Ariana González, Clementina González *, Rafael Hernández-Guzmán, Eduardo Mendoza
Instituto de Investigaciones sobre los Recursos Naturales, Universidad Michoacana de San Nicolás de Hidalgo, San Juanito Itzícuaro s/n Col. Nueva Esperanza, 58337 Morelia, Michoacán, Mexico
*Corresponding author: clementina.gonzalez@umich.mx (C. González)
Received: 24 February 2021; accepted: 9 November 2021
Abstract
Landscape connectivity between protected natural areas and their surroundings is essential to maintain wildlife movement and to promote gene flow and genetic diversity. The grayish opossum mouse (Tlacuatzin canescens) was used for modeling functional landscape connectivity between the Chamela-Cuixmala Biosphere Reserve, an important biological reserve with large extensions of tropical dry forest in the Mexican Pacific coast, and surrounding vegetation patches. The model was estimated through graph and circuit theory, using a resistance matrix and the calculation of the minimum area of suitable habitat patches. Thirty-eight patches of suitable habitat for T. canescens and 60 potential corridors were identified. Three patches adjacent to the CCBR played the most important role in maintaining the connectivity of the tropical dry forest in the region. In contrast, suitable habitat patches with the lowest connectivity were embedded in a landscape matrix composed of areas for cattle raising and agriculture, increasing the loss and isolation of forest patches. Our results highlight not only the importance of maintaining large patches of suitable habitat, but also smaller patches which might play a significant role as stepping stones, promoting habitat connectivity for T. canescens and similar species.
Keywords: Deciduous tropical forest; Didelphidae; Protected natural areas; Resistance matrices; Small mammals
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Modelando la conectividad funcional del ratón tlacuache (Tlacuatzin canescens) en un bosque tropical caducifolio altamente amenazado de la costa del Pacífico mexicano
Resumen
La conectividad del paisaje entre áreas naturales protegidas y sus alrededores es esencial para mantener el movimiento de la fauna, promover el flujo y la diversidad genética. Usamos al ratón tlacuache (Tlacuatzin canescens) para modelar la conectividad funcional entre la Reserva de la Biosfera Chamela-Cuixmala, que mantiene una importante extensión de bosque tropical caducifolio (BTC) en la costa del Pacífico mexicano y los parches de vegetación circundante. El modelo de conectividad funcional se realizó a través de la teoría de grafos y circuitos, utilizando una matriz de resistencias y el área mínima de parches de hábitat adecuado. Se identificaron 38 parches de hábitat adecuado para T. canescens y 60 corredores potenciales. Tres parches adyacentes a la CCBR jugaron el papel más importante para mantener la conectividad del BTC. En contraste, los parches de hábitat adecuados menos conectados se encuentran inmersos en una matriz compuesta por áreas dedicadas a la ganadería y agricultura, incrementando la pérdida y aislamiento de parches de bosque. Nuestros resultados resaltan no solo la importancia de mantener grandes parches de hábitat adecuado, sino también parches más pequeños que podrían desempeñar un papel importante como peldaños, promoviendo la conectividad del hábitat para T. canescens y especies similares.
Palabras clave: Bosque tropical caducifolio; Didelphidae; Áreas naturales protegidas; Matrices de resistencia; Pequeños mamíferos
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Habitat loss and degradation due to human activities are 2 of the main threats for tropical forests and the main causes of biodiversity loss (Flores-Casas & Ortega-Huerta, 2019; Laurance et al., 2012; Vieira-De Matos et al., 2019; Wilson et al., 2015). It has been estimated that between 1990 and 2015, 10% of the global extent of tropical forests was lost due to deforestation (FAO, 2015; Keenan et al., 2015). This loss of natural habitats affects directly forest-dependent vertebrates by decreasing landscape connectivity, reducing food supply availability and areas for shelter and mating (Merrick & Koprowski, 2017; Morales-Díaz et al., 2019; Theobald et al., 2012). Moreover, habitat loss might increase vertebrate exposure to external threats such as diseases and exotic species (de la Peña et al., 2003; Passamani & Fernández, 2011). The effects of these threats can be exacerbated when an animal’s limited dispersal ability and an unsuitable surrounding landscape matrix combine to increase populations isolation (Brooker & Brooker, 2002; Luck & Daily, 2003; Moore et al., 2008). Therefore, the study of animal’s habitat connectivity stands out as a topic of major relevance to understand the response of wildlife to anthropic perturbations. Habitat connectivity can be measured based on the continuity of suitable landscape features (i.e., structural connectivity) or by incorporating animal’s characteristics such as its ability to disperse through different land use and vegetation covers (Robichaux & Yetman, 2000; Taylor et al., 2006; Tischendorf & Fahrig, 2000; With et al., 1997). Thus, functional connectivity considers the animal’s physical cost of moving through portions of the landscape with different degrees of suitability for the species (Adriaensen et al., 2003; Moilanen & Hanski, 2001; Shah & McRae, 2008).
Tropical dry forests (TDFs) are amongst the most biodiverse and endemism-rich ecosystems, but also amongst the natural habitats most heavily impacted by deforestation and fragmentation (Bullock et al., 1995; Janzen, 1988; Miles et al., 2006). Mexico has the largest extent of tropical dry forest in the Americas, covering an area of 181,461 km2 which accounts for 38% of their total extent in the continent (Portillo-Quintero & Sánchez-Azofeifa, 2010). However, dry forests are being lost at accelerated rates. The area that originally covered dry forests in Mexico has been reduced by ~ 70%, and its annual deforestation rate is 2%, one of the highest among the country’s main ecosystems (García, 2006; Trejo & Dirzo, 2000). It is estimated that only 30% of the TDF in the country maintains a good level of conservation and approximately only a 10% is protected (Trejo, 2010; Trejo & Dirzo, 2002). Whereas protected areas help to reduce deforestation in their interior, they are much more limited in terms of reducing the loss of natural habitats in their vicinity (Bennett, 2004; Bruner et al., 2001; Cuenca & Echeverria, 2017; De Clerck et al., 2010; Garmendia et al., 2013). Thus, original habitats within protected areas face an increasing level of isolation which greatly affect the dispersal of individuals and genes of a great variety of taxa (Calabrese & Fagan, 2004; Ricketts, 2001). Among the consequences of connectivity loss are the reduction of genetic diversity within populations and an increase of genetic differentiation among populations due to reduced gene flow and genetic drift (Hutchinson & Templeton, 1999). These consequences in the TDF are particularly serious due to the high biodiversity it harbors. For example, 183 species of mammals have been recorded in TDFs of Mexico, which corresponds to 35% of all mammal species in the country, from which 23% are endemic (Ceballos & García, 1995; Ceballos & Oliva, 2005).
Most mammal species inhabiting TDFs are herbivorous, have small body sizes, short generational times, and small home ranges and some of them bear some level of physiological adaptation to deal with water shortage during the dry season (Ceballos & Miranda, 2000; Stoner & Timm, 2004). For example, some species, including marsupials, are able to reduce their body temperature through diurnal torpor and to store fat in their tails (Lovegrove et al., 1999). However, in spite of these adaptations, mammals can be sensitive to changes brought about by forest fragmentation showing responses at spatial scales of a few meters (Corry, 2005). Many of these small mammal species are involved in fundamental biotic interactions for forest regeneration such as seed dispersal and pollination (Arreola-Gómez & Mendoza, 2020; Ghazoul, 2005; Howe & Smallwood, 1982; Lobova et al., 2009). Therefore, the lack of connectivity throughout the dry forest can have important repercussions not only in terms of the viability of wild mammal populations but also for the regeneration of the forest itself.
Even though numerous ecological and conservation studies have been conducted in the region of Chamela, Jalisco in the Pacific coast of Mexico, to our knowledge, none have made an assessment of habitat connectivity. This study is aimed at analyzing the level of functional habitat connectivity for the endemic grayish mouse opossum (Tlacuatzin canescens) in a heterogeneous landscape originally covered by TDF. T. canescens is an excellent system to modelling connectivity because is a small species with relatively limited dispersal abilities, highly dependent on trees for its displacement and strongly associated to dry forests. Therefore, deforestation likely represents a main threat for its survival. Specifically, we assessed the level of functional connectivity for T. cansescens between the Chamela-Cuixmala Biosphere Reserve (CCBR) and dry forest remnants in its vicinity using graph and circuit theory; identified dry forest patches having the greatest role in maintaining connectivity with the CCBR; and identified potential corridors that can help to maintain habitat connectivity for T. canescens. Given the ecological characteristics of the study species, particularly its dependence on arboreal vegetation and its limited dispersal abilities, and given the marked loss of TDF that has affected the study region, we hypothesize that functional connectivity for the species in areas where the original habitat has been transformed to more open vegetation covers, should be limited. Moreover, given the heterogeneity in the features of the landscape we expect the level of habitat connectivity loss to vary across the study area.
Materials and methods
Tlacuatzin canescens (synonym Marmosa canescens, J.A. Allen, 1893) is a marsupial species endemic to Mexico that belongs to the family Didelphidae (Ceballos & Arroyo-Cabrales, 2013). Tlacuatzin is the only endemic genus of marsupials recognized in Mexico. The distribution of this species spans along the Mexican Pacific coast, from Sonora to Chiapas, including the Balsas river basin, from sea level up to 2,300 m asl (González-Christen & Rodríguez, 2014; Voss & Jansa, 2009; Zarza et al., 2003). T. canescens has a great ability to move through the forest canopy but has a more limited ability for displacement on the forest floor (Zarza et al., 2003). There is a lack information on the species demography but a recent study, conducted in the dry forest of the state of Colima, Mexico, found that T. canescens accounted for 85.7% of the captures of small mammals, reaching an estimated density of 0.7 – 8.0 individuals/ha (Kennedy et al., 2013). This species is not listed in the Mexican Official Norm 059, which is the national compendium of species of conservation concern, and in the Red List is classified as a species of least concern (Lorenzo & González-Ruiz, 2018; Martin, 2017). However, the strong connection of this species with the dry forest suggests that they are likely under threat due to habitat loss and degradation.
This study was conducted in the Chamela-Cuixmala region, that supports one of the most preserved areas of tropical dry forest in Mexico. In this region 72 mammal species have been recorded, from which 18 are endemic (accounting for 60% of endemic mammal genera in the country), and at least 31% of these species are classified as threatened (Ceballos & García, 1995; Ceballos et al., 2010). The study focused on the Chamela-Cuixmala Biosphere Reserve (CCBR) and a surrounding buffer area of 20 km which totalized 154,836 ha (Supplementary material: Fig. S1). This buffer encompasses at least 10 times the average maximum dispersion distance calculated for similar species (1,904.7 m; Table 1) and duplicates the average buffer suggested to maintain viable populations of a didelphid species similar to T. cansescens (Alexandre et al., 2010). Besides, within this buffer most of the historical records for the species have been registered (data not shown). Moreover, it has been suggested that a 10-20 km buffer around a protected area is large enough to identify substantial variation in vegetation cover, but close enough that changes in land cover heterogeneity in the surrounding area likely influence population and ecosystem processes in the protected area (Hansen & Defries, 2007; Seiferling et al., 2012). The surrounding buffer included some villages (Supplementary material: Fig. S1), and the main land uses and cover types in the zone are tropical dry forest, mangrove, crop lands (i.e. sorghum, corn, citrus, bananas, watermelons, and vegetables) and pasture for intensive cattle raising. Beyond the surrounding buffer TDF is interrupted by substantial changes in natural vegetation and by large agricultural fields and pastures for cattle. It has been estimated that 47,200 ha of original vegetation were lost from 1986 to 2017 in this region (Hernández-Guzmán et al., 2019). Moreover, transition models indicate that the Chamela-Cuixmala Biosphere Reserve (CCBR) is vulnerable to land cover changes occurring in the surrounding environments (Flores-Casas & Ortega-Huerta, 2019).
We developed a resistance model based on the approach used by Beier et al. (2009) and Correa-Ayram et al. (2014). Resistance models involve defining a species’ suitable habitat and assigning values that represent the resistance to species movement through the surrounding landscape matrix (Sawyer et al., 2011). Cells with high resistance values represent areas where individuals are unlikely to move under typical conditions due to high energetic, survival, or other ecological costs involved (Adriaensen et al., 2003). We selected landscape variables that have potentially a strong effect on the movement of T. canescens. Those variables were: land use and land cover, slope (degrees), road type (width), and river drainage order. In order to rank these variables based on their importance to limit T. canescens dispersal, we sent a questionnaire requesting the opinion of mammal´s experts from different Mexican universities and research institutes, however only 4 researches replied to our request (Table 2). These experts were asked to rank each of the variables we included in our analysis in terms of their resistance to displacement from 1 (minimum resistance) to 100 (maximum resistance; Beier et al., 2009; Freeman et al., 2019). We averaged the ranks assigned by the experts to build our resistance model (RM) as follows:
RM = LULC + SLP + ORD + Rt
where LULC = land use and land cover; SLP = terrain slope; ORD = river drainage order; Rt = road type.
The land use and land cover map was produced through the unsupervised classification of Landsat OLI (Operational Land Imager) images from 2018 using the isocluster algorithm. This algorithm makes use of an iterative process where the user sets the number of clusters to be identified. A set of N clusters are then arbitrarily located in the band space and pixels are assigned to their nearest cluster location. Once all the pixels have been assigned, a new mean location is computed. This algorithm makes use of a full Maximum Likelihood procedure providing a very robust cluster assignment (Eastman, 2016). All the pixels were assigned to 30 spectral classes that were in turn reclassified into the following general informational classes: aquatic surfaces, exposed soils, tropical dry forest, rivers, roads, and other types of vegetation (including mangrove and evergreen forest). Human settlements and river classes were digitized on-screen over high-resolution images available from Google earth Pro, while roads were extracted from the Mexican Institute of Transport and added to the final thematic map.
We derived the slope layer (SLP) from a digital elevation model (DEM) downloaded from the National Institute of Statistics and Geography (INEGI; https://www.inegi.org.mx/app/geo2/elevacionesmex/), with a 30 m of spatial resolution. Slope values were reclassified to have the following intervals: 0° – 5°, 5° – 15°, 15° – 30°, and 30° – 65° (Table 2). We used the same DEM to derive the river drainage order layer (ORD). During a preprocessing procedure all the terrain depressions were identified and removed. We applied the D8 (Deterministic 8) algorithm (Jenson & Domingue, 1988) to assess the flow direction and flow accumulation. To define the river drainage order we used a constant threshold value following Strahler (1957). Order 1 corresponded to links without runoff, order 2 resulted from intercepting 2 links of order 1, order 3 resulted from intercepting 2 links of order 2, and so on (Table 2). Road type layers (Rt) were downloaded from the webpage of the Mexican Institute of Transport (IMT; https://www.gob.mx/imt/acciones-y-programas/red-nacional-de-caminos). These layers were originally vectorial but were rasterized to be classified based on their width into the following categories: 3.5 m; 3.5 – 5 m; 5 – 6 m; 6 – 7 m, and 7 – 8 m (Table 2).
Table 1
Home range values used to estimate the maximum dispersion distance (MDD) of T. canescens. We used the home ranges of the species to calculate their maximum dispersal distance. MDD = 40 * linear dimension of home range (Bowman et al., 2002). T. canescens has an estimated body weight of 20 – 60 g (Arreola-Gómez & Mendoza, 2020).
| Gender | Body weight (g) | Home range (m2) | MDD (m) | Reference |
| Marmosa | 60 | 3,800 | 2,465.6 | Melo et al., 2017 |
| Gracilianus | 30 – 45 | 1,800 | 1,696.8 | Olifiers et al., 2004; Pires et al., 2010 |
| Monodelphis | 83 | 1,500 | 1,551.6 | Gordon, 2003; Melo et al., 2017 |
| Average | 2,366.7 | 1,904.7 |
Table 2
Resistance values assigned by experts to feed the resistance model. In columns 1 – 4 individual resistance values are shown and the average in the last column.
| Variables | Resistance values | ||||
| 1 | 2 | 3 | 4 | Average | |
| 1. Land use and cover | |||||
| a) Water | 100 | 100 | 70 | 80 | 87.5 |
| b) Tropical dry forest | 1 | 10 | 1 | 1 | 3.3 |
| c) Exposed soil | 85 | 80 | 50 | 70 | 71.3 |
| d) Other vegetation (mangrove / evergreen forest) | 10 | 10 | 1 | 55 | 19.0 |
| e) Rural zone | 95 | 90 | 80 | 90 | 88.8 |
| 2. Roads (width in meters) | |||||
| a) 7 – 8 | 97 | 80 | 50 | 80 | 76.8 |
| b) 6 – 7 | 96 | 80 | 50 | 80 | 76.5 |
| c) 5 – 6 | 94 | 80 | 45 | 80 | 74.8 |
| d) 3.5 – 5 | 92 | 80 | 30 | 60 | 65.5 |
| e) 3.5 | 90 | 60 | 30 | 20 | 50.0 |
| 3. Slope (degrees) | |||||
| a) 0 – 5 | 1 | 10 | 1 | 10 | 5.5 |
| b) 5 – 15 | 5 | 10 | 1 | 10 | 6.5 |
| c) 15 – 30 | 15 | 10 | 1 | 10 | 9.0 |
| d) 30 – 65 | 25 | 20 | 1 | 20 | 16.5 |
| 4. Rivers (categories) | |||||
| a) Order 1 | 85 | 100 | 70 | – | 85.0 |
| b) Order 2 | 90 | 100 | 80 | – | 90.0 |
| c) Order 3 | 99 | 100 | 90 | – | 96.3 |
| d) Order 4 | 100 | 100 | 90 | – | 96.7 |
All the layers used were standardized to have the same grid cell size and projection (30 m pixel size and UTM13N, respectively), maintaining the same number of columns and rows. The average of individual resistance values assigned by the experts was included in the final cumulative resistance model. All the processes were made using ArcMap 10.3.1 (ESRI).
To define suitable habitat patches for T. canescens we used as a criterion the size of the area needed to support a population of 500 individuals of this species. This size has been used as a rule of thumb to define the conditions needed for a population to increase its probability of long-term viability (Jamieson & Allendorf, 2012). To estimate the extent of habitat needed to support such population size we used the middle point (2.6 ind/ha) of the abundance estimates reported for the species (Ceballos, 1990; Kennedy et al., 2013). Thus, the estimated area of habitat needed was 192.3 ha. We used this value to define the minimum size of suitable habitat patches of dry forest for T. canescens.
We modelled habitat connectivity by using 2 complementary approaches, the circuit theory and the graph theory through the least cost path. We used Circuitscape v4.0 (McRae & Beier, 2007) to estimate connectivity within the study area. For this, 2 input elements were required for the selection of patches to connect and the resistance surface. As a result, we obtained a displacement probability of T. canescens between patches. This analysis was complemented by calculating the least cost paths among habitat patches (LCP; Cost Distance tool in ArcMap 10.3) using graph theory, which allows to find the only route that generates less cost for the displacement of the species. The potential corridors obtained through the LCP were limited by the maximum dispersion distance (MDD) of the focal species. The value of MDD for T. canescens was estimated by calculating the average home ranges of closely related species (Bowman et al., 2002; Melo et al., 2017; Table 1). Thus, the maximum length of potential biological corridors was limited to 1,905 m and its width to10 km (corresponding approximately to 4 times the home range of the species).
To assess landscape connectivity, we calculated the Integral Index of Connectivity (IIC) and the Probability of Connectivity (PC) using the software Conefor Sensinode 2.6 (Saura & Torné, 2012). These indices quantify the extent to which landscape connectivity is modified if a particular forest patch is removed (Saura et al., 2011). The application of IIC and PC was evaluated by dIIC and dPC (depending on the selected metric). These node importance indices measure the probability of connectivity loss caused by the removal of a patch from the landscape. dIIC and dPC are composed of the sum of dIICflux + dIICconnector and dpCflux + dPCconnector respectively. dIICflux and dPCflux indicate how well a node is connected to other nodes in the landscape without considering its contribution to intrapopulation connectivity. The dIICconnector and dPCconnector show whether a node contributes to the connectivity between other nodes as a stepping stone. Both, IIC and PC range from 0 to 1, with larger values corresponding to improved connectivity (Saura & Pascual-Hortal, 2007).

Results
Tropical dry forest was the dominant vegetation coverage with ~ 95,980 ha (62%) throughout the entire study area. The second largest class was exposed soils with ~ 44,820 ha (28.9%), mainly composed of induced grassland and agricultural lands. Exposed soils were mainly located in the eastern part of the study area, as well as to the north and northeast of the CCBR. Mangrove, riparian vegetation, and evergreen forest were grouped under the category “other types of vegetation” and occupied ~ 9,930 ha (6.4%). Finally, the rest of the categories occupied less than 3% of the study area.
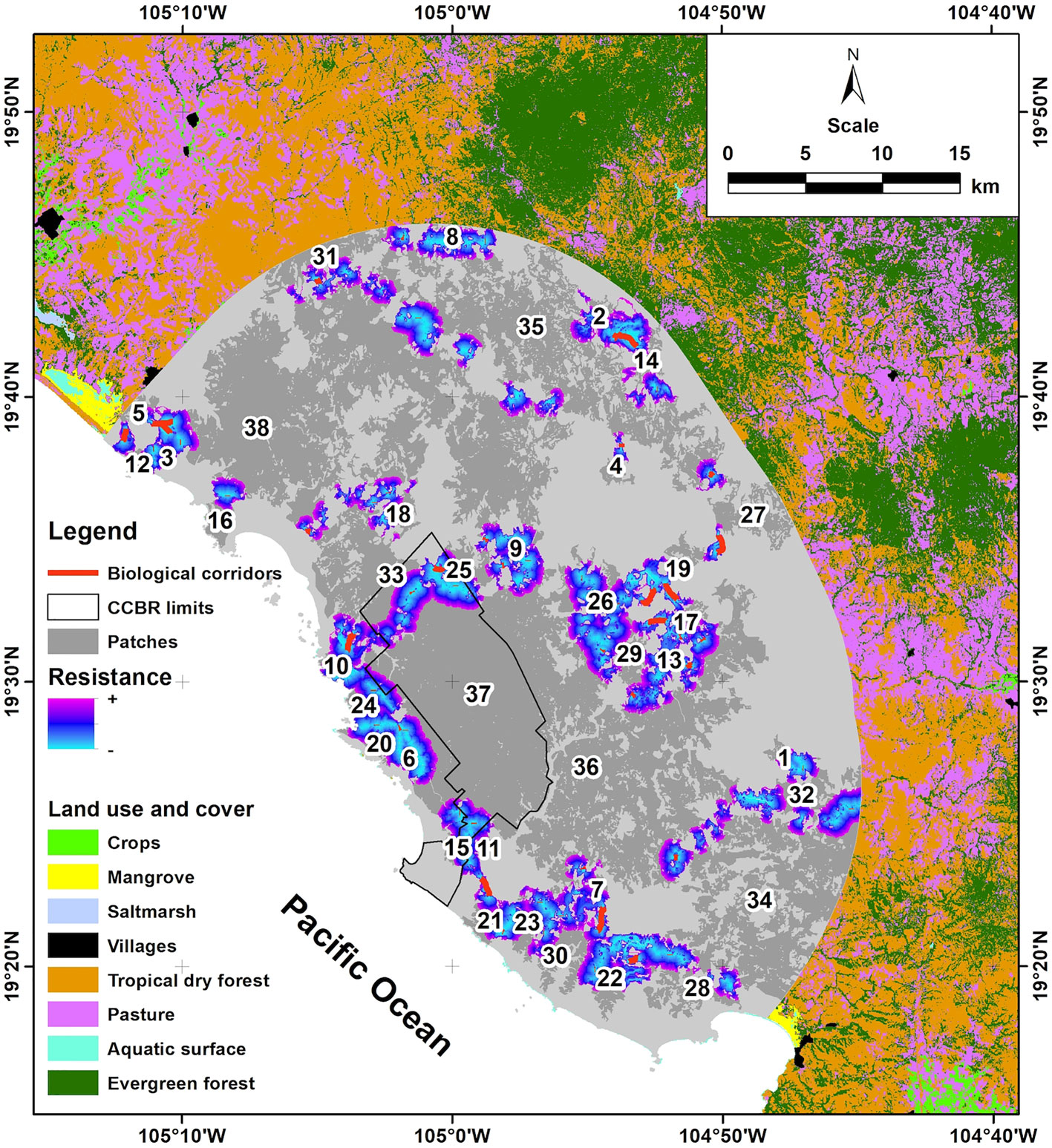
We identified 38 suitable patches of tropical dry forest habitat for T. canescens which accounted for a total area of ca. 84,100 ha. The smallest patch had 197.5 ha, the largest 18,884 ha and the average size was 2,213.2 ha. The habitat patch 37 had 16,675 ha and contained the largest portion of the CCBR (13,142 ha). Moreover, we identified 60 potential corridors to connect these patches (Fig. 1; Supplementary material: Table S1). The average Euclidean distance among patches was 244.5 m and the average least cost path was 367.5 m. The longest least cost path (1,716 m) connected patches 7 and 30 and required to cross a large expanse of exposed soil. The least connected zone was located at the eastern side of the study area which was characterized by a landscape dominated by forest remnants mixed with agrosystems. In this zone the distance among habitat patches was longer than the MDD of the focal species. We obtained 2 models of current flow connecting suitable habitat patches (Fig. 2). The highest flow probability occurred around the largest patches (36, 37, and 38) and showed a reduction around patches having internal perforations (28, 32, 34, and 35). Overall, the highest flow probability was associated with the presence of the CCBR.
The Integral Index of Connectivity (IIC) and the Probability of Connectivity (PC) are shown in Table S2 (Supplementary material). The highest values of dIIC corresponded to patches 38 (45.5), 37 (44.5), and 36 (29.8) which also had the largest extents of tropical dry forest. Habitat patches 38 and 37 also had the highest values of dIICflux (30.7 and 30.0, respectively) whereas patches 36 and 38 had the largest dIICconnector values. Finally, we found that patches 37, 38 and 36 provided the greatest contribution to patch connectivity (49.0, 49.0, and 33.3) whereas patches 38 and 37 had the largest values of dPCflux (5.3 and 4.1, respectively). The habitat patch 37 had the largest value of dPCconnector (12.4) followed by patches 38 and 36 (8.7 and 8.3, respectively).
Discussion
Despite the fact that the study area is primarily rural and human settlements occupy less than 1% (Flores-Casas & Ortega-Huerta, 2019; Hernández-Guzmán et al., 2019), it is characterized by showing a high level of disturbance due to the conversion of natural areas to agrosystems. The methods used in this study allowed the evaluation of tropical dry forest connectivity among a protected area and their surroundings through the identification of the most important patches and potential corridors for the focal species. The results of our models for T. cansescens highlight not only the importance of the maintenance of large patches of suitable habitat but also of some smaller patches that can play a role of stepping stones, favoring connectivity. The region with the best functional connectivity for T. canescens was concentrated at the western part of the study area, where the Chamela-Cuixmala Biosphere Reserve (CCBR) is located (patch 37) together with patches 36 and 38. A high connectivity probability was detected among those patches due to their size and their proximity with other patches. However, we did not obtain any potential corridor that directly connected the CCBR with the large patches 36 and 38, partially because the Cuixmala river likely functions as a natural barrier for species displacement. Consequently, intermediate patches, such as 29 and 33, are important for functional connectivity due to their role as stepping stones.
Natural reserves are one of the most important strategies for biodiversity conservation (Bruner et al., 2001; Saura et al., 2019). However, to ensure their effectiveness they require avoid becoming “conservation islands” (Calabrese & Fagan, 2004; Ricketts, 2001). In Mexico, many natural reserves are located in the proximity of growing cities, in regions undergoing intense land cover changes, or near touristic sites. Therefore, fine resolution landscape modelling outside natural reserves, which takes into account the different elements that favor fragmentation and isolation of the natural habitat for different organisms, is essential (Ricketts, 2001; Turchin, 1998; Vandermeer & Carvajal, 2001). This is particularly important for mammals, since 25% of their species are categorized as endangered, and the populations of 52% of known mammals are in decline, including species categorized as “least concern” as a consequence of increased habitat fragmentation and loss (Schipper et al., 2008; Theobald et al., 2012).
Despite the northwestern part of the study area supports larger patches favoring long-distance dispersal of our focal species, there is a potential risk for future connectivity loss. This, due to the fact that primary and several secondary roads are located in that area which can favor the establishment and growth of human settlements (Supplementary material Fig. S1; Decout et al., 2012; Forman & Alexander, 1998). Moreover, an increased traffic in these roads can greatly limit animal dispersal and become an important source of mortality since translocation experiments have shown that small mammals tend to return to their sites of origin, most of the time avoiding crossing roads (Bowne et al., 1999; Delgado-Trejo et al., 2018; Mader, 1984; Merriam et al., 1989).
In contrast to these findings, in the northern part (patches 8, 31, and 35), as well as in the southeastern part of the study area (patches 28, 32 and 34) a higher risk of connectivity loss was shown. This risk exists because there is an ongoing perforation process inside those patches that can lead to fragment size reduction, and thus to landscape fragmentation, increasing the loss and isolation of original habitat. Although this reduction in connectivity may be influenced by the location of the patches in the limits of the established buffer, we think that the effect is minimal due to the fact that the buffer is naturally limited by evergreen forest and large extensions of agricultural fields and pastures for cattle. Likewise, at the eastern part of the study area there is a region with low connectivity and high resistance due to extensive areas for cattle raising and agriculture (e.g., patches 4, 19, and 27). As a result, those isolated patches are under high probabilities of losing connectivity in the short and medium term. Some studies have shown that agrosystems may favor connectivity for some small mammal species, depending on the type of crop, the percentage of coverage they offer and the degree of habitat specialization of the species (Benedek & Sîrbu, 2018; Cruz-Lara et al., 2004; Fahring et al., 2011; Mellink, 1985). However, although some species have been shown to be able to cross areas with unfavorable land use and cover, they may not necessarily inhabit this type of cover, at least not in the long term (Birney et al., 1976; Ruefenacht & Knight, 1995). Tlacuatzin canescens, due to its arboreal and low dispersal habits, is likely a species sensitive to habitat modification not able to cross large tracts of unfavorable habitat (Zarza et al., 2003). This could put T. canescens and other similar specialists’ species inhabiting TDF remnants immersed in agrosystems at risk of local extinction. This effect could be reduced if agrosystems such as corn, coconut, and mango plantations offer enough permeability for the species to disperse. Nevertheless, it is important to take into consideration that providing areas to disperse in the form of corridors or permeable matrices should not be considered as a replacement for the protection of large extensions of primary forest that are needed for the survival of this and other species (Rosenberg et al., 1997). The functional connectivity model we developed could help to guide conservation efforts to benefit not only populations of the study species, but a set of co-distributed populations of small mammals having similar life story attributes and ecological traits as those of T. canescens (Supplementary material: Table S3; Ceballos & Miranda, 2000; Wilson & Reeder, 2005).
We acknowledge the lack of empirical evidence to support the construction of surface resistance models for this and many other species of interest, so that the resistance allocation to landscape variables during the functional connectivity modeling was built on the basis of expert knowledge (Foltête, 2018; Wade et al., 2015; Zeller et al., 2011). In addition, the functional connectivity model requires knowledge of the movement patterns of the species of interest. This is challenging since the movement patterns of many small mammal species are unknown, limiting the possibility to have more accurate models (Bowman et al., 2002; Moilanen, 2011; Wikelski et al., 2007). As long as key data on small mammal ecology is missing, we will need to continue relying on indirect methods, as an alternative to deal with the need to support conservation decisions, which are extremely important to maintain wild population of vertebrates and the functioning of ecological systems. We also acknowledge that modifying the size of the buffer the results could change, since connectivity measures based on graph theory require habitat quality thresholds in order to define habitat patches (Moilanen, 2011). However, we consider that the buffer stablished for the analyzes of this study is adequate since it integrates several criteria, such as the dispersal capacity and the historical records of the species, the minimum area to maintain viable populations in similar species, as well as the recommendations on the buffer area that have been suggested to study protected natural areas (Hansen & Defries, 2007; Seiferling et al., 2012). In addition, the buffer used allowed the data to be analyzed with a fine resolution but computationally manageable.
To mitigate the effects of fragmentation, a highly desirable alternative is to maintain the functional connectivity of the landscape by establishing multiple redundant connections between conservation areas (Villers-Ruiz & Trejo-Vázquez, 1998). However, due to conservation constraints an alternative to protect the species is the selection of least cost paths between the protected areas and the patches to be connected. This can provide the basis to plan the purchasing and managing of sites adjacent to conservation areas and to focus efforts of stakeholders (e.g., landowners and the National Commission of Natural Protected Areas, CONANP). Therefore, it is urgent to conduct studies that help to identify priority areas that guarantee maintaining functional connectivity between the protected areas and adjacent patches, as well as promoting alternatives such as live fences and elevated bridges as alternative routes for the dispersal of species.
Acknowledgements
To M. Hidalgo-Mihart for his contributions during all stages of this project; Francisco A. Sánchez Almaguer for help during field work; A. Rizo and A. Guerrero from the Universidad Autónoma del Estado de Morelos, M. Hidalgo-Mihart from the Universidad Juárez Autónoma de Tabasco and I. Íñiguez from Universidad de Guadalajara for answering the questionnaire to assign resistance values for the model construction. We also thank logistic support by Katherine Renton and the staff of the “Estación de Biología Chamela” of the Universidad Nacional Autónoma de México (UNAM). Funds were provided by a research grant (2015-1250) from CONACyT to CG, as well as a scholarship (622232) from CONACyT to AG. This work constitutes partial fulfillment of the A. González’s master in science (Maestría en Ecología Integrativa) by the Universidad Michoacana de San Nicolás de Hidalgo.
References
Adriaensen, F., Chardon, J. P., De Blust, G., Swinnen, E., Villalba, S., Gulinck, H. et al. (2003). The application of ‘least-cost’ modelling as a functional landscape model. Landscape and Urban Planning, 64, 233–247. https://doi.org/10.1016/S0169-2046(02)00242-6
Alexandre, B., Crouzeilles, R., & Grelle, C. E. V. (2010). How can we estimate buffer zones of protected areas? A proposal using biological data. Natureza & Conservação, 8, 165–170. https://doi.org/10.4322/NATCON.00802010
Arreola-Gómez, R., & Mendoza, E. (2020). Marsupial visitation to the inflorescences of the endemic Agave cupreata in western Mexico. Western North American Naturalist, 80, 563–568. https://doi.org/10.3398/064.080.0417
Beier, P., Majka, D. R., & Newell, S. L. (2009). Uncertainty analysis of least-cost modeling for designing wildlife linkages. Ecological Applications, 19, 2067–2077. https://doi.org/10.1890/08-1898.1
Benedek, A. M., & Sîrbu, I. (2018). Responses of small mammal communities to environment and agriculture in a rural mosaic landscape. Mammalian Biology, 90, 55–65. https://doi.org/10.1016/j.mambio.2018.02.008
Bennett, G. (2004). Integrating biodiversity conservation and sustainable use: lessons learned from ecological networks. Gland, Switzerland, and Cambridge, UK: IUCN.
Birney, E. C., Grant, W. E., & Baird, D. D. (1976). Importance of vegetative cover to cycles of Microtus populations. Ecology, 57, 1043–1051. https://doi.org/10.2307/1941069
Bowman, J., Jaeger, J. A., & Fahrig, L. (2002). Dispersal distance of mammals is proportional to home range size. Ecology, 83, 2049–2055. https://doi.org/10.1890/0012-9658
(2002)083[2049:DDOMIP]2.0.CO;2
Bowne, D. R., Peles, J. D., & Barrett, G. W. (1999). Effects of landscape spatial structure on movement patterns of the hispid cotton rat (Sigmodon hispidus). Landscape Ecology, 14, 53–65. https://doi.org/10.1023/A:1008025827895
Brooker, L., & Brooker, M. (2002). Dispersal and population dynamics of the Blue-breasted Fairy-wren, Malurus pulcherrimus, in fragmented habitat in the Western Australian wheatbelt. Wildlife Research, 29, 225–233. https://doi.org/10.1071/WR01113
Bruner, A. G., Gullison, R. E., Rice, R. E., & Da Fonseca, G. A. (2001). Effectiveness of parks in protecting tropical biodiversity. Science, 291, 125–128. https://doi.org/10.1126/science.291.5501.125
Bullock, S. H., Mooney, H. A., & Medina, E. (1995). Seasonally dry tropical forests. Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511753398
Calabrese, J. M., & Fagan, W. F. (2004). A comparison-shopper’s guide to connectivity metrics. Frontiers in Ecology and the Environment, 2, 529–536. https://doi.org/10.1890/1540-9295(2004)002[0529:ACGTCM]2.0.CO;2
Ceballos, G. (1990). Comparative natural history of small mammals from tropical forests in western Mexico. Journal of Mammalogy, 71, 263–66. https://doi.org/10.2307/1382182
Ceballos, G., & Arroyo-Cabrales, J. (2013). Lista actualizada de los mamíferos de México 2012. Revista Mexicana de
Mastozoología, 2, 27–80. https://doi.org/10.22201/ie.2007
4484e.2012.2.1.20
Ceballos, G., & García, A. (1995). Conserving Neotropical biodiversity: the role of dry forests in western Mexico. Conservation Biology, 9, 1349–1356. https://doi.org/10.
1046/j.1523-1739.1995.09061349.x
Ceballos, G., & Miranda, A. (2000). A field guide to the mammals of the Jalisco Coast, Mexico. México D.F.: Fundación Ecológica de Cuixmala AC/ Universidad Nacional Autónoma de México.
Ceballos, G. &, Oliva, G. (2005). Los mamíferos silvestres de México. México D.F.: Fondo de Cultura Económica.
Ceballos, G., Martínez, L., García, A., & Espinoza, E. (2010). Áreas prioritarias para la conservación de las selvas secas del Pacífico mexicano. In G. Ceballos, L. Martínez, A. García, E. Espinoza, J. Bezaury-Creel, & R. Dirzo (Eds.), Diversidad, amenazas y áreas prioritarias para la conservación de las selvas secas del Pacífico de México (pp. 387–392). México D.F.: Fondo de Cultura Económica/ Conabio.
Correa-Ayram, C. A., Mendoza, M. E., Pérez-Salicrup, D. R., & López-Granados, E. (2014). Identifying potential conservation areas in the Cuitzeo Lake basin, Mexico by multitemporal analysis of landscape connectivity. Journal for Nature Conservation, 22, 424–435. https://doi.org/10.1016/j.jnc.2014.03.010
Corry, R. C. (2005). Characterizing fine-scale patterns of alternative agricultural landscapes with landscape pattern indices. Landscape Ecology, 20, 591–608. https://doi.org/10.1007/s10980-004-5036-8
Cruz-Lara, L. E., Lorenzo, C., Soto, L., Naranjo, E., & Ramírez-Marcial, N. (2004). Diversidad de mamíferos en cafetales y selva mediana de las Cañadas de La Selva Lacandona, Chiapas, México. Acta Zoológica Mexicana, 20, 63–81.
Cuenca, P., & Echeverría, C. (2017). How do protected landscapes associated with high biodiversity and population levels change? Plos One, 12, e0180537. https://doi.org/10.1371/journal.pone.0180537
De Clerck, F. A., Chazdon, R., Holl, K. D., Milder, J. C., Finegan, B., Martínez-Salinas, A. et al. (2010). Biodiversity conservation in human-modified landscapes of Mesoamerica: past, present and future. Biological Conservation, 143, 2301–2313. https://doi.org/10.1016/j.biocon.2010.03.026
de la Peña, N. M., Butet, A., Delettre, Y., Paillat, G., Morant, P., Le Du, L. et al. (2003). Response of the small mammal community to changes in western French agricultural landscapes. Landscape Ecology, 18, 265–278. https://doi.org/10.1023/A:1024452930326
Decout, S., Manel, S., Miaud, C., & Luque, S. (2012). Integrative approach for landscape-based graph connectivity analysis: a case study with the common frog (Rana temporaria) in human-dominated landscapes. Landscape Ecology, 27, 267–279. https://doi.org/10.1007/s10980-011-9694-z
Delgado-Trejo, C., Herrera-Robledo, R., Martínez-Hernández, N., Bedolla-Ochoa, C., Hart, C. E., Alvarado-Díaz, J. et al. (2018). Vehicular impact as a source of wildlife mortality in the Western Pacific Coast of Mexico. Revista Mexicana de Biodiversidad, 89, 1234–1244. https://doi.org/10.22201/ib.20078706e.2018.4.2084.
Eastman, J. R. (2016). TerrSet Manual. Clark labs. Worcester, MA: Clark University.
Fahrig, L., Baudry, J., Brotons, L., Burel, F. G., Crist, T. O., Fuller, R. J. et al. (2011). Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecology Letters, 14, 101–112. https://doi.org/
10.1111/j.1461-0248.2010.01559.x
FAO, Forest Resources Division FD (2015). “México – Evaluación de los recursos forestales mundiales – Informe Nacional 2015” http://www.fao.org/documents/card/es/c/154c5a2b-882d-4d3e-b040-7dd52a778d17/
Flores-Casas, R., & Ortega-Huerta, M. A. (2019). Modelling land cover changes in the tropical dry forest surrounding the Chamela-Cuixmala biosphere reserve, Mexico. International Journal of Remote Sensing, 40, 6948–6974. https://doi.org/10.1080/01431161.2019.1597305
Foltête, J. C. (2018). A parcel-based graph to match connectivity analysis with field action in agricultural landscapes: Is node removal a reliable method? Landscape and Urban Planning, 178, 32–42. https://doi.org/10.1016/j.landurbplan.2018.05.016
Forman, R. T., & Alexander, L. E. (1998). Roads and their major ecological effects. Annual Review of Ecology and Systematics, 29, 207–231. https://doi.org/10.1146/annurev.ecolsys.29.1.207
Freeman, B., Roehrdanz, P. R., & Peterson, A. T. (2019). Modeling endangered mammal species distributions and forest connectivity across the humid Upper Guinea lowland rainforest of West Africa. Biodiversity and Conservation, 28, 671–685. https://doi.org/10.1007/s10531-018-01684-6
García, A. (2006). Using ecological niche modeling to identify diversity hotspots of the herpetofauna of Pacific lowlands and adjacent interior valleys of Mexico. Biological Conservation, 130, 25–46. https://doi.org/10.1016/j.biocon.2005.11.030
Garmendia, A., Arroyo-Rodríguez, V., Estrada, A., Naranjo, E. J., & Stoner, K. E. (2013). Landscape and patch attributes impacting medium-and large-sized terrestrial mammals in a fragmented rain forest. Journal of Tropical Ecology, 29, 331–344. https://doi.org/10.1017/S0266467413000370
Ghazoul, J. (2005). Pollen and seed dispersal among dispersed plants. Biological Reviews, 80, 413–443. https://doi.org/10.1017/s1464793105006731
González-Christen, A., & Rodríguez, N. V. (2014). Primer registro de Tlacuatzin canescens, (Mammalia, Didelphimorphia, Marmosidae) en Veracruz, México. Therya, 5, 845–854. https://doi.org/10.12933/therya-14-221
Gordon, C. L. (2003). A first look at estimating body size in dentally conservative marsupials. Journal of Mammalian Evolution, 10, 1–21. https://doi.org/10.1023/A:1025545023221
Hansen, A. J., & DeFries, R. (2007). Ecological mechanisms linking protected areas to surrounding lands. Ecological Applications, 17, 974–988. https://doi.org/10.1890/05-1098
Hernández-Guzmán, R., Ruiz-Luna, A., & González, C. (2019). Assessing and modeling the impact of land use and changes in land cover related to carbon storage in a western basin in Mexico. Remote Sensing Applications: Society and Environment, 13, 318–327. https://doi.org/10.1016/j.rsase.2018.12.005
Howe, H. F., & Smallwood, J. (1982). Ecology of seed dispersal. Annual Review of Ecology and Systematics, 13, 201–228. https://doi.org/10.1146/annurev.es.13.110182.001221
Hutchison, D. W., & Templeton, A. R. (1999). Correlation of pairwise genetic and geographic distance measures: inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution, 53, 1898–1914. https://doi.org/10.2307/2640449
Jamieson, I. G., & Allendorf, F. W. (2012). How does the 50/500 rule apply to MVPs? Trends in Ecology and Evolution, 27, 578–584. https://doi.org/10.1016/j.tree.2012.07.001
Janzen, D. H. (1988). Tropical dry forests. In E. O. Wilson (Ed.), Biodiversity (pp. 130–137). Washington DC: National Academic Press.
Jenson, S. K., & Domingue, J. O. (1988). Extracting topographic structure from digital elevation data for geographic information system analysis. Photogrammetric Engineering and Remote Sensing, 54, 1593–1600.
Keenan, R. J., Reams, G. A., Achard, F., de Freitas, J. V., Grainger, A., & Lindquist, E. (2015). Dynamics of global forest area: results from the FAO Global Forest Resources Assessment 2015. Forest Ecology and Management, 352, 9–20. https://doi.org/10.1016/j.foreco.2015.06.014
Kennedy, M. L., Schnell, G. D., Romero-Almaraz, M. L., Malakouti, B. S., Sánchez-Hernández, C., Bes, T. L. et al. (2013). Demographic features, distribution, and habitat selection of the gray mouse opossum (Tlacuatzin canescens) in Colima, Mexico. Acta Theriologica, 58, 285–298. https://doi.org/10.1007/s13364-012-0117-6
Laurance, W. F., Useche, D. C., Rendeiro, J., Kalka, M., Bradshaw, C. J., & Sloan, S. P. (2012). Averting biodiversity collapse in tropical forest protected areas. Nature, 489, 290–294. https://doi.org/10.1038/nature11318
Lobova, T. A., Geiselman, C. K., & Mori, S. A. (2009). Seed dispersal by bats in the Neotropics. New York Botanical Garden.
Lorenzo, C., & González-Ruiz, N. (2018). Mammals in the Mexican Official Norm NOM-059-SEMARNAT-2010. Therya, 9, 69–72. https://doi.org/10.12933/therya-18-565
Lovegrove, B. G., Körtner, G., & Geiser, F. (1999). The energetic cost of arousal from torpor in the marsupial Sminthopsis macroura: benefits of summer ambient temperature cycles. Journal of Comparative Physiology B, 169, 11–18. https://doi.org/10.1007/s003600050188
Luck, G. W., & Daily, G. C. (2003). Tropical countryside bird assemblages: richness, composition, and foraging differ by landscape context. Ecological Applications, 13, 235–247. https://doi.org/10.1890/1051-0761(2003)013[0235:TCBARC]2.0.CO;2
Mader, H. J. (1984). Animal habitat isolation by roads and agricultural fields. Biological Conservation, 29, 81–96. https://doi.org/10.1016/0006-3207(84)90015-6
Martin, G. M. (2017). Tlacuatzin canescens. The IUCN Red List of Threatened Species 2017, e.T12813A22177663. https://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T12813A22177663.en
McRae, B. H., & Beier, P. (2007). Circuit theory predicts gene flow in plant and animal populations. Proceedings of the National Academy of Sciences, 104, 19885–19890. https://doi.org/10.1073/pnas.0706568104
Mellink, E. (1985). Agricultural disturbance and rodents: three farming systems in the Sonoran Desert. Journal of Arid Environments, 8, 207–222. https://doi.org/10.1016/S0140-1963(18)31282-5
Melo, G. L., Sponchiado, J., Cáceres, N. C., & Fahrig, L. (2017). Testing the habitat amount hypothesis for South American small mammals. Biological Conservation, 209, 304–314. https://doi.org/10.1016/j.biocon.2017.02.031
Merriam, G., Kozakiewicz, M., Tsuchiya, E., & Hawley, K. (1989). Barriers as boundaries for metapopulations and demes of Peromyscus leucopus in farm landscapes. Landscape Ecology, 2, 227–235. https://doi.org/10.1007/BF00125093
Merrick, M. J., & Koprowski, J. L. (2017). Circuit theory to estimate natal dispersal routes and functional landscape connectivity for an endangered small mammal. Landscape Ecology, 32, 1163–1179. https://doi.org/10.1007/s10980-017-0521-z
Miles, L., Newton, A. C., DeFries, R. S., Ravilious, C., May, I., Blyth, S. et al. (2006). A global overview of the conservation status of tropical dry forests. Journal of Biogeography, 33, 491–505. https://doi.org/10.1111/j.1365-2699.2005.01424.x
Moilanen, A. (2011). On the limitations of graph-theoretic connectivity in spatial ecology and conservation. Journal of Applied Ecology, 48, 1543–1547. https://doi.org/10.1111/j.1365-2664.2011.02062.x
Moilanen, A., & Hanski, I. (2001). On the use of connectivity measures in spatial ecology. Oikos, 95, 147–151. https://www.jstor.org/stable/3547357
Moore, R. P., Robinson, W. D., Lovette, I. J., & Robinson, T. R. (2008). Experimental evidence for extreme dispersal limitation in tropical forest birds. Ecology Letters, 11, 960–968. https://doi.org/10.1111/j.1461-0248.2008.01196.x
Morales-Díaz, S. P., Álvarez-Añorve, M. Y., Zamora-Espinoza, M. E., Dirzo, R., Oyama, K., & Ávila-Cabadilla, L. D. (2019). Rodent community responses to vegetation and landscape changes in early successional stages of tropical dry forest. Forest Ecology and Management, 433, 633–644. https://doi.org/10.1016/j.foreco.2018.11.037
Olifiers, N., Vieira, M. V., & Grelle, C. E. V. (2004). Geographic range and body size in Neotropical marsupials. Global Ecology and Biogeography, 13, 439–444. https://www.jstor.org/stable/3697574
Passamani, M., & Fernández, F. A. S. (2011). Abundance and richness of small mammals in fragmented Atlantic Forest of southeastern Brazil. Journal of Natural History, 45, 553–565. https://doi.org/10.1080/00222933.2010.534561
Pires, M. M., Martins, E. G., Silva, M. N. F., & Dos Reis, S. F. (2010). Gracilinanus microtarsus (Didelphimorphia: Didelphidae). Mammalian Species, 42, 33–40. https://doi.org/10.1644/851.1
Portillo-Quintero, C. A., & Sánchez-Azofeifa, G. A. (2010). Extent and conservation of tropical dry forests in the Americas. Biological Conservation, 143, 144–155. https://doi.org/10.1016/j.biocon.2009.09.020
Ricketts, T. H. (2001). The matrix matters: effective isolation in fragmented landscapes. American Naturalist, 158, 87–99. https://doi.org/10.1086/320863
Robichaux, R. H., & Yetman, D. (2000). The tropical deciduous forest of Alamos: biodiversity of a threatened ecosystem in Mexico. Tucson: University of Arizona Press.
Rosenberg, D. K., Noon, B. R., & Meslow, E. C. (1997). Biological corridors: form, function, and efficacy. Bioscience, 47, 677–687. https://doi.org/10.2307/1313208
Ruefenacht, B., & Knight, R. L. (1995). Influences of corridor continuity and width on survival and movement of deermice Peromyscus maniculatus. Biological Conservation, 71, 269–274. https://doi.org/10.1016/0006-3207(94)00036-P
Saura, S., Bertzky, B., Bastin, L., Battistella, L., Mandrici, A., & Dubois, G. (2019). Global trends in protected area connectivity from 2010 to 2018. Biological Conservation, 238, 108183. https://doi.org/10.1016/j.biocon.2019.07.028
Saura, S., & Pascual-Hortal, L. (2007). A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landscape and Urban Planning, 83, 91–103. https://doi.org/10.1016/j.landurbplan.2007.03.005
Saura, S., & Torné, J. (2012). Conefor 2.6 user manual. Madrid: Universidad Politécnica de Madrid.
Saura, S., Vogt, P., Velázquez, J., Hernando, A., & Tejera, R. (2011). Key structural forest connectors can be identified by combining landscape spatial pattern and network analyses. Forest Ecology and Management, 262, 150–160. https://doi.org/10.1016/j.foreco.2011.03.017
Sawyer, S. C., Epps, C. W., & Brashares, J. S. (2011) Placing linkages among fragmented habitats: Do least-cost models reflect how animals use landscapes? Journal of Applied Ecology, 48, 668–678. https://doi.org/10.1111/j.1365-2664.2011.01970.x
Schipper, J., Chanson, J. S., Chiozza, F., Cox, N. A., Hoffmann, M., Katariya, V. et al., (2008). The status of the world’s land and marine mammals: diversity, threat, and knowledge. Science, 322, 225–230. https://doi.org/10.1126/science.1165115
Seiferling, I. S., Proulx, R., Peres-Neto, P. R., Fahrig, L., & Messier, C. (2012). Measuring protected-area isolation and correlations of isolation with land-use intensity and protection status. Conservation Biology, 26, 610–618. https://doi.org/10.1111/j.1523-1739.2011.01674.x
Shah, V. B., & McRae, B. H. (2008). Circuitscape: a tool for landscape ecology. In Proceedings of the 7th Python in Science Conference. Pasadena, California, USA: SciPy 7, 62-66.
Stoner, K. E., & Timm, R. M. (2004). Tropical dry-forest mammals of Palo Verde: Ecology and conservation in a changing landscape. In G. W. Frankie, A. Mata, & B. S. Vinson (Eds.), Biodiversity conservation in Costa Rica: learning the lessons in a seasonal dry forest (pp. 48–66). Berkley: University of California Press.
Strahler, A. N. (1957). Quantitative analysis of watershed geomorphology. Eos, Transactions American Geophysical
Union, 38, 913–920. https://doi.org/10.1029/TR038i006p00
913
Taylor, P., Fahrig, L., & With, K. (2006). Landscape connectivity: a return to the basics. In K. Crooks, & M. Sanjayan (Eds.), Connectivity Conservation, Conservation Biology (pp. 29–43). Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511754821
Theobald, D. M., Reed, S. E., Fields, K., & Soule, M. (2012). Connecting natural landscapes using landscape permeability model to prioritize conservation activities in the United States. Conservation Letters, 5, 123–133. https://doi.org/10.1111/j.1755-263X.2011.00218.x
Tischendorf, L., & Fahrig, L. (2000). On the usage and measurement of landscape connectivity. Oikos, 90, 7–19. https://doi.org/10.1034/j.1600-0706.2000.900102.x
Trejo, I. (2010). Las selvas secas del Pacífico mexicano. In G. Ceballos, L. Martínez, A. García, E. Espinoza, J. Bezauty-Creel, & R. Dirzo (Eds.), Diversidad, amenazas y áreas prioritarias para la conservación de las selvas secas del Pacífico de México (pp. 41–51). México D.F.: Fondo de Cultura Económica/ Conabio.
Trejo, I., & Dirzo, R. (2000). Deforestation of seasonally dry tropical forest: a national and local analysis in Mexico. Biological Conservation, 94, 133–142. https://doi.org/10.1016/S0006-3207(99)00188-3
Trejo, I., & Dirzo, R. (2002). Floristic diversity of Mexican seasonally dry tropical forests. Biodiversity and Conservation, 11, 2063–2084. https://doi.org/10.1023/A:1020876316013
Turchin, P. (1998). Quantitative analysis of movement: measuring and modeling population redistribution of plants and animals. Sunderland, MA: Sinauer Associates.
Vandermeer, J., & Carvajal, R. (2001). Metapopulation dynamics and the quality of the matrix. American Naturalist, 158, 211–220. https://doi.org/10.1086/321318
Vieira-De Matos, T. P., Vieira-De Matos, V. P., De Mello, K., & Averna, R. V. (2019). Protected areas and forest fragmentation: sustainability index for prioritizing fragments for landscape restoration. Geology, Ecology and Landscapes, 5, 19–31. https://doi.org/10.1080/24749508.2019.1696266
Villers-Ruiz, L., & Trejo-Vázquez, I. (1998). Climate change on Mexican forests and natural protected areas. Global Environmental Change, 8, 141–157. https://doi.org/10.1016/S0959-3780(98)00012-0
Voss, R. S., & Jansa, S. A. (2009). Phylogenetic relationships and classification of didelphid marsupials, an extant radiation of the New World metatherian mammals. Bulletin of the American Museum of Natural History, 2009, 1–177.
Wade, A. A., McKelvey, K. S., & Schwartz, M. K. (2015). Resistance-surface-based wildlife conservation connectivity modeling: Summary of efforts in the United States and guide for practitioners. Gen. Tech. Rep. RMRS-GTR-333. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. https://doi.org/10.2737/RMRS-GTR-333
Wikelski, M., Kays, R. W., Kasdin, N. J., Thorup, K., Smith, J. A., & Swenson, G. W. (2007). Going wild: what a global small-animal tracking system could do for experimental biologists. Journal of Experimental Biology, 210, 181–186. https://doi.org/10.1242/jeb.02629
Wilson, M. C., Chen, X. Y., Corlett, R. T., Didham, R. K., Ding, P., R. D. Holt et al. (2015). Habitat fragmentation and biodiversity conservation: key findings and future challenges. Landscape Ecology, 31, 219–227. https://doi.org/10.1007/s10980-015-0312-3
Wilson, D. E., & Reeder, D. M. (2005). Mammal species of the world: a taxonomic and geographic reference. Baltimore, MD: The Johns Hopkins University Press.
With, K. A. (1997). The application of neutral landscape models in conservation biology. Conservation Biology, 11, 1069–1080. https://www.jstor.org/stable/2387389
Zarza, H., Ceballos, G., & Steele, M. A. (2003). Marmosa canescens. Mammalian Species, 725, 1–4. https://doi.org/10.1644/0.725.1
Zeller, K. A., Nijhawan, S., Salom-Pérez, R., Potosme, S. H., & Hines, J. E. (2011). Integrating occupancy modeling and interview data for corridor identification: a case study for jaguars in Nicaragua. Biological Conservation, 144, 892–901. https://doi.org/10.1016/j.biocon.2010.12.003