Joelcio Freitas a, b, *, Elton John de Lírio c, d, Quélita dos Santos-Moraes a, Victor Santos-Miranda e, Renara Nichio-Amaral a, Favio González f, Anderson Alves-Araújo a, g
a Universidade Estadual de Feira de Santana, Av. Transnordestina s/n., Feira de Santana, 44036-900 Bahia, Brazil
b Instituto Nacional da Mata Atlântica, Avenida José Ruschi, 04, Centro, Santa Teresa, 29650-000 Espírito Santo, Brazil
c Universidade de São Paulo, Instituto de Biociência, Departamento de Botânica, Rua do Matão 277, Edifício Sobre-as-ondas (Herbário), São Paulo, 05508-900 São Paulo, Brazil
d Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rua Pacheco Leão, 915, Jardim Botânico, Rio de Janeiro, 22460-030 Rio de Janeiro, Brazil
e Universidade Federal do Espírito Santo, Departamento de Ciências Agrárias e Biológicas, Rodovia BR 101 Norte, Km 60, São Mateus, 29932-540 Espírito Santo, Brazil
f Universidad Nacional de Colombia, Facultad de Ciencias, Instituto de Ciencias Naturales, AA 7495, Bogotá, Colombia
g Universidade Federal da Bahia, Instituto de Biologia, Depto. Botânica, R. Barão de Jeremoabo s/n, Campus Universitário de Ondina, Salvador, 40170-115 Bahia, Brazil
*Corresponding author: joelciofr@gmail.com (J. Freitas)
Received: 6 February 2022; accepted: 5 July 2022
Abstract
Two new occurrences of Aristolochia in Brazil are reported here. Aristolochia cremersii, hitherto known from French Guiana, was collected in Oriximiná/Pará, and A. argentina, with previously known distribution in Argentina, Bolivia and Paraguay, was recorded in Bodoquena/Mato Grosso do Sul. The latter species was identified based on photographic records. We provide descriptions, morphological comments, a preliminary extinction risk assessment (with one of them applying the rapid Least Concern method) and illustration/images of the 2 species, and finally discuss the use of photographs as valuable scientific records.
Keywords: Environmental photography; Hexandrae; Piperales; Rapid Least Concern; Taxonomy
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Nuevos registros de Aristolochia (Aristolochiaceae) de Brasil: la importancia de la ciencia ciudadana como herramienta para estudiar la distribución geográfica, la conservación y las interacciones insecto-planta
Resumen
Aquí se reportan 2 nuevas presencias de Aristolochia en Brasil. Aristolochia cremersii, hasta ahora conocida solo de la Guayana Francesa, fue registrada en Oriximiná/Pará y A. argentina, con una distribución previamente conocida en Argentina, Bolivia y Paraguay, fue registrada en Bodoquena/Mato Grosso do Sul. Esta última especie fue determinada con base en registros fotográficos. Presentamos descripciones y comentarios morfológicos, una evaluación del riesgo de extinción (una de ellas aplicando el método rápido de preocupación menor) e ilustraciones/imágenes de las 2 especies. Finalmente, se discute el uso de fotografías como importantes registros científicos aportados por la ciencia ciudadana, para el avance del conocimiento de la biodiversidad.
Palabras clave: Fotografía ambiental; Hexandrae; Piperales; Método rápido preocupación menor; Taxonomía
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Aristolochiaceae (Piperales) comprises 4 genera: Aristolochia L., Asarum L., Saruma Oliver and Thottea Rottbøll, and is distributed in tropical, subtropical and temperate zones (Wanke et al., 2006). In Brazil, the family is represented by 83 species of Aristolochia occurring in the 5 terrestrial biomes found in the country: the Caatinga, the Cerrado, the Amazon Forest, the Atlantic Forest and the Pampas (BFG, 2015; 2022; Freitas et al., 2019; Freitas, Lirio et al., 2020). The species occurring in Brazil can be recognized as vines or lianas, or more rarely, herbaceous perennials reaching up to 40 cm in height. They often have corky and xylopodic stems, solitary flowers or arranged in cymose or racemose inflorescences. The flowers are monochlamydeous and possess a monosymmetric and highly elaborate perianth formed by 3 fused petaloid sepals, which completely surround a gynostemium formed by the fusion of styles and stamens. The inferior ovary formed by 6 carpels and 6 locules often becomes a septicidal, acropetal capsule upon successful insect pollination (Freitas & Alves-Araújo, 2017; González, 1990).
All South American species of Aristolochia belong to the subgenus Aristolochia and to the subsection Hexandrae. This subsection is divided into series Hexandrae F. González and Thyrsicae F. González. In turn, Aristolochia series Hexandrae comprises subseries Hexandrae F. González, characterized by axillary, solitary flowers, and subseries Anthocaulicae F. González, characterized by ramiflorous or cauliflorous racemes with flowers subtended by small bracts and internodes generally shortened (González, 1990, 1991, 1997, 1998). Brazil is one of the hotspots for the diversification of the latter 2 subseries in the New World.
This work aims to report for the first time the occurrence of Aristolochia cremersii Poncy (from subseries Anthocaulicae) and A. argentina Grisebach (from subseries Hexandrae) in Brazil, as a result of ongoing taxonomic and phylogenetic studies of the genus in South America. Whereas the former species had a restricted occurrence in French Guiana (Feuillet & Poncy, 1998), the latter was so far known to occur in Argentina, Bolivia and Paraguay (González et al., 2015). These reports allow us to expand the geographical distribution and to better assess the conservation status of these 2 species. Furthermore, we highlight the importance of photographic records in scientific works especially if the collection of physical material for subsequent deposition as herbarium vouchers is not possible or becomes highly invasive.
Material and methods
Samples of the following herbaria were studied: ALCB, B, BHCB, BM, CAY*, CEPEC, COAH, COL, CONN*, CORD*, CVRD, DUKE*, ESA, F, G, GH, GOET*, HBG*, HSTM, HTSA* (acronym not included in the Index Herbariorum), HUA, HUCP*, HUEFS, IAN, INPA, JAUM, K, L, MA, MBM*, MBML, MEDEL, MG, MO, MPU*, NY, P, R, RB, SCZ*, SP, UFACPZ, U*, UEC*, UPTC, US and VIES (acronyms according to Thiers [2022]). Herbaria indicated with an asterisk (*) were accessed through digital images made available online (GPI, 2022; INCT, 2022; REFLORA, 2022). We also used records available at iNaturalist
(www.inaturalist.org).
The species were identified using the comprehensive keys available in the literature (Ahumada, 1967; Araújo, 2013; Capellari, 1991; Feuillet & Poncy, 1998; González, 1990, 1994; González et al., 2015; Hoehne, 1927, 1942; Nascimento, 2008) and through confirmation of original descriptions and type specimens (at the website of JSTOR (http://plants.jstor.org). Descriptions of the general morphological characters of the species follow Harris and Harris (2001) whereas specific flower and fruit characters of Aristolochia are given according to González (1990, 1994).
IUCN criteria (IUCN, 2012, 2022) were used to assess the risk of global extinction of both species, with extent of occurrence (EOO) and area of occupation (AOO) calculated using the Geospatial Conservation Assessment Tool (GeoCat) (Bachman et al., 2011). Exceptionally, Aristolochia argentina had the AOO calculated through the Rapid Least Concern tool (Bachman et al., 2020), given its high number of records. The Quantum-GIS 2.12 software was used to draw the distribution maps.
Description
Aristolochia cremersii Poncy, Adansonia 4: 339. 1988. Type: French Guiana. Sommet Tabulaire, 25 August 1980 (flower), G. Cremers 6455 (Holotype: P00152012!; isotypes: CAY010508!; CAY010509!; P00152013!).
Lianas with corky stems to 7 cm in diam; branches with internodes 12-14.5 cm long, glabrous; pseudostipules absent. Leaves with petiole 4-6 cm long, and blade broadly ovate, 9-15.5 × 7-14 cm, chartaceous, discolor, adaxial surface dark green, glabrous, abaxial surface grayish, with filiform, multicellular, moniliform to articulate trichomes, base truncate to slightly cordate (sinus ca. 8-12 mm deep), slightly peltate, apex acute to acuminate, basal primary veins 3 (5). Inflorescences in cauliflorous racemes 15-40 cm long, each with 15-25 flowers, each subtended by a minute, triangular bract to 1.5 x 1 mm, and internodes 5-15 mm long. Peduncle + ovary 4.2-10 cm long, slender, < 1 mm in diam. Perianth slightly curved, glabrous on its outer surface; utricle orbicular to obovate, 4-6 × 3-4 mm; syrinx 1.5 mm long, inequilateral; tube funnel-shaped, 1.5-2.5 cm long, 3-4 mm proximal diameter, 6 mm distal diameter; limb bilabiate, upper lip narrowly ovate to linear, 2.3-3.5 × 0.2-0.4 cm, convex, the inner surface with whitish trichomes on its lower portion and fimbriae on its distal margin, not papillate, apex emarginate, caudate, acumen absent, lower lip ovate to oblong, 5-8 × 2-4 mm, concave, apex emarginate. Capsule cylindrical to narrowly cylindrical, 3-5 × 1.4-1.5 cm, formed by 6 fused carpels that lack prominent midveins, shortly rostrate, rostrum < 1 mm long. Seeds ovoid, 4-5 × 2.5-3 mm, concave-convex, verrucose, non-winged, raphe prominently cylindrical.
Taxonomic summary
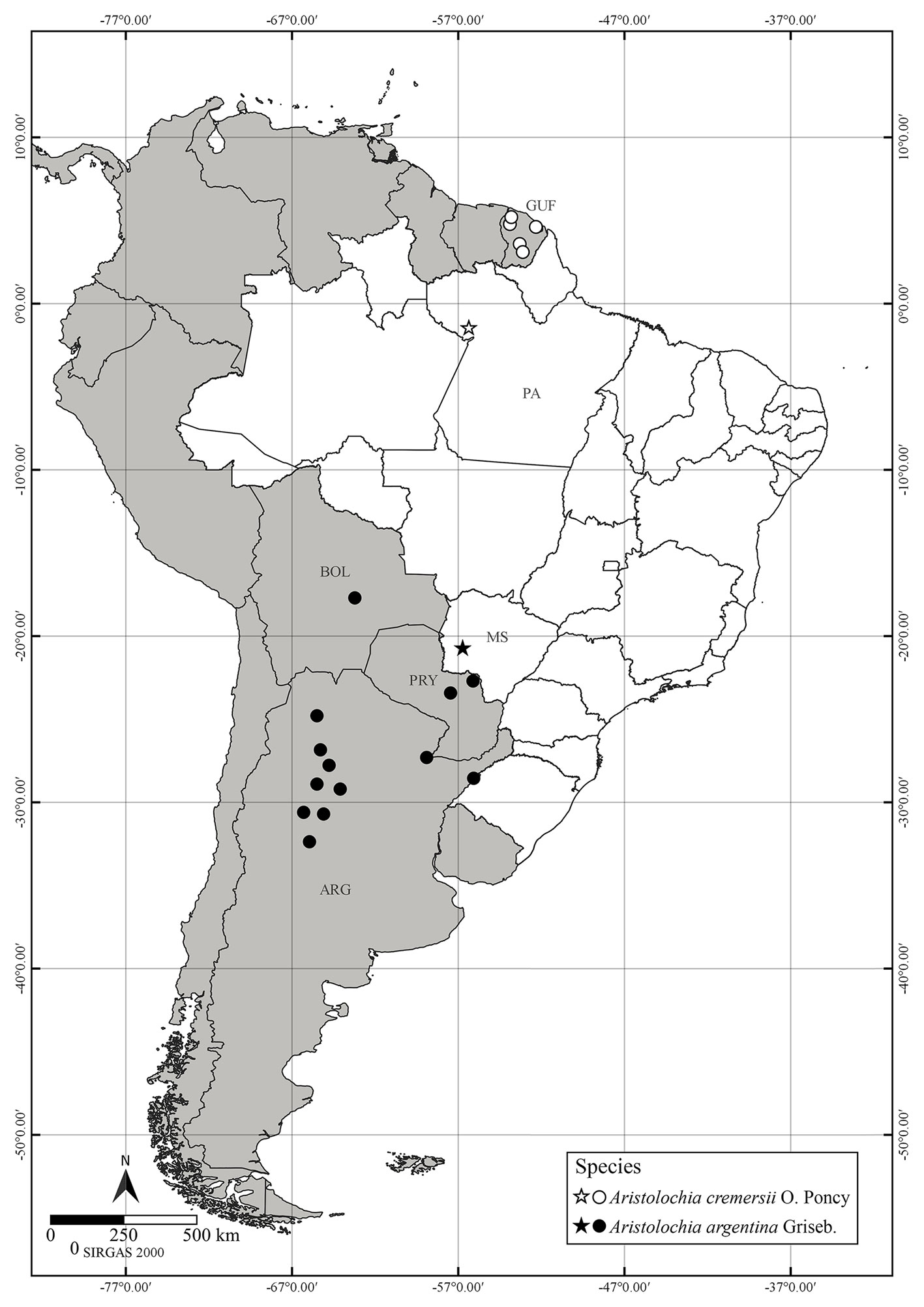
Distribution, habitat and conservation status: Aristolochia cremersii (Fig. 1), hitherto known from French Guiana (Feuillet & Poncy, 1998), is reported here to occur naturally in the state of Pará, municipality of Oriximiná (Brazil) (Fig. 2). The species is here categorized as endangered (EN) B2ab (i, ii, iii, iv, v), following the IUCN criteria (IUCN 2012, 2022). The species has an EOO of 79,604.889 km² and an AOO of 24 km², occurring in 6 localities; increasing fires, deforestation and mining are the main and immediate threats. The plant was recollected in a mining area of Oriximiná (OH Knowles 1596) in 1990. Since then, the species has not been recollected in the area, despite extensive fieldwork by the local botanist plant recollector João Batista Fernandes da Silva in Oriximiná/Pará (pers. commun.) and by the first author of the present work. It is possible that the species has become regionally extinct in Brazil.
Specimens examined: Brazil: Pará, Oriximiná, Mineração Rio do Norte, Porto Trombetas, Estrada da Vila Caranã. 18 October 1990 (flower), O.H. Knowles 1596 (HTSA, INPA). French Guiana: Route de Kaw, Piste du Placer Trésor, 08 February 1999 (flower), O. Poncy 1200 (CAY, P); Camp Pararé, Station de l’Arataye, Bassin de l’Approuague, 14 November 1978 (fruit), O. Poncy 217 (CAY, P); Säul and vicinity, Mont Galbao, NW peak, 03º36’ N, 53º16’ W, 525-700 m, 11 September 1994 (flower; fruit), B. M. Boom 10803 (CAY, MO, US); Region de Paul Isnard, Montagne Lucifer Sur les pentes de la montagne, 250 m, 9 November 1982 (fruit), C. Feuillet 308 (CAY, US); Basin du Maroni, 15 November 2010 (flower) H. Richard & J. Ateni 333 (CAY).
Remarks
Aristolochia cremersii belongs to Aristolochia subser. Anthocaulicae as its inflorescences are cauliflorous racemes with highly reduced subtending bracts (González, 1990, 1991, 1995). Notably, the species has long and loose inflorescences with elongate internodes, a trait atypical in the subseries. Among the neotropical species, it is similar to A. flava Poncy, from which it differs by the upper lip of perianth lacking papillae on its inner surface and the ovate to oblong shape of its lower lip 5-8 × 2-4 cm (vs. the inner surface of the upper lip papillate and the broadly ovate lower lip, 2 × 3-3.5 cm, in A. flava), the peduncle + ovary 4.2-10 cm long (vs. 2.5-3.5 cm in A. flava), and the emarginate apex of the upper perianth lip (vs. obtuse in A. flava).
Aristolochia argentina Griseb., Abh. Königl. Ges. Wiss. Göttingen 19: 156. 1874. Type: Argentina: “Santiago del Estero, in sepibus praediorum prope urbem”, December 1871 (flower), P. G. Lorentz 56 (Lectotype: GOET000266; isolectotypes: CORD00004799, CORD00004800, GOET 000267).
Vines; branches with internodes 6-13.1 cm long, glabrous; pseudostipules present, circular, 1-1.5 cm. Leaves with petiole 2-3.5 cm long, and blade ovate to broadly ovate, 3-7 × 2.5-8.4 cm, membranaceous, adaxial and abaxial surfaces glabrous, base cordate with sinuses 6-29 mm in depth, not peltate, apex obtuse to slightly emarginate, basal primary veins 3 (5). Flowers axillary, solitary, subtended by expanded (not reduced) leaves. Peduncle + ovary 3.5-5.5 cm long, the ovary thickened to 1.4 mm in diam. Perianth strongly curved between the utricle and the tube, glabrous on its outer surface, beige to greenish with vinaceous grooves; utricle widely obovoid, 10-15 × 5-9 mm; tube funnel-shaped, 1-1.8 cm long, 2-3 mm in proximal diameter, 6-8 mm in distal diameter, at an angle of ca. 45° with respect to the tube; limb unilabiate, broadly ovate, 1-2.5 × 1-2.2 cm, yellowish green to dark green, base constricted, apex obtuse. Capsule cylindrical, 2-3.1 × 0.9-1.7 cm, striate, smooth, slightly rostrate, rostrum > 5 mm long. Seeds ovoid, 4-5 × 2.5-3 mm, flat, slightly papillary, with a short, peripheral wing and a prominent raphe.
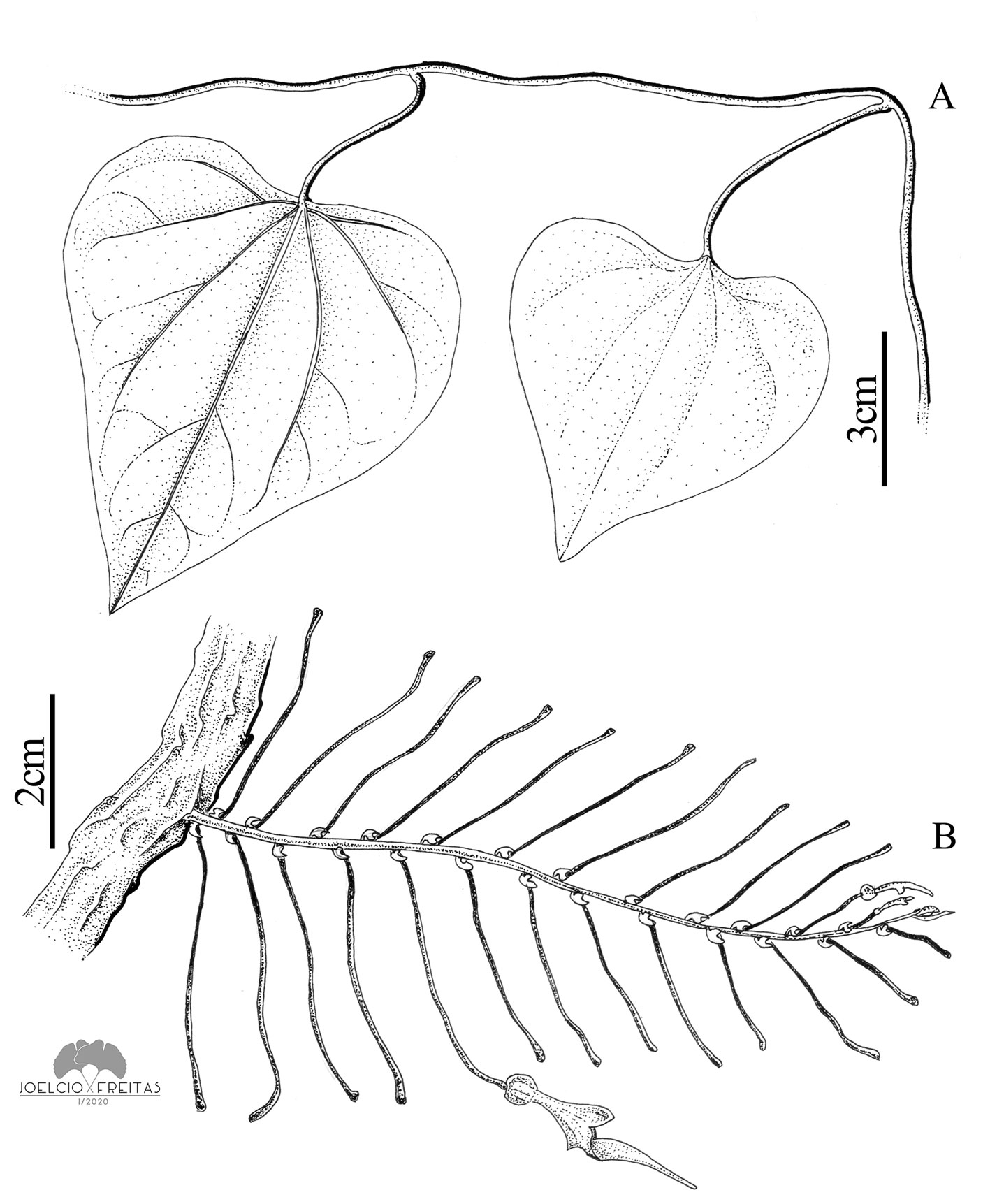
Taxonomic summary
Distribution, habitat and conservation status: previously known from Argentina (Catamarca, Chaco, Córdoba, Corrientes, Jujuy, La Rioja, Salta, San Luis, Santiago del Estero and Tucumán), Bolivia (Santa Cruz) and Paraguay (Amambay and Concepción), the present record of A. argentina (Fig. 3) expands its distribution area to Brazil (state of Mato Grosso do Sul, municipality of Bodoquena) (Fig. 2). The photographic record was carried out on 5 May 2017, near the Boca da Onça Waterfall, during a vacation trip. Aristolochia argentina was classified as Least Concern (LC) according to IUCN criteria. The species has a wide area of geographical distribution, with an EOO of 1,191,124.190 km², AOO of 5,900 km², 99 records in 16 localities, and without significant threats. The cut-off threshold for Rapid Least Concern (Bachman et al., 2020) was established as a minimum of 5 regions, so that the species can be considered as LC.
Specimens examined: Argentina: Catamarca, Dept. Ancasti, a ± 13 km de Ancasti, rumbo a Icaño. 27 February 2004 (flower), Barboza et al. 861 (CORD); Chaco, Colonia Benítez. 1 April 1972 (flower), A.G. Schulz 2571 (MO); Córdoba, Dept. Cruz del Eje. 25 January 1952 (flower), A.T. Hunziker 9720 (CORD); Corrientes. Depto. Santo Tomé, RN 14, San Benito. 16 January 1947 (flower), Huidrobo 4463 (MO); Jujuy. Dept. Ledesma, Calilegua. 3 June 1943 (flower), Bartlett 20342 (NY); La Rioja, Dept. G. Belgrauv, Olta. 23 September 1944 (flower), M.S. Gomez 69 (CONN); Salta, without location. 15 November 1929 (flower), Venturi 9834 (MO); San Luis. Depto. Ayacucho, Luján. 02 December 1944 (flower), Varela 721 (F); Santiago del Estero, Dep. Ojo de Agua. 4 March 1989 (flower), T.M. Pedersen 15169 (CORD, MBM, L); Tucumán, Manantial. 17 October 1945 (flower), Villa 535 (MO); Bolivia: Santa Cruz, Prov. Cordillera, Alto Parapetí “Hacienda Yapuimbia”. 01 September 1985 (flower), R. Michel & S.G. Beck 487 (CONN); Brazil: Mato Grosso do Sul, Bodoquena, Cachoeira Boca da Onça. 05 May 2017 (flower), L.C. Marinho https://www.gbif.org/occurrence/2802635120; https://www.inaturalist.org/observations/97550230 (iNaturalist); Paraguay: Amambay, Sierra de Amambay, ad margines silvarum prope Aramburú. 25 July 1910 (flower), T. Rojas 9731 (MO); Concepción, without location. 12 December 2008 (flower), M. Bolson sn (HUCP).
Remarks
Aristolochia argentina belongs to Aristolochia subser. Hexandrae due to its solitary flowers subtended by expanded leaves (González, 1991). Among the neotropical species, it is similar to A. triangularis Chamisso, but differs from it by the leaf blade with a deeply cordate base (vs. base often truncate or very slightly cordate in A. triangularis), the flowering shoots with expanded subtending leaves (vs. flowering shoots often reduced to small racemes with reduced subtending leaves in A. triangularis), the perianth limb constricted at its base (vs. base not constricted in A. triangularis) and the capsules with a smooth surface (vs. warty surface in A. triangularis).
Importance of field photographs to document floral traits in Aristolochia
Photographs are used in field work mainly to record the habitat in which the plant occurs, as well as threats (IUCN, 2022), and traits that become lost in herbarium specimens. The latter is particularly true for the extremely elaborate, convoluted and colorful perianth of Aristolochia, an organ that is crucial for species identification or discovery of new taxa, as was shown in detail recently (Freitas, González et al., 2020; González & Monzón-Sierra, 2022). The Flora do Brasil Online 2020 (BFG, 2018, 2022), a project that aims to monograph all plant species registered in Brazil, makes it possible that experts from each group provide photographs on the website. These images are intended to facilitate species determination by broad communities, since the website is freely accessible and easy to use.
Photographs also have an important value to follow vegetation changes over time, as they provide accurate time and location data. This resource can also be used to record ecological interactions, such as predators, herbivores, floral visitors, and fruit and seed dispersers (González & Pabón-Mora, 2017). In the field of history and human behavior, photographs can be used to study the botanists’ view of their object of study (Watts, 2017).
Field photographs of material collected and deposited in herbaria have been increasingly used in recent years. However, making these pictures available for users of herbaria and linking them to the vouchers is a current challenge. Some plant collectors have already attached field photographs to herbarium sheets. As examples we can cite the collections of F. Sennen (1861-1937), mainly deposited in the BC herbarium (http://www.ibb.csic.es/herbari/JPEG/BC81104.jpg), and H. A. Liogier (1916-2009), mainly deposited in the NY and S herbaria (Watts, 2017). Recently, Gómez-Bellver et al. (2020) recommended the use of photographs to complement the information of herbarium samples of species difficult to collect or press, such as palm trees, cycads, toxic plants and/or cacti, or plant groups whose drying process strongly distort taxonomic characters (e.g., color and shape), or whose collections become highly invasive and destructive. Some initiatives to fulfill such endeavor, such as SpeciesLink (INCT, 2022) and Tropicos (Missouri Botanical Garden, 2022), are currently linking photographs with vouchers in the database and making them available online.
An additional advantage of field photographs, even less explored, is the possibility of the photograph itself being used as a record, even if it is not associated with any material deposited in collections. Gómez-Bellver et al. (2020) discuss the use of “photo vouchers”, a term coined by Funk et al. (2018) to refer to records that were made only through photos without collection of biological samples. A photograph is not a substitute for a voucher specimen of biological material because it does not allow the gathering of micromorphological, molecular or phytochemical traits. Thus, they are not considered as specimens in the light of the Article 8 of the Code of Botanical Nomenclature (Turland et al., 2018). Despite such limitations, some authors argue that the use of photo vouchers is a valuable, non-invasive alternative in some cases such as when a physical voucher is not possible either due to the lack of a collecting permit or when the plant is protected by law, critically endangered or represented in the locality by a single individual, a good quality photograph, in which vegetative and reproductive characters are evident, can be a useful alternative (Funk et al., 2018; Gómez-Bellver et al., 2020). “Photo vouchers” are already represented in herbaria, as is the case of Dudleya saxosa subsp. collomiae (Rose ex Morton) Moran, in the Desert Botanical Garden Herbarium (DES) (http://swbiodiversity.org/seinet/collections/individual/index.php?occid=8553404) and Achillea maritima (L.) Ehrendorfer & Y.P.Guo (BC-PV-844027), that represents a small population of 6 individuals in Tarragona (Catalonia, Spain) (Gómez-Bellver et al., 2020).

Initiatives such as Discover Life (2022) and the SpeciesLink Photo Library (INCT, 2022) allow photographs of biodiversity to be provided online in their platforms. In the Vinícius Dittrich Photo Library – FVD (2022), for example, there is a record of A. argentina (FVD 37) captured by the botanist V. Dittrich in 2015, in Santiago de Chiquitos, Robore, Santa Cruz, Bolivia. The photo was the only record of the plant, because he did not have a collecting permit for that site (V. Dittrich, pers. comm., 2015).
Tools such as iNaturalist and PlantNet, an additional app for plant identification and recording, have shown to be highly useful by documenting biodiversity through photographic records from citizen science (Bonnet et al., 2020). Citizen science is a powerful ally for ecological, conservation and taxonomic studies, as it makes available reliable data from nature sympathizers that provide novel geographic records and little-known life history traits, or even that help the discovering of new species (Bonnet et al., 2020; Chau, 2022; Gonella et al., 2015; Neill & Kieschnick, 2021).
The iNaturalist, which at the time of writing, has achieved more than 100 million observations, contributed by more than 2 million citizens, can be used on any cell phone with a camera, and photos and information can be added to the application even without access to the internet, or can be uploaded directly on the website if photos were captured with a photographic camera. iNaturalist records can be identified by the photographer, and if another user of the platform later confirms the identification, that record gains the status of “scientific record” and is automatically inserted in the Global Biodiversity Information Facility (GBIF, 2022), an international platform widely used by botanists and conservationists committed to the assessment of species extinction risk (Heberling & Isaac, 2018). Such an initiative contributes to the advancement of knowledge on biodiversity, through citizen science, and makes available critical records from protected areas with restrictive collection permits.
We use the Aristolochia argentina record as an additional example to demonstrate that photographs with proper metadata and referenced locations clearly display the necessary morphological characters for species identifications and increase knowledge on geographic distribution, local ecology and conservation. In this case, the identification of the species was possible through reproductive characters, including the slightly constricted limb, the solitary flowers, and the smooth fruits, all of them clearly visible in the aforementioned photos (FVD 37 [https://specieslink.net/search/records/col/470] and https://www.inaturalist.org/observations/97550230), which left us with no doubts about the identity of the species in question. The interaction of A. argentina with Troidini butterflies is shown in figure 3G. Caterpillars in this group feed exclusively on Aristolochia foliage, sequester the aristolochic acids present in the leaves (toxic to most herbivores) and use them to become toxic and unpalatable for predators (von Euw et al., 1968). Unfortunately, identification of larvae at the species level is not possible. However, the presence of a retractile paired organ called osmeterium (Fig. 3H), located between the head and the anterior larval segments, and activated upon perturbation, is a synapomorphy of the species of tribe Troidini (Reed & Sperling, 2022), and allows their identification at the tribe level.
Although some of these new records can bring crucial information to science, some care and recommendations need to be taken by the photographer, specially providing coordinates. A suitable solution is to include coordinates when the photo is taken (e.g., this can be done directly in iNaturalist and does not require internet access). Another important recommendation is that the use of images as information for scientists depends on the presence of diagnostic taxonomic characters. And lastly, photographs can be initial records of the species and an additional tool for further in-depth studies, yet hardly replacing an herborized specimen, which is always encouraged when possible.
We expect that this study fosters the use of photographic records to increase Aristolochia knowledge. When collections are not possible due to the non-granting of a collecting permit, tools for preparation of herbarium material are not at hand, or species are not abundant in nature or are protected, photographic records should be used as non-invasive but yet highly informative data. This tool also becomes of great relevance and highly attractive for citizen science in megadiverse countries such as Brazil and Colombia, where funding for science and jobs for botanists are lacking or decreasing.
Acknowledgements
We would like to thank Lucas Cardoso Marinho and Vinícius Dittrich for providing images of Aristolochia argentina and the curators of the consulted/visited herbaria for making samples and images available for this study. JF and EJL thank the National Council for Scientific and Technological Development (CNPq) for the granting of a doctoral (grant number 141415/2016-9) and post-doctoral scholarships, respectively, JF also thanks CNPq (within the scope of the Programa de Capacitação Institucional – PCI/INMA) of the Brazilian Ministry of Science, Technology and Innovation (MCTI) for the fellowship granted (grant number 302136/2022-3); and to the National Geographic Society and the International Association of Plant Taxonomy (IAPT) for granting support for field expeditions. AAA also thanks the Research and Innovation Support Foundation of Espírito Santo for the provision of financial support (FAPES Nº 18/2018, TO 525/2018).
References
Ahumada, L. Z. (1967). Revisión de las Aristolochiaceae argentinas. Opera Lilloana, 16, 1–148.
Araújo, A. A. M. (2013). Aristolochiaceae Juss. na Mata Atlântica do Nordeste, Brasil (Master Thesis). Univesidade Federal do Pernambuco, Recife, Brazil.
Bachman, S., Moat, J., Hill, A. W., de la Torre, J., & Scott, B. (2011). Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. In V. Smith, & L. Penev (Eds.), e-Infrastructures for data publishing in biodiversity science. Zookeys, 150, 117–126. https://doi.org/10.3897/zookeys.150.2109
Bachman, S., Walker, B. E., Barrios, S., Copeland, A., & Moat, J. (2020). Rapid Least Concern: towards automating Red List assessments. Biodiversity Data Journal, 8, e47018. https://doi.org/10.3897/BDJ.8.e47018
BFG (The Brazil Flora Group) (2015). Growing knowledge: An overview of Seed Plant diversity in Brazil. Rodriguésia, 66, 1085–1113. https://doi.org/10.1590/2175-7860201566411
BFG (The Brazil Flora Group) (2018). Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia, 69, 1513–1527. https://doi.org/10.1590/2175-7860201869402
BFG (The Brazil Flora Group) (2022). Brazilian Flora 2020: Leveraging the power of a collaborative scientific network. Taxon, 71, 178–198. https://doi.org/10.1002/tax.12640
Bonnet, P., Champ, P., Goëau, H., Stöter, F. R., Deneu, B., Servajean, M. et al. (2020). Pl@ntNet Services, a Contribution to the Monitoring and Sharing of Information on the World Flora. Biodiversity Information Science and Standards, 4, e58933. https://doi.org/10.3897/biss.4.58933
Capellari-Jr., L. (1991). Espécies de Aristolochia L. (Aris-
tolochiaceae) ocorrentes no estado de São Paulo (Master Thesis). Universidade Estadual de Campinas, Campinas.
Chau, M. M. (2022). Rapid response to a tree seed conservation challenge in Hawai‘i through crowdsourcing, citizen science, and community engagement. Journal of Sustainable Forestry, 41, 605–623. https://doi.org/10.1080/10549811.2020.1791186
Discover Life. (2022). Accessed 14 January 2022. https://www.discoverlife.org
Feuillet, C., & Poncy, O. (1998). Aristolochiaceae. In A. R. A. Görts-van Rijn, & M. J. Jansen-Jacobs (eds.), Flora of the Guianas, ser. A. Phanerogams, Fascicle 10. Royal Botanic Gardens, Kew.
FVD (Fototeca Vinícius Dittrich). (2022). Accessed 14 January 2022. https://specieslink.net/col/FVD
Freitas, J., & Alves-Araújo, A. (2017). Flora do Espírito Santo: Aristolochiaceae. Rodriguésia, 68, 1505–1539. https://doi.org/10.1590/2175-7860201768501
Freitas, J., González, F., Poncy, O., Feuillet, C., & Alves-Araújo, A. (2020). Floral geometric morphometrics unveils a new cauliflorous species of Aristolochia (Aristolochiaceae) from the Guiana Shield. Phytotaxa, 474, 1–14. https://doi.org/10.11646/phytotaxa.474.1.1
Freitas, J., Lírio, E. J., Barros, F., & González, F. (2020) Aristolochiaceae in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. https://doi.org/10.47871/jbrj2021004
Freitas, J., Lírio, E. J., Guimarães, E. F., & Alves-Araújo, A. (2019). Two new records and morphological discussions of Aristolochia (Aristolochiaceae) from Rio de Janeiro state, Brazil. Journal of the Botanical Research Institute of Texas, 13, 235–240. https://doi.org/10.17348/jbrit.v13.i1.848.
Funk, V. A., Edwards, R., & Keeley, S. (2018). The problem with (out) vouchers. Taxon, 67, 3–5. https://doi.org/10.12705/671.1
GBIF (Global Biodiversity Information Facility). (2022). GBIF Secretariat 2022. Accessed 14 January 2022. https://www.gbif.org
Gómez-Bellver, C., Ibáñez, N., López-Pujol, J., Nualart, N., & Susanna, A. (2020). How photographs can be a complement of herbarium vouchers: A proposal of standardization. Taxon, 68, 1321–1326. https://doi.org/10.1002/tax.12162
Gonella, P. M., Rivadavia, F., & Fleischmann, A. (2015). Drosera magnifica (Droseraceae): the largest New World sundew, discovered on Facebook. Phytotaxa, 220, 257–267. https://doi.org/10.11646/phytotaxa.220.3.4
González, F. (1990). Aristolochiaceae. Flora de Colombia. Monografia n° 12. Bogotá: Universidad Nacional de Colombia, Instituto de Ciencias Naturales.
González, F. (1991). Notes on the systematics of Aristolochia subsect. Hexandrae. Annals of the Missouri Botanical Garden, 78, 497–503. https://doi.org/10.2307/2399576
González, F. (1994). Aristolochiaceae. In G. Harling & L. Andersson (Eds.), Flora of Ecuador. Monograph No. 51 (pp. 1-42). Copenhague: Council for Nordic Publications in Botany.
González, F. (1997). Hacia una filogenia de Aristolochia y sus congéneres neotropicales. Caldasia, 19, 115–130. http://dx.
doi.org/10.15446/caldasia
González, F. (1998). Two new species of Aristolochia (Aristolochiaceae) from Brazil and Peru. Brittonia, 50, 5–10. https://doi.org/10.2307/2807710
González, F., & Pabón-Mora, N. L. (2017). Inflorescence and floral traits of the Colombian species of Tristerix (Loranthaceae) related to hummingbird pollination. Anales del Jardín Botánico de Madrid, 74, e061, 1–14.https://doi.org/10.3989/ajbm.2474
González, F., & Monzón-Sierra, J. (2022). An updated synopsis of Aristolochia (Aristolochiaceae) in Guatemala. Brittonia, 74, 1–26. https://doi.org/10.1007/s12228-022-09704-0
González, F., Ospina, J. C., & Zanotti, C. (2015). Sinopsis y novedades taxonómicas de la familia Aristolochiaceae para la Argentina. Darwiniana, Nueva Serie, 3, 38–64. https://doi.org/10.14522/darwiniana.2015.31.644
GPI (Global Plants Initiative). (2022). Accessed 16 January 2022. http://plants.jstor.org
Harris, J. G., & Harris, M. W. (2001). Plant identification terminology: an illustrated glossary. 2nd ed. Spring Lake, NJ: Spring Lake Publishing,
Heberling, J. M., & Isaac, B. L. (2018). iNaturalist as a tool to expand the research value of museum specimens. Applications in Plant Sciences, 6, e1193. https://doi.org/10.1002/aps3.1193
Hoehne, F. C. (1927). Monographia illustrada das aristolochiaceas brasileiras. Memórias do Instituto Oswaldo Cruz, 20, 67–175.
Hoehne, F. C. (1942). Aristolochiaceas. Instituto de Botânica, São Paulo. Flora Brasílica, 15, 1–123.
IBGE (Instituto Brasileiro de Geografia e Estatistica). (2022). Accessed 16 January 2022. https://cidades.ibge.gov.br/brasil/
panorama
ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade). (2022). Accessed 16 January 2022. https://www.icmbio.gov.br/portal/unidadesdeconservacao/biomas-brasileiros
INCT (Instituto Nacional de Ciência e Tecnologia). (2022). Herbário Virtual da Flora e dos Fungos – SpeciesLink Network. Accessed 16 January 2022. http://inct.splink.org.br
IUCN (International Union for Conservation of Nature). (2012). IUCN Red List Categories and Criteria: Version 3.1. Second edition. Gland, Switzerland and Cambridge, UK: IUCN. https://portals.iucn.org/library/sites/library/files/documents/RL-2001-001-2nd.pdf
IUCN (International Union for Conservation of Nature). (2022). Guidelines for using the IUCN red list categories and criteria. Version 15.1. Prepared by the Standards and Petitions Subcommittee. https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf
Missouri Botanical Garden. (2022). Tropicos.org. Missouri Botanical Garden. Accessed 15 January 2022. http://www.tropicos.org
Nascimento, D. S. (2008). Estudo taxonômico da família Aristolochiaceae Juss. do Sul do Brasil (Master Thesis). Universidade Federal do Paraná, Curitiba.
Neill, A. K., & Kieschnick, S. R. (2021). Noccaea perfoliata or Microthlaspi perfoliatum (Brassicaceae), new to the Flora of Texas, U.S.A. Journal of the Botanical Research Institute of Texas, 15, 309–315. https://doi.org/10.17348/jbrit.v15.i1.1065
Poncy, O. (1988). Studies on the flora of the Guianas. Quatre espèces nouvelles d’Aristolochia de Guyane française, et remarques nomenclaturales concernant une espèce d’Aublet. Bulletin du Muséum National d’Histoire Naturelle, sér. 4, 10. sect. B, Adansonia, 4, 337–344.
Reed, R. D., & Sperling, F. A. H. (2022). Papilionidae. The swallowtail butterflies. Accessed 26 June 2020. http://tolweb.org/Papilionidae/12177
REFLORA. (2022). Herbário Virtual Reflora. Accessed 20 January 2022. http://reflora.jbrj.gov.br/reflora/herbarioVirtual
Thiers, B. (2022). Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium 2022. Accessed 16 January 2022. http://sweetgum.nybg.org/ih
Turland, N. J., Wiersema, J. H., Barrie, F. R., Greuter, W., Hawksworth, D. L., Herendeen, P. S. et al. (Eds.). (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten: Koeltz Botanical Books. https://doi.org/10.12705/Code.2018
von Euw, J., Reichstein, T., & Rothschild, M. (1968). Aristolochic acid in the swallowtail butterfly Pachlioptera aristolochiae. Israel Journal of Chemistry, 6, 659–670. https://doi.org/
10.1002/ijch.196800084
Wanke, S., González, F., & Neinhuis, C. (2006). Systematics of pipevines: Combining morphological and fast-evolving molecular characters to investigate the relationships within subfamily Aristolochioideae (Aristolochiaceae). International Journal of Plant Sciences, 167, 1215–1227. https://doi.org/10.1086/508024
Watts, B. (2017). The value of plant science field photographs (Master of Science in Library and Information Science Thesis). University of California, Los Angeles.




