Mario C. Lavariegaa, *, Emilio Martínez-Ramíreza, Rocio N. Santiago-Oliverab, Gabriel Isaías Cruz-Ruízc, Rosa María Gómez-Ugalded, Miguel Briones-Salasa
a Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Unidad Oaxaca, Instituto Politécnico Nacional, Hornos 1003, 71230 Santa Cruz Xoxocotlán, Oaxaca, Mexico
b Universidad Autónoma Benito Juárez de Oaxaca, Av. Universidad s/n, Ex-Hacienda 5 Señores, 68120 Oaxaca, Oaxaca, Mexico
c Departamento de Sistemática y Ecología Acuática, El Colegio de la Frontera Sur, Unidad Chetumal, Av. Centenario Km 5.5, 77014 Chetumal, Quintana Roo, Mexico
d Instituto Tecnológico del Valle de Oaxaca, Tecnológico Nacional de México, Ex-Hacienda de Nazareno, Santa Cruz Xoxocotlán, 71233 Oaxaca, Oaxaca, Mexico
*Corresponding author: mlavariegan@ipn.mx (M.C. Lavariega)
Received: 16 November 2018; accepted: 12 June 2020
Abstract
Land otters are predators at the top of the food chain in the rivers where they live and mold the biotic communities at lower trophic levels. The neotropical otter, Lontra longicaudis, is widely distributed in the Americas, but populations are being decimated by hunting, habitat loss, water pollution, and roadkills. An otter population was located in southern Tehuacán-Cuicatlán Biosphere Reserve (TCBR), Mexico. Surveys were conducted in the dry season to determine otter presence, to record physical-chemical data and to collect scats to determine their populational densities and feeding habits. River width and depth, dissolved oxygen and elevation were the significant variables that explained their presence. In the rivers where otters were present, we estimated densities between 0.19 and 0.22 otters/km. We found 12 prey taxa, mainly freshwater fish. In this protected area, the neotropical otter is restricted to large rivers at low elevations, and populations are low. Although otters have diverse feeding habits, they mainly prey upon Poeciliopsis spp., which constituted 60% of the scats. Density estimates comprise the dry season and were lower than previous surveys, therefore it is necessary to conduct yearly monitoring to identify trends and to implement actions focused on their conservation and management.
Keywords: Density; Feeding habits; Geographic distribution; Habitat; Mustelidae; Protected areas
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Ecología de la nutria neotropical (Lontra longicaudis) en el sur de la Reserva de la Biosfera Tehuacán-Cuicatlán, México
Resumen
Las nutrias son los depredadores tope de los ríos que habitan moldeando las comunidades bióticas de los niveles tróficos más bajos. La nutria neotropical Lontra longicaudis, tiene la distribución geográfica más amplia en América, pero sus poblaciones han sido diezmadas a causa de la cacería, pérdida de hábitat, contaminación de los cuerpos de agua y atropellamientos. En el sur de la Reserva de la Biosfera Tehuacán-Cuicatlán (RBTC), México, una población de nutrias fue localizada. Se realizaron muestreos en la temporada seca para determinar su presencia, registrar datos fisicoquímicos, para colectar heces y determinar sus densidades poblacionales, y hábitos alimentarios. La anchura y profundidad de los ríos, oxígeno disuelto y la altitud de los ríos fueron variables significativas que explicaron su presencia. En los ríos con presencia de nutrias, se estimaron densidades entre 0.19 and 0.22 nutrias/km. Se hallaron 12 taxones presa, principalmente peces nativos. En esta área natural protegida, la nutria está restringida a los ríos grandes en elevaciones bajas y con poblacionales reducidas. Aunque las nutrias tienen una dieta diversa, ellas depredaron principalmente Poeciliopsis spp., que constituyeron 60% en las heces. Las densidades estimadas corresponden a la estación seca y fueron menores que en estudios previos, por tanto, es necesario realizar monitoreos anuales para identificar tendencias e implementar acciones enfocadas en su conservación y manejo.
Palabras clave: Densidad; Hábitos alimentarios; Distribución geográfica; Hábitat; Mustelidae; Áreas protegidas
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
Otters are at the top of the food chain in the rivers they inhabit. Here, they mold the biotic communities at lower trophic levels (Kruuk, 2006). Otters are widely distributed carnivores, currently, there are 13 species, most of them distributed in Asia and Africa (Kruuk, 2006). In continental America, 4 species have survived. Of these, the neotropical otter has the widest geographic distribution, from the coastal plains to central and southern Mexico, and throughout Central America to Argentina and the coastal plains of Peru (de Almeria & Ramos, 2017; Lavière, 1999; Rheingantz et al., 2017). Despite its wide distribution, it is becoming a threatened species because its populations are decreasing due to hunting, habitat loss, water bodies pollution and roadkills (IUCN, 2016; Rheingantz & Trinca, 2015).
Most of the scientific knowledge about the neotropical otter ecology refers to its feeding habits (Rheingantz et al., 2017). It has been accepted that the neotropical otter is a generalist predator basing their feeding on the local abundance of their prey (de Almeira & Ramos, 2017; Gallo-Reynoso et al., 2008; Monroy-Vilchis & Mundo, 2009), preferring those prey that are large with little mobility (Rheingantz et al., 2012, 2017). Fish and crustaceans predominate in their diet but can include birds, reptiles and small mammals (de Almeira & Ramos, 2017; Lavière, 1999; Rheingantz et al., 2017). Other aspects of the ecology and conservation of the neotropical otter, such as population size, habitat features, and how anthropogenic activities impact the species have been scarcely explored (Alarcón & Simões-Lopes, 2003; de Almeria & Ramos, 2017).
Indirect methods through counting scats have been the most common proxy for estimating population parameters of the neotropical otters (Rheingantz et al., 2017). With this method, densities have been estimated between 0.001 and 3.0 otters/km; the lowest values corresponding to a highly disturbed site in the Estado de México, Mexico, whereas the highest are from open areas in the Pantanal, Brazil (Guerrero-Flores et al., 2013; Kruuk, 2006). Studies on their habitat have shown otters prefer sites with wide and deep body waters, low anthropogenic disturbance, high understory vegetation cover, and high availability of sites for dens (Alarcon & Simões-Lopes, 2003; Carrillo-Rubio & Lafón, 2004). Therefore, the neotropical otter is considered a good indicator of the health of the riparian ecosystems (Gomez et al., 2014; Lavière, 1999).
Neotropical otters were first reported in the Grande River of the Tehuacán-Cuicatlán Biosphere Reserve (TCBR), Oaxaca, Mexico, in 2006 (Botello et al., 2006). At the time, their density was estimated at 0.51 otters/km and their food was 89% fish, mainly native fish of the species Paraneetroplus bulleri, Rhamdia guatemalensis and Profundulus punctatus (Duque-Dávila et al., 2013). However, environmental features determining otter presence were not explored, whereas densities and feeding habits should be updated for conservation planning and management. Consequently, surveys were conducted during the dry season in the main rivers of the TCRB to determine otter presence, to record physical-chemical data and to collect scats to determine their density and feeding habits. We expected the presence of otters in the TCRB would be explained by habitat features and chemical variables. We did not expect differences in feeding habits and abundance concerning previous studies in the region.
Materials and methods
The TCBR is shared by the States of Puebla and Oaxaca in south-central Mexico. In Oaxaca, the TCBR covers 2,962.7 km2 (60.44%) in 31 municipalities distributed in 6 districts (97o41’ – 97o49’ W, 17 o31’ – 18o16’ N; Fig. 1). The weather is hot and semiarid to very arid (Trejo, 2004). The main vegetation types are tropical deciduous forest, oak forest, shrub with predominance of bushes, and cacti. There are areas designated for agriculture and livestock grazing (Arriaga et al., 2000). Fresh water fish fauna in this reserve consists of 14 species, 12 native and 2 introduced. Two native species important for aquaculture have been extirpated (Martínez-Ramírez et al., 2013).
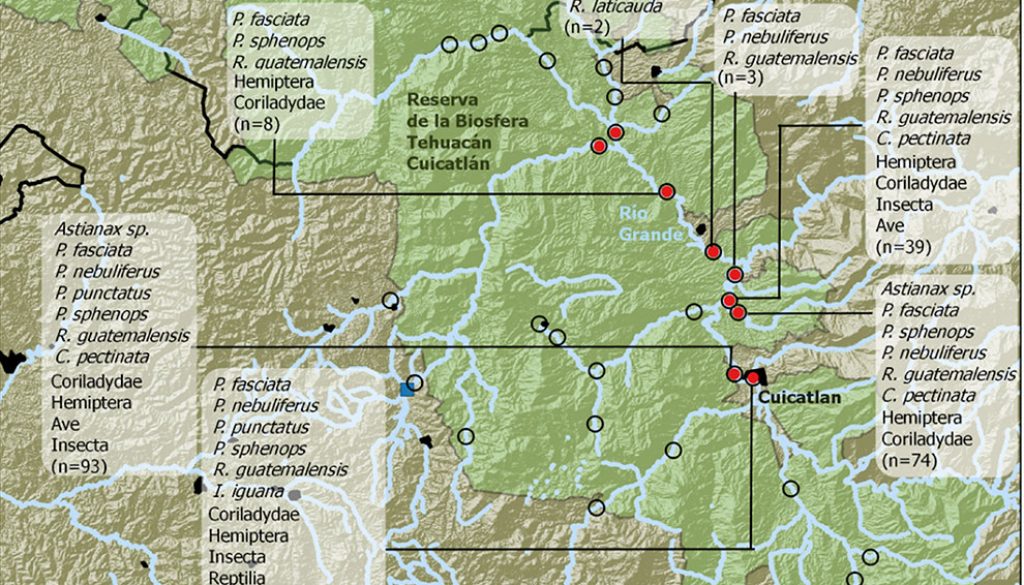
To determine the presence of otters in rivers of the Oaxaca TCBR, 2 surveys were conducted in October and November 2015. During the visits, we defined 32 transects along the riverbanks to look for evidence of otters, such as tracks and scats (Fig. 2). In each transect, we recorded physical-chemical data of the water and the environment (Table 1). For each sample, we recorded the geographic position. Some tracks were preserved in fast-setting plaster, while scats were placed in previously labeled paper bags on which site information was recorded (Aranda, 2000). Although otters usually deposit their feces in latrines, during surveys feces were mostly found solely. The plaster tracks were deposited in the Collection of Mammals of the Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional (CIIDIR) Unidad Oaxaca, Instituto Politécnico Nacional (OAX.MA.026.0497).

Table 1
Environmental variables of rivers in southern Tehuacán-Cuicatlán Biosphere Reserve.
| Variable | Description (unit) | Variable type |
| Otter | Otter presence | Response variable binomial |
| Elevation | Elevation (meters) | Continuous explanatory variable |
| River width | River width mean (meters) | Continuous explanatory variable |
| River depth | River depth mean (meters) | Continuous explanatory variable |
| Dissolved oxygen | Dissolved oxygen (mg/L) | Continuous explanatory variable |
| Vegetation cover | Vegetation cover index | Nominal explanatory variable (ordinal); 4 levels: 1 (null) to 4 (dense) |
| Disturbance | Index of alteration of the aquatic environment | Nominal explanatory variable (ordinal); 4 levels 1 (few) a 4 (much) |
| Vegetation and land use cover | Vegetation and land use cover according to INEGI (2013) and field observations | Nominal explanatory variable (categorical) |
Otter density was determined in 3 transects where sufficient scats were found: 1) Cuicatlán Grande River, 2) Quiotepec Grande River, and 3) Quiotepec Cacahuatal River. Twelve surveys were conducted in these transects between December 2015 and February 2016, corresponding to the dry season. Transects were walked slowly in the morning (07:00 – 11:00 hr) to find scats; both old and fresh scats were recorded. In order to avoid overestimating densities derived from the counting of old scats, we walked the transects 2 consecutive days, one to collect older scats (to “clean” the site) and the second one to collect fresh scats. Fresh scats were determined by possessing a dark-green color, presence of mucous and oiliness with a strong fish odor.
All scats from each sampled location were collected regardless of the number of individuals depositing their scats in each site and were used to obtain the feeding habits of otters. The collected scats were washed with a mixture of water and soap and passed through a 1 mm mesh. The dry solid remains of the scats were observed in a stereomicroscope (10 and 40×) to separate the different food items. The taxa were determined using specialized keys (Palma, 2013) and by comparison with specimens from the Collection of Continental Fish of the CIIDIR Unidad Oaxaca (OAX.PEC.122.0302).
The presence of otters was analyzed with generalized linear models using a binomial distribution (link = logit) (Zuur et al., 2009). As the response variable, we used the presence/absence of otters in sites surveyed. The environmental and physical-chemical variables, as explanatory variables, were centered to avoid intersects of 0. With the transformed variables, a model was fit with each of the 7 variables in 7 separate models. The analyses were performed with lm4 package for R (R Core Team, 2013).
Otter density was estimated with the Gallo-Reynoso (1996) and Eberhardt and Van Etten (1956) indexes, modified by Duque-Dávila et al. (2013). The Gallo-Reynoso index is calculated with the equation: otters/km = (ns/df)/tl, whereas the Eberhardt and Van Etten index is calculated with the equation: otters/km = (ns/(ds*df))/tl. In both indexes, ns is the number of scats, ds is the time since the deposit of the scats, df is the defecation rate, tl is the transect longitude. The defecation rate used was 3 scats/day as reported by Gallo-Reynoso (1996).
The number of prey taxa was obtained by counting the taxa found in the scats. The frequency of appearance of the food consumed by the otters was calculated with the quotient of the number of scats in which the prey taxon appeared and the total number of scats multiplied by 100 (Macías-Sánchez & Aranda, 1999). For fish, in which a hypural plate corresponds to one individual, we calculated the frequency of each fish species relative to the total number of hypural plates. In addition, we calculated the frequency of each species of fish in each scat. We measured the feeding niche breath with the Levins index modified by Krebs (1989) with the equation: B = ((1/Σ(fr)2)-1)n-1, where B is the Levin´s index, fr is the relative frequency of each type of food, and n is the total number of prey. When values are lower than 0.6 the diet of the species is determined by a few prey, i.e., it is a specialist predator, whereas values higher than 0.6 indicate a generalist predator (Feisinger et al., 1981).
Results
Of the 32 transects examined along the riverbanks in the southeastern TCBR during the dry season 2015-2016, we found evidence (scats, tracks, and direct observations) of otters in only 9 of them (28.1%; Fig. 2). The records of otters were localized along the Grande River in a section between the municipalities of Nanahuatipam and Cuicatlán. We found no evidence in the northern or southern parts of this stretch, nor in the river tributaries, except for the Cacahuatal River (Fig. 2).
The general linear models show positive and significant correlation of otter presence with the river width (p = 0.026), river depth (p = 0.016), and dissolved oxygen concentration (p = 0.039). On the other hand, we found a negative effect with elevation (p = 0.031). No nominal variable was significant as a determinant of the otter presence (p > 0.05; Table 2).
With the Gallo-Reynoso index, overall estimated density was 0.19 otters/km (n = 13; s.d. = 0.19), while with the Eberhardt and Van Etten index it was 0.22 otter/km (n = 9; s.d. = 0.19). According to the Gallo-Reynoso index, the Quiotepec Cacahuatal River had the highest density (0.28 otters/km), followed by the Quiotepec Grande River (0.15 otters/km), whereas for the Eberhardt and Van Etten index, the position of these transects are inverted, with 0.29 otters/km for the Quiotepec Grande River and 0.25 otters/km for the Quiotepec Cacahuatal River. The Cuicatlán Grande River presented the lower density for both indexes, 0.15 and 0.13 otters/km, respectively.
In 270 otter scats analyzed, we found remains of 12 prey taxa: fish (7 taxa), reptiles (2 taxa), birds (1 taxon), hemipteran (1 taxon), and megalopteran (1 taxon). Only native fish (Actinopterygii) were found, distributed in 5 families and 4 orders. In the southeastern part of the TCBR, fish were the main otter food, found in 94.1% of the scats, while in 4.4%, there were insects. Reptiles and birds appeared in 0.9% and 0.6% of the scats, respectively (Table 3).
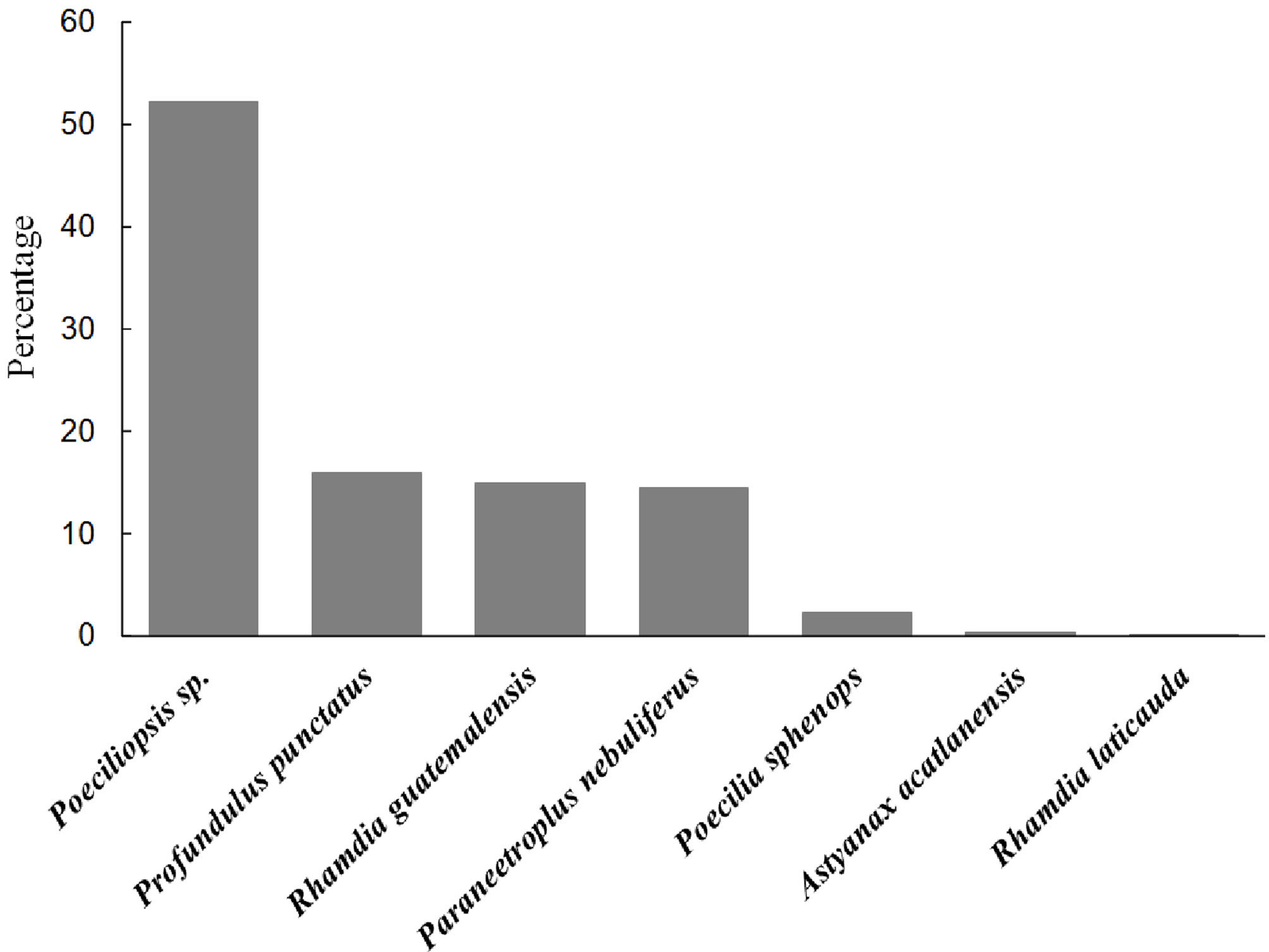
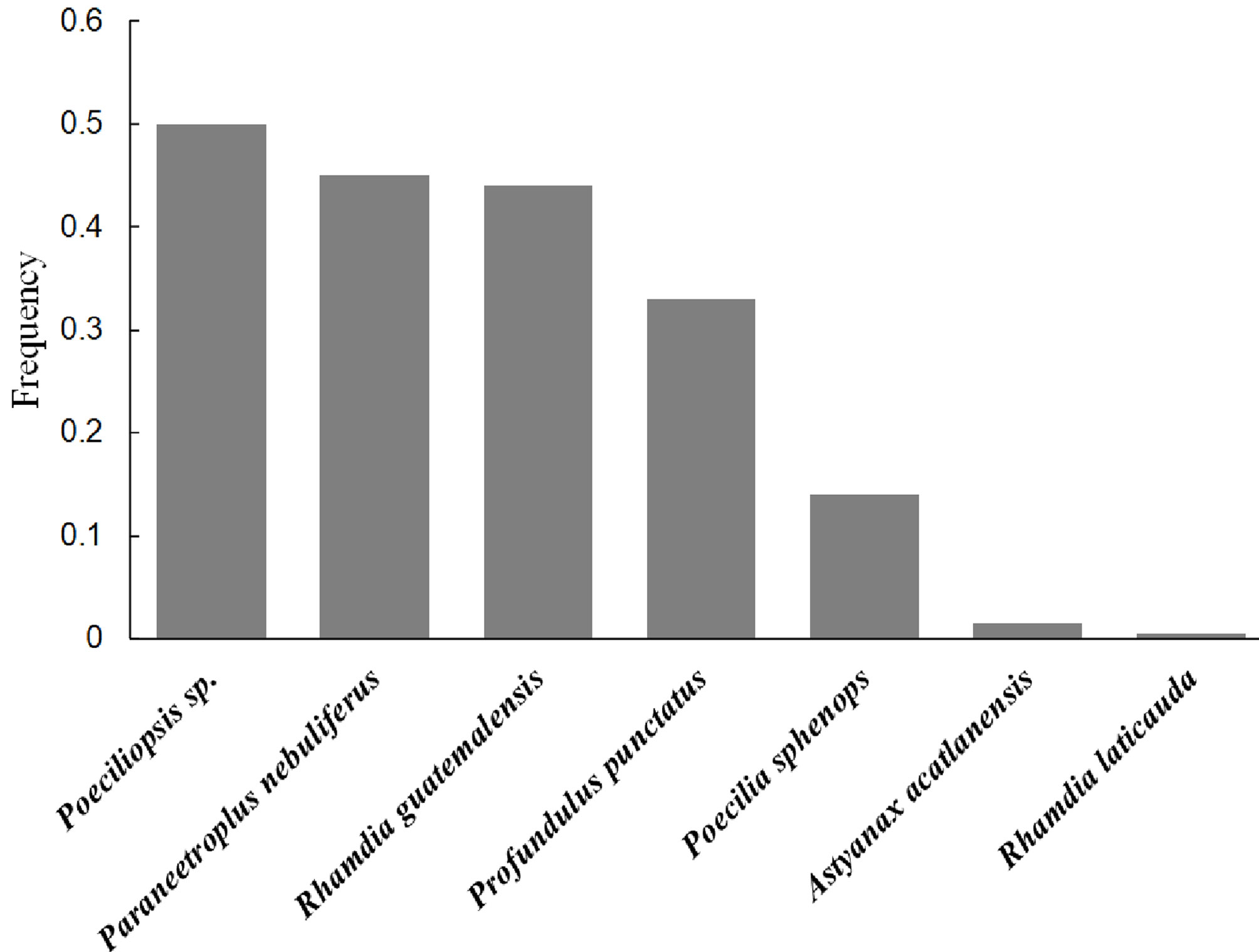
Of the 270 scats analyzed, there were hypural plates in 190 (n = 985). Based on the count of these plates (1 plate = 1 individual), of the 985 preyed individuals, half of them were Poeciliopsis sp. (52.3%), and the other 3 most frequent species (Profundulus punctatus, Rhamdia guatemalensis, and Paraneetroplus nebuliferus) combined made up 42% (Fig. 3). The order of importance of these species varied slightly if we consider their frequency in the scats because we found hypural plates of Poeciliopsis sp., P. nebuliferus and R. guatemalensis, in half of the analyzed scats, and plates of P. punctatus in one-third of the scats (Fig. 4). The feeding niche breadth measured with the Levins index was 0.22.
Table 2
Results of the generalized linear models investigating the effect of environmental variables on the Neotropical otter presence in southern Tehuacán-Cuicatlán Biosphere Reserve.
| Variable | Estimate | Standard error | z value | Pr (> |z|) | AICc |
| Cover | 37.94 | ||||
| Few | 1.38 | 1.2845 | 1.079 | 0.280 | |
| Moderate | 0.13 | 1.3758 | 0.097 | 0.923 | |
| Dense | -17.17 | 2465.3259 | -0.007 | 0.994 | |
| Vegetation type | 43.485 | ||||
| Gallery | 0.56 | 1.5469 | 0.362 | 0.7175 | |
| Xerophilous | 1.2528 | 1.2320 | 1.017 | 0.3092 | |
| Dry forest | 1.2528 | 1.3758 | 0.911 | 0.3625 | |
| Disturbance | 42.934 | ||||
| Moderate | 0.4055 | 1.4434 | 0.281 | 0.779 | |
| Much | -0.6931 | 1.3463 | -0.515 | 0.607 | |
| Complete | -15.8729 | 2399.5450 | -0.007 | 0.995 | |
| Elevation | -10.993 | 5.110 | -2.151 | 0.0314 | 17.059 |
| River width | 1.1199 | 0.5027 | 2.228 | 0.02588 | 33.401 |
| River depth | 2.0756 | 0.8608 | 2.411 | 0.0159 | 26.692 |
| Dissolved oxygen | 1.1989 | 0.5808 | 2.064 | 0.039 | 33.7562 |
Table 3
Prey items in neotropical otter scats in the dry season from southern Tehuacán-Cuicatlán Biosphere Reserve.
| Phylum | Class | Order | Family | Species | Percentage |
| Arthropoda | Insecta | Hemiptera | 1.37 | ||
| Megaloptera | Coriladydae | 3.03 | |||
| Cordata | Actinopterygii | Characiformes | Characidae | Astyanax acatlanensis | 0.39 |
| Cyprinodontiformes | Poeciliidae | Poecilia sphenops | 4.30 | ||
| Poeciliopsis sp. | 61.19 | ||||
| Profundulidae | Profundulus punctatus | 1.56 | |||
| Perciformes | Cichlidae | Paraneetroplus nebuliferus | 13.00 | ||
| Siluriformes | Heptapteridae | Rhamdia guatemalensis | 13.59 | ||
| Rhamdia laticauda | 0.10 | ||||
| Reptilia | Squamata | Iguanidae | Iguana iguana | 0.10 | |
| Ctenosaura pectinata | 0.78 | ||||
| Aves | 0.59 |
Discussion
We found that the neotropical otter is present along the Grande River, the main river of the southeastern part of the TCBR, extending the range more northward than previously reported (Duque-Dávila et al., 2013). The surveys conducted in the tributaries of the Grande River did not show evidence of otter presence, except for the Cacahuatal River. Ruiz-Velásquez et al. (2014) reported evidence of the neotropical otter in the Tepelmeme River. This river joins the Grande River, but in the transect along the Tepelmeme River we found no evidence of otters, except where this river connects with the Grande River. Like the Tepelmeme River, other transects located at elevations above 800 m asl, did not have evidence of otter presence. In relation to this observation, the generalized linear models showed elevation was a variable determining otter presence. Overall, most of the records of otters are at elevations below 1,500 m asl, and our records fit this pattern (Lavière, 1999).
We found river width and depth to be positively correlated to otter presence. Similar findings were reported previously for rivers in Chihuahua, northern Mexico, and Pantanal, Brazil, where neotropical otters selected deep and wide rivers (Carrillo-Rubio & Lafón, 2004; Muãnis & Oliveira, 2011). Large and deep-water bodies can have more prey than small water bodies. On the other hand, we found a weak relationship between otter presence and dissolved oxygen concentration.
Although, otters can tolerate anthropogenic disturbances, being capable of inhabiting in agricultural and cattle ranching landscapes and even near urban areas (Rheingantz et al., 2017); high abundances are found in sites with particular characteristics, such as deep and wide water bodies, and low human presence (Laviere et al., 1999). In our study, average otter abundance in the dry season was between 0.19 and 0.22 otters/km, according to the indexes of Gallo-Reynoso (1996) and Eberhardt and Van Etten (1956), respectively. These estimates are in the interval observed for the species in Mexico (0.001 to 0.97 otters/km; Arellano et al., 2012; Briones-Salas et al., 2008; Casariego-Madorell et al., 2006; Duque-Dávila et al., 2013; Gallo-Reynoso, 1996; González-Christen et al., 2013; Guerrero-Flores et al., 2013). However, in a previous study conducted in the area, with the same method and in the same season, Duque-Dávila et al. (2013) reported an abundance of 0.79 otters/km, with the Gallo-Reynoso index, which is nearly 4 times that reported here. This decrease in abundance is more notable if we consider the rate of finding scats, 2.37 fresh scats per km, reported by Duque-Dávila et al. (2013) in 2006 against 0.30 fresh scats found in our study. In contrast, in Lake Catemaco (Veracruz), which has one of the highest densities reported for Mexico, González-Christen et al. (2013) reported an average of 4.1 fresh scats per km between November and January. Likewise, we found differences in specific estimates for the transects studied, densities in the 2 transects in Quiotepec were higher than the transect in Cuicatlán. Differences could be attributable to the lower habitat perturbation in Quiotepec, which is a small-sized settlement (270 inhabitants; INEGI, 2010), in contrast with Cuicatlán (3,558 inhabitants; INEGI, 2010). Duque-Dávila et al. (2013), during their surveys, observed moderate levels of disturbance in the study area with scant garbage management and sewage discharge. We also observed these conditions, particularly in Cuicatlán. Pollution and high human density are probably affecting the otters as previously observed (Gomez et al., 2014).
From the scats collected in the southern TCBR, we determined that the neotropical otter feeds on 12 types of prey, including insects, reptiles, birds and mainly fish. Particularly for Mexico, ca. 67 taxa had been identified as food for the neotropical otter (Briones-Salas et al., 2013; Casariego-Madorell et al., 2006; Duque-Dávila et al., 2013; Gallo-Reynoso et al., 2008; Macías-Sánchez & Aranda, 1999; Santiago-Plata et al., 2013; this study). Therefore, in the southern part of TCRB, it feeds on 26.8% of the total known prey. Although otters feed on a diverse spectrum of prey, the feeding niche breadth of the neotropical otter in this study was low (0.22), indicating a specialized predatory behavior in the dry season, with Poeciliopsis sp. present in 61% of the scats.
In this study, fish had the highest proportion in the scats (94.1%), and it was slightly higher than that reported by Duque-Dávila et al. (2013). Although, our study is restricted to the dry season, in the 2 studies, the proportion of fish is higher than that reported in other studies conducted in rivers of Mexico, where fish was 29.5 to 54.1% of their diet (Briones-Salas et al., 2013; Casariego-Madorell et al., 2006; Macías-Sánchez & Aranda, 1999). Also, it was notable that, in both the Duque-Dávila et al. (2013) study and ours, crayfish were absent. This group usually constitutes a high or remarkably high proportion of otter diets (Briones-Salas et al., 2013; Casariego-Madorell et al., 2006; Macías-Sánchez & Aranda, 1999). Although our surveys were carried out only in the dry season, we discard the absence of crayfish in scat samples is season dependent. The most plausible explanation is the barrier caused by the dams Miguel Alemán (Temascal) and Miguel de la Madrid (Cerro de Oro) constructed in 1949 to 1955 and in the 1980s, respectively. In interviews, local inhabitants mentioned that crayfish were part of the animal community in rivers in the southern part of TCBR (Martínez-Ramírez, 2017), but currently dams constitute barriers for migration of these species of crayfish to the Grande River and could explain their absence in otter scats.
The fish Poeciliopsis sp., P. nebuliferus, P. punctatus and R. guatemalensis, are a major part of otter diet in this site of the TCBR, appearing in 50% of the scats. Poeciliopsis sp. is a small species with a total length of 1 to 5.1 cm (Lucinda, 2003; Miller et al., 2009; Velázquez-Velázquez et al., 2015); it has been observed to be an abundant species in the rivers (Velázquez-Velázquez et al., 2007). In contrast, P. punctatus, R. guatemalensis and P. nebuliferus are larger species: the first measures 3.2 to 10.0 cm (González, 2008; Huber, 1996; Velázquez-Velázquez et al., 2015), the second is 20 cm long, and the third is often 12 cm long, but can grow up to 47 cm long (Anzueto et al., 2013; Bockmann & Guazzelli, 2003; Conkel, 1993). Consequently, in the southern part of the TCBR, the high frequencies of this species in otter diet is due to high abundances (Poeciliopsis sp.) or large size (R. guatemalensis and P. nebuliferus). Moreover, the presence of P. punctatus, a species that inhabits high elevations, above 1,000 m in this watershed, suggests that they move along the tributaries, in turn explaining the registers of otters (Martínez-Ramírez, 2017; Ruiz-Velásquez et al., 2014).
The fish species consumed by otters in the southern TCBR have been identified as water quality bioindicators (Huidobro-Campos, 2000). P. punctatus inhabits clean rivers with low human impact. R. guatemalensis, R. laticauda, and P. nebuliferus are more abundant in sites far from urban areas. Poecilia sphenops is usually tolerant to urban and industrial pollution and low concentrations of dissolved oxygen. Astyanax acatlanensis is considered a tolerant species able to live in water with high concentrations of heavy metals, with urban, agricultural and industrial pollution, although in the study sites higher abundance of this species has been registered in sites far from human settlements (Martínez-Ramírez, 2017). In the area there are 2 species of Poeciliopsis, P. gracilis and P. fasciata, the former is tolerant to urban and industrial pollution, while the latter is more abundant in sites distant from human settlements. This indicates that the otters are consuming species that are common in the main river (Grande River), where the populations of fish are located far from urban areas and they venture into the meridional parts of the TCBR where there are human settlements.
The presence of the neotropical otter in the southern part of TCBR was explained by elevation, river depth, and width, and dissolved oxygen concentrations. Although otter feeding habits are diverse, 4 species of fish constitute the basis of their diet, selected for their size or abundance. Finally, we detected lower otter abundance relative to previous studies. Therefore, it is necessary to continue studies on their abundance to plan their long-term conservation.
Since our results apply only to the dry season, and abundance estimates were lower than previous surveys, it is necessary to conduct yearly studies to identify trends and to implement actions focused on their conservation and management (de Almeida & Ramos, 2017).
Acknowledgements
To Municipal authorities of Santiago Chazumba, San José Miahuatlán, San Antonio Nanahuatipam, Santa María Tecomavaca, San Juan de los Cués, San Martín Toxpalan, Santa María Ixcatlán, San Miguel Huautla, Santiago Apoala, Santa María Texcatitlán, San Juan Bautista Cuicatlán, San Juan Bautista Atatlahuca, San Juan Bautista Coixtlahuaca, San Miguel Tequixtepec, San Juan de los Cués and Tepelmeme Villa de Morelos. We thank E. Morales, A. López, and J. Viveros for their support during surveys; C. Pinacho for their comments and suggestions; M. S. García Velasco and Diane Miyoshi reviewed the English version. The Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio) and Instituto Politécnico Nacional (SIP-2015-RE/054) for the support throughout the project LI007 “Diagnóstico de las especies invasoras de peces en el área oaxaqueña de la Reserva de la Biosfera Tehuacán-Cuicatlán”, and the Instituto Politécnico Nacional through “Relación trófica entre especies exóticas y nativas de peces en la parte oaxaqueña de la Reserva de la Biosfera Tehuacán-Cuicatlán” (SIP 20164780) project.
References
Alarcon, G. G., & Simões-Lopes, P. C. (2003). Preserved versus degraded coastal environments: a case study of the Neotropical otter in the environmental protection area of Anhatomirim, Southern Brazil. IUCN Otter Specialist Group Bulletin, 20, 6–18.
Anzueto, M. J., Velázquez, E., Gómez, A. E., Quiñonez, R. M., & Joyce, B. (2013). Peces de la Reserva de la Biosfera Selva El Ocote, Chiapas, México. Informe técnico. Tuxtla Gutiérrez: Universidad de Ciencias y Artes de Chiapas.
Aranda, M. (2000). Huellas y otros rastros de los mamíferos grandes y medianos de México. Xalapa: Instituto de Ecología, A.C.
Arellano, N., Sánchez, E., & Mosqueda, M. A. (2012). Distribución y abundancia de la nutria neotropical (Lontra longicaudis annectens) en Tlacotalpan, Veracruz. Acta Zoológica Mexicana (n.s.), 8, 270–279. https://doi.org/10.21829/azm.2012.282832
Arriaga, L., Espinoza, J. M., Aguilar, C., Martínez, E., Gómez, L., & Loa, E. (Coord.). (2000). Regiones terrestres prioritarias de México. Ciudad de México: Comisión Nacional para el Conocimiento y uso de la Biodiversidad.
Bockmann, F. A., & Guazzelli, G. M. (2003). Heptapteridae (Heptapterids). In R. E. Reis, S. O. Kullander, & C. J. Ferraris, Jr. (Eds.), Checklist of the freshwater fishes of South and Central America (pp. 406–431). Porto Alegre: EDIPUC.
Botello, F., Salazar, J. M., Illoldi, P., Linaje, M., Monroy, G., Duque, D. et al. (2006). Primer registro de la nutria neotropical de río (Lontra longicaudis) en la Reserva de la Biosfera de Tehuacán-Cuicatlán, Oaxaca, México. Revista Mexicana de Biodiversidad, 77, 133-135. http://doi.org/10.22201/ib.20078706e.2006.001.323
Briones-Salas, M., Cruz, J., Gallo-Reynoso, J. P., & Sánchez-Cordero, V. (2008). Abundancia de la nutria neotropical de río (Lontra longicaudis annectens Major, 1897) en el río Zimatán en la costa de Oaxaca, México. In C. Lorenzo, E. Medinilla, & J. Ortega (Eds.), Avances en el estudio de los mamíferos de México II (pp. 354–376). Ciudad de México: Asociación Mexicana de Mastozoología, A.C. (AMMAC).
Briones-Salas, M., Peralta-Pérez, M. A., & Arellanes, E. (2013). Análisis temporal de los hábitos alimentarios de la nutria neotropical (Lontra longicaudis) en el río Zimatán en la costa de Oaxaca, México. Theyra, 4, 311–326. https://doi.org/10.12933/therya-13-138
Carrillo-Rubio, E., & Lafón, A. (2004). Neotropical river otter micro-habitat preference in west-central Chihuahua, Mexico. IUCN Otter Specialist Group Bulletin, 21, 10–15.
Casariego-Madorell, M., List, R., & Ceballos, G. (2006). Aspectos básicos sobre la ecología de la nutria de río (Lontra longicaudis annectes) para la costa de Oaxaca. Revista Mexicana de Mastozoología, 10, 71–74. https://doi.org/10.22201/ie.20074484e.2006.10.1.143
Conkel, D. (1993). Cichlids of North and Central America. New Jersey: T.F.H. Publications.
de Almeida, L. R., & Ramos, M. J. (2017). Ecology and biogeography of the Neotropical otter Lontra longicaudis: existing knowledge and open questions. Mammal Research, 62, 313–321. https://doi.org/10.1007/s13364-017-0333-1
Duque-Dávila, D., Martínez-Ramírez, E., Botello, F. J., & Sánchez-Cordero, V. (2013). Distribución, abundancia y hábitos alimentarios de la nutria (Lontra longicaudis annectens Major, 1897) en el río Grande, Reserva de la Biosfera Tehuacán-Cuicatlán, Oaxaca, México. Therya, 4, 281–296. https://doi.org/10.12933/therya-13-128
Eberhardt, L., & Van Etten, R. C. (1956). Evaluation of the pellet group count as a deeer census method. The Journal of Wildlife Management, 20, 70–74. https://doi.org/10.2307/3797250
Feisinger, P., Spears, E., & Poole, R. (1981). A simple measure of niche breath. Ecology, 62, 27–32. https://doi.org/10.2307/1936664
Gallo-Reynoso, J. P. (1996). Distribution of the neotropical river otter (Lontra longicaudis annectens Major, 1897) in the río Yaqui, Sonora. IUCN Otter Group Specialist Bulletin, 13, 27–31.
Gallo-Reynoso, J. P., Ramos-Rosas, N. N., & Rangel-Aguilar, O. (2008). Depredación de aves acuáticas por la nutria neotropical (Lontra longicaudis annectens), en el río Yaqui, Sonora, México. Revista Mexicana de Biodiversidad, 79, 275–279. http://dx.doi.org/10.22201/ib.20078706e.2008.001.502
Gomez, J. J., Túnez, J. I., Fracassi, N., & Cassini, M. H. (2014). Habitat suitability and anthropogenic correlates of Neotropical river otter (Lontra longicaudis) distribution. Journal of Mammalogy, 95, 824–833. https://doi.org/10.1644/
13-MAMM-A-265
González, A. A. (2008). Estudio morfométrico y osteológico del género Profundulus (Cyprinodontiformes: Profundulidae) (Masters thesis). Querétaro: Universidad Autónoma de Querétaro.
González-Christen, A., Delfín-Alonso, C. A., & Sosa-Martínez, A. (2013). Distribución y abundancia de la nutria neotropical (Lontra longicaudis annectens Major, 1897), en el Lago de Catemaco, Veracruz, México. Therya, 4, 201–217. https://doi.org/10.12933/therya-13-125
Guerrero-Flores, J. J., Macías-Sánchez, S., Mundo, V., & Méndez-Sánchez, F. (2013). Ecología de la nutria (Lontra longicaudis) en el municipio de Temascaltepec, estado de México: estudio de caso. Therya, 4, 231–242. https://doi.org/10.12933/therya-13-127
Huber, J. H. (1996). Killi-Data 1996. Updated checklist of taxonomic names, collecting localities and bibliographic references of oviparous Cyprinodont fishes (Atherinomorpha, Pisces). Paris: Société Française d’Ichtyologie, Muséum National d’Histoire Naturelle.
Huidobro-Campos, L. (2000). Peces. In G. De la Lanza, S. Espino, & J. L. Carbajal (Eds.), Organismos indicadores de la calidad del agua y de contaminación (bioindicadoras) (pp. 195–263). Ciudad de México: Secretaría de Medio Ambiente Recursos Naturales y Pesca/ Comisión Nacional del Agua/ Universidad Nacional Autónoma de México/ Plaza Valdés y Editores.
INEGI (Instituto Nacional de Estadística, Geografía e Informática). (2010). National census 2010 [Access in 2016]. Available at: https://www.inegi.org.mx/programas/ccpv/2010/
INEGI (Instituto Nacional de Estadística, Geografía e Informática). (2013). Land cover and use of Mexico, scale 1:25000 [Access in 2016]. Available at: https://www.inegi.org.mx/temas/usosuelo/
IUCN (International Union for Conservation of Nature). (2016). Red List of Threatened Species, IUCN-SSC [Access in 2016]. Available at: www.redlist.org
Krebs, C. J. (1989). Ecological methodology. New York: Harper Collins.
Kruuk, H. (2006). Otters: ecology, behavior and conservation. Oxford: Oxford University Press.
Lavière, S. (1999). Lontra longicaudis. Mammalian Species, 609, 1–5.
Lucinda, P. H. F. (2003). Poeciliidae (Livebearers). In R. E. Reis, S. O. Kullander, & C. J. Ferraris, Jr. (Eds.), Checklist of the freshwater fishes of South and Central America (pp. 555–581). Porto Alegre, Brazil: EDIPUCRS.
Macías-Sánchez, S., & Aranda, M. (1999). Análisis de la alimentación de la nutria Lontra longicaudis (Mammalia: Carnivora) en un sector del río Los Pescados, Veracruz, México. Acta Zoológica Mexicana (n.s.), 76, 49–57.
Martínez-Ramírez, E. (2017). Proyecto LI007 Diagnóstico de las especies invasoras de peces en el área oaxaqueña de la Reserva de la Biosfera Tehuacán-Cuicatlán. Informe técnico. Ciudad de México: Comisión Nacional para el Conocimiento y uso de la Biodiversidad.
Martínez-Ramírez, E., Cruz Arenas, E., Cruz-Ruíz, G. I., & Gómez-Ugalde, R. M. (2013). Los peces de la Reserva de la Biosfera Tehuacán-Cuicatlán, Región Oaxaca. In M. Briones-Salas, G. Manzanero-Medina, & G. González-Pérez (Eds.), Estudios en zonas áridas de Oaxaca. Homenaje al Dr. Alejandro Flores Martínez (pp. 130–144). Ciudad de Oaxaca: CIIDIR-IPN Unidad Oaxaca.
Miller, R. R., Minckley, W. L., & Norris, S. M. (2009). Peces dulceacuícolas de México. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad/ Sociedad Ictiológica Mexicana, A.C./ El Colegio de la Frontera Sur/ Consejo de los Peces del Desierto México-Estados Unidos.
Monroy-Vilchis, O., & Mundo, V. (2009). Nicho trófico de la nutria neotropical (Lontra longicaudis) en un ambiente modificado, Temascaltepec, México. Revista Mexicana de Biodiversidad, 80, 801–808. https://doi.org/10.1016/j.rmb.2017.07.001
Muãnis, M. C., & Oliveira, L. F. B. (2011). Habitat use and food niche overlap by Neotropical otter, Lontra longicaudis, and giant otter, Pteronura brasiliensis, in the Pantanal wetland, Brazil. Otter Specialist Group Bulletin, 28, 76–85.
Palma, A. (2013). Guía para la identificación de macroinvertebrados acuáticos. Santiago, Chile: Departamento de Ecología y Medio Ambiente, Instituto de Filosofía y Ciencias de la Complejidad.
R Core Team. (2013). R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria [Access in 2016]. Available in: http://www.R-project.org/
Rheingantz, M. L., Oliveira-Santos, L. G. R., Waldemarin, H. F., & Pellegrini, E. (2012). Are otters generalists or do they prefer larger, slower prey? Feeding flexibility of the Neotropical Otter Lontra longicaudis in the Atlantic Forest. IUCN Otter Specialist Group Bulletin, 29, 80–94.
Rheingantz, M. L., Santiago-Plata, V. M., & Trinca, C. S. (2017). The Neotropical otter Lontra longicaudis: a comprehensive update on the current knowledge and conservation status of this semiaquatic carnivore. Mammal Review, 47, 291–305. https://doi.org/10.1111/mam.12098
Rheingantz, M. L., & Trincam, C. S. (2015). Lontra longicaudis. The IUCN Red List of Threatened Species 2015: e.T12304A21937379. [Access in 2015]. Available at: http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T12304
A21937379.en
Ruiz-Velásquez, E., Andrés-Reyes, J. V., & Santos-Moreno, A. (2014). Registros notables de tres especies de mamíferos del estado de Oaxaca, México. Revista Mexicana de Biodiversidad, 85, 325–327. https://doi.org/10.7550/rmb.
33961
Santiago-Plata, V. M., Valdez-Leal, J. D., Pacheco-Figueroa, C. J., Cruz-Burelo, F., & Moguel-Ordóñez, E. J. (2013). Aspectos ecológicos de la nutria neotropical (Lontra longicaudis annectens) en el camino La Veleta en la Laguna de Términos, Campeche, México. Therya, 2, 265–280. https://doi.org/10.12933/therya-13-131
Trejo, I. (2004). Clima. In A. J. García-Mendoza, J. M. Ordóñez, & M. Briones-Salas (Eds), Biodiversidad de Oaxaca (pp. 67–85). Ciudad de México: Instituto de Biología, UNAM/ Fondo Oaxaqueño para la Conservación de la Naturaleza/ World Wildlife Fund.
Velázquez-Velázquez, E., Gómez-González, A. E., Vega-Cendejas, M. E., Rivera-Velázquez, G., & Domínguez-Cisneros, S. E. (2007). Peces del sistema estuarino Carretas-Pereyra, Reserva de la Biosfera La Encrucijada, Chiapas. Lacandonia, 1, 45–54.
Velázquez-Velázquez, E., Maza-Cruz, M., Gómez-González, A. E., & Navarro-Alberto, J. A. (2015). Length-weight relationships for 32 fish species in the Grijalva River Basin, Mexico. Journal of Applied Ichthyology, 31, 413–414. https://doi.org/10.1111/jai.12676
Zuur, F., Ieno, E. N., Walker, N. J., Saveliev, A. A., & Smith, G. M. (2009). Mixed effects models and extensions in ecology with R. New York: Springer.