Alicia Franco-Ramírez a, Jesús Pérez-Moreno a, b, *, Gabriela Sánchez-Viveros c, Carlos R. Cerdán-Cabrera c, Juan J. Almaraz-Suárez a, Víctor M. Cetina-Alcalá d, Alejandro Alarcón a
a Colegio de Posgraduados, Campus Montecillo, Microbiología, Edafología, Carretera México-Texcoco Km 36.5, 56230 Montecillo, Texcoco, Estado de México, Mexico
b Chinese Academy of Sciences, Kunming Institute of Botany, 132 Lanhei Road, Heilongtan, Kunming, Yunnan 650201, Kunming, China
c Universidad Veracruzana, Facultad en Ciencias Agrícolas, Circuito Gonzalo Aguirre Beltrán s/n Zona Universitaria, 91000 Xalapa, Veracruz, Mexico
d Colegio de Posgraduados, Campus Montecillo, Programa Forestal, Carretera México-Texcoco Km 36.5, 56230 Montecillo, Texcoco, Estado de México, Mexico
*Corresponding author: jepemo@yahoo.com.mx (J. Pérez-Moreno)
Received: 10 October 2019; accepted: 19 October 2020
Abstract
Traditionally, it is thought that arbuscular mycorrhizae establish a symbiosis with the roots of numerous angiosperm and some gymnosperm families. However, the mobilization and transfer of macro- and micronutrients to Pinaceae via arbuscular mycorrhizal fungi (AMF) has not been reported so far. The present work evaluated whether arbuscular mycorrhizae are able to establish, mobilize, and transfer nutrients in the neotropical species of Pinaceae, Pinus greggii. Seedlings were inoculated with 3 AMF consortia isolated from an agricultural site, a Cupressus lusitanica forest and a Pinus hartwegii forest. Evidence of mobilization and transfer of macro- and micronutrients in plants inoculated with the 3 consortia was evaluated. A greater Mg, Mn, and Zn mobilization and transfer was observed in plants inoculated with the pine forest AMF consortium after 7 months. In addition to these positive effects, AMF root colonization of 10 to 15% and 21 to 36% was observed depending on the AMF consortia after 2 and 7 months. In the present work, we report for the first time that AMF mobilize and transfer N, P, K, Ca, Mg, Fe, Mn, Zn, Cu, and B to a member of Pinaceae, indicating that this mycorrhizal symbiosis is more complex than previously believed.
Keywords: Glomeromycota; Pinaceae; Neotropic; Nutrient mobilization and transfer; Arbuscular mycorrhiza
Movilización y transferencia de nueve macro y micronutrientes a plántulas de Pinuss greggii a través de hongos micorrízico arbusculares
Resumen
Tradicionalmente, se considera que las micorrizas arbusculares establecen simbiosis con las raíces de las plantas de numerosas familias de angiospermas y algunas gimnospermas. Sin embargo, la movilización y transferencia de macro y micronutrientes en Pinaceae a través de micorrizas arbusculares no se ha registrado hasta ahora. El presente trabajo evaluó si las micorrizas arbusculares son capaces de establecerse, movilizar y transferir nutrientes en la pinácea neotropical Pinus greggii. Las plántulas de este árbol se inocularon con 3 consorcios de hongos micorrícico arbusculares (HMA) aislados de un sitio agrícola, un bosque de Cupressus lusitanica y un bosque de Pinus hartwegii. Se registró la movilización y transferencia de macro y micronutrientes en plantas inoculadas con los 3 consorcios evaluados. La movilización y transferencia fue mayor para Mg, Mn y Zn en plantas inoculadas con el consorcio de hongos del bosque de pino después de 7 meses. Además de estos efectos positivos, se observó una colonización de micorriza arbuscular de 10 a 15% y de 21 a 36% dependiendo de los consorcios de HMA después de 2 y 7 meses, respectivamente. En el presente trabajo, se registra por primera vez que los HMA movilizan y transfieren N, P, K, Ca, Mg, Fe, Mn, Zn, Cu y B a un miembro de Pinaceae, lo que indica que esta simbiosis micorrícica es más compleja de lo que se creía anteriormente.
Palabras clave: Glomeromycota; Pinaceae; Neotrópico; Movilización y transferencia nutrimental; Micorriza arbuscular
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Under natural conditions, it has been estimated that around 85% of terrestrial plants form mycorrhizal symbioses (Smith & Read, 2008). Arbuscular mycorrhizal fungi (AMF) are native to all terrestrial ecosystems and can be found in almost all soils (Jansa et al., 2006). These fungi are members of the phylum Mucoromycota, subphylum Glomeromycotina (Spatafora et al., 2016) and are important components in the soil rhizosphere because they serve multiple functions in ecosystems, favor plant growth, and facilitate nutrients absorption, including P, N, and water (Sharif & Claassen, 2011; Siqueira et al., 2002). AMF have been reported in most vascular plants including mainly angiosperms, and also in early diverging lineages such as liverworts and hornworts (Bonfante & Genre, 2008; Wang et al., 2010). In addition, some gymnosperms are known to establish arbuscular mycorrhiza, including cycads (Fisher & Vovides, 2004; Muthukumar & Udaiyan, 2002) and some ancient conifers whose origin is dated to the late and middle Carboniferous, respectively, more than 300 million years ago (Strullu-Derrien et al., 2018). The fossil record has shown that extinct voltzialean conifers from middle Triassic, around 220 million years ago, belonging to the genus Notophytum presented arbuscular mycorrhiza as well, showing that this symbiotic fungal association was likely important in early transitional conifers (Harper et al., 2015). Some living gymnosperms, members of the family Araucariaceae, which were widespread globally during the Jurassic Period from 199 to 145 million years ago, also harbor AMF (Padamsee et al., 2016). There have also been reports of AMF colonization in other gymnosperms belonging to Pinaceae (Cázares & Trappe, 1993; Smith et al., 1998; Wagg et al., 2008), Cupressaceae (Aalipour et al., 2020; Bush, 2008), Taxaceae (López-García et al., 2013), and Podocarpaceae (Russell et al., 2002).
In the particular case of Pinaceae, the presence of AMF vesicles has been recorded in the seedlings root of species belonging to Abies (Cázares & Trappe, 1993; Smith et al., 1998), Pinus (Smith et al., 1998; Wagg et al., 2008, 2011), Pseudotsuga (Cázares & Trappe, 1993; Dučić et al., 2009; Salgado et al., 2013; Wagg et al. 2011), and Tsuga (Cázares & Smith, 1995). Although the presence of AMF has been documented previously in Pinaceae, its functional importance in terms of a screening of both macro and micronutrient mobilization and transfer to their host is lacking in the family, which includes numerous species of forest importance worldwide. In the present work, we studied the effect of the inoculation with 3 AMF consortia on the growth, macronutrient (N, P, K, Ca, and Mg), and micronutrient (Fe, Mn, Zn, Cu, and B) content of the Neotropical pine species Pinus greggii. Mycorrhizal colonization was also evaluated 2 and 7 months after inoculation.
Materials and methods
Rhizospheric soil was collected from 3 sites located in the community of San Pablo Ixayoc, Texcoco, Estado de México, over an elevational gradient on the western slope of the Tláloc Mountains: at 2,650 m in an agricultural area where maize is cultivated; at 2,700 m in Cupressus lusitanica forest; and at 3,600 m in Pinus hartwegii forest. The rhizospheric soil from each site was used as inoculum to propagate the AMF from each ecosystem. Sterile river sand, 500 g of rhizospheric soil, and corn and common grass (Brachiaria decumbens) seeds were added to 2 kg-capacity pots. These systems were set up to have a more representative AMF diversity than originally present in each soil ecosystem, since frequently a number of AMF prevail in natural ecosystems as intraradical or extraradical mycelium rather than as spores. Five pots were set-up for each of the 3 rhizospheric soils over a 3 month period after which the AMF species present as spores were identified.
The identification of the AMF morphospecies was conducted taking into account the characteristics of the spores such as color, size, number, and ornamentation of the wall layers and shape and coupling of the supporting hypha following the methods and data base INVAM (2020). Photographs were taken under a light microscope (Olympus, Model BX51). Each of the 3 inocula was labelled as the agricultural inoculum consortium (AIC), the cedar inoculum consortium (CIC), and the pine inoculum consortium (PIC). The reason to choose 3 contrasting AMF consortia was to evaluate if AMF communities coming from contrasting ecosystems would produce differential plant growth and macro and micronutrient effects.
We used Pinus greggii as a model pine species due to its rapid growth and therefore methodological feasibility; seeds were obtained from a plantation in central Mexico (Toluca, Estado de México). Prior to sowing, the seeds of P. greggii were soaked in distilled water for 24 h to eliminate germination inhibiting compounds. The water was changed every 7 h to allow for the oxygenation of the embryos. The seeds were sterilized with 30% H2O2 for 20 min and rinsed with sterile distilled water under aseptic conditions. Once disinfected, the seeds were washed again for 15 min with sterile distilled water. Seeds were planted in a plastic container at 0.5 cm depth. Once germinated, before the emergence of true leaves, when they only had cotyledons, seedlings were transplanted into 140 cm3 plastic tubes containing the substrate consisting of a mixture of river sand, crushed pine bark, and forest soil at a 2:2:1 ratio. This substrate was sterilized with steam for 9 h at 125 °C, then after 24 h the process was repeated. Before transplanting seedlings, the tubes were filled at their base with a layer of sterilized “tezontle” (porous volcanic rock) to allow the flow of water during the experiment, and the rest was filled with sterilized substrate. The AMF inoculum consisted of 5 g of soil and roots per plant, of each consortium propagated as described above, according to treatments. This inoculum was added at transplantation time in order to place it in contact with pine roots. To avoid contamination from other AMF or ectomycorrhizal propagules, and “cross contamination” among treatments, experimental devices previously described by Carrasco-Hernández et al. (2011) were used. During the first 2 months after germination, a Captan solution was applied at a dose of 2 gL-1 of water every third day, for 20 days, followed by 1 application per week until the lignification of the stem occurred to avoid “damping off”, a disease commonly caused by Phytophthora sp., Pythium sp., and Fusarium circinatum (García-Díaz et al., 2017). The plants remained under greenhouse conditions for 210 days before harvesting. The height, shoot and root dry weight, and mycorrhizal colonization were evaluated. A nutrient analysis of both roots and shoots was performed for N, P, K, Ca, Mg, Fe, Mn, Zn, and B.
Nutrient analyses were performed on the 10 plants used for the dry weight evaluation. The N was determined by the semimicro-Kjeldahl method (Bremner, 1975). Total P was determined according to Allen et al. (1997); K was extracted with ammonium acetate and measured by flame photometry. Ca, Mg, Fe, Cu, Mn, Zn, and B, were determined using atomic absorption spectrophotometry (Varian, Spectra-AA220).
Two harvests were carried out, 2 and 7 months after sowing. At harvest, the height of the plants was measured from the neck of the roots to the upper region of the apical bud. Each plant was extracted from the containers, and the root system was cut from the stem to the neck of the root. Subsequently, they were washed under running water to extract the largest amount of the root system. Sieves (0.180 and 0.085 mm) were used to reduce the loss of short roots. Next, to determine their dry weight, both the stems and the root system were dried at 80 ºC for 48 h to a constant weight. This process was performed in 10 plants per treatment.
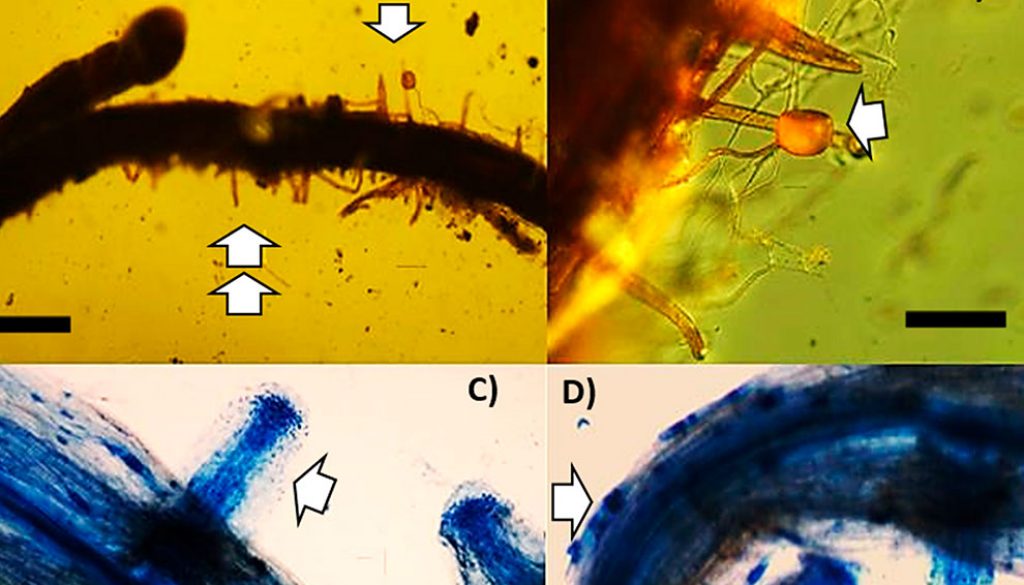
Firstly, to avoid any influence of possible ectomycorrhizal colonization in the results, evaluation of possible ectomycorrhizal colonization was conducted with stereomicroscopy; when necessary, root cross-sections were made by hand to look for Hartig net, mantle and external mycelium under optical light field microscopy. Since no ectomycorrhizal colonization was detected in any of the treatments, in both harvest times, the evaluation of arbuscular mycorrhizal colonization was carried out. An adaptation of the clearing and staining method proposed by Phillips and Hayman (1970) was used. The roots of 5 P. greggii seedlings per treatment were placed in sterilizable plastic capsules in a beaker containing 10% KOH and incubated overnight. The following day, the samples were decanted and rinsed with running water. This process was repeated for 5 consecutive days. Next, H2O2 was applied for 1 h, decanted, and then rinsed with running water. Subsequently, 10% HCL was added for 1 h and decanted, and then, 0.05% trypan blue dye was applied in lactoglycerol for 24 h. The roots were cut into 1 cm fragments that were mounted on slides. Microscopic analysis was performed using light field optical microscopy to quantify the following AMF structures: hyphae, vesicles, and arbuscules, using the magnified intersections method (McGonigle et al., 1990).
The experimental design used 4 completely randomized treatments, including an uninoculated control and 3 treatments of plants inoculated with consortia of AMF isolated from agricultural soil, soil from a Cupressus lusitanica forest, and soil from a Pinus hartwegii forest, as described above. Fifteen replicates were planted for each treatment and harvest time; treatments were harvested 2 and 7 months after sowing, respectively; thus, the experiment had 4 treatments × 2 harvest times × 15 replicates = 120 experimental units, each consisting of a seedling.
For the variables height, shoot and root dry weight, and nutritional content, variance analysis and comparison of means were performed using Tukey’s test (p ≤ 0.05) with the program Statistical Analysis System (SAS, 2002). The mycorrhizal colonization data were arcsine transformed to (x/100)1/2 to meet the criteria of normality, following Moreira-Souza et al. (2003).
Results
From the 3 AMF consortia used, 13 total species were identified. Acaulospora and Scutellospora were the predominant genera. In the AIC, CIC, and PIC we found 5, 6, and 5 AMF species, respectively (Fig. 1). Funneliformis mosseae and Scutellospora cerradensis were found in both the agricultural area and in the Cupressus lusitanica forest. Archaespora sp. was found in both the Cupressus lusitanica and Pinus hartwegii forests (Table 1).
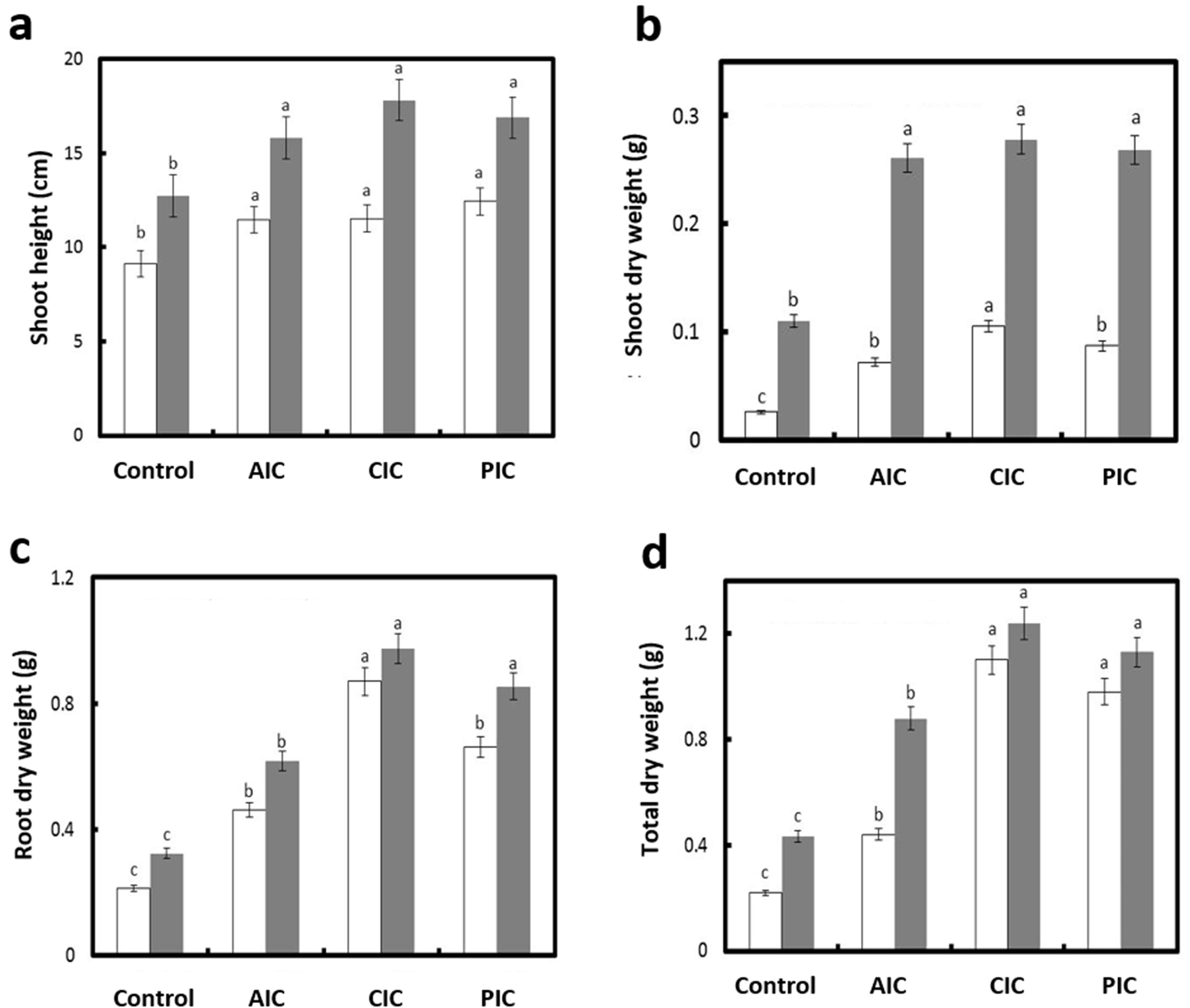
In general, P. greggii plants inoculated with the 3 AMF consortia showed increases in terms of growth and nutritional content compared with non-inoculated plants. We observed an increase in height in inoculated plants compared to non-mycorrhized plants, which was independent of the evaluation time and the type of mycorrhizal consortium (Fig. 2a). We observed an increase (F = 107520; p ˂ 0.0001) in the dry weight of the shoots, especially 7 months after sowing, in inoculated plants compared to non-inoculated plants, regardless of the consortium (Fig. 2b).
We observed an increase in the biomass of root dry weight 7 months after sowing in plants inoculated with AMF from the Cupressus lusitanica and Pinus hartwegii forests compared to those from the agricultural soil (Fig. 2c). In the 3 cases, the root dry weight values were higher (F = 3932.01; p ˂ 0.0001) than those registered in non-inoculated plants (Fig. 2c). A similar trend was observed (F = 6010.41; p ˂ 0.0001) for total dry weight (Fig. 2d).
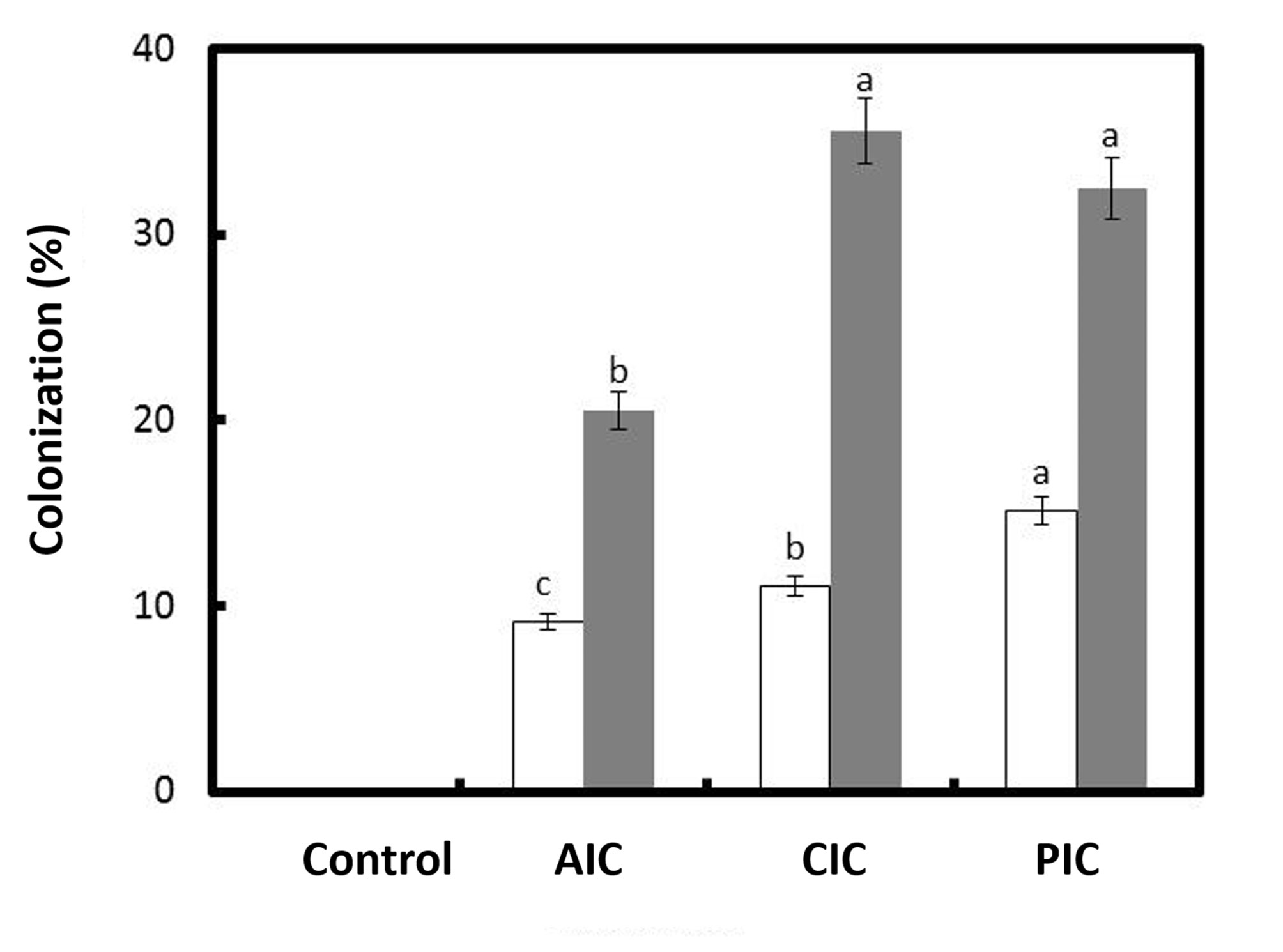
Differences were observed in terms of mycorrhizal colonization 2 months (F = 45518.6; p ˂ 0.0001) and 7 months (F = 17113.1; p ˂ 0.0001) after sowing. Colonization values were 2.2 to 3.2 times higher 7 months after sowing, depending on the AMF consortium. The inoculated plants had mycorrhizal colonization values ranging from 20.5% to 35.5%, depending on the consortium (Fig. 3). The presence of hyphae, vesicles, and arbuscules was observed, as well as the germination of AMF spores. In non-inoculated plants, no mycorrhizal colonization was observed (Fig. 4).
A higher content of the macronutrients N (F = 4321.32; p ˂ 0.0001), P (F = 1608.47; p ˂ 0.0001), K (F = 87999.4; p ˂ 0.0001), Ca (F = 1763.90; p ˂ 0.0001), and Mg (F = 27362.0; p ˂ 0.0001) was observed in the shoots when comparing inoculated to non-inoculated plants, regardless of the source of inoculum. A similar trend was recorded for these nutrients in the roots: N (F = 3721.45; p ˂ 0.0001), P (F = 4095.15; p ˂ 0.0001), K (F = 21485.2; p ˂ 0.0001), Ca (F = 447629; p ˂ 0.0001), and Mg (F = 48417.6; p ˂ 0.0001 (Table 2). The same tendency was observed for the shoot contents of the micronutrients Fe (F = 1898.65; p ˂ 0.0001), Mn (F = 114297; p ˂ 0.0001), Zn (F = 230.18; p ˂ 0.0001), Cu (F = 2585.22; p ˂ 0.0001), and B (F = 213141; p ˂ 0.0001) and for the root contents of the same micronutrients: Fe (F = 3587035; p ˂ 0.0001), Mn (F = 4568.71; p ˂ 0.0001), Zn (F = 117666; p ˂ 0.0001), Cu (F = 30964.3; p ˂ 0.0001), and B (F = 3299607; p ˂ 0.0001). These trends were particularly evident for N, Fe, Zn, and Cu inoculated with AMF from the Pinus hartwegii forests (Table 3). Macro and micronutrients shoot:root ratios of inoculated plants versus non-inoculated plants allowed us to interpret the efficiency of nutrient transport resulting from the AMF. Based on these ratios, we observed the greatest transport for the micronutrients Zn and B in plants inoculated with AMF from Pinus hartwegii forests (Table 4); for K, Ca, and Mg in plants inoculated with agricultural soil; and for Fe, Mn, Cu, and B in plants inoculated with AMF from Cupressus lusitanica forests.
Discussion
Mycorrhizal fungi contribute to the growth and development of vascular plants. Multiple studies have shown that mycorrhizae also protect plants against soil pathogens. The increase in plant growth in terms of height and biomass after inoculation with AMF has been widely documented in a number of angiosperm families, but not in Pinaceae. In the present work, Pinus greggii plants inoculated with AMF had increased height and greater shoot and root biomass compared to the non-inoculated plants independently of the source of inoculum. Interestingly, the AMF consortia that originated from both forests produced a greater root and total dry weight compared with the consortium that originated from agricultural land. This can be explained in terms of the differential inter- and intraspecific beneficial effect of AMF on their associated host plants, which has been widely documented (Smith & Read, 2008; Varma et al., 2017).

AMF are characterized by intra- and intercellular growth in the root cortex and by the formation of hyphae and external hyphae. In the present work, 7-months after sowing arbuscular colonization ranged from 20.5% to 35.5% in the Pinus greggii plants inoculated with the AMF consortia. Additionally we found presence of hyphae, vesicles, and arbuscules. In general, fossil evidence of mycorrhizal colonization is scarce; however, some records have provided valuable phylogenetic clues. In gymnosperms, multiple AMF developing in the rooting system of Notophytum krauselii, an extinct conifer, were found in Antarctica (Bomfleur et al., 2013). This evidence shows that the establishment of associations of AMF with gymnosperms dates back to at least to the early Mesozoic, the period during which most of the modern conifer families first appeared. Interestingly, the roots of the 3 extant gymnosperm families, Araucariaceae, Podocarpaceae, and Phyllocladaceae, possess similar small nodular roots containing Glomeromycota that enter from the soil through epidermal cells (Padamsee et al., 2016; Russell et al., 2002). Thus, arbuscular mycorrhizas have been documented in fossil Mesozoic cycads and conifers, showing that they were present in the diverse ancient gymnosperm trees and shrubs of the mid-northern latitude and high southern latitude polar forests that developed in the ‘greenhouse’ climates of the Mesozoic and Cenozoic eras (Strullu- Derrien et al., 2018). However, arbuscular mycorrhizas have not been extensively studied in modern gymnosperms. New data on arbuscular mycorrhiza diversity in living species is therefore crucial in order to understand the phylogeny of arbuscular mycorrhiza. Currently, arbuscular mycorrhiza colonization has already been studied in gymnosperms from the structural and phylogenetic perspectives (e.g., Cázares & Trappe, 1993; Padamsee et al., 2016; Russell et al., 2002; Wagg et al., 2008, 2011). However, studies related to the functional role of arbuscular mycorrhizal symbiosis in this important plant group, for example in terms of nutrient transfer, is still in its infancy.
Table 1
Arbuscular mycorrhizal fungi (AMF) species identified in the 3 evaluated inoculum consortia.
|
AMF species |
Agricultural area |
Cupressus lusitanica forest |
Pinus hartwegii forest |
|
Acaulospora excavata |
nf |
+ |
nf |
|
Acaulospora laevis |
nf |
nf |
+ |
|
Acaulospora mellea |
nf |
+ |
nf |
|
Acaulospora sp. |
nf |
nf |
+ |
|
Archaeospora sp. |
nf |
+ |
+ |
|
Claroideoglomus etunicatum |
nf |
+ |
nf |
|
Glomus sp. |
nf |
nf |
+ |
|
Gigaspora sp. |
+ |
nf |
nf |
|
Funneliformis mosseae |
+ |
+ |
nf |
|
Paraglomus sp. |
nf |
nf |
+ |
|
Scutellospora cerradensis |
+ |
+ |
nf |
|
Scutellospora pellucida |
+ |
nf |
nf |
|
Septoglomus constrictum |
+ |
nf |
nf |
nf = not found; + = recorded
Table 2
Pinus greggii shoot and root macronutrient content in plants inoculated with 3 consortia of arbuscular mycorrhizal fungi, 210 days after sowing.
|
Treatment |
N |
P |
K |
Ca |
Mg |
|
(mg per plant) |
|||||
|
Shoot |
|||||
|
Non-inoculated plants |
5.09 ± 0.23c |
0.19 ± 0.30b |
1.41 ± 0.04c |
1.22 ± 0.08c |
1.35 ± 0.05c |
|
Pi with AIC |
21.38 ± 0.65b |
0.79 ± 0.80a |
2.39 ± 0.21b |
5.98 ± 0.73b |
4.63 ± 0.08b |
|
Pi with PIC |
32.55 ± 0.36a |
0.89 ± 0.21a |
2.87 ± 0.28b |
6.10 ± 0.69a |
4.20 ± 0.07a |
|
Pi with CIC |
22.13 ± 0.71b |
0.99 ± 0.19a |
3.15 ± 0.36a |
5.75 ± 0.09b |
4.81 ± 0.06b |
|
Root |
|||||
|
Non-inoculated plants |
6.74 ± 0.21c |
0.23 ± 0.02c |
1.11 ± 0.03c |
1.80 ± 0.06b |
2.15 ± 0.04b |
|
Pi with AIC |
18.50 ± 0.58b |
0.67 ± 0.09b |
1.78 ± 0.08b |
5.24 ± 0.72a |
3.44 ± 0.05a |
|
Pi with PIC |
23.79 ± 0.61a |
0.64 ± 0.05b |
2.19 ± 0.19a |
5.64 ± 0.21a |
3.90 ± 0.03a |
|
Pi with CIC |
19.75 ± 0.51b |
0.76 ± 0.08a |
2.63 ± 0.24a |
5.32 ± 0.82a |
3.45 ± 0.02a |
|
Total |
|||||
|
Non-inoculated plants |
11.83 ± 0.91c |
0.42 ± 0.12b |
2.52 ± 0.07c |
3.02 ± 0.06b |
3.5 ± 0.21b |
|
Pi with AIC |
39.88 ± 0.99b |
1.46 ± 0.06a |
4.17 ± 0.12b |
11.22 ± 0.82a |
8.07 ± 0.63a |
|
Pi with PIC |
56.34 ± 1.03a |
1.53 ± 0.04a |
5.06 ± 0.08a |
11.74 ± 0.79a |
8.1 ± 0.56a |
|
Pi with CIC |
41.88 ± 0.97b |
1.75 ± 0.21a |
5.78 ± 0.09a |
11.07 ± 0.81a |
8.26 ± 0.81a |
Pi = Plants inoculated, AIC = agricultural inoculum consortium, PIC = Pinus hartwegii forest inoculum consortium, CIC = Cupressus lusitanica forest inoculum consortium. Values are averages ± standard error of the mean, n = 10. Data in the same column, for each plant compartment, with the same letter are not different according to Tukey test (p ≤ 0.0001).
Table 3
Pinus greggii shoot and root micronutrient content in plants inoculated with 3 consortia of arbuscular mycorrhizal fungi, 210 days after sowing.
|
Treatment |
Fe |
Mn |
Zn |
Cu |
B |
|
(mg per plant) |
|||||
|
Shoot |
|||||
|
Non-inoculated plants |
0.12 ± 0.01d |
0.36 ± 0.21b |
0.28 ± 0.03c |
0.30 ± 0.02c |
0.42 ± 0.02b |
|
Pi with AIC |
0.28 ± 0.02c |
0.71 ± 0.38a |
0.54 ± 0.02b |
0.69 ± 0.50b |
0.72 ± 0.51a |
|
Pi with PIC |
0.49 ± 0.02a |
0.94 ± 0.45a |
0.85 ± 0.03a |
0.84 ± 0.71a |
0.90 ± 0.82a |
|
Pi with CIC |
0.37 ± 0.02b |
0.87 ± 0.39a |
0.53 ± 0.03b |
0.79 ± 0.52b |
0.83 ± 0.52a |
|
Root |
|||||
|
Non-inoculated plants |
0.18 ± 0.02c |
0.24 ± 0.02b |
0.36 ± 0.02c |
0.45 ± 0.03c |
0.55 ± 0.03b |
|
Pi with AIC |
0.36 ± 0.03b |
0.55 ± 0.03a |
0.47 ± 0.02b |
0.64 ± 0.22b |
0.75 ± 0.22a |
|
Pi with PIC |
0.69 ± 0.3a |
0.68 ± 0.023a |
0.73 ± 0.03a |
0.81 ± 0.71a |
0.84 ± 0.31a |
|
Pi with CIC |
0.43 ± 0.02b |
0.55 ± 0.020a |
0.59 ± 0.05b |
0.73 ± 0.59b |
0.77 ± 0.26a |
|
Total |
|||||
|
Non-inoculated plants |
0.30 ± 0.01c |
0.6 ± 0.02c |
0.64 ± 0.05c |
0.75 ± 0.03c |
0.97 ± 0.71b |
|
Pi with AIC |
0.64 ± 0.3b |
1.26 ± 0.81b |
1.01 ± 0.32b |
1.33 ± 0.82b |
1.47 ± 0.82a |
|
Pi with PIC |
1.18 ± 0.9a |
1.62 ± 0.92a |
1.58 ± 0.71a |
1.65 ± 0.91a |
1.74 ± 0.77a |
|
Pi with CIC |
0.80 ± 0.05b |
1.42 ± 0.80a |
1.12 ± 0.36b |
1.52 .± 0.69a |
1.60 ± 0.58a |
Pi = Plants inoculated, AIC = agricultural inoculum consortium, PIC = Pinus hartwegii forest inoculum consortium, CIC = Cupressus lusitanica forest inoculum consortium. Values are averages ± standard error of the mean, n=10. Data in the same column, for each plant compartment, with the same letter are not different according to Tukey test (p ≤ 0.0001).
Table 4
Pinus greggii macro and micronutrients shoot:root ratios in plants 210 days after sowing inoculated with 3 consortia of mycorrhizal fungi.
|
Treatment |
N |
P |
K |
Ca |
Mg |
Fe |
Mn |
Zn |
Cu |
B |
|
Pi with AIC |
||||||||||
|
Pi with PIC |
1.36 |
1.39 |
1.31 |
1.08 |
1.07 |
0.71 |
1.38 |
1.16 |
1.03 |
1.07 |
|
Pi with CIC |
1.12 |
1.30 |
1.19 |
1.08 |
1.21 |
0.86 |
1.58 |
1.11 |
1.08 |
1.07 |
|
Nip |
0.75 |
0.82 |
0.82 |
0.67 |
0.62 |
0.66 |
0.15 |
0.77 |
0.66 |
0.76 |
|
Pi with AIC: Nip |
1.53 |
1.43 |
1.63 |
1.70 |
2.16 |
1.16 |
8.6 |
1.48 |
1.62 |
1.36 |
|
Pi with PIC: Nip |
1.81 |
1.69 |
1.59 |
1.61 |
1.72 |
1.07 |
9.2 |
1.50 |
1.56 |
1.40 |
|
Pi with CIC: Nip |
1.49 |
1.58 |
1.45 |
1.61 |
1.95 |
1.30 |
10.5 |
1.44 |
1.63 |
1.40 |
Pi = Plants inoculated, AIC = agricultural inoculum consortium, PIC = Pinus hartwegii forest inoculum consortium, CIC = Cupressus lusitanica forest inoculum consortium, Nip = non-inoculated plants. Values are average shoot:root ratios for each nutrient in the mentioned treatments, n = 10.
In the present work, the mobilization and transfer of a wide range of macro- and micronutrients via AMF in Pinaceae is reported for the first time. Inoculation with all 3 AMF consortia in the Neotropical pine P. greggii showed a beneficial effect in terms of growth, colonization, and content of macro- and micronutrients in comparison with non-inoculated plants. Although nutrient mobilization and transfer by AMF has been extensively demonstrated in angiosperms, as far as we know, reports exploring the transfer in a wide range of nutrient in gymnosperms is lacking. This work reports the mobilization and transfer of an extensive variety of macronutrients and micronutrients within the shoots and roots in a member of Pinaceae for the first time, including N, K, Ca, Mg, Fe, Mn, Zn, Cu, and B. Previously, only Dučić et al. (2009) have suggested a positive influence of arbuscular mycorrhiza on P uptake in Pseudotsuga menziesii; and Smith et al. (1998) demonstrated that the P content in the needles of colonized P. menziesii seedlings grown with a VAM host was about twice as high as in non-colonized seedlings grown along with the same VAM host. In contrast, these authors found that tissue N did not differ between the same treatments.
In the present work, Mg, Mn, and Zn were mobilized in the shoots of plants inoculated with AMF. Mn contributes to the functioning of various biological processes, including photosynthesis, via the synthesis of chlorophyll, respiration, and the assimilation of nitrogen. Mn also participates in the formation of chloroplasts, activates the growth of plants, promotes cellular lengthening in the roots, and confers resistance to pathogens (Alejandro et al., 2020; Lambers et al., 2015).
The transfer of Mn in angiosperms inoculated with mycorrhizal fungi has been reported. For example, Bethlenfalvay and Franson (1989) recorded high concentrations of Mn in the shoots of barley plants (Hordeum vulgare). The same authors reported increases in Mn and increased growth in wheat plants (Triticum durum) inoculated with the AMF Glomus monosporum. On the other hand, Arines et al. (1989) found that in red clover (Trifolium pratense), the total Mn transfer increased in plants inoculated with the mycorrhizal fungi Gigaspora aurigloba and Glomus tenue compared with non-inoculated plants. In the present work, the difference in the Mn content between inoculated and non-inoculated trees was greater in the roots than in the shoots, possibly because the mycorrhizae altered the spatial distribution of this nutrient.
The lower absorption of Mn in the roots than in the shoots observed in mycorrhized plants can be explained by the existence of a fungal mechanism that controls the absorption of Mn or by the effect of the fungi on the rhizosphere and surrounding soil. Previously, a decrease in Mn toxicity in the presence of AMF in soybeans has been documented (Garcia et al., 2020; Nogueira & Cardoso, 2003). Angiosperms colonized by AMF are often more resistant to excess Mn than plants not colonized by this fungus. Mg is an essential nutrient for plants and is critical for a wide range of functions. Mg is involved in photosynthesis and is a basic component of chlorophyll. Xiao et al. (2014) observed greater biomass in the roots and shoots in orange plants (Poncirus trifoliata) and concluded that inoculation with the mycorrhizal fungus Funneliformis mosseae had positive effects on the growth and physiology under Mg-deficient conditions. Hassan et al. (2017) observed that plants inoculated with mycorrhizae had significantly higher content of dry and fresh root weight and chlorophyll content than plants without mycorrhizae.
Mycorrhizal colonization increased Mg uptake but decreased K uptake. Xiao et al. (2014) suggested that the mycorrhizal fungus Glomus versiforme can improve the growth and distribution of Mg in orange seedlings grown in soil low in Mg. These authors reported that the concentrations of Mg in the shoots and roots, biomass yield, and chlorophyll content increased with the inoculation of 3 species of mycorrhizal fungi, especially Glomus versiforme.
In the present work, significant nutritional transport of 10 macro- and micronutrients was observed, primarily Mg and Mn in the shoots of plants inoculated with the AMF consortium from the Pinus hartwegii forest. This work demonstrates for the first time the functional importance of AMF in terms of growth and nutrient mobilization and transfer in a member of Pinaceae. AMF allowed for the mobilization and transfer of 10 nutrients, primarily Mg, Mn, and Zn, to the roots and shoots of the gymnosperm Pinus greggii. Plants of P. greggii inoculated with AMF produced more biomass than non-inoculated plants. The total colonization of P. greggii varied depending on the source of inoculum 7 months after inoculation. Greater colonization was observed in Pinus plants inoculated with the mycorrhizal consortia from Cupressus forests. Additionally, this is the first study to present pictures of the arbuscules formed by arbuscular mycorrhizal fungi in Pinaceae.

The presence of arbuscules, which we have documented photographically, shows that P. greggii establishes a functional mutualist symbiosis with the AMF, as the exchange of nutrients occurs in this structure. These results indicate that Pinus greggii improves its nutritional status in the early stages of its development by associating with AMF; thus, inoculation with these fungi should be considered if reforestation activities of pine forests are desired.
Acknowledgments
The first author thanks Conacyt for the financial support of her Doctorate in Sciences. Jesús Pérez-Moreno acknowledges the financial support from Conacyt 2018-07-01EXTV and COMECyT to develop a sabbatical stay in the Kunming Institute of Botany, Kunming, China. We deeply appreciate the valuable suggestions and comments from two anonymous reviewers and the editor, which substantially improved the manuscript quality.
References
Aalipour, H., Nikbakht, A., Etemadi, N., Rejali, F., & Soleimani, M. (2020). Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Scientia Horticulturae, 261, 108923. https://doi.org/10.1016/j.scienta.2019.108923
Alejandro, S., Höller, S., Meier, B., & Peiter, E. (2020). Manganese in plants: from acquisition to subcellular allocation. Frontiers in Plant Science, 11, 300. https://doi.org/10.3389/fpls.2020.00300
Allen, S. E., Grimshaw, H. M., Parkinson, J. A., & Quarmbym, C. (1997). Chemical analysis of ecological materials. Oxford, UK: Blackwell Scientific Publications.
Arines, J., Vilariño, A., & Sainz, M. (1989). Effect of different inocula of vesicular-arbuscular mycorrhizal fungi on the content and concentration of manganese in red clover plants (Trifolium pratense L.). New Phytologist, 112, 215-219. https://doi.org/10.1111/j.1469-8137.1989.tb02376.x
Bethlenfalvay, G. J., & Franson, R. L. (1989). Manganese toxicity alleviated by mycorrhizae in soybean. Journal of Plant Nutrition, 12, 953–970. https://doi.org/10.1080/
01904168909364006
Bomfleur, B., Decombeix, A. L., Escapa, I. H., Schwendemann, A. B., & Axsmith, B. (2013). Whole-plant concept and environment reconstruction of a Telemachus conifer (Voltziales) from the Triassic of Antarctica. International Journal of Plant Sciences, 174, 425–444. http://dx.doi.org/
10.1086/668686.
Bonfante, P., & Genre, A. (2008). Plants and arbuscular mycorrhizal fungi: an evolutionary developmental perspective. Trends in Plant Science, 13, 492–498. http://dx.
doi.org/10.1016/j.tplants.2008.07.001
Bremner, J. M. (1975). Total Nitrogen. In C. A. Black (Ed.), Methods of soil analysis. Agronomy Part 2 (pp. 1149-1178). Madison, Wisconsin: American Society of Agronomy.
Bush, J. K. (2008). The potential role of mycorrhizae in the growth and establishment of Juniperus seedlings. In Van Auken, O. W. (Ed.) Western North American Juniperus communities. Ecological Studies, Vol. 196. New York: Springer. https://doi.org/10.1007/978-0-387-34003-6_6
Carrasco-Hernández, V., Pérez-Moreno, J., Espinosa-Hernández, V., Almaraz-Suárez, J., Quintero-Lizaola, R., & Torres-Aquino, M. (2011). Contenido de nutrientes e inoculación con hongos ectomicorrízicos comestibles en dos pinos neotropicales. Revista Chilena de Historia Natural, 84, 83–96.
http://dx.doi.org/10.4067/S0716-078X2011000100006
Cázares, E., & Smith, J. E. (1995). Occurrence of vesicular-arbuscular mycorrhizae in Pseudotsuga menziesii and Tsuga heterophylla seedlings grown in Oregon Coast Range soils. Mycorrhiza, 6, 65–67. https://doi.org/10.1007/s005720050108
Cázares, E., & Trappe, J. M. (1993). Vesicular endophytes in roots of the Pinaceae. Mycorrhiza, 2, 153–156. https://doi.org/10.1007/BF00210584
Dučić, T., Berthold, D., Langenfeld-Heyser, R., Beese, F., & Polle, A. (2009). Mycorrhizal communities in relation to biomass production and nutrient use efficiency in two varieties of Douglas fir (Pseudotsuga menziesii var. menziesii and var. glauca) in different forest soils. Soil Biology and Biochemistry, 41, 742–753. https://doi.org/10.1016/j.soilbio.2009.01.013
Fisher, J. B., & Vovides, A. P. (2004). Mycorrhizae are present in cycad roots. The Botanical Review, 70, 16–23. https://doi.org/10.1663/0006-8101(2004)070
Garcia, K. G. V., Mendes-Filho, P. F., Pinheiro, J. I., Carmo do, J. F., Pereira, A. P. A., Martins, C. M. et al. (2020). Attenuation of Manganese-induced toxicity in Leucaena leucocephala colonized by arbuscular mycorrhizae. Water Air Soil Pollution, 231, 22. https://doi.org/10.1007/s11270-019-4381-9
García-Díaz, S., E., Arnulfo, A., Alvarado-Rosales, D., Cibrián-Tovar, D., Méndez-Montiel, J. T., Valdovinos-Ponce, G. et al. (2017). Efecto de Fusarium circinatum en la germinación y crecimiento de plántulas de Pinus greggii en tres sustratos. Agrociencia, 51, 895–908. https://www.redalyc.org/articulo.oa?id=30253817006
Harper, C. J., Taylor, T. N., Krings, M., & Taylor, E. L. (2015). Arbuscular mycorrhizal fungi in a voltzialean conifer from the Triassic of Antarctic. Review of Palaeobotany and Palynology, 215, 76–84. https://doi.org/10.1016/J.REVPALBO.2015.01.005
Hassan, Z. M., Narges, K. A., & Faezeh, G. (2017). Influence of mycorrhizal fungi on growth, chlorophyll content, and potassium and magnesium uptake in maize. Journal of Plant Nutrition, 40, 2026–2032. https://doi.org/10.1080/01904167.
2017.1346119
INVAM. (2020). International culture collection of vesicular-arbuscular mycorrhizal fungi. West Virginia University. Retrieved on June 2, 2020, from: http://invam.caf.wvu.edu/
Jansa, J., Wiemken, A., & Frossard, E. (2006). The effects of agricultural practices on arbuscular mycorrhizal fungi. In E. Frossard, W.E.H. Blum, B.P. Warkentin (Eds.), Function of soils for human societies and the environment (pp. 89–115). London: Geological Society of London. https://doi.org/10.1144/GSL.SP.2006.266.01.08
Lambers, H., Hayes, P. E., Laliberté, E., Oliveira, R. S., & Turner, B. L. (2015). Leaf Manganese accumulation and Phosphorus-acquisition efficiency. Trends in Plant Science, 20, 83–90. https://doi.org/10.1016/j.tplants.2014.10.007
López-García, Á., Hempel, S., de D. Miranda, J., Rillig, M. C., Barea J. M., & Azcón-Aguilar, C. (2013). The influence of environmental degradation processes on the arbuscular mycorrhizal fungal community associated with yew (Taxus baccata L.), an endangered tree species from Mediterranean ecosystems of Southeast Spain. Plant Soil, 370, 355–366. https://doi.org/10.1007/s11104-013-1625-0
McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L., & Swan, J. A. (1990). A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytologist, 115, 495–501. http://doi.wiley.com/10.1111/j.1469-8137.1990.tb00476.x
Moreira-Souza, M., Trufem, S. F. B., Gomes-da Costa, S. M., & Cardoso, E. J. B. N. (2003). Arbuscular mycorrhizal fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza, 3, 211–215. https://doi.org/10.1007/s00572-
003-0221-1
Muthukumar, T., & Udaiyan, K. (2002). Arbuscular mycorrhizas in cycads of southern India. Mycorrhiza, 12, 213–217. https://doi.org/10.1007/s00572-002-0179-4
Nogueira, M. A., & Cardoso, E. J. B. N. (2003). Eficacia micorrízica toxicidad del manganeso en la soja afectada por el tipo de suelo y el endófito. Scientia Agricola, 60, 329–335. https://doi.org/10.1590/S0103-90162003000200018
Padamsee, M., Johansen, R. B., Stuckey, S. A., Williams, S. E., Hooker, J. E., Burns, B. R. et al. (2016). The arbuscular mycorrhizal fungi colonising roots and root nodules of New Zealand kauri Agathis australis. Fungal Biology, 120, 807–817. https://doi.org/10.1016/j.funbio.2016.01.015
Phillips, J. M., & Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions British Mycological Society, 55, 158–161. https://doi.org/10.1016/S0007-1536%2870%2980110-3
Russell, A. J., Bidartondo, M. I., & Butterfield, B. G. (2002). The root nodules of the Podocarpaceae harbour arbuscular mycorrhizal fungi. New Phytologist, 156, 283–295. https://doi.org/10.1046/j.1469-8137.2002.00504.x
Salgado, M. E., Barroetaveña, C., & Rajchenberg, M. (2013). Pseudotsuga menziesii invasion in native forests of Patagonia, Argentina: What about mycorrhizas? Acta Oecologica, 49, 5–11. https://doi.org/10.1016/j.actao.2013.01.018
SAS. (2002). The SAS system for windows, ver. 9.0. SAS Institute Inc, Cary, North Carolina. USA.
Sharif, M., & Claassen, N. (2011). Action mechanisms of arbuscular mycorrhizal fungi in phosphorus uptake by Capsicum annuum L. Pedosphere, 21, 502–511. https://doi.org/10.1016/S1002-0160(11)60152-5
Siqueira, J. O., Lambais, M. R., & Sturmer, S. L. (2002). Fungos micorrízicos arbusculares: características, associação simbiótica e aplicação na agricultura. Biotecnologia Ciência y Desenvolvimento, 25, 12–21.
Smith, J., Johnson, K. A., & Cázares, E. (1998). Mycorrhizal colonization of seedlings of Pinaceae and Betulaceae following spore inoculation with Glomus intraradices. Mycorrhiza, 7, 279–285. https://doi.org/10.1007/s005720050193
Smith, S. E., & Read, D. J. (2008). Mycorrhizal symbiosis. New York: Academic Press.
Spatafora, J. W., Chang, Y., Benny, G. L., Lazarus, K., Smith, M. E., Berbee, M. L. et al. (2016). A Phylum-level phylogenetic classification of Zygomycete fungi based on genome-scale data. Mycologia, 108, 1028–1046. https://doi.org/10.3852/16-042
Strullu-Derrien, C., Selosse, M. A., Kenrick, P., & Martin, F. M. (2018). The origin and evolution of mycorrhizal symbioses: from palaeomycology to phylogenomics. New Phytologist, 220, 1012–1030. https://doi.org/10.1111/nph.15076
Varma, A., Prasad, R., & Tuteja, N. (Eds.). (2017). Mycorrhiza, function, diversity, state of the art. Cham, Switzerland: Springer.
Wagg, C., Maderia, P., & Peterson, R. (2011). Arbuscular mycorrhizal fungal phylogeny-related interactions with a non-host. Symbiosis, 53, 41–46. https://doi.org/10.1007/s13199-011-0107-5
Wagg, C., Pautler, M., Hugues, B., Massicotte, R., & Peterson, L. (2008). The co-occurrence of ectomycorrhizal, arbuscular mycorrhizal, and dark septate fungi in seedlings of four members of the Pinaceae. Mycorrhiza, 18, 103–110. https://doi.org/10.1007/s00572-007-0157-y
Wang, B., Yeun, L. H., Xue, J. Y., Liu, Y, Ané, J. M., & Qiu, Y. L. (2010). Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytologist, 186, 514–525. https://doi.org/10.1111/j.1469-8137.2009.03137.x
Xiao, J. X., Hu, C. Y., Chen, Y. Y., Yang, B., & Hua, J. (2014). Effects of low magnesium and an arbuscular mycorrhizal fungus on the growth, magnesium distribution and photosynthesis of two citrus cultivars. Scientia Horticulturae, 177, 14–20. https://doi.org/10.1016/j.scienta.2014.07.016