejo Reserva Particular do Patrimônio Natural Fazenda Almas. Recife, Pernambuco: Associação Plantas do Nordeste/APNE.
Birkebak, J. M. (2010). The genus Leucocoprinus in Washington. Mycotaxon, 112, 83–102. https://doi.org/10.5248/112.83
Borror, D. J. (1960). Dictionary of word roots and combining fors. Mountain View, California: Mayfield Publishing Company.
Campi, M. G. G., Bonzi, B. R. M., Rivas, A. M. I. F., & Niveiro, N. (2015). El género Leucocoprinus Pat. (Agaricaceae-Agaricomycetes) en el norte de Argentina y Paraguay. Iheringia, Série Botânica, 70, 309–320.
Candusso, M., & Lanzoni, G. (1990). Lepiota s.l. Fungi Europaei. Saronno: Libreria editrice Giovanna Biella Ι.
Capelari, M., & Gimenes, L. J. (2004). Leucocoprinus brunneoluteus, uma nova espécie de Agaricaceae. Hoehnea, 31, 331–335.
Dennis, R. W. G. (1952). Lepiota and allied genera in Trinidad, British West Indies. Kew Bulletin, 4, 459–499. https://doi.org/10.2307/4117800
Ferreira, A. J. & Cortez, V. G. (2012). Lepiotoid Agaricaceae (Basidiomycota) from São Camilo State Park, Paraná State, Brazil. Mycosphere, 3, 962–976. https://doi.org/10.5943/mycosphere/3/6/11
Guzmán, G., & Guzmán-Dávalos, L. (1992). A checklist of the Lepiotaceous Fungi. Champaign: Koeltz Scientific Books USA.
He, M. Q., Zhao, R. L., Hyde, K. D., Begerow, D., Kemler, M., Yurkov, A. et al. (2019) Notes, outline and divergence times of Basidiomycota. Fungal Diversity, 99, 105–367. https://doi.org/10.1007/s13225-019-00435-4
Heinemann, P. (1973). Leucocoprinées nouvelles d’Afrique centrale. Bulletin du Jardin Botanique National de Belgique, 43, 7–13. https://doi.org/10.2307/3667558
Heinemann, P. (1977a). Leucocoprinées nouvelles d’Afrique centrale II. Bulletin du Jardin Botanique National de Belgique, 47, 83–86. https://doi.org/10.2307/3667983
Heinemann, P. (1977b). Leucocoprinus (Agaricaceae). Flore Ilustrée des Champignon d’Afrique Centrale, 5, 87–101.
IBGE (Instituto Brasileiro de Geografia e Estatística). (2004) Mapas de cobertura vegetal dos biomas brasileiros. Ministério do Meio Ambiente, Ministério do Planejamento, Orçamento e Gestão Instituto Brasileiro de Geografia e Estatística, Diretoria de Geociências, Brasilia.
IUCN (International Union for Conservation of Nature). (2019). Guidelines for Using the IUCN Red List categories and Criteria. Standards and Petitions Subcommittee. Version 14. Prepared by the Standards and petitions Subcommittee. https://www.iucnredlist.org/resources/redlistguidelines
Justo, A., Angelini, C., & Bizzi, A. (2021). The genera Leucoagaricus and Leucocoprinus in the Dominican Republic. Mycologia, 113, 348–389. https://doi.org/10.1080/00275514.2020.1819142
Justo, A., Angelini, C., Bizzi, A., & Vizzini, A. (2020). Three new cryptic Caribbean species in the Leucocoprinus heniemannii complex (Agaricaceae, Agaricales). Mycological Progress, 19, 1445–1457. https://doi.org/10.1007/s11557-020-01638-9
Kumar, T. K. A., & Manimohan, P. (2004). A new species of Leucocoprinus from India. Mycotaxon, 90, 393–397.
Kumar, T. K. A, & Manimohan, P. (2009). The genera Leucoagaricus and Leucocoprinus (Agaricales, Basidiomycota) in Kerala State, India. Mycotaxon, 108, 385–428. https://doi.org/10.5248/108.385
Largent, D. L. (1986). How to identify mushrooms to genus i: macroscopic features. Eureka, California: Mad River Press.
Leal, I. R., Tabarelli, M., da Silva, J. M. C. (2003). Ecologia e Conservação da Caatinga. Recife, Pernambuco: Editora Universitária da UFPE.
Lima, I. B., & Barbosa, M. R. V. (2014). Composição Florística da RPPN Fazenda Almas, no Cariri Paraibano, Paraíba, Brasil. Revista Nordestina de Biologia, 23, 49–67.
Mohr, P, & Ludwig, E. (2004). Vier neue Arte naus den Gattungen Leucoagaricus und Leucocoprinus MIT bräunlichen bis rußfarbigen Tönungen in den Hutfarben. Feddes Repertorium, 115, 20–34. https://doi.org/10.1002/fedr.200311022
Nascimento, S. S., & Alves, J. J. A. (2008). Ecoclimatologia do Cariri Paraibano. Revista Geográfica Acadêmica, 2, 28–41.
Nascimento, C. C., & Alves, M. H. (2014). New records of Agaricaceae (Basidiomycota, Agaricales) from Araripe National Forest, Ceará State. Mycosphere, 5, 319–332. https://doi.org/10.5943/mycosphere/5/2/6
Online Auction Color. (2004). The online Auction Color Chart. Stanford, California: Online Auction Color Co.
Pegler, D. N., & Rayner, R. W. (1969). A contribution to the agaric flora of Kenya. Kew Bulletin, 3, 347–412. https://doi.org/10.2307/4117177
Pegler, D. N. (1983). Agaric flora of the Lesser Antilles. Kew Bull Additional Series, 9, 1–668.
Raithelhuber, J. (2004). Nueva flora micológica argentina: Stuttgart: J.H. Raithelhuber.
Rick, J. (1920). Contributio ad monographiam Agaricacearum Brasiliensium. Brotéria, Série Botânica, 18, 48–63.
Rother, M. S., & Silveira, R. M. B. (2008). Família Agaricaceae (Agaricales, Basidiomycota) no Parque Estadual de Itapuã, Viamão, Rio Grande do Sul, Brasil. Revista Brasileira de Biociências, 6, 259–268.
Rother, M. S., & Silveira, R. M. B. (2009). Leucocoprinus Pat. (Agaricaceae, Basidiomycota) no Parque Estadual de Itapuã, Viamão, RS, Brasil. Acta Botanica Brasilica, 23, 720−728. https://doi.org/10.1590/S0102-33062009000300011
Singer, R. (1951 [1949]). The Agaricales (mushrooms) in modern taxonomy. Lilloa, 22, 5–832.
Singer, R. (1953). Type studies on Basidiomycetes VI. Lilloa, 26, 57–159.
Singer, R. (1986). The Agaricales in modern Taxonomy. 4th ed. Koenigstein: Koeltz Scientific Books.
Smith, H. V. (1981). Some species of Leucocoprinus which grow in greenhouses. Michigan Botanist, 20, 45–52.
Sousa, R. F., Fernandes, M. F., & Barbosa, M. P. (2008). Vulnerabilidades, semi-aridez e desertificação: cenários de riscos no Cariri Paraibano. OKARA: Geografia em Debate, 2, 128–206.
Thiers, B. (2021). Index Herbariorum. A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/
Vellinga, E. C. (2001). Leucocoprinus. In M. E. Noordeloos, T. H. W. Kuyper, & E. C. Vellinga (Eds.), Flora Agaricina Neerlandica 5 (pp. 76–84). Balkema: A. A. Publishers, Lisse/ Abingdon/ Exton (PA), Tokyo.
Vellinga, E. C. (2004a). Genera in the family Agaricaceae: evidence from nrITS and nrLSU sequence. Mycological Research, 108, 354–377. https://doi.org/10.1017/S0953756204009700
Vellinga, E. C. (2004b.) Ecology and distribution of lepiotaceous fungi (Agaricaceae). Nova Hedwigia, 78, 273–299. https://doi.org/10.1127/0029-5035/2004/0078-0273
Vellinga, E. C., Sysouphanthong, P., & Hyde, K. D. (2011). The family Agaricaceae: phylogenies and two new white-spored genera. Mycologia, 103, 494–509. https://doi.org/10.3852/10-204
Velloso, A., Sampaio, E. V. S. B., & Pareyn, F. G. C. (2002). Escorregões propostas para o bioma Caatinga. Instituto de Conservação Ambiental The Nature Conservancy do Brasil. Recife, Pernambuco: Associação Plantas do Nordeste.
Wartchow, F. (2018). Breve história da sistemática de Agaricaceae (Fungi) e distribuição no Brasil. Pesquisa e Ensino em Ciências Exatas e da Natureza, 2, 130–147. https://doi.org/10.29215/pecen.v2i2.1067
Wartchow, F., Putzke, J., & Cavalcanti, M. A. Q. (2008). Agaricaceae Fr. (Agaricales, Basidiomycota) from areas of Atlantic Forest in Pernambuco, Brazil. Acta Botanica Brasilica, 22, 287–299. https://doi.org/10.1590/S0102-33062008000100026
Wijayawardene, N. N., Hyde, K. D., Al-Ani, L. K. T., Tedersoo, L., Haelewaters, D., Rajeshkumar, K. C. et al. (2020). Outline of Fungi and fungus-like taxa. Mycosphere, 11, 1060–1456. https://doi.org/10.5943/mycosphere/11/1/8




