Biodiversity, distribution, ecology and management of non-native weeds in Mexico: a review
Francisco J. Espinosa-García(a)⁎ ✉ , José Luis Villaseñor(b)
Abstract
The current knowledge on the richness, ecology, distribution and management of non-native flowering weeds in Mexico and some data on their possible environmental and economic impact are briefly reviewed. We reviewed 216 refereed publications, most indexed international articles. Most publications refer to management sensu lato (34.9%), floristics (19.5%), ecology (21.5%), and detection of new non-native weeds (13.3%). The most complete research area is floristics, along with species inventories with their incidence at the state level. The publications, although interesting and of high quality, are disjointed and rarely coordinated with decision makers, general public or policy makers. It is estimated that there are about 700 wild non-native species in Mexico; 80% naturalized and we estimate that there are between 58 and 180 invasive weed species that cause environmental or socioeconomic damage. The 700 species represent 2.8% of the 23,000 species of Mexican flora. Although there is no overall estimate of the cost of the losses caused by weeds introduced for Mexico, it is argued that it is high in terms of agriculture, environment and human health. A number of measures are suggested to generate the scientific knowledge needed to prevent and/or sustainably manage invasive weed invasions.
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:
Invasive weeds in Mexico; Estimated socio-economic impact; Exotic plant species; Management of invasive weeds;
Biodiversidad, distribución, ecología y manejo de malezas alóctonas en México: una revisión
Resumen
Se revisan brevemente los conocimientos actuales sobre la riqueza, ecología, distribución y manejo de las malezas con flores alóctonas en México y algunos datos sobre su posible impacto ambiental y económico. Revisamos 216 publicaciones, la mayoría artículos internacionales indizados; la mayoría se refieren a manejo sensu lato (34.9%), después florística (19.5%), ecología (21.5%) y detección de malezas exóticas nuevas (13.3%). El área de investigación más completa es florística, junto con inventarios de especies con su distribución a escala estatal. El resto de las investigaciones, aunque interesantes y de alta calidad, están desarticuladas y raras veces se coordinan con tomadores de decisiones, el público en general o los políticos. Se estima que hay cerca de 700 especies alóctonas silvestres en México; alrededor del 80% se ha naturalizado y estimamos que habría entre 58 y 180 especies invasoras. Las 700 especies representan el 2.8% de las 23,000 especies de la flora mexicana. Aunque faltan estimaciones del costo de las pérdidas causadas por las malezas introducidas para México, se argumenta que es alto en términos agropecuarios, ambientales y en la salud humana. Se sugieren una serie de medidas para generar el conocimiento científico necesario para prevenir y/o manejar sustentablemente las invasiones de plantas invasoras.
Palabras clave:
Malezas invasoras en México; Impacto socioeconómico estimado; Especies de malezas alóctonas; Manejo de malezas invasoras;
Introduction
Non-native, also known as exotic, species are increasingly found in all ecosystems, mainly in those directly disturbed by humans. A small fraction of the bulk of non-native plant species introduced in a country become invaders ( Williamson & Fitter, 1996 ), causing human health, environmental, or economic damage, mainly at the local scale ( Powell, Chase, & Knight, 2011 ). Although the biological invasion process is well described ( Richardson et al., 2000 ), including the major pathways naturalized plants follow in new ranges ( van Kleunen et al., 2015 ), the prediction of which non-native species will behave as invasive in the new range is not accurate enough. The complexity of biological invasions has not allowed a full theoretical understanding of the phenomenon ( Kueffer, Pysek, & Richardson, 2013 ), in spite of an exponential growth of research on biological invasions since 1990 (44,111 scientific papers published in the 2010–2015 period out of 70,400 studies published between 1900 and 2015) (ISI Web of Science search of “biological invasions” in December, 2015). Thus, management decisions ranging from prevention to mitigation have been based on a mixture of empirical and scientifically based information as not enough scientific knowledge is available for the myriad of conditions in which biological invasions occur.
The environmental and economic damage caused by invasive species is of such magnitude, at least 1.4 trillion US dollars globally ( Diversitas, 2010 ), that it has prompted international attention through global initiatives that foster scientific research and societal attention to manage and prevent biological invasions. For example, the Global Invasive Species Programme (GISP) (1997–2010) sponsored by DIVERSITAS, was founded to provide support to the Convention of Biological Diversity (CDB). GISP proposed, among other things, a framework to investigate the ecology and the socioeconomic factors involved in biological invasions and their management ( Mooney, 2005 ). For Mexico, the CBD and GISP were instrumental for the National Commission on Biodiversity (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Conabio) in fostering the National Strategy on Invasive Species in Mexico, prevention, control and eradication ( Comité Asesor Nacional sobre Especies Invasoras, 2010 ). Under these worldwide conditions of scientific and empirical knowledge on invasive species, we briefly review the research regarding non-native weeds in Mexico published up to 2015. We then evaluate the advances represented in the Mexican scientific literature on non-native weeds considering the strategic objectives on prevention, early detection and rapid response, containment and mitigation proposed by the National Strategy on invasive species in Mexico: strategic objective 1, “Prevent, detect and reduce the risk of introduction, establishment and dispersal of invasive species”; strategic objective 2, “Establish control and eradication programs for invasive species’ populations, which minimize or eliminate their negative impacts and favor ecosystem restoration and conservation”; strategic objective 3, “Inform the public in an appropriate and efficient way to achieve a broad civil support and participation within their reach in actions to prevent, control and eradicate invasive species”.
Methods
We used references within previous accounts on research and management of non-native plant species in Mexico ( Espinosa-García, 2009; Espinosa-García, Villaseñor, & Vibrans, 2009 ), as well as the authors’ personal libraries, and a key word search in the ISI Web of Science database encompassing published literature from 1950 to 2015. The keywords used for the search were “invasive plant* AND Mexico”; “invasive weed* AND Mexico”; “exotic plant* AND Mexico”; “exotic weed* AND Mexico”; “introduced plant* AND Mexico”; “introduced weed* AND Mexico”; “alien plant* AND Mexico”; “alien weed* AND Mexico”. In most cases these searches were limited to authors or coauthors with Mexican addresses, but we excluded studies performed in other countries with participation of authors from Mexican institutions. From personal libraries we included publications on non-native species in Mexico from researchers without Mexican addresses (e.g. Williams, 2010 ). We estimate that we included most refereed publications not indexed in the Web of Science, although it is very likely that some were missed. We are not including most B.Sc. and M.Sc. thesis, non-refereed-congress proceedings or abstracts (e.g. Memories of the Mexican Weed Society (ASOMECIMA) meetings), and technical reports from field research stations which are usually not refereed nor included in ISI Web of Science. Most of these publications report important research on weeds (mainly management) but the distinction between native and exotic weed species is usually lacking and they have very limited accessibility.
Results
We found 229 papers, chapters or books on: non-native algae (7), ferns (6) and flowering plants (216) published between 1939 and 2015. Here we review the publications of non-native wild flowering plants.
Publication topics
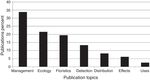
The publications’ topics are represented in Figure 1 . Most papers (34.5%) deal with management sensu lato , including restoration, and chemical, cultural or biological control; 21.5% with several ecological topics; 19.5% of papers deal with floristics; 13% with detection of new non-native weeds, usually accompanied by the distribution range when the non-native was discovered; 8% deal with actual and potential distribution of naturalized or invasive plants; 6% deal with the economic or ecological impact of non-native weeds; and finally 3% deal with non-native species uses or ethnobotany. Percentages add up to 105.5% as there were publications included in more than 2 topics. Publications that covered more than one topic were assigned to their predominant one; if 2 or more predominated equally, then the publications were considered in more than one topic.
Publications on non-native species throughout time
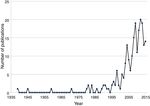
Schinus molle L., the pirú or pirul tree, was introduced in the New Spain, now Mexico, by the viceroy Antonio de Mendoza in the XVI century; the introduction, dispersal and uses were documented by Alzate and Ramírez 1791, Gómez 1889–1890, Jiménez 1873, Herrera 1896 and López y Parra 1899 (authors cited in Corkidi, Cacho, & Búrquez, 1991 ). Although no other purposeful introduction of a species was documented, botanists in Mexico have been well aware of introduced wild species since the early 20th century. For example, in “La Flora Excursoria en el Valle Central de México” ( Reiche, 1926 ) several naturalized species are reported as coming from the “old world” such as Avena fatua L. and Helminthotheca echioides (L.) Gaertn (=Picris echioides L.). However, this kind of publications did not go beyond mentioning the continent of origin of the naturalized species. Thus, we concentrated on publications dedicated predominantly on non-native naturalized and/or invasive species or to those that compare native with non-native flora. Adding up the results of the ISI Web of Science and the bibliographic resources of the authors, we found 229 published articles or book chapters dealing with non-native algae (7), ferns (6) and flowering plants (216) in a period going from 1939 through 2015. The first two papers on invasive plants were published by the prominent agronomist Gabriel Itie ( Cotter & Osborne, 1996 ). The first paper was published on Urochloa mutica (Forssk.) T.Q. Nguyen (= Panicum purpurescens Raddi), a grass introduced as forage and later escaped and became naturalized ( Itié, 1939). In 1945 a paper appeared on Melinis repens (Willd.) Zizka, an African grass introduced in the 1930s to be tested as forage ( Itié, 1945 ). This grass failed as a forage crop but escaped, mainly along roads and highways. It is now an invasive plant that has colonized roadsides all over Mexico encroaching on semi-desert scrublands, orchards and vegetation adjacent to roads and highways ( Díaz-Romo et al., 2012; Melgoza-Castillo, Balandrán-Valladares, Mata-González, & Pinedo-Álvarez, 2014 ). There were no publications on non-native plants up to 1959, when Dr. Jerzy Rzedowsky directed his scholarly attention to invasive species documenting the spread of the invasive Salsola kali L. var. tenuifolia Tausch (Rzedowski, 1959 ). His attention continued to date, recognizing invasive species together with his wife in the Floras published by Rzedowski and Calderon-de Rzedowski (Flora Fanerogámica del Valle de México and Flora del Bajío), and detecting new non-native species ( Calderón-de Rzedowski & Rzedowski, 2004; Calderón-de Rzedowski et al., 2001; Rzedowski & Calderón-de Rzedowski, 1985, 2005; Rzedowski, Vibrans, & Calderón-de Rzedowski, 2003 ). In 1971, a pioneer work on allelopathy of a non-native species, S. molle was published (Anaya & Gómez-Pompa, 1971 ). We detected no new publications until the 1980s, where 7 papers where published, one on urban floristics distinguishing native and non-native weeds ( Rapoport, Díaz-Betancourt, & López-Moreno, 1983 ), 2 on non-native species detection ( Espinosa-García, 1981; Lot, Novelo, & Cowan, 1980 ), 2 on chemical control ( Magallanes, Ortiz, & Rojas-Garcidueñas, 1986; Tamayo-Esquer & Gaillardon, 1989 ) and 2 on ecological aspects of Nicotiana glauca Graham (Hernández, 1981) and Eichhornia crassipes (C. Mart.) Solms. ( Niño-Sulkowska & Lot, 1983). In the1990s, after Rzedowski and Calderón-de Rzedowski (1990) published an important paper on African weeds naturalized in Mexico, a paper on the dispersal of S. molle by birds (Corkidi et al., 1991 ) together with another important paper on the sinanthropic Asteraceae in Mexico ( Rzedowski, 1993 ), a fluctuating increase in publication is observed through 2015 ( Fig. 2 ). Although the publication increase is notorious since 1999, it is far from the huge increase in international publications. The fluctuations are probably related to the activity of a few individuals who have a primary, but not unique, interest on non-native plants. For example, the authors publishing new reports of non-native species ( Table 2 ) are or were primarily agronomists, taxonomists, specialists in floristics, ethnobotanists or ecologists.
Figure 1
Percentage of publciations on non-native weedy flowering plants in Mexico in 1939–2015 on major research topics. Percentages add up to 105.5% as there were publications included in more than 2 topics. Sources: ISI Web of Knowledge; Espinosa-García (2009); Espinosa-García et al. (2009) ; FJE and JLV personal libraries.
Figure 2
Refereed publications on non-native flowering plants published by authors with Mexican address (n = 216). Data obtained from 216 publications obtained from the sources mentioned in Figure 1.
Floristics and detection of new non-native species for Mexico
Dealing with invasive weeds requires extensive knowledge of the identity and distribution of the non-native plant species that have entered a country, established, naturalized, and became invasive (i.e. cause damage) or pests (i.e. noxious or destructive). Although the number of publications on floristics or detection of new non-native weeds is not the majority (20% of total publications), the families with introduced species in Mexico is well known ( Espejo-Serna, López-Ferrari, & Ugarte, 2004 ), as well as the list of most non-native species following the papers by Villaseñor and Espinosa-García (1998, 2004) and Espinosa-García et al. (2009) .
In 2004, 618 non-native species were reported for Mexico ( Villaseñor & Espinosa-García, 2004 ); with 22 species added in 2009 ( Espinosa-García et al., 2009 ). In this review, 31 previously undetected species have been added ( Table 1 ), but recent reviews of the non-native weedy Poaceae and Asteraceae ( Sánchez-Ken, Zita-Padilla, & Mendoza-Cruz, 2012; Villaseñor, Ortiz, Hinojosa-Espinosa, & Segura-Hernández, 2012 ), changed the non-native status of 39 species, but reported 40 previously undetected wild species, many of them formerly known as ornamentals. Additionally, JLV has detected 26 more species in his database (not reported here). These data add up to 698 non-native species registered for Mexico, but recent publications (not included in this review) enlist additional non-native species with unknown wild or cultivated status. We are currently revising the entire list of wild non-native species in Mexico for a future publication, but we estimate that the actual figure for wild non-native species for Mexico to be in the order of 700–750 species.
Table 1
Recently detected non-native weed species not enlisted in Villaseñor and Espinosa-García (2004) . Update from the list published in Espinosa-García et al. (2009) .
| Family | Species | Origin | Comments |
| Alismataceae | Sagittaria sagittifolia L. | Europe | EDOMEX h t t p & # 5 8 ; & # 4 7 ; & # 4 7 ; w w w & # 4 6 ; e d o m e x ico.gob.mx/sma/se/BIO_INTERNET/monografias.html seen on 25/04/2007; h t t p & # 5 8 ; & # 4 7 ; & # 4 7 ; w w w & # 4 6 ; i s s g & # 4 6 ; o r g & # 4 7 ; d a t a b a s e & # 4 7 ; s p e c i e s & # 4 7 ; d i s t r i b u t i o n & # 9 5 ; d i s p l a y & # 4 6 ; a s p & # 6 3 ; s i & # 6 1 ; 8 5 8 & # 38;ri=18917&pc=*&sts=sss&status=Alien#Alien seen on 5/03/2007 |
| Apiaceae | Cuminum cyminum L. | Mediterranean | CHIH, VER (Williams, 2010) |
| Asteraceae | Arctotheca prostrata (Salisb.) Britten | South Africa | DF ( Hinojosa-Espinosa & Villaseñor, 2015 ) |
| Asteraceae | Carduus nutans L. | Eurasia | HGO (Williams, 2010) |
| Asteraceae | Crepis capillaris (L.) Wallr. | Europe | VER. Naturalizada, ruderal y en agostaderos ( Rzedowski & Calderón-de Rzedowski, 2005 ) |
| Brassicaceae | Chorispora tenella (Pall.) DC. | Eurasia | SON (Van Devender & Reina, 2007) |
| Brassicaceae | Sisymbrium erysimoides | Mediterranean | EDOMEX, Heike Vibrans (Vibrans 8076, MEXU, CHAPA) (Vibrans, 2010) |
| Caesalpiniaceae | Senna polyantha (Collad.) H.S. Irwin & Barneby | Africa | BC 11 estados (Villaseñor & Espinosa-García, unpublished data) |
| Chenopodiaceae | Dysphania botrys (L.) Mosyakin & Clemants = Chenopodium botrys L. | Mediterranean | CHIH (Williams, 2010) |
| Chenopodiaceae | Salsola paulsenii Litv. | Asia | CHIH (Williams, 2010) |
| Convolvulaceae | Convolvulus crenatifolius Ruiz & Pavón | South America | DF, EDOMEX, GTO, HGO, JAL, MICH, QRO ( Carranza-González, 2005) |
| Convolvulaceae | Ipomoea wrightii A. Gray. | Unknown | CAM, JAL, SON, TAB, YUC ( Williams, 2010) ( http://bios.conabio.gob.mx/especies/6076916 ) |
| Euphorbiaceae | Phyllanthus urinaria L. | Sri Lanka and southeast Asia. | CHIS, OAX, VER (Williams, 2010) |
| Fabaceae | Abrus precatorius L. | India | CAM, VER, YUC. (Villaseñor & Espinosa-García, unpublished data) |
| Fabaceae | Albizia lebbeck (L.) Benth. | Tropical Asia | CAM, SIN, TAM (Williams, 2010) |
| Fabaceae | Lotus corniculatus L. | Eurasia | BC (Williams, 2010) |
| Fumariaceae | Fumaria officinalis L. | Europe | SON (Van Devender & Reina, 2007) |
| Lamiaceae | Vitex agnus-castus L. | Mediterranean | NL (Williams, 2010) |
| Liliaceae | Nothoscordum inodorum (Ait.) Nicholson | South America | MICH (Williams, 2010) |
| Malvaceae | Abutilon theophrasti Medik. | Southern Asia | EDOMEX (Domínguez & Pitalúa, unpublished data; Vibrans, unpublished data), SON ( Van Devender & Reina, 2007) |
| Mimosaceae | Acacia tortuosa (L.) Willd. | Tropical Asia | COL, DGO, HGO, JAL, MICH, OAX, SLP, TAM, SLP (Villaseñor & Espinosa-García, unpublished data) |
| Moraceae | Morus alba L. | China | COAH, SON, TAMS (Williams, 2010) |
| Papaveraceae | Fumaria officinalis L. | Europe | SON (Williams, 2010) |
| Papaveraceae | Glaucium corniculatum (L.) Rudolph | Eurasia | SON (Van Devender & Reina, 2007) |
| Poaceae | Cenchrus brownii Roem. & Schult. | South Africa or Tropical America | All México (Villaseñor & Espinosa-García, unpublished data) |
| Poaceae | Chloris barbata Sw. | Africa | VER (Williams, 2010) |
| Poaceae | Cortaderia selloana (Schult. & Schult. f.) Asch. & Graebn. | South America | Widely cultivated, feral in EDOMEX (Vibrans 8055, CHAPA) |
| Poaceae | Eragrostis ciliaris (L.) R. Br. | Cosmopolitan | BC, CAM, CHIS, COL, GRO, JAL, MEX, MICH, MOR, NAY, OAX, PUE, QROO, SLP, SIN, SON, TAB, TAM, VER, YUC ( Martin et al., 1998; h t t p ://mobot.mobot.org/cgi-bin/search_vast#meso seen on November 13, 2007) |
| Poaceae | Eragrostis echinochloidea Stapf | Africa | SON (Van Devender & Reina, 2007) |
| Poaceae | Hyparrhenia cymbaria (L.) Stapf, | Africa | Vibrans, García-Moya, Clayton, & Sánchez-Ken (2014) |
| Poaceae | Hyparrhenia variabilis Stapf | Africa | Vibrans et al. (2014) |
| Poaceae | Panicum coloratum L. | Africa | NL (Williams, 2010) |
| Poaceae | Setaria pumila (Poir.) Roem. & Schult. | Eurasia | NL, SON (Williams, 2010) |
| Poaceae | Themeda quadrivalvis (L.) Kuntze | Africa | MOR ( Sánchez-Ken, Cerros-Tlatilpa, & Vibrans, 2013 ) |
| Polygonaceae | Polygonum nepalense Meisn. | Asia | EDOMEX (Vibrans & Alipi, 2008 , Vibrans 8036, MEXU, CHAPA) |
| Polygonaceae | Rumex dentatus L. | Eurasia | SON, TAM (Villaseñor & Espinosa-García, unpublished data) |
| Polygonaceae | Rumex stenophyllus Ledeb. | Europe | SON (Martin et al., 1998) |
| Ranunculaceae | Ranunculus sardous Crantz | Old World | PUE ( Hanan-Alipi, Mondragón-Pichardo, & Vibrans, 2005 ) |
| Rosaceae | Prunus persica (L.) Batsch | Asia | SON (Van Devender & Reina, 2007) |
| Rubiaceae | Pentodon pentandrus (Schumach. & Thonn.) Vatke | Brazil | TAM, VER, NL (Villaseñor & Espinosa-García, unpublished data) |
| Scrophulariaceae | Verbascum blattaria L. | Eurasia | CHIH (Williams, 2010) |
| Simaroubaceae | Ailanthus altissima (Mill.) Swingle | China | CHIH, COAH (Williams, 2010) |
| Solanaceae | Solanum sisymbriifolium Lam. | South America | CAM, CHIH, JAL, SON, VER ( Martin et al., 1998; Van Devender & Reina, 2007 ) |
| Verbenaceae | Glandularia tenuisecta (Briq.) Small | South America | SON (Martin et al., 1998) |
| Verbenaceae | Verbena brasiliensis Vell. | South America | NAY, NL (Williams, 2010) |
It is likely that several newly detected species have been present in Mexico long before and had not been detected due to under exploration or because they were overlooked or misidentified in herbarium collections ( Villaseñor et al., 2012). For example, Williams (2010) reported 24 non-native species for Mexico not listed by Villaseñor and Espinosa-García (2004) based on herbarium records and field work. Herbarium records were also of crucial importance in the studies of Sánchez-Ken et al. (2012) and Villaseñor et al. (2012) . Other potential sources of newly naturalized non-native species are the cultivated or ornamental species becoming feral. Ornamental, forage or crop escapees constitute a large proportion of naturalized non-native species in various floras ( Dehnen-Schmutz, Touza, Perrings, & Williamson, 2007; Mack, 2000 ), but they are often overlooked when they become feral. Thus, we suspect that between one third and one half of the detected species between 2004 and 2015 arrived recently to Mexican territory.
Assuming that the non-native status change for 39 species ( Sánchez-Ken et al., 2012 ) is confirmed, and the actual species number is ca. 700, then 121 new non-native species for Mexico were added between 2004 and 2015; this means that, on average, around 13 species per year were recorded. This number doubles the estimate by Espinosa-García et al. (2009) and is much higher than the 5 species per year calculated for the 1930–2008 period in the United States Pacific Northwest ( Stohlgren, Barnett, Jarnevich, Flather, & Kartesz, 2008 ).
The detection of most new non-native species has been mainly the result of the activity of few professional botanists with long trajectory in floristics and/or taxonomy, such as J. Rzedowski and G. Calderón-de Rzedowski, Heike Vibrans, José Luis Villaseñor and Tom Van Devender among others ( Table 2 ). This activity is adequate but insufficient, as Mexico is a large country (1,964,375 km2 ) and a formal early detection program is needed.
Table 2
Publications reporting previously undetected non-native species from 1939 to 2015.
| 1939 | Itié (1939), Urochloa mutica (Forssk.) T.Q. Nguyen (Poaceae) |
| 1945 | Itié (1945), Melinis repens (Willd.) Zizka (Poaceae) |
| 1959 | Rzedowski (1959), Salsola kali L. var. teniufolia Tausch (Amaranthaceae) |
| 1980 | Lot, Novelo & Cowan (1980), Phyllanthus fluitans Benth. ex Müll.Arg. (Euphorbiaceae) |
| 1981 | Espinosa-García (1981), Kickxia elatine (L.) Dumort. ssp. crinita (Mabille) Greuter (Plantaginaceae).; Polygonum convolvulus L. (Convolvulaceae) |
| 1990 | Rzedowski, & Calderón-de Rzedowski (1990), Several species of African origin |
| 1993 | Rzedowski (1993), Several Asteraceae species |
| 1995 | Vibrans (1995), Silene noctiflora L. (Caryophyllaceae) |
| 1996 | Vibrans (1996), Bellis perennis L., Guizotia abyssinica (Lf) Cass., Hypochaeris radicata L. (Asteraceae) |
| 1997 | Van Devender, Felger, Búrquez (1997), Several species in the Sonoran Desert |
| 1998 | Martin, Yetman, Fishbein, Jenkins, Van Devender & R. Wilson (1998), Several species in the Sonoran Desert |
| 2001 | Estrada & Yen (2001), Lespedeza cuneata (Dum.Cours.) G.Don (Fabaceae) |
| 2003 | Colmenero-Robles & Fernández-Nava (2003) Corchorus sp1 y sp2 (Tiliaceae) |
| 2003 | Rzedowski, Vibrans & G. Calderón-de Rzedowski (2003), Senecio inaequidens DC. (Asteraceae) |
| 2003 | Vibrans (2003) , Several species of Brassicaceae. |
| 2005 | Carranza-González (2005), Convolvulus crenatifolius Ruiz & Pavón (Convolvulaceae) |
| 2005 | Hanan-Alipi, Mondragón-Pichardo & Vibrans (2005), Ranunculus sardous Crantz. (Ranunculaceae) |
| 2005 | Rzedowski & Calderón-de Rzedowski (2005), Crepis capillaris Wallr. (Asteraceae). |
| 2007 | Van Devender & Reina (2007) . Sonoran Noteworthy Records, several species. |
| 2008 | Martínez, Mora-Olivo & Daniel (2008) , Hygrophila polysperma Anderson (Acanthaceae), |
| 2008 | Vibrans & Hanan-Alipi (2008), Polygonum nepalense Meisn. (Polygonaceae). |
| 2009 | Dimmitt, M. A., & T. R. Van Devender (2009), Brassica tournefortii Gouan. (Brassicaceae) |
| 2010 | Williams (2010) , twenty four exotic species not reported by Villaseñor and Espinosa-García (2004) |
| 2013 | Sánchez-Ken, G., J., Cerros-Tlatilpa, & Vibrans (2013) , Themeda quadrivalvis (L.) Kuntze (Poaceae) |
| 2014 | Mora-Olivo and Sánchez-Del Pino (2014) , Alternanthera philoxeroides Griseb. (Amaranthaceae) |
| 2014 | Vibrans, García-Moya, Clayton & Sánchez-Ken (2014), Hyparrhenia variabilis (L.) Stapf. and Hyparrhenia cymbaria Stapf. (Poaceae). |
| 2015 | Hinojosa-Espinosa & Villaseñor (2015), Arctotheca prostrata (Salisb.) Britten (Asteraceae). |
Other important sources of new non-native species detection are the papers dealing with floristics that distinguish native and non-native species. There are floristic accounts concerned exclusively with non-native species ( Garcillán, de la Luz, Rebman, & Delgadillo, 2013; González-Elizondo, González-Elizondo, Tena-Flores, López-Enriquez, & Bacon, 2009; León-de la Luz, Domínguez, & Domínguez, 2009; Serrano-Cárdenas, Balderas-Aguilar, & Pelz-Marín, 2009; Villaseñor et al., 2012 ). An increasing number of floristic studies in specific ecosystems or regions have contributed to the knowledge of the distribution of non-native species. For specific ecosystems or landscapes: cities and surrounding areas ( Alfaro-Rodríguez & Arriaga, 2009; Corral-Díaz & Pelayo, 2009; Díaz-Betancourt, 1999; Martínez-de la Cruz et al., 2015; Vibrans, 1998 ); agroecosystems ( Martínez-Díaz & Jiménez-León, 2009; Sánchez-Blanco & Guevara-Féfer, 2013 ); pastures or grazing lands ( Gómez-Sánchez, Suárez-Martínez, & Martínez-Montes, 2011; Harker, García-Rubio, & Riojas-López, 2008; Lira-Noriega, Guevara, Laborde, & Sánchez-Ríos, 2007 ); wetlands ( Lot, 2012; Mora-Olivo, Villaseñor, & Martínez, 2013 ); savanna ( Ortiz-Díaz, Tun-Garrido, Arnelas-Seco, & García-Gil, 2014 ); and cloud forest and their edges ( López-Pérez, Tejero-Diez, Torres-Díaz, & Luna-Vega, 2011 ).
Some recent floristic accounts from regions of Nuevo León ( Guzmán-Lucio et al., 2013) and Guerrero ( Morales-Saldaña, Martínez-Ambriz, & Valencia, 2015 ), exemplify the increasing awarenes about the importance of non-native species among botanists and ecologists. A similar phenomenon is occurring with taxonomists, reviewing genera with native and non-native species ( Martínez-Bernal, Duno-de Stefano, & Lorena-Can, 2011; Saarela, Peterson, & Valdes-Reyna, 2014; Sánchez-del Pino, Espadas, & Pool, 2013 ).
The question of how many undetected non-native species are to be discovered in Mexico, remains to be answered after further floristic exploration and an implementation of an early detection and rapid response program operated by personnel familiarized with the local floras. However, a positive and significant correlation between native and non-native weed richness, and between non-weed native species and native weed richness has been found ( Espinosa-García, Villaseñor, & Vibrans, 2004b; Villaseñor, 2013 ). The estimated number of weeds in Mexico in 2012 was 683 introduced and 2,523 native species ( Villaseñor, 2013 ). Mexico has 23,314 documented species of flowering plants although this number could reach 29,000 ( Villaseñor, 2016 ). Considering that the non-native species represent between 2.6 and 10.3 (mean = 5.2 + 1.75%) of the Mexican States’ flora (data calculated from Villaseñor & Espinosa-García, 2004 ), and that the actual estimates of native plus non-native flora is between 24,014 and 24,064, we should expect to have approximately 1,260 non-native weed species (in the range between 834 and 1676). Thus, between 134 and 976 non-native weed species may yet to be documented in Mexico or are expected to arrive.
The estimates on the number of naturalized species that become invasive pests vary from 10% ( Williamson & Fitter, 1996), 12% ( Caley, Groves, & Barker, 2008 ) to 15–30% (Rejmánek & Randall, 2004 ). Thus, assuming that 20% ( Villaseñor & Espinosa-García, 2004 ) of the 700–750 non-native weeds in Mexico have naturalized (560–600), then an estimated number of invasive pest species in Mexico would be between 56 and 180. From the results of this review and our personal experience, we can clearly detect 37 highly problematic species, which need to be contained or have their negative effects mitigated ( Table 3 ). This list includes 8 invasive weeds that were identified formerly ( de Ita, Torres, Calderón, Luna, & Peralta, 1992 ). In a previous work, 33 invasive or invasive plant pests of high priority for Mexico were identified by a panel of scientists, NGOs’ personnel and government officials ( Aguilar et al., 2008 ); many of these species were not yet present in Mexico or some had been detected recently. Thus, the list of Table 3 and that of Aguilar et al. (2008) show little agreement. In a very recent contribution, Vibrans (2016) mentioned a number of highly problematic species that probably should be added to those in Table 3: Asphodelus fistulosus L., Canna indica L., Cyperus papyrus L., Dactyloctenium aegyptium (L.) Willd., Digitaria sanguinalis (L.) Scop., Digitaria velutina (Forsk.) Beauv., Eragrostis curvula (Schrader) Ness, Hyparrhenia hirta (L.) Stapf, Leonotis nepetifolia and Ricinus communis. Vibrans mentioned other non-native species that may be problematic in reduced areas, and others with widespread distribution but without evidence of causing environmental or economic damage. The UNIBIO survey of the biological invasions of plants ( http://www.unibio.unam.mx/invasoras/ ) listed 700 species of introduced plants, out of which 226 were classified as invasive, a greater number than the upper estimate obtained from the literature reviewed. The invasive species attributed high (133) and extreme invasiveness (7) represents 62% of the UNIBIOS’ invasive species pool. UNIBIO’s page, however, does not explain how the invasive category was assigned by the 67 floristic experts consulted to build the database because such category should be assigned according to their definition: “Una especie invasora es aquella que, como consecuencia de las actividades humanas, se ha expandido fuera de su rango de distribución natural, ha aumentado su densidad dentro de comunidades naturales de especies nativas y/o tiene impactos negativos en la biodiversidad de dichas comunidades” (an invasive species is one that, as a result of human activities, has expanded outside its natural range, has increased its density within natural communities of native species and/or has negative impacts on the biodiversity of these communities).
Table 3
Preliminary list of major invasive species detected in Mexico. The list was obtained from the results of this review and the authors’ experience. The suggested management options were determined based on the distribution extent: EDRR (early detection and rapid response) for incipient colonization of new ranges; local eradication for early stages of colonization; containment for non-eradicable species well established in an area but absent in other susceptible areas; mitigation for non-eradicable species occupying all the susceptible areas in the new range.
| Family | Scientific name | Introduction mode | Management options |
| Amaranthaceae | Althernanthera phyloxeroides | Accidental introduction | EDRR, Containment |
| Amaranthaceae | Atriplex spp | Forage escapee | Containment |
| Amaranthaceae | Kochia scoparia | Forage escapee | Mitigation |
| Amaranthaceae | Salsola tragus | Forage escapee | Mitigation |
| Apocinaceae | Cryptostegia grandiflora | Ornamental escapee | Containment, Local eradication |
| Apocynaceae | Vinca major | Ornamental escapee | Containment |
| Araceae | Zantedeschia aethiopica | Ornamental escapee | Containment, Local eradication |
| Asteraceae | Centaurea melitensis | Accidental introduction | Containment, Local eradication |
| Asteraceae | Senecio inaequidens | Accidental introduction | Containment, Mitigation |
| Asteraceae | Taraxacum officinale | Accidental introduction | Mitigation |
| Brassicaceae | Brassica tournefortii | Accidental introduction | Containment |
| Casuarinaceae | Casuarina equisetifolia | Ornamental escapee | Containment, Local eradication |
| Convolvulaceae | Convolvulus arvensis | Accidental introduction | Mitigation, Biological control |
| Cyperaceae | Cyperus esculentus | Accidental introduction? | Mitigation, Biological control |
| Poaceae | Arundo donax | Utilitarian introduction | Containment, Local eradication, Biological control |
| Poaceae | Avena fatua | Accidental introduction | Mitigation, Biological control |
| Poaceae | Brachiaria mutica | Forage escapee | Mitigation |
| Poaceae | Bromus rubens | Accidental introduction | Containment, Mitigation |
| Poaceae | Cynodon dactylon | Forage escapee | Mitigation, Biological control |
| Poaceae | Digitaria decumbens | Forage escapee | Mitigation |
| Poaceae | Echinochloa crusgalli | Accidental introduction | Mitigation, Biological control |
| Poaceae | Echinochloa pyramidalis | Forage escapee | Mitigation, Cultural control |
| Poaceae | Hyparrhenia cymbaria | Accidental introduction | Eradication, Containment |
| Poaceae | Hyparrhenia rufa | Forage escapee | Mitigation |
| Poaceae | Hyparrhenia variabilis | Accidental introduction | Eradication, Containment |
| Poaceae | Megathirsus maximus | Forage escapee | Mitigation |
| Poaceae | Melinis repens | Forage escapee | Mitigation, Cultural control |
| Poaceae | Pennisetum ciliaris | Forage escapee | Containment, Mitigation |
| Poaceae | Pennisetum clandestinum | Ornamental and forage escapee | Mitigation |
| Poaceae | Phalaris minor | Accidental introduction | Mitigation, Containment for herbicide resistant varieties |
| Poaceae | Rottboellia cochinchinensis | Accidental introduction | Containment, Mitigation, Biological control |
| Poaceae | Schismus barbatus | Accidental introduction | Containment, Mitigation, Biological control |
| Poaceae | Sorghum halepense | Accidental introduction? | Mitigation, Biological control |
| Poaceae | Themeda quadrivalvis | Accidental introduction | Eradication, Containment |
| Polygonaceae | Polygonum convolvulus | Accidental introduction | Eradication, Containment |
| Pontederiaceae | Eichhornia crassipes | Ornamental escapee | Containment, Mitigation, Biological control |
| Tamaricaceae | Tamarix chinensis | Ornamental escapee | Containment, Local eradication |
Aside of the important divergences of UNIBIO’s definition with the commonly accepted definitions in scientific literature (e.g. Richardson et al., 2000) or in official publications (CDB, 2009; DOF, 2015 ), the change in density should be determined by periodical observations of the same site. Also, the negative impacts should be documented. Since no indications of the qualification or quantification procedures for damage and density change are provided, the invasive and degree of invasiveness categorizations should be taken with caution.
In Mexico there is a small number of introduced species that are causing serious damage to agricultural and livestock systems and other ecosystems. This is the case of the Sonoran agricultural area where most of the problematic weeds were introduced purposely as forage or ornamental (Martínez-Díaz & Jiménez-León, 2009). These are grasses like buffelgrass ( Cenchrus ciliaris L.), rose Natal grass (M. repens [=Rhynchelytrum repens (Willd.) C. E. Hubb.]), kikuyu grass ( Pennisetum clandestinum Hochst. ex Chiov.), Johnson grass ( Sorghum halepense (L.) Pers.), and those causing the “Africanization of Americas tropics” ( Parsons, 1972 ). Amaranthaceae introduced for forage are some salt-bush species Atriplex spp., fireweed or burning bush Kochia scoparia (L.) Schrad., and tumbleweed Salsola tragus L. Notorious ornamentals that had escaped and naturalized are water hyacinth ( E. crassipes), white arum hyacinth (Zantedeschia aethiopica (L.) Spreng.), vinca or periwinkle ( Vinca major L.) and the rubber vine (Cryptostegia grandiflora R. Br.) ( Pérez-Panduro, 1998; Rodríguez-Estrella, Pérez-Navarro, Granados, & Rivera, 2010 ). In a publication on agricultural weeds of northern Mexico and the Mexican plateau ( de Ita et al., 1992 ), 10 of 22 species cited are non-native ( A. fatua, Chenopodium album L. Bosc ex Moq., Convolvulus arvensis L., Cynodon dactylon (L.) Pers., Cyperus esculentus L., Echinochloa crusgalli (L.) P. Beauv., Phalaris minor Retz., Rumex crispus L., S. halepense, and Taraxacum officinale G. H. Weber ex Wigg.).
Distribution
Most introduced species have a restricted distribution in Mexico compared with native weeds; moreover, the distribution pattern of introduced weeds at the state scale is similar to that of native non-weeds, that is, most species are reported from 1–2 states ( Espinosa-García, Villaseñor, & Vibrans, 2004a ). This pattern has to be confirmed because the number of botanists interested in non-native weeds is limited. However, we pose the hypothesis that most introduced species with wide distribution are a problem in cultivated or natural areas such as those species mentioned in the previous section.
Biogeographical studies of the Mexican flora including non-native weeds are few ( Luna-Vega, 2008 ). Most temperate non-native species come from Eurasia and the Mediterranean region, whereas most tropical ones come from Africa, via South America ( Rzedowski, 1993; Rzedowski & Calderón-de Rzedowski, 1990; Villaseñor & Espinosa-García, 2004 ). The diversity of native and introduced weed species is closely correlated with the diversity of native non-weed species ( r = 0.7–0.9) ( Espinosa-García et al., 2004b; Villaseñor, 2013 ). This agrees with the global pattern in which “the rich get richer” that implies that the regions with more native diversity have more introduced species ( Stohlgren, Barnett, & Kartesz, 2003 ). However, the distribution of the invasive pest species is probably not strongly related to native plant biodiversity but with the invasibility of the territory and the propagule pressure for each region ( del Val et al., 2015 ). The potential distribution patterns of the invasive pest species according to their invasivity degree are still to be described in order to sustainably manage the areas threatened by these species.
Studies on the current or potential distribution of specific non-native species in Mexico range from distribution patterns at the local scale, for example a university campus ( Zavala-Hurtado, Portilla-Gutiérrez, Ayala-Fernández, & Bravo-Rivera, 2003 ), to the national scale, modeling the potential distribution of the introduced Asteraceae in Mexico and the actual and potential distribution of S. molle ( Ramírez-Albores, Bustamante, & Badano, 2016; Villaseñor et al., 2012 ). The distribution of most non-native species has been recorded at the state scale ( Espinosa-García et al., 2009; Villaseñor & Espinosa-García, 1998 ), but few detailed actual and potential distributions of non-native species are know: buffel grass ( C. ciliaris ) in Sonora and the Baja California Peninsula ( Arriaga, Castellanos, Moreno, & Alarcón, 2004 ); testing the effectiveness of various potential distribution models with Brassica tournefortii Gouan and Schismus arabicus Nees in the Sonoran Desert ( Sánchez-Flores, 2007; Sánchez-Flores, Rodríguez-Gallegos, & Yool, 2008 ); Hypochaeris radicata L. actual distribution in Mexico city ( Hinojosa-Espinosa & Cruz-Durán, 2008 ); Arundo donax L., tracing its multiple introductions and identifying the origin of the genotypes that invaded the Río Bravo Basin ( Tarin et al., 2013); and Atriplex semibaccata R. Br., B. tournefortii, Bromus rubens L., Centaurea melitensis L., C. dactylon, S. tragus, Schismus barbatus, and Tamarix chinensis Lour (=T. ramossisima Ledeb.), in Baja California ( Palma-Ordaz & Delgadillo-Rodríguez, 2014 ). A special mention is for the Villaseñor et al. (2012) comprehensive work for the potential distribution for 30 of the 61 species of the non-native Asteraceae in the whole country performed with ecological niche models using MaxEnt.
Actual and potential distribution studies are very important for management prioritization, early detection, eradication, containment and mitigation. The knowledge on actual or potential distribution of non-native weeds in Mexico is fragmented and disarticulated with decision makers. However, the completion of the analysis of the distribution of non-native weeds and its linkage to decision makers is included in the National Strategy for Invasive Species ( Comité Asesor Nacional sobre Especies Invasoras, 2010 ) and in the recent National Biodiversity Strategy for Mexico ( Conabio, 2016 ). A major effort is needed to promote these studies with the co-participation of researchers and decision makers.
Ecology
Ecological publications cover a wide range of topics, often focusing on one aspect of a single species, with the notable exception of the publications of Patricia Moreno-Casasola and her research group, focusing on the invasion of a marsh by Echinochloa pyramidalis (Lam.) Hitch. & Chase. They have documented the invasion ( Castillo & Moreno-Casasola, 1996 ), explored the impact of cattle ranching practices on the invasion ( Travieso-Bello, Moreno-Casasola, & Campos, 2005 ), and studied the relationships of hydroperiod and water physicochemical properties with competition among hydrophytes in the marsh ( López-Rosas & Moreno-Casasola, 2012; Peralta-Pelaez, Moreno-Casasola, & López-Rosas, 2014 ). This group used these studies and others in restoration ecology ( López-Rosas, Moreno-Casasola, & Espejel-González, 2015 ) to reverse the invasion by E. pyramidalis with partial success and they continue the restoration efforts (see the management section). Focusing in one ecological aspect of a single invasive species is frequent in Mexican ecologists: reproductive ecology of N. glauca (Hernández, 1981); demography of E. crassipes (Niño-Sulkowska & Lot, 1983 ); ecophysiology of gas exchange of C. ciliaris ( De la Barrera & Castellanos, 2007 ); oil contamination effects on M. repens and C. ciliaris ( Reynoso-Cuevas, Gallegos-Martínez, Cruz-Sosa, & Gutiérrez-Rojas, 2008 ); population genetics of ruderal and cultivated populations of C. ciliaris ( Gutiérrez-Ozuna, Eguiarte, & Molina-Freaner, 2009 ); fate of M. repens in the soil seed bank of Neo-Tropical pastures ( Maza-Villalobos, Lemus-Herrera, & Martínez-Ramos, 2011 ); fitness of M. repens growing under different regimes of soil humidity ( Díaz-Romo et al., 2012 ); reproductive ecology of the orchid Oncidium poikilostalix (Kränzl.) M.W. Chase & N.H. Williams ( García-González, Damon, Iturbide, & Olalde-Portugal, 2013 ), and invasion by clonal spread of Kalanchoe delagoensis Eckl. & Zeyh. ( Guerra-García, Golubov, & Mandujano, 2015 ). Competition between herbicide-resistant and herbicide-susceptible accessions of P. minor has been studied (Torres-García et al., 2015 ) as well as the herbicide resistance traits presented by this grass in wheat fields ( Cruz-Hipólito, Domínguez-Valenzuela, Osuna, & De Prado, 2012; García-Franco et al., 2014 ).
Other authors have focused on communities or groups of invasive species: for example, how changes in the hydrological regime and salinity have caused T. chinensis invasion of the Colorado River delta and how the reversal of those changes allow the regeneration of the native plant communities ( Glenn et al., 1998; Nagler et al., 2005 ); life-history diversity of native and non-native species in heterogeneous vegetation ( Pérez-García, Meave, Villaseñor, Gallardo-Cruz, & Lebrija-Trejos, 2010 ); incidence of non-native weed species along an altitudinal gradient ( Sánchez-González & López-Mata, 2005 ); plant richness analysis of areas where non-native grasses are abundant ( Cano-Santana, Castillo-Arguero, Martínez-Orea, & Juárez-Orozco, 2008 ); incidence of non-native species in small patches in fragmented forests ( Arroyo-Rodríguez, Pineda, Escobar, & Benitez-Malvido, 2009 ); population genetics of Sinorhizobium spp. mutualists isolated from Medicago spp. ( Silva, Kan, & Martínez-Romero, 2007 ); and seed bank ecology of agricultural fields in the tropical dry forest ( Meave, Flores-Rodríguez, Pérez-García, & Romero-Romero, 2012 ). Few authors have focused on non-native weed ecology either by studying the combination of factors influencing non-native richness ( Espinosa-García et al., 2004b ) or by combining several ecological variables to build a model to identify risk areas in Mexico ( del Val et al., 2015). Santibañez-Andrade, Castillo-Arguero, and Martínez-Orea (2015) determined that the importance index of weeds, native and non-native, is an important aid to asses the conservation status of temperate forests.
There is a group of studies testing hypotheses on invasiveness by comparing populations of Mexican native species that are invasive elsewhere in the world such as Ageratina adenophora (Spreng.) King & H.Rob. and Chromolaena odorata (L.) R.M.King & H.Rob. For example, the EICA (Evolution of Increased Competitive Ability) hypothesis and the Novel Weapons hypothesis (NWH) were tested with both species ( Feng et al., 2009, 2011; Inderjit et al., 2011; Qin et al., 2013; Zheng et al., 2015 ). EICA postulates that change in resource allocation in invasive species produces high competitive ability, which in turn determines a successful invasion; the NWH postulates that invasive success is determined by biochemical weapons (allelopathy) of the invasive that are novel for the invaded range native plants. The comparison of native and invasive populations of A. adenophora and C. odorata made by the previously cited authors suggest that both increased competitive ability and the possession of novel weapons operate in the success of these species as invasives.
Research on the ecology of native species that are invasive elsewhere has also been published, such as the demography of Prosopis glandulosa Torr. (Golubov et al., 1999); bacterial symbionts of Mimosa spp. (Elliott et al., 2009 ) and the relationship of herbivory with terpenoid variability at local and regional scales for Mikania micrantha Kunth and the specialist insect herbivore Stolas punicea (Boheman, 1850) ( Bravo-Monzón, Ríos-Vásquez, Delgado-Lamas, & Espinosa-García, 2014, 2016 ).
Although the operative definition of invasive species conveys the introduction of the plant to a new country, i.e. an exotic or alien plant, there are cases where a native plant species is introduced to a new range within a country. These plants are called “translocated”, but others call them “invasives” in agreement with the Convention on Biological Diversity definition, as it is the case of the authors that contributed to the theory of ecological invasions. Ruellia nudiflora (Engelm. & A.Gray) Urb. is a native species in many states of Mexico, but not in Yucatan, where it behaves as invasive. Víctor Parra-Tabla and his research group have compared Yucatan’s native Ruellia species with R. nudiflora on germination, seedling survival, reproductive phenology and niche width ( Cervera & Parra-Tabla, 2009; Munguía-Rosas, Parra-Tabla, Ollerton, & Carlos Cervera, 2012; Vargas-Mendoza, Ortegon-Campos, Manufo-Zapata, Herrera, & Parra-Tabla, 2015 ). Roberto Lindig-Cisneros’ research group has worked with “translocated” or “invasive” Phragmites australis (Cav.) Trin. ex Steud., and Typha dominguensis Pers. in wetlands. Both species have a widespread original range, the former a neartic one and the later in the Americas, but these species colonize wetlands behaving like invasives displacing native species particularly after perturbations in the nutrient regime by eutrophication ( P. australis) or fire (T. dominguensis) ( Escutia-Lara & Lindig-Cisneros, 2012; López-Arcos, Gómez-Romero, Lindig-Cisneros, & Zedler, 2012 ). Another case of a translocated species is Stenocereus griseus (Haw.) Buxb., introduced as crop in a part of Oaxaca, but now becoming feral and apparently displacing Escontria chiotilla (F.A.C.Weber ex K.Schum.) Rose, in the wild ( Ramírez-Galindo, Barbosa-Martínez, & Ponce-de León, 2011 ).
We found publications in which native species are labeled as “native invasive” species Tithonia tubiformis (Jacq.) Cass. and T. rotundifolia (P. Mill.) S.F. Blake ( Tovar-Sánchez et al., 2012) and as invasive species, Ferocactus latispinus (Haw.) Britton & Rose and Viguiera dentata (Cav.) Spreng. ( Aquino-Soto, Zavala-Hurtado, Pérez-Moreno, & Camargo-Ricalde, 2012 ). In contrast with the possibly invasive or translocated species, in the Tithonia spp., F. latispinus and V. dentata cases there is no evidence of a condition of insularity (such as wetlands), peninsularity (such as Yucatán) or other physiographic traits that could function as barriers. Barriers are important in isolating the invasive species from their natural enemies; the release from natural enemies is supposed to be one of the main mechanisms that allow invasive species to become more competitive in the new range by reallocating to competition resources previously used for defense against natural enemies ( Moles et al., 2012 ). Thus, we suggest that the term “native invasive” is inadequate to qualify the mentioned Tithonia species or their hybrid in Mexico, and that F. latispinus and V. dentata should not be labeled as invasive in Puebla.
Economic and environmental impact of non-native weeds
There are numerous agricultural publications in Mexico, not included in this review, showing the negative effects of weeds on crops, either by causing severe harvest reduction by competition or as refuge for crop pests and pathogens. However, most of these studies are mainly at a small landowner scale without separating the effects of native vs. non-native weeds, and as far as we know, none quantifies the economic impact of weeds at a regional or national scale. A general review of the potential impacts caused by invasive plants impacts was briefly addressed by Aguirre-Muñoz and Mendoza-Alfaro (2009) , based mainly on the information of Espinosa-García et al. (2009) . Cost estimates of weed effects and control were presumed high ( Espinosa-García et al., 2009 ) by observing weed-associated losses in United States, where 34,660 million dollars was the combined cost (losses and control) of weeds in 2003 ( Pimentel et al., 2002; Pimentel, Zúñiga, & Morrison, 2005; Westbrooks, 1998 ). However, it is difficult to extrapolate costs due to the big differences in weed flora ( Espinosa-García et al., 2009 ) and the diversity of agricultural production models prevalent in both countries. Still, potential yield losses of 38–73% (average 52%) in corn produced in United States were estimated for a non-weed control condition ( Dille, Sikkema, Everman, Davis, & Burke, 2015 ); the magnitude of the loss is probably similar for conventional corn production in Mexico. Some invasive weeds are a big problem in Mexico. For example, Johnson grass ( S. halepense ) is a highly competitive perennial grass that aside from causing strong yield reductions in several crops in northern Mexico and the Bajío ( Gámez-González et al., 2002 ), also hosts Claviceps africana and the pepper huasteco geminivirus ( Garzón-Tiznado et al., 2002; Montes-Belmont, Flores-Moctezuma, & Nava-Juarez, 2013 ). Again, there is no national cost estimation for Johnson grass or any other weed, especially the invasive species mentioned here (see the section “Floristics and detection of new non-native species for Mexico)” except for the water hyacinth, E. crassipes (Pérez-Panduro, 1998 ). Four hundred and fourty one million dollars were the annual estimated losses in weed control and the direct and indirect effects caused by the water hyacinth (see Espinosa-García et al., 2009 ). Additional non-native weeds, such as Marrubium vulgare L., have been reported as hosts of the African cluster bug Agnoscelis puberula Spinola, 1837 ( Ortega-León, Thomas, & Soriano, 2006; Thomas, Eger, Jones, & Ortega-León, 2003 ). L. nepetifolia (L.) R. Br., formerly used as ornamental, is now a ruderal weed in almost all Mexico ( Villaseñor & Espinosa-García, 1998 ), and it is an important virus reservoir for phytopathogenic virus ( Piedra-Ibarra, de la Torre-Almaraz, Zúniga, Xoconostle-Cazares, & Ruiz-Medrano, 2005 ).
Aside from direct and indirect economic losses caused by non-indigenous weeds, it is very difficult to monetarily evaluate several collateral costs such as harmful effects on human health, water and soil pollution by herbicides, and disruption of ecosystem services ( Bejarano-González, 2002; Hansen et al., 2013 ). In the USA, chemical control for all pests cost $5,000 million dollars per year, meaning $20,000 in savings in crop yields ( Pimentel et al., 2005 ). Environmental pollution and human health costs are not included and there is no clear means of identifying or quantifying the additional cost.
Costs associated to non-native weed in Mexico must be significant considering the expenses in other countries, especially since ecological and human health problems related to pesticides are bigger and more expensive in third world countries ( Jeschke et al., 2014; Pimentel et al., 2002 ). In Mexico, 15,719 ton of herbicides were used in 1995 ( Inegi-Semarnap, 1999); 4,472 tons of the most popular herbicides (2,4-D, paraquat, atrazine, picloram, and glyphosate) were used in 1999. Sales figures for such herbicides in 1999 (except Atrazine) were 537 million MXP ( Bejarano-González, 2002 ). It is impossible to calculate the share linked to introduced species; 22% of weeds are introduced, but almost half of the 24 species cited as problematic for intensive agriculture in the Mexican plateau and northern Mexico are introduced ( de Ita et al., 1992 ). In addition, agrochemical companies jealously guard their sales figures, making it difficult to estimate the actual weed control costs related directly to productivity and those indirectly related with negative effects on ecosystem services, human health and soil and water contamination.
Quantification of environmental and economic impacts becomes very difficult when the invasive species has an important economic use. This is the case of invasive African grasses introduced as forage such as E. pyramidalis (German grass), a forage plant introduced in the Mexican tropics for cattle raising that became invasive in marshes ( López-Rosas, Moreno-Casasola, & Mendelssohn, 2005, 2006 ), C. ciliaris (buffel grass) that has been sown in Sonora and other northern Mexican states for cattle raising ( Durán-Puga et al., 2011; Marshall, Friedel, van Klinken, & Grice, 2011; Quero-Carrillo, Enríquez-Quiroz, Morales-Nieto, & Miranda-Jiménez, 2010; Ramírez, Haenlein, García-Castillo, & Núñez-González, 2004 ). However, this grass became wild and, being prone to fire, has caused the elimination of non-fire adapted vegetation ( Búrquez-Montijo, Miller, & Martínez-Yrizar, 2002; Franklin et al., 2006; Van Devender, Espinosa-García, Harper-Lore, & Hubbard, 2009 ). The grasses identified by Parsons (1972) as those causing the africanization of the Americas tropics: Megathirsus maximus (Jacq.) B.K. Simon & S.W.L. Jacobs (Guinea grass), Urochloa mutica (Forssk.) T.Q. Nguyen (= Brachiaria mutica; Pará grass), Melinis minutiflora P. Beauv. (molasses grass.), Hyparrhenia rufa (Nees) Stapf (jaraguá), P. clandestinum Hochst. ex Chiov. (Kikuyu grass), Digitaria eriantha Steud. (=D. decumbens Stent; Pangola grass), have also become invasive. The effect of grasses, with little or no forage value, has been documented in Mexico; they affect local biodiversity by direct displacement or interference with seedling establishment; for example, M. repens in grasslands in Durango ( Herrera-Arrieta, Pámanes-García, Herrera-Corral, Chairez-Hemandez, & Cortés-Ortiz, 2011 ), several invasive grasses in humid montane forests ( Ortega-Pieck, López-Barrera, Ramírez-Marcial, & García-Franco, 2011 ), and E. pyramidalis in a tropical freshwater marsh (López-Rosas et al., 2006 ). The removal of some of those grasses in abandoned tropical pastures allows the establishment and growth of native trees ( Román-Dañobeytia et al., 2012 ).
In agricultural systems, invasive grasses affect crop growth, for example, C. dactylon, S. halepense ( Gámez-González et al., 2002 ), and Rottboellia cochinchinensis (Lour.) Clayton ( Contreras-Ramos et al., 2013; Tucuch-Cauich, Orona-Castro, Almeyda-León, & Aguirre-Uribe, 2013 ). In Oasis, the invasive vine C. grandiflora , makes the habitat unsuitable for birds and reptiles ( Rodríguez-Estrella et al., 2010 ). The environmental effects of E. crassipes are mixed, as it may harbor Anopheles albimannus C. R. G. Wiedemann, 1820, a malarial vector ( Savage, Rejmankova, Arredondo-Jiménez, Roberts, & Rodríguez, 1990 ), some free living amoebae pathogenic to humans ( Ramírez, Robles, & Martínez, 2010 ), reduce water quality ( Gutiérrez, Huerto, Saldana, & Arreguín, 1996; Lind & Davalos-Lind, 2002 ), or become inhospitable to small migratory birds ( Villamagna, Murphy, & Karpanty, 2012 ), but E. crassipes also may harbor a great diversity of invertebrates and function as preferred foraging habitat for coots ( Hernández et al., 2015; Rocha-Ramírez, Ramírez-Rojas, & Chávez-López, 2007; Rocha-Ramírez, Robles-Valderrama, & Ramírez-Flores, 2014; Román-Contreras, Rocha-Ramírez, & Cházaro-Olvera, 2008; Villamagna, Murphy, & Trauger, 2010 ). Water hyacinth also removes heavy metals and insecticides from water (see management and use section).
Environmental impacts are difficult to measure and do not necessarily increase proportionally with invasive species biomass ( Jeschke et al., 2014 ), however, a new classification scheme for invasive species environmental impacts ( Blackburn et al., 2014 ) could be useful to evaluate invasive impact for Mexican conditions. Considering that many non-native species never become invasive, and that even when they are invasive some of them cause environmental change directly (drivers) while others just prosper in an already changed one (passengers) ( MacDougall & Turkington, 2005 ), it is imperative to develop or adapt a classification scheme suited for Mexico that distinguish the actual or potential ecological role of the evaluated species in heterogeneous regions. Apart from the expensive cost of losing ecosystem services (water retention, recreation, pollination, pest control and soil conservation), native biodiversity is also impacted. For example, loss of native vegetation, directly or through fire, has been documented for buffelgrass and Sahara mustard ( B. tournefortii ), invasive species occupying huge regions in northwestern Mexico ( Búrquez-Montijo et al., 2002; Dimmitt & Van Devender, 2009 ).
Summarizing, the negative effects of invasive species on the environment, productive systems and human health are important and diverse, but complete damage quantification for most invasive species is lacking, and it is imperative to allocate economic and human resources to prevent and mitigate the noxious effects of these non-native species.
Management of non-native weeds
Proper management of non-native weeds includes prevention, early detection, eradication, containment and mitigation. All these tasks should be integrated in a National weed management strategy able to deal with native and non-native weeds at various spatial and time scales ( Espinosa-García & Vibrans, 2009 ). The national strategy is only viable if all the stakeholders, individuals and institutions are well aware of the needs for weed control and the effects of weed invasions ( Espinosa-García, 2009 ). In spite of having a National Invasive Species Strategy ( Comité Asesor Nacional sobre Especies Invasoras, 2010 ), the individualistic weed management model is still prevalent among producers and the articulation among government institutions, producers and academia is still poor, but it has some improvement since 2008 ( Espinosa-García, 2009 ). For example, prior to 2008, there was not a single eradication campaign for invasive weeds in spite of published reports of quarantine weeds growing in Mexico such as Polygonum convolvulus (Espinosa-García, 1981) or Rotttboellia conchinchinensis ( Esqueda-Esquivel, 2000; Medina-Pitalúa & Domínguez-Valenzuela, 2001 ). Then, after Juan Carlos Delgado and his team of the “Comité Estatal de Sanidad Vegetal de Guanajuato” detected several P. convolvulus infestations in wheat fields in Guanajuato ( Delgado-Castillo, 2010 ), an official eradication campaign, promoted by Delgado, was started, resulting in a very strong reduction of the weed in the infested fields ( Vibrans & Delgado, 2010 ). Eradication campaigns have followed since in several states for P. convolvulus, Cuscuta indecora Choisy, R. cochinchinensis and Themeda quadrivalvis , although in many cases eradication was not achieved and in some states the quarantined weeds have increased their density (see internet reports of “Campañas contra Malezas Cuarentenarias” or “Campañas contra malezas reglamentadas” for Mexico or for the “Comités Estatales de Sanidad Vegetal”, for example h t t p & # 5 8 ; & # 4 7 ; & # 4 7 ; w w w .gob.mx/senasica/acciones-y-programas/malezas-reglamentadas ).
Advances in Mexico on general management issues have been concentrated in diagnostic studies involving the identification of threatening species ( Aguilar et al., 2008; Peña-Jiménez & Neyra-González, 1998; Semarnat, 2016 ); biodiversity estimates, distribution and possible impacts of non-native weeds for Mexico ( Espinosa-García et al., 2009 ); policies on invasives (Arriaga, 2009 ); and management strategies and cooperation at national and international scales ( Espinosa-García & Van Devender, 2009; Espinosa-García & Vibrans, 2009 ). There are also methodological proposals exemplified with taxonomic groups, specific regions or countries or invasive species, mainly for species prioritization for management actions ( Sánchez-Blanco, Sánchez-Blanco, Mario Sousa, & Espinosa-García, 2012 ), distribution modeling ( Sánchez-Flores, 2007; Sánchez-Flores et al., 2008 ), and infestation monitoring modeling ( Sonnentag et al., 2011).
Control and use of non-native weeds
Weed control generally involves drastic abundance reduction or elimination from a generally small area, under an individualistic weed management model that promotes the misuse or abuse of herbicides and poor control of noxious or invasive weeds ( Espinosa-García & Vibrans, 2009 ). The prevalent weed control mode has been chemical (not covered in this review), although weed herbicide resistance and the negative effects on non-target species have fostered control efforts by biological, mechanical, solarization ( Lira-Saldívar et al., 2004 ) or integrated control, mainly for some invasive weeds.
Most publications on weed management deal with biological control of E. crassipes, C. arvensis, S. halepense, A. donax (Goolsby et al., 2011) and Althernanthera phyloxeroides (Mart.) Griseb. ( Lara-Villalón, Mora-Olivo, Sánchez-Ramos, & Martínez-Ávalos, 2014 ). Water hyacinth is the most studied invasive weed, testing insects ( Aguilar, Camarena, Center, & Bojórquez, 2003; Martínez-Jiménez, Gutiérrez-López, Huerto-Delgadillo, & Ruiz-Franco, 2001; Martínez-Morales, Estrada-Venegas, Equihua-Martínez, & Valdez-Carrasco, 2014; Pérez-Panduro, 1998 ), fungi ( Martínez-Jiménez & Charudattan, 1998; Martínez-Jiménez & Gutiérrez-López, 2001; Martínez-Jiménez, Gutiérrez-López, et al., 2001; Martínez-Jiménez, Brown et al., 2001 ) or both kinds of natural enemies for control purposes ( Martínez-Jiménez & Gómez-Balandra, 2007 ). Even with some successes in biological control, water hyacinth still is one of the most troublesome invasive species that is mostly managed with mechanical control ( Mangas-Ramírez & Elías-Gutiérrez, 2004 ) that many times reduces temporarily water hyacinth abundance, making the removal of this plant a permanent activity or business for those that own and operate the machinery needed for this task. Chemical control has been applied also, but concerns about herbicide toxicity in water have limited this kind of management (see Santibañez-Aguilar, Ponce-Ortega, González-Campos, Serna-González, & El Hawagi, 2013 ). Moreover, the seed bank of E. crassipes in the sediments provides new recruits when successful removal of the floating plants has been achieved ( Santibañez-Aguilar et al., 2013 ). Some attempts or proposals have been made to use water hyacinth as forage, mulch or as a phytoremediation agent to remove heavy metals or organophosphorus and organochlorine pesticides from water ( Fileto-Perez et al., 2015; Mercado-Borrayo, Heydrich, Pérez, Quiroz, & Hill, 2015; Rodríguez, Avila-Pérez, & Barceló-Quintal, 1998; Santibañez-Aguilar et al., 2013; Tejeda, Zarazua, Avila-Pérez, Carapia-Morales, & Martínez, 2010 ). In spite of calls for integrated management of water hyacinth ( Gutiérrez et al., 1996 ) and many proposed uses for this plant, the problems caused by E. crassipes continue. A recent proposal for biorefining networks to process E. crassipes biomass for profit might contribute strongly to prevent or mitigate water hyacinth damage to water bodies ( Santibañez-Aguilar et al., 2013 ), but still that proposal has yet to become a reality. The theme of finding uses or beneficial environmental effects for invasive plants has also been repeated for tamarisk ( Tamarix ramosissima Ledeb.) ( MacGregor-Fors, Ortega-Álvarez, Barrera-Guzman, Sevillano, & del Val, 2013 ), field bindweed, Johnson grass, natal grass and other escaped forage grasses (see section ahead). Still a cost-benefit analysis of the effects of these invasives could show that the negative effects will probably outweigh the positive ones.
Chemical control of field bindweed or correhuela ( C. arvensis ), a highly problematic weed, has been frequently studied (e.g. Tamayo-Esquer & Gaillardon, 1989); also cultural control (Robles et al., 2006 ) and biological control with mites ( Rodríguez-Navarro, Flores-Macías, & Torres-Martínez, 2008; Rodríguez-Navarro, Rodríguez-Morell, Alemán-Martínez, Flores-Macías, & Torres-Martínez, 2011; Rodríguez-Navarro, Torres-Martínez, & Olivares-Orozco, 2004 ) have been studied, but as far as we know, the biological control for this weed has not been generalized. The use of C. arvensis also has been explored for phytoremediation by heavy metal accumulation ( Cruz-Jiménez et al., 2005; Gardea-Torresdey, Peralta-Videa, Montes, de la Rosa, & Corral-Díaz, 2004; Montes, Peralta-Videa, Parsons, Corral Díaz, & Gardea-Torresdey, 2013; Montes-Holguin et al., 2006 ). A similar pattern of studies as that found with correhuela is the one with Johnson grass ( S. halepense ) with studies on chemical control ( Esqueda-Esquivel, Uresti-Durán, & Hernández-Aragón, 2015; Magallanes et al., 1986; Vera-Núñez, Grageda-Cabrera, Altamirano-Hernández, & Peña-Cabriales, 2010 ); biological control ( Iracheta-Cárdenas, Galan-Wong, & Pereyra-Alferez, 1995; Martínez-Mendoza et al., 2012 ); and use as forage ( Castro-González, Alayon-Gamboa, Ayala-Burgos, & Ramírez-Aviles, 2008; Gutiérrez et al., 2008; Ramírez-Vera et al., 2012 ).
- minutiflora is so widely distributed that novel attempts for its use have been evaluated: first as repellent for Rhipicephalus microplus (Canestrini, 1888) (= Boophilus microplus ), a cattle tick pest in the tropics ( Fernández-Ruvalcaba et al., 2003; Fernández-Ruvalcaba, Preciado-de la Torre, Cruz-Vázquez, & García-Vázquez, 2004; Muro-Castrejón, Cruz-Vázquez, Fernández-Ruvalcaba, & Torres, 2004 ), and later again as forage ( Durán-Puga et al., 2011; Murillo-Ortiz, Mellado-Bosque, Herrera-Torres, Reyes-Estrada, & Carrete-Carreon, 2014; Ramírez et al., 2009 ).
As Mexico is within the area of origin for important invasive plants elsewhere, we found five publications prospecting for natural enemies of invasive species mainly in Australia: Sida acuta Burm. f. and Sida rhombifolia L. ( Gillett, Harley, Kassulke, & Miranda, 1991 ); Mimosa pigra L. ( Heard et al., 2010; Heard, Mira, Fichera, & Segura, 2012 ); Parkinsonia aculeata L. ( Brown, Segura, Santiago-Jiménez, Rota, & Heard, 2011 ); Lantana camara L. ( Manners, Palmer, Burgos, McCarthy, & Walter, 2011 ) and Jatropha gossypiifolia L. ( Heard, Dhileepan, Bebawi, Bell, & Segura, 2012 ). In adition, a neotropical nematode was detected as a promising biocontrol against native Melastomataceae: Clidemia hirta (L.) D.Don, Miconia calvescens DC., and Tibouchina herbacea (DC.) Cogn. (Oliveira et al., 2013).
Many non-native weeds naturalized long time ago are now used by Mexican ethnic groups as medicinal plants, for rituals, or emergency food ( Blancas, Casas, Pérez-Salicrup, Caballero, & Vega, 2013; Blancas et al., 2010; Blanckaert et al., 2007; Flores & Kantun-Balam, 1997; Sánchez-González, Granados-Sánchez, & Simón-Nabor, 2008 ). A special case is that of the pirú ( S. molle ), a tree naturalized long ago and widespread in the Mexican plateau, which has cultural and medicinal importance in Mexico ( Ramírez-Albores & Badano, 2013 ). The invasive label for the pirú is valid if the invasive definition is based on the formation of new populations 100 m or more away of the original population ( Richardson et al., 2000 ), but that label does not hold considering the requisite of proof of economic or environmental damage or threat ( CDB, 2009; DOF, 2015 ). Although allelopathic effects were suggested for pirú ( Anaya & Gómez-Pompa, 1971 ), and the tree is frequently found growing in several ecosystems, there is no evidence of local extirpations or extinctions caused by this tree. Perhaps we are witnessing a naturalization case more than an invasion by this non-native tree.
Research on management of invasive plants for restoration purposes
Although few papers have been published on the management of invasive plants for restoration purposes, a series of studies in a wetland invaded by E. pyramidalis is remarkable. These studies include description, damage evaluation, invasive dynamics and restoration methods ( López-Rosas & Moreno-Casasola, 2012; López-Rosas et al., 2006; Moreno-Casasola et al., 2016 ). Other restoration programs, particularly in the Humid Forests Biome and tropical dry forests, include invasive weeds removal (particularly grasses and ferns) to facilitate the establishment of desired plant species ( López-Barrera et al., 2016; Martínez-Garza, Osorio-Beristain, Alcalá-Martínez, Valenzuela-Galván, & Mariano, 2016; Román-Dañobeytia et al., 2012; Williams-Linera & Álvarez-Aquino, 2016 ). In the cases where the invasive species prospers only after the environment has been disturbed, i.e. passanger species ( MacDougall & Turkington, 2005 ), the restoration occurs after the disturbance factors are removed. For example, native trees return displacing T. chinensis in the Colorado River delta when the hidological and salinity regimes return to pre-invasion levels ( Nagler et al., 2005).
General overview of the scientific knowledge on non-native species
The National Strategy on Invasive Species for Mexico emphasizes that scientific information is imperative to achieve an adequate knowledge and management of plant invasions and non-native weed species (strategic transversal actions: knowledge and information). This review shows that there are significant advances in the scientific knowledge on non-native species across many topics, mainly the check-list of the species present in Mexico and their incidence at a state scale. There has also been a constant addition of previously undetected species to the checklist of non-native weeds in Mexico, but these contributions result from the activity of few researchers that divide their interests between invasive species and several other academic fields. Although this approach has yielded acceptable results, it should be complementary of a dedicated and permanent early detection program led by federal agencies in which botanists and the general public participate (e.g. Citizen Science, http://www.naturalista.mx/ ).
Very few studies on the detailed distribution, actual or potential, are available for invasive weeds, but none of them have been used to take preventive or management actions by policy or decision makers. The information on weed distribution is imperative for risk analysis and management, but it is lacking for most non-native weeds in Mexico. Similarly, the information on risk analysis to prevent the entrance of invasive species or to prioritize attention to the invasive species already present in Mexico is still incomplete and missing for most species present in the country. The knowledge of the impacts by non-native species on the environment, economy or human health, is necessary for risk analysis and prioritization of management. However, as seen in this review, this knowledge is limited to a few species, studied locally and has not been quantified at the regional or national scales, except for the water hyacinth ( Pérez-Panduro, 1998 ), although this quantification should be updated. A particularly difficult challenge is that of the control of the widely used invasive species that escape cultivation causing noxious effects, such as the grasses introduced for forage reviewed previously. Most of these grasses are very important for cattle rising, and the control measures are strongly opposed by ranchers. For example, there are numerous studies on the distribution, ecology and negative effects of buffel grass ( C. ciliaris ), but the areas planted with this grass have not diminished. Furthermore, the Mexican official research institution for agriculture and cattle rising (INIFAP) promotes C. ciliaris cultivation by developing new buffel varieties. The conflicts caused by useful-noxious invasive species point to the need of coordination and cooperation among government agencies and to the research needed to provide alternatives to the people that depend on those invasive species.
Regarding the ecological research on invasive species, the environments that they invade and the plant invasion processes, these research topics in Mexico are still limited, disarticulated and clearly insufficient for the challenges posed by a mega-diverse country as Mexico. With the exception of the marsh invaded by E. pyramidalis , there are few continued efforts to study and restore environments invaded by invasive weeds. Taken as a group, the restoration studies involving invasive weeds are also disarticulated and performed by very few specialized researchers in restoration ecology.
The largest set of publications reviewed here is concerned with invasive management, mainly about biological control concentrated in few high-impact invasive species such as E. crassipes, S. halepense or C. arvensis . Unfortunately most management studies concentrate in one invasive weed and one control agent. The problems posed by non-native species are complex and varied, but they cannot and should not be treated with an approach exclusively focused on an invasive species. Instead, an ecosystemic approach should be used to prevent and treat biological invasions. For example, a review of Australian ecosystem responses to invasive weed removal, indicated that in most cases recolonization of the sites with other weeds, native or non-native, was observed and in few cases plant recolonization was conductive to the local ecosystem restoration ( Reid, Morin, Downey, French, & Virtue, 2009 ).
The scientific knowledge generated by Mexican researchers for plant invasions in Mexico is not enough, although most of it is of high quality, and the goals concerning scientific knowledge set for 2020 in the National Strategy on Invasive species probably will not be met.
A valid question can be raised on the need for developing scientific information on invasives and plant invasions in Mexico when thousands of papers on these topics are available in the international scientific literature. Unfortunately, although much of the international literature is useful to understand general patterns and process in biological invasions, the state of the art on understanding invasions is not well integrated ( Jeschke, 2014 ). Moreover, a recent review on the scientific support for the main hypotheses that try to explain the nature of invasive species, the invaded ecosystems and the interactions between invaders and ecosystems, found incomplete support, and in many cases evidence that does not support or contradict those hypothesis ( Moles et al., 2012 ). The reasons offered for the incomplete support or contradictory evidence, are the idiosyncratic nature of each invasive species and the invaded environments. This means that the experimental results vary depending on the taxonomic and/or ecological group to which invasive species belong. Also, results on ecosystem invasibility or ecosystemic impacts are also idiosyncratic, change with time and depend on the history of the invasion and the invaded ecosystem ( Kueffer et al., 2013; Moles et al., 2012 ). To face the idiosyncratic and dynamic nature of invasive species, invaded ecosystems and the interactions between them, integrated studies with selected model species and model ecosystems repeated around the world have been proposed, as well as meta-analysis of the thousands of papers on invasives and invasions ( Kueffer et al., 2013).
The Mexican research on non-native weeds and invasions has made some contributions to the management and policy on invasive plants and invasions, but most of the management has been empirically based, reproducing the empirical management mostly developed in the first world. For example, the widely accepted scheme of early detection and eradication of invasive weeds and the cost associated to this scheme has been developed in Australia and the United States ( Mendoza et al., 2009; Panetta, 2015; Rejmánek & Pitcairn, 2002 ). Risk analysis in Mexico for unwanted weeds is based mainly in the Australian scheme ( Panetta, 1993; Pheloung, Williams, & Halloy, 1999 ), which has been adapted for other countries such as the United States ( Gordon, Tancig, Onderdonk, & Gantz, 2011 ). A preliminary effort to contribute to risk analysis for non-native species already present species in Mexico was developed for Fabaceae ( s.l.) species (Sánchez-Blanco et al., 2012).
Perspectives on invasive weed research
Given the incomplete knowledge of plant invasions and their idiosyncratic and dynamic nature, invasive weed management in Mexico should continue adjusting the empirical knowledge generated abroad and devising new empirical approaches derived from the scientific knowledge developed specifically for Mexican ecosystems affected by invasions. For example, among the many areas in which Mexican and International research can be integrated is that of determination of vulnerability to invasions. A map predicting the vulnerability to invasions for Mexico was obtained overlapping maps with propagule availability, vegetation type, anthropic disturbance and native plant species richness ( del Val et al., 2015 ); the correlation of the four variables with non-native plant incidence was obtained previously by international researchers, and the confirmation of the correlations was obtained for Mexico ( Espinosa-García et al., 2004b ). A further improvement of this model can be obtained by indicating which vulnerable regions are prone to be invaded by weeds imported from different parts of the world in a similar way as the work done for India by modeling susceptibility of its regions to weeds from North America, South America, Europe, Africa and Australia ( Adhikari, Tiwary, & Barik, 2015 ). The improved model would be very useful for prevention and surveillance in early detection programs.
The publications included in this review are of high quality showing that Mexico has an important, but not sufficient, number of researchers able to generate the scientific knowledge important for the prevention and management of the non- native weeds. However, the prevalent pattern of the Mexican research on non-native weeds for all research topics reflects research produced by few academic groups, but mostly disarticulated and few times connected with decision makers. Thus, a primary task to meet the scientific knowledge needs to prevent and/or manage biological invasions in Mexico is to engage young researchers on invasions and coordinate them with the existent researchers to investigate a highly selected series of topics identified in the National Strategy for Biological Invasions (see Espinosa-García, 2009; Espinosa-García et al., 2009 ). This can be achieved opening new academic positions in universities and research institutions and through competitive research funds administrated by the National Commission on the Knowledge and use of Biodiversity (Conabio) or the National Commission of Science and Technology (Conacyt).
Another key task is capacity building at all levels, from the general public to recognize and report invasive species, to postgraduate students specializing in invasive species and biological invasions. The existing and future Mexican researchers should integrate also with international researchers to study model invaded ecosystems and model invasive species to advance the theory on biological invasions.
Final observations
Summarizing, the last few years we had made considerable advances collecting basic information on non-native weeds. Introduced species’ impact in Mexico is perhaps lower than that in countries that are our main trade partners. However, there are species such as water lily and several African grasses, which cause considerable damage to agricultural and natural landscapes. An urgent task is a full assessment of the actual and potential impact of non-native species on Mexican ecosystems to prioritize the weeds and regions that require immediate attention. Of course, this task cannot be completed without a previous full knowledge of the identity, abundance and distribution of the weeds introduced in Mexico.
Acknowledgements
The authors thank Miguel Martínez Ramos for the invitation to elaborate this review. We also thank Yolanda M. García Rodríguez for help in the references formatting and to Heberto Ferreira and Alberto Valencia for their help with databases and computing. We acknowledge the corrections and comments from two anonymous reviewers of an earlier version of this manuscript. This work was supported by IIES-UNAM-POFJEG.
References
Adhikari, D., Tiwary, R., & Barik, S. K. (2015). Modelling Hotspots for Invasive Alien Plants in India. Plos One, 10(7). doi: 10.1371/journal.pone.0134665
Aguilar, J. A., Camarena, O. M., Center, T. D., & Bojórquez, G. (2003). Biological control of waterhyacinth in Sinaloa, Mexico with the weevils Neochetina eichhorniae and N.bruchi. Biocontrol, 48(5), 595-608. doi: 10.1023/a:1025707603627
Aguilar, V., Aguirre, A., Alarcón, J., Bomer, A., Contreras, S., Del Val, E., . . . Zimmerman, H. (2008). Especies invasoras de alto impacto a la biodiversidad. Prioridades en México. Jiutepec, Morelos, México: IMTA, The Nature Conservancy, Conabio, Aridamérica, GECI.
Aguirre Muñoz, A., R. Mendoza Alfaro et al. 2009. Especies exóticas invasoras: impactos sobre las poblaciones de flora y fauna, los procesos ecológicos y la economía, In Capital natural de México, vol. II: Estado de conservación y tendencias de cambio. Conabio, México, pp. 277-318.
Anaya, A. L., & Gómez-Pompa, A. (1971). Inhibición del crecimiento producido por el “pirú” (Schinus molle L.) Revista de la Sociedad Mexicana de Historia Natural., 32, 99-109.
Aquino Soto, V. H., Zavala Hurtado, J. A., Pérez Moreno, J., & Camargo Ricalde, S. L. (2012). Estacionalidad de bacterias y hongos en la rizósfera de dos especies de plantas en el Valle semiárido de Zapotitlán, Puebla. Revista Mexicana de Ciencias Agrícolas, 3(6), 1231-1245.
Arriaga, L. (2009). The Invasive Species Problem in Mexico: Current Policy Response. In Van Devender T., F.J. Espinosa-García, B.L. Harper-Lore , T. Hubbard (Ed.), Invasive Plants on the Move. Controlling them in North America. (pp. 33-42). Tucson, Arizona, USA: Arizona-Sonora Desert Museum.
Arriaga, L., Castellanos, A. E., Moreno, E., & Alarcón, J. (2004). Potential ecological distribution of alien invasive species and risk assessment: a case study of buffel grass in arid regions of Mexico. Conservation Biology, 18(6), 1504-1514. doi: 10.1111/j.1523-1739.2004.00166.x
Arroyo-Rodríguez, V., Pineda, E., Escobar, F., & Benitez-Malvido, J. (2009). Value of Small Patches in the Conservation of Plant-Species Diversity in Highly Fragmented Rainforest. Conservation Biology, 23(3), 729-739. doi: 10.1111/j.1523-1739.2008.01120.x
Bejarano-González, F. (2002). La espiral del veneno. Guía crítica ciudadana sobre plaguicidas. México, D.F.: Red de Acción sobre Plaguicidas y Alternativas en México.
Blackburn, T. M., Essl, F., Evans, T., Hulme, P. E., Jeschke, J. M., Kuehn, I., . . . Bacher, S. (2014). A Unified Classification of Alien Species Based on the Magnitude of their Environmental Impacts. Plos Biology, 12(5). doi: 10.1371/journal.pbio.1001850
Blancas, J., Casas, A., Pérez-Salicrup, D., Caballero, J., & Vega, E. (2013). Ecological and socio-cultural factors influencing plant management in Nahuatl communities of the Tehuacan Valley, Mexico. Journal of Ethnobiology and Ethnomedicine, 9. doi: 10.1186/1746-4269-9-39
Blancas, J., Casas, A., Rangel-Landa, S., Moreno-Calles, A., Torres, I., Pérez-Negron, E., . . . Dávila, P. (2010). Plant Management in the Tehuacan-Cuicatlan Valley, Mexico. Economic Botany, 64(4), 287-302. doi: 10.1007/s12231-010-9133-0
Blanckaert, I., Vancraeynest, K., Swennen, R. L., Espinosa-García, F. J., Piñero, D., & Lira-Saade, R. (2007). Non-crop resources and the role of indigenous knowledge in semi-arid production of Mexico. Agriculture Ecosystems & Environment, 119(1-2), 39-48. doi: 10.1016/j.agee.2006.06.015
Bravo-Monzón, A. E., Rios-Vásquez, E., Delgado-Lamas, G., & Espinosa-García, F. J. (2014). Chemical diversity among populations of Mikania micrantha: geographic mosaic structure and herbivory. Oecologia, 174(1), 195-203. doi: 10.1007/s00442-013-2748-y
Bravo-Monzón, A. E., Rios-Vásquez, E., Delgado-Lamas, G., & Espinosa-García, F. J. (2016). Differential herbivory of the specialist beetle Stolas punicea on chemical phenotypes of its host Mikania micrantha. Biocontrol Science and Technology, 26(3), 419-425. doi: 10.1080/09583157.2015.1118436
Brown, J. W., Segura, R., Santiago-Jiménez, Q., Rota, J., & Heard, T. A. (2011). Tortricid moths reared from the invasive weed Mexican palo verde, Parkinsonia aculeata, with comments on their host specificity, biology, geographic distribution, and systematics. Journal of Insect Science, 11(7), 1-17.
Búrquez-Montijo, A., Miller, M. E., & Martínez-Yrizar, A. (2002). Mexican grasslands, thornscrub, and the transformation of the Sonoran Desert by invasive exotic buffelgrass (Pennisetum ciliare). In T. B. (Ed.), Invasive exotic species in the Sonoran Region. (pp. 126-146 ). Tucson. Arizona, U.S.A.: University of Arizona Press.
Calderón de Rzedowski, G., & Rzedowski, J. (2004). Manual de malezas de la región de Salvatierra, Guanajuato. Flora del Bajío y Regiones Adyacentes, 20, 11-315.
Caley, P., Groves, R. H., & Barker, R. (2008). Estimating the invasion success of introduced plants. Diversity and Distributions, 14(2), 196-203. doi: 10.1111/j.1472-4642.2007.00440.x
Cano-Santana, Z., Castillo-Arguero, S., Martínez-Orea, Y., & Juarez-Orozco, S. (2008). Analysis of plant richness and conservation value of the three areas added to the Pedregal de San Angel Ecological Reserve, Federal District (Mexico). Boletin de la Sociedad Botanica de Mexico, 82, 1-14.
Carranza-González, E. (2005). Registro de Convolvulus crenatifolius Ruiz & Pavón (Convolvulaceae) en México. Acta Botanica Mexicana(73), 59-68.
Castillo, S. A., & Moreno-Casasola, P. (1996). Coastal sand dune vegetation: an extreme case of species invasion. Journal of Coastal Conservation, 2(1), 13-22.
Castro-González, A., Alayon-Gamboa, J. A., Ayala-Burgos, A., & Ramírez-Aviles, L. (2008). Effects of Brosimum alicastrum and Lysiloma latisiliquum mixtures on voluntary intake, nutrient digestibility and nitrogen balance in sheep fed tropical pastures. Animal Feed Science and Technology, 141(3-4), 246-258. doi: 10.1016/j.anifeedsci.2007.06.033
CDB. (2009). What are Invasive Alien Species? Retrieved 22/11/2016, 2016, from https://www.cbd.int/invasive/WhatAreIAS.shtml
Cervera, J. C., & Parra-Tabla, V. (2009). Seed germination and seedling survival traits of invasive and non-invasive congeneric Ruellia species (Acanthaceae) in Yucatan, Mexico. Plant Ecology, 205(2), 285-293. doi: 10.1007/s11258-009-9617-0
Colmenero Robles, J. A. & Fernández Nava, R. (2003). New records of Corchorus (Tiliaceae) for Mexico. SIDA, Contributions to Botany, 1299-1309.
Comité Asesor Nacional sobre Especies Invasoras. (2010). Estrategia nacional sobre especies invasoras en México, prevención, control y erradicación. México, D.F., México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Comisión Nacional de Áreas Protegidas, Secretaría de Medio Ambiente y Recursos Naturales.
Contreras-Ramos, S. M., Rodríguez-Campos, J., Saucedo-García, A., Cruz-Ortega, R., Macías-Rubalcava, M. L., Hernández-Bautista, B. E., . . . Luisa Anaya, A. (2013). Mutual effects of Rottboellia cochinchinensis and maize grown together at different densities. Agronomy Journal, 105(6), 1545-1554. doi: 10.2134/agronj2013.0068
Corkidi, L., Cacho, S., & Búrquez, A. (1991). Dispersión del pirú (Schinus molle L., Anacardiaceae) por aves en Teotihuacán, México. Acta Botanica Mexicana(15), 17-22.
Cotter, J., & Osborne, M. A. (1996). Agronomia afranceada (sic): the French contribution to Mexican agronomy, 1880-1940. Science Technology & Society, 1(1), 25-49.
Cruz-Jiménez, G., Peralta-Videa, J. R., de la Rosa, G., Meitzner, G., Parsons, J. G., & Gardea-Torresdey, J. L. (2005). Effect of sulfate on selenium uptake and chemical speciation in Convolvulus arvensis L. Environmental Chemistry, 2(2), 100-107. doi: 10.1071/en05028
De Ita, G., Torres, G., Calderón, O., Luna, E., & Peralta, F. (1992). Malezas comunes en cultivos agrícolas. Descripción, distribución, importancia económica y control. México, D.F.: Secretaría de Agricultura y Recursos Hidráulicos.
De la Barrera, E., & Castellanos, A. E. (2007). High temperature effects on gas exchange for the invasive buffel grass (Pennisetum ciliare L. Link). Weed Biology and Management, 7(2), 128-131. doi: 10.1111/j.1445-6664.2007.00248.x
Dehnen-Schmutz, K., Touza, J., Perrings, C., & Williamson, M. (2007). The Horticultural Trade and Ornamental Plant Invasions in Britain. Conservation Biology, 21(1), 224-231. doi: 10.1111/j.1523-1739.2006.00538.x
Del-Val, E., Balvanera, P., Castellarini, F., Espinosa-García, F. J., Murguía, M., & Pacheco, C. (2015). Identifying areas of high invasion risk: a general model and an application to Mexico. Revista Mexicana De Biodiversidad, 86(1), 208-216. doi: 10.7550/rmb.44743
Díaz Romo, A., Flores Ancira, E., De Luna Jiménez, A., Luna Ruiz, J. d. J., Frías Hernández, J. T., & Olalde Portugal, V. (2012). Biomasa aérea, cantidad y calidad de semilla de Melinis repens (Willd.) Zizka, en Aguascalientes, México.. Revista Mexicana De Ciencias Pecuarias, 3(1), 33-47.
Dille, J. A., Sikkema, P. H., Everman, W. J., Davis, V. M., & Burke, I. C. (2015). Perspectives on corn yield losses due to weeds in North America. Retrieved January 10, 2016, 2016. http://wssa.net/wp-content/uploads/WSSA-2015-Corn-Yield-Loss-poster-updated-calc.pdf
Dimmitt, M. A., & Van Devender, T. R. (2009). Sahara mustard (Brassica tournefortii): A New, Serious Threat to Low Desert Ecosystems in the southwestern United States and northwestern Mexico. In Van Devender T., F.J. Espinosa-García, B.L. Harper-Lore, T. Hubbard (Ed.), Invasive Plants on the Move. Controlling them in North America (pp. 241-250). Tucson, Arizona, U.S.A.: Arizona-Sonora Desert Museum.
Diversitas. (2010, 27 January, 2016). Global Invasive Species Programme-Diversitas. from http://www.diversitas-international.org/activities/past-projects/global-invasive-species-programme-gisp
DOF. (2015). DECRETO por el que se reforman y adicionan diversas disposiciones de la Ley General del Equilibrio Ecológico y la Protección al Ambiente, y de la Ley General de Vida Silvestre. México, D.F.: Diario Oficial de la Federación. DOF: 09/01/2015
Durán Puga, N., Ruiz Corral, J. A., González Eguiarte, D. R., Núñez Hernández, G., Padilla Ramírez, F. J., & Contreras Rodríguez, S. H. (2011). Development cardinal temperatures of the planting-emergence stage for 11 forage grasses. Revista Mexicana de Ciencias Pecuarias, 2(3), 347-357.
Elliott, G. N., Chou, J. H., Chen, W. M., Bloemberg, G. V., Bontemps, C., Martínez‐Romero, E., . . . James, E. K. (2009). Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N‐limited conditions. Environmental microbiology, 11(4), 762-778.
Escutia-Lara, Y., & Lindig-Cisneros, R. (2012). Dynamics of Phragmites australis and Schoenoplectus americanus in response to the addition of phosphorus and nitrogen in experimental wetlands. Botanical Sciences, 90(4), 459-467.
Espinosa-García, F. J. (1981). Adiciones a la flora arvense del Valle de México. Boletin de la Sociedad Botanica de Mexico, 41, 27-32.
Espinosa-García, F. J. (2009). Invasive Weeds in Mexico: Overview of Awareness, Management and Legal Aspects. In S. J. Darbyshire & R. Prasad (Eds.), Proceedings of the Weeds Across Borders 2008 Conference, Banff, Alberta. (pp. 17–29). Lethbridge, AB. Canada.: Alberta Invasive Plants Council.
Espinosa-García, F. J., T. R. Van Devender. (2009). Cooperation among North American Countries Dealing with Weeds: Advances and Challenges. In F. J. E.-G. Van Devender T., B.L. Harper-Lore, T. Hubbard (Ed.), Invasive Plants on the Move. Controlling them in North America. (pp. xxi-xxvii). Tucson, Arizona, U.S.A.: Arizona-Sonora Desert Museum.
Espinosa-García, F. J., & Vibrans, H. (2009). The Need of a National Weed Management Strategy in Mexico. In T. Van Devender, F. J. Espinosa-García, B. L. Harper-Lore & T. Hubbard (Eds.), Invasive Plants on the Move. Controlling them in North America (pp. 23-32). Tucson, Arizona, U.S.A.: Arizona-Sonora Desert Museum.
Espinosa-García, F. J., Villaseñor, J. L., & Vibrans, H. (2004a). Geographical patterns in native and exotic weeds of Mexico. Weed Technology, 18, 1552-1558. doi: 10.1614/0890-037x(2004)018[1552:gpinae]2.0.co;2
Espinosa-García, F. J., Villaseñor, J. L., & Vibrans, H. (2004b). The rich generally get richer, but there are exceptions: Correlations between species richness of native plant species and alien weeds in Mexico. Diversity and Distributions, 10(5-6), 399-407. doi: 10.1111/j.1366-9516.2004.00099.x
Espinosa-García, F. J., Villaseñor, J. L., & Vibrans, H. (2009). Biodiversity, Distribution, and Possible Impacts of Exotic Weeds in Mexico. In Van Devender T., F. J. Espinosa-García, B.L. Harper-Lore, T. Hubbard (Ed.), Invasive Plants on the Move. Controlling them in North America (pp. 43-52). Tucson, Arizona, USA: Arizona-Sonora Desert Museum.
Esqueda E., V. (2000). Las malezas del cultivo de arroz (Oryza sativa L.) en México. Revista Mexicana de la Ciencia de la Maleza Número Especial, 63-81.
Esqueda-Esquivel, V. A., Uresti-Durán, D., & Hernández-Aragón, L. (2015). Alternativas al fenoxaprop-etil para el control del zacate Johnson (Sorghum halepense) en arroz de riego. Ecosistemas y Recursos Agropecuarios, 2(6), 317-325.
Estrada C, E., & Yen M, C. (2001). Lespedeza cuneata (Fabaceae), a first record of its occurrence in Mexico. SIDA, Contributions to Botany, 741-743.
Feng, Y.-L., Lei, Y.-B., Wang, R.-F., Callaway, R. M., Valiente-Banuet, A., Li, Y.-P., & Zheng, Y.-L. (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences, 106(6), 1853-1856.
Feng, Y.-L., Li, Y.-P., Wang, R.-F., Callaway, R. M., Valiente-Banuet, A., & Inderjit. (2011). A quicker return energy-use strategy by populations of a subtropical invader in the non-native range: a potential mechanism for the evolution of increased competitive ability. Journal of Ecology, 99(5), 1116-1123. doi: 10.1111/j.1365-2745.2011.01843.x
Fernández-Ruvalcaba, M., Preciado-De-La Torre, F., Cruz-Vázquez, C., & García-Vázquez, Z. (2004). Anti-tick effects of Melinis minutiflora and Andropogon gayanus grasses on plots experimentally infested with Boophilus microplus larvae. Experimental and Applied Acarology, 32(4), 293-299. doi: 10.1023/B:APPA.0000023233.63268.cc
Fernández-Ruvalcaba, M., Preciado-de la Torre, J. F., Crdoba-Juarez, G., García-Vázquez, Z., Rosario-Cruz, R., & Saltijeral-Oaxaca, J. (2003). Animal bait effect on the recovery of Boophilus microplus larvae from experimentally infested grass in Morelos, Mexico. Parasitologia Latinoamericana, 58(1-2), 54-58.
Flores, S. J., & Kantun Balam, J. (1997). Importance of plants in the Ch’A Chaak Maya ritual in the Peninsula of Yucatan. Journal of Ethnobiology, 17(1), 97-108.
Franklin, K. A., Lyons, K., Nagler, P. L., Lampkin, D., Glenn, E. P., Molina-Freaner, F., . . . Huete, A. R. (2006). Buffelgrass (Pennisetum ciliare) land conversion and productivity in the plains of Sonora, Mexico. Biological Conservation, 127(1), 62-71. doi: 10.1016/j.biocon.2005.07.018
Gámez González, H., Zavala García, F., Maiti, R. K., Moreno Limón, S., Lozano Del Río, D. E., & Martínez Lozano, S. (2002). Effect of extracts of Cynodon dactylon L. and Sorghum halepense L. on cultivated plants. Crop Research (Hisar), 23(2), 382-388.
García-González, A., Damon, A., Iturbide, F. A., & Olalde-Portugal, V. (2013). Reproduction of Oncidium poikilostalix (Orchidaceae), potentially invading coffee plantations in Soconusco, Chiapas, Mexico. Plant Ecology and Evolution, 146(1), 36-44. doi: 10.5091/plecevo.2013.674
Garcillán, P. P., De La Luz, J. L. L., Rebman, J. P., & Delgadillo, J. (2013). Naturalized non-native plants of Baja California penninsula, Mexico. Botanical Sciences, 91(4), 461-475.
Gardea-Torresdey, J. L., Peralta-Videa, J. R., Montes, M., de la Rosa, G., & Corral-Díaz, B. (2004). Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: impact on plant growth and uptake of nutritional elements. Bioresource Technology, 92(3), 229-235. doi: 10.1016/j.biortech.2003.10.002
Garzón-Tiznado, J. A., Acosta-García, G., Torres-Pacheco, I., González-Chavira, M., Rivera-Bustamante, R. F., Maya-Hernández, V., & Guevara-González, R. G. (2002). Presence of geminivirus, pepper huasteco virus (PHV), texas pepper virus-variant Tamaulipas (TPV-T), and Chino del tomate virus (CdTV) in the states of Guanajuato, Jalisco and San Luis Potosi, Mexico. Revista Mexicana de Fitopatologia, 20(1), 45-52.
Gillett, J. D., Harley, K. L., Kassulke, R. C., & Miranda, H. J. (1991). Natural enemies of Sida acuta and S. rhombifolia (Malvaceae) in Mexico and their potential for biological control of these weeds in Australia. Environmental Entomology, 20(3), 882-888.
Glenn, E., Tanner, R., Méndez, S., Kehret, T., Moore, D., García, J., & Valdes, C. (1998). Growth rates, salt tolerance and water use characteristics of native and invasive riparian plants from the delta of the Colorado River, Mexico. Journal of Arid Environments, 40(3), 281-294. doi: 10.1006/jare.1998.0443
Golubov, J., Mandujano, M. D. C., Franco, M., Montaña, C., Eguiarte, L. E., & López‐Portillo, J. (1999). Demography of the invasive woody perennial Prosopis glandulosa (honey mesquite). Journal of Ecology, 87(6), 955-962.
González-Elizondo, M. S., M. González-Elizondo, J. A. Tena-Flores, I.L. López-Enriquez, & J. R. Bacon. 2009. Invasive Alien Plants in Durango, México. In Van Devender T., F.J. Espinosa-García, B.L. Harper-Lore, T. Hubbard. (Eds.) Invasive Plants on the Move. Controlling them in North America. Arizona-Sonora Desert Museum, Tucson, Arizona, U.S.A. ISBN 978-1-886679-28-3
Gordon, D., Tancig, K., Onderdonk, D., & Gantz, C. (2011). Assessing the invasive potential of biofuel species proposed for Florida and the United States using the Australian Weed Risk Assessment. Biomass and Bioenergy, 35(1), 74-79.
Guerra-García, A., Golubov, J., & Mandujano, M. C. (2015). Invasion of Kalanchoe by clonal spread. Biological Invasions, 17(6), 1615-1622. doi: 10.1007/s10530-014-0820-0
Gutiérrez-Ozuna, R., Eguiarte, L. E., & Molina-Freaner, F. (2009). Genotypic diversity among pasture and roadside populations of the invasive buffelgrass (Pennisetum ciliare L. Link) in north-western Mexico. Journal of Arid Environments, 73(1), 26-32. doi: 10.1016/j.jaridenv.2008.09.007
Gutiérrez, D., Mendoza, S., Serrano, V., Bah, M., Pelz, R., Balderas, P., & León, F. (2008). Proximate composition, mineral content, and antioxidant properties of 14 Mexican weeds used as fodder. Weed Biology and Management, 8(4), 291-296. doi: 10.1111/j.1445-6664.2008.00307.x
Gutiérrez, E., Huerto, R., Saldana, P., & Arreguín, F. (1996). Strategies for waterhyacinth (Eichhornia crassipes) control in Mexico. Hydrobiologia, 340(1-3), 181-185. doi: 10.1007/bf00012752
Hanan-Alipi, A. M., J. Mondragón-Pichardo & H. Vibrans. 2005. Ranunculus sardous Crantz. En: H. Vibrans (ed.), Malezas de México, http://www.conabio.gob.mx/malezasdemexico/ranunculaceae/ranunculus-sardous/fichas/pagina1.htm.
Hansen, A. M., Quintanilla, L. G. T., Pacheco, H. M., Canela, M. V., Márquez, L. C. G., Garcés, R. A. G., & Antonio, A. H. (2013). Atrazina: un herbicida polémico. Revista Internacional de Contaminación Ambiental, 29, 65-84.
Heard, T. A., Dhileepan, K., Bebawi, F., Bell, K. L., & Segura, R. (2012). Jatropha gossypiifolia L. – bellyache bush. Biological Control of Weeds in Australia, 324-333.
Heard, T. A., Elliott, L. P., Anderson, B., White, L., Burrows, N., Mira, A., . . . Segura, R. (2010). Biology, host specificity, release and establishment of Macaria pallidata and Leuciris fimbriaria (Lepidoptera: Geometridae), biological control agents of the weed Mimosa pigra. Biological Control, 55(3), 248-255. doi: 10.1016/j.biocontrol.2010.08.001
Heard, T. A., Mira, A., Fichera, G., & Segura, R. (2012). Nesaecrepida infuscata: a biological control agent of the invasive plant Mimosa pigra. Biocontrol, 57(4), 573-580. doi: 10.1007/s10526-011-9431-1
Hernández, H. M. (1981). Sobre la ecología reproductiva de Nicotiana glauca: una maleza de distribución cosmopolita. Boletin de la Sociedad Botanica de Mexico, 41, 47-74.
Hernández, S. V. M., Ruiz, L. J. R., Aguilar, J. G., de la Cruz, J. A. A., Moreno, J. M., Morales, M. G., . . . Campillo, L. G. (2015). Freshwater mollusk species richness in the Rio Grijalva-Villahermosa and Rio Tonala, Lagunas del Carmen-Machona Watersheds from Tabasco, Mexico. Hidrobiologica, 25(2), 239-247.
Herrera-Arrieta, Y., Pámanes-García, D. S., Herrera-Corral, J., Chairez-Hemandez, I., & Cortés-Ortiz, A. (2011). Changes of Vegetation and Diversity in Grasslands along 28 Years of Continuous Grazing in the Semi-Arid Durango Region, North Mexico. Journal of Animal and Veterinary Advances, 10(22), 2913-2920.
Hinojosa-Espinosa, O., & Cruz-Durán, R. (2008). Nota sobre la presencia de Hypochaeris radicata L.(Asteraceae: Lactuceae) en la flora del Distrito Federal, México. Boletin de la Sociedad Botanica de Mexico(82), 63-65.
Hinojosa-Espinosa, O., & Villaseñor, J. L. (2015). Arctotheca prostrata (Asteraceae: Arctotideae), una especie sudafricana ahora en México. Botanical Sciences, 93(4), 877-880.
Inderjit, Evans, H., Crocoll, C., Bajpai, D., Kaur, R., Feng, Y.-L., . . . Callaway, R. M. (2011). Volatile chemicals from leaf litter are associated with invasiveness of a Neotropical weed in Asia. Ecology, 92(2), 316-324. doi: 10.1890/10-0400.1
INEGI-SEMARNAP. (1999). Estadísticas del Medio Ambiente, México, 1997, pp. 316-320. México, D. F.: Secretaría de Ecología, Medio Ambiente, Recursos Naturales y Pesca Retrieved from http://www.semarnat.gob.mx/estadisticas_2000/naturaleza/estadistica-am/informe/acrobat/capitulo3-3-5.pdf
Iracheta-Cárdenas, M. M., Galan-Wong, L. J., & Pereyra-Alferez, B. (1995). Spore production by Helminthosporium triseptatum and its use for biological control of johnsongrass (Sorghum halepense). Revista Latinoamericana de Microbiologia, 37(2), 101-108.
Itié, G. (1939). Introducción del zacate pará , Panicum purpurascens Raddi. en México y área de dispersión del mismo. Revista de la Sociedad Mexicana de Historia Natural, 1(1), 29-32.
Itié, G. (1945). Un Zacate emigrante (Tricholaena rosea Nees). . Boletin de la Sociedad Botanica de Mexico, 2, 19-20.
Jeschke, J. M. (2014). General hypotheses in invasion ecology. Diversity and Distributions, 20(11), 1229-1234. doi: 10.1111/ddi.12258
Jeschke, J. M., Bacher, S., Blackburn, T. M., Dick, J. T. A., Essl, F., Evans, T., . . . Kumschick, S. (2014). Defining the Impact of Non-Native Species. Conservation Biology, 28(5), 1188-1194. doi: 10.1111/cobi.12299
Kueffer, C., Pysek, P., & Richardson, D. M. (2013). Integrative invasion science: model systems, multi-site studies, focused meta-analysis and invasion syndromes. New Phytologist, 200(3), 615-633. doi: 10.1111/nph.12415
León de la Luz, J. L., M. Domínguez-L., and R. Domínguez-C. 2009. Invasive Weeds in Baja California Sur. In Van Devender T., F.J. Espinosa-García, B.L. Harper-Lore and T. Hubbard. (Eds.) Invasive Plants on the Move. Controlling them in North America. University of Arizona Press, Tucson, Arizona, U.S.A
Lind, O. T., & Davalos-Lind, L. O. (2002). Interaction of water quantity with water quality: the Lake Chapala example. Hydrobiologia, 467(1-3), 159-167. doi: 10.1023/a:1014902630410
Lira-Saldivar, R. H., Salas, M. A., Cruz, J., Coronado, A., Hernández, F. D., Guerrero, E., & Gallegos, G. (2004). Solarization and goat manure on weeds management and melon yield: (with 1figure & 2 tables). Phyton (Buenos Aires), 73, 205-211.
López-Arcos, D., Gómez-Romero, M., Lindig-Cisneros, R., & Zedler, P. H. (2012). Fire-mobilized nutrients from hydrophyte leaves favor differentially Typha domingensis seedling growth. Environmental and Experimental Botany, 78, 33-38. doi: 10.1016/j.envexpbot.2011.12.023
López-Barrera, F., García-Franco, J. G., Rojas-Soto, O., Aguirre, A., Landgrave, R., Ortega-Pieck, A., . . . Rojas-Santiago, B. B. (2016). Ecología de la restauración del bosque nublado en el centro de Veracruz. In C. Eliane & C. Martínez-Garza (Eds.), Experiencias mexicanas en la restauración de los ecosistemas (Vol. 1, pp. 103-129). Cuernavaca, México: Universidad Nacional Autónoma de México, Centro Regional de Investigaciones Multidisciplinarias; Universidad Autónoma del Estado de Morelos; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
López-Rosas, H., & Moreno-Casasola, P. (2012). Invader versus natives: Effects of hydroperiod on competition between hydrophytes in a tropical freshwater marsh. Basic and Applied Ecology, 13(1), 40-49. doi: 10.1016/j.baae.2011.10.004
López-Rosas, H., Moreno-Casasola, P. & I. Mendelssohn, I. (2005). Effects of an African grass invasion on vegetation, soil and interstitial water characteristics in a tropical freshwater marsh in La Mancha, Veracruz (Mexico). Journal of Plant Interactions 1: 187-195.
López-Rosas, H., Moreno-Casasola, P., & Mendelssohn, I. (2006). Plant invasion of a tropical freshwater marsh: response of the invaded community to experimental disturbances. Wetlands, 26(2), 593-604.
Lot, A., Novelo, A., & Cowan, C. P. (1980). Hallazgo en México de una euforbiacea acuática (Phyllanthus fluitans) originaria de sudamérica. Boletin de la Sociedad Botanica de Mexico, 39, 83-90.
MacGregor-Fors, I., Ortega-Alvarez, R., Barrera-Guzman, A., Sevillano, L., & del-Val, E. K. (2013). Tama-risk? Avian responses to the invasion of saltcedars (Tamarix ramosissima) in Sonora, Mexico. Revista Mexicana De Biodiversidad, 84(4), 1284-1291. doi: 10.7550/rmb.33904
Mack, R. N. (2000). Cultivation Fosters Plant Naturalization by Reducing Environmental Stochasticity. Biological Invasions, 2(2), 111-122. doi: 10.1023/a:1010088422771
Magallanes, M. E., Ortiz, G., & Rojas Garcidueñas, M. (1986). Algunos efectos causados por el herbicida fluazifop-butil sobre la fisiología del zacate Johnson (Sorghum halepense (L.) Pers.). Turrialba, 36(4), 469-472.
Mangas-Ramírez, E., & Elías-Gutiérrez, M. (2004). Effect of mechanical removal of water hyacinth (Eichhornia crassipes) on the water quality and biological communities in a Mexican reservoir. Aquatic Ecosystem Health & Management, 7(1), 161-168.
Manners, A. G., Palmer, W. A., Burgos, A., McCarthy, J., & Walter, G. H. (2011). Relative host plant species use by the lantana biological control agent Aconophora compressa (Membracidae) across its native and introduced ranges. Biological Control, 58(3), 262-270. doi: 10.1016/j.biocontrol.2011.05.013
March Mifsut, I., & Martínez Jiménez, M. (Eds.). (2008).Especies invasoras de alto impacto a la biodiversidad: prioridades en México. Instituto Mexicano de Tecnología del Agua. México.
Marshall, N. A., Friedel, M., van Klinken, R. D., & Grice, A. C. (2011). Considering the social dimension of invasive species: the case of buffel grass. Environmental Science & Policy, 14(3), 327-338. doi: 10.1016/j.envsci.2010.10.005
Martin, P., Yetman, D., Fishbein, M., Jenkins, P., Van Devender, T., & Wilson, R. (1998). Gentry’s Rio Mayo plants: the tropical deciduous forest and environs of northwest Mexico: University of Arizona Press, Tucson.
Martínez, M., Mora-Olivo, A., & Daniel, T. F. (2008). Hygrophila polysperma (Acanthaceae), una maleza acuática registrada por primera vez para la flora mexicana. Revista Mexicana de Biodiversidad, 79, 285-289.
Martínez-Garza, C., Osorio-Beristain, M., Alcalá-Martínez, R. E., Valenzuela-Galván, D., & Mariano, N. (2016). Ocho años de restauración experimental en las selvas estacionales de México. In C. Eliane & C. Martínez-Garza (Eds.), Experiencias mexicanas en la restauración de los ecosistemas (Vol. 1, pp. 385-405). Cuernavaca, México: Universidad Nacional Autónoma de México, Centro Regional de Investigaciones Multidisciplinarias; Universidad Autónoma del Estado de Morelos; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Martínez-Mendoza, E. K., Mena-Violante, H. G., Mendez-Inocencio, C., Oyoque-Salcedo, G., Cortez-Madrigal, H., Olalde-Portugal, V., & Angoa-Pérez, M. V. (2012). Effects of Bacillus subtilis extracts on weed seed germination of Sorghum halepense and Amaranthus hybridus. African Journal of Microbiology Research, 6(9), 1887-1892. doi: 10.5897/ajmr11.554
Martínez-Morales, O., Estrada-Venegas, E. G., Equihua-Martínez, A., & Valdez-Carrasco, J. (2014). Morfología de Neochetina eichhorniae (Warner) (Coleoptera: Curculionidae). Acta zoológica mexicana, 30(2), 247-267.
Martínez Jiménez, M., & Charudattan, R. (1998). Survey and evaluation of Mexican native fungi for potential biocontrol of waterhyacinth. Journal of Aquatic Plant Management, 36(JULY), 145-148.
Martínez Jiménez, M., & Gómez Balandra, M. A. (2007). Integrated control of Eichhornia crassipes by using insects and plant pathogens in Mexico. Crop Protection, 26(8), 1234-1238. doi: 10.1016/j.cropro.2006.10.028
Martínez Jiménez, M., & Gutiérrez López, E. (2001). Host range of Cercospora piaropi and Acremonium zonatum, potential fungal biocontrol agents for waterhyacinth in Mexico. Phytoparasitica, 29(2), 175-177.
Martínez Jiménez, M., Gutiérrez López, E., Huerto Delgadillo, R., & Ruiz Franco, E. (2001). Importation, rearing, release and establishment of Neochetina bruchi (Coleoptera Curculionidae) for the biological control of waterhyacinth in Mexico. Journal of Aquatic Plant Management, 39, 140-143.
Maza-Villalobos, S., Lemus-Herrera, C., & Martínez-Ramos, M. (2011). Successional trends in soil seed banks of abandoned pastures of a Neotropical dry region. Journal of Tropical Ecology, 27, 35-49. doi: 10.1017/s0266467410000611
Medina Pitalúa, J. L. & Domínguez Valenzuela, J. A. (2001). Rottboellia cochinchinensis en México: una maleza fuera de la ley. Revista Mexicana de la Ciencia de la Maleza 1, 15-18.
Meave, J. A., Flores-Rodríguez, C., Pérez-García, E. A., & Antonio Romero-Romero, M. (2012). Edaphic and seasonal heterogeneity of seed banks in agricultural fields of a tropical dry forest region in Southern Mexico. Botanical Sciences, 90(3), 313-329.
Melgoza Castillo, A., Balandrán Valladares, M. I., Mata-González, R., & Pinedo Álvarez, C. (2014). Biología del pasto rosado Melinis repens (Willd.) e implicaciones para su aprovechamiento o control: Revisión. Revista Mexicana De Ciencias Pecuarias, 5(4), 429-442.
Mendoza, R., Cudmore, B., Orr, R., Fisher, J., Balderas, S. C., Courtenay, W., . . . Damián, M. A. (2009). Directrices trinacionales para la evaluación de riesgos de las especies acuáticas exóticas invasoras: Comisión para la Cooperación Ambiental de América del Norte, Montreal.
Mercado-Borrayo, B. M., Heydrich, S. C., Pérez, I. R., Quiroz, M. H., & Hill, C. P. D. (2015). Organophosphorus and Organochlorine Pesticides Bioaccumulation by Eichhornia crassipes in Irrigation Canals in an Urban Agricultural System. International Journal of Phytoremediation, 17(7), 701-708. doi: 10.1080/15226514.2014.964841
Moles, A. T., Flores-Moreno, H., Bonser, S. P., Warton, D. I., Helm, A., Warman, L., . . . Thomson, F. J. (2012). Invasions: the trail behind, the path ahead, and a test of a disturbing idea. Journal of Ecology, 100(1), 116-127. doi: 10.1111/j.1365-2745.2011.01915.x
Montes-Belmont, R., Flores-Moctezuma, H. E., & Nava-Juarez, R. A. (2003). Alternate hosts of Claviceps africana Frederickson, Mantle and de Millano, causal agent of sorghum “ergot” in the state of Morelos, Mexico. Revista Mexicana de Fitopatologia, 21(1), 63-66.
Montes-Holguin, M. O., Peralta-Videa, J. R., Meitzner, G., Martínez-Martínez, A., De la Rosa, G., Castillo-Michel, H. A., & Gardea-Torresdey, J. L. (2006). Biochemical and spectroscopic studies of the response of Convolvulus arvensis L. to chromium (III) and chromium (VI) stress. Environmental Toxicology and Chemistry, 25(1), 220-226. doi: 10.1897/05-089r.1
Montes, M. O., Peralta-Videa, J. R., Parsons, J. G., Corral Díaz, B., & Gardea-Torresdey, J. L. (2013). Spectroscopic determination of the toxicity, absorption, reduction, and translocation of Cr(vi) in two magnoliopsida species. International Journal of Phytoremediation, 15(2), 168-187. doi: 10.1080/15226514.2012.687017
Mooney, H. A. (2005). Invasive alien species: a new synthesis (Vol. 63): Island press.
Moreno-Casasola, P., López-Rosas, H., Vázquez-Benavides, J., López-Barrera, F., Espejel-González, V. E., & Sánchez-Higueredo, L. (2016). Restauración de un popal: estado de la vegetación y nivel de inindación después de siete años del manejo de una gramínea invasora en Veracruz. In C. Eliane & C. Martínez-Garza (Eds.), Experiencias mexicanas en la restauración de los ecosistemas (Vol. 1, pp. 433-455). Cuernavaca, México: Universidad Nacional Autónoma de México, Centro Regional de Investigaciones Multidisciplinarias; Universidad Autónoma del Estado de Morelos; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Munguia-Rosas, M. A., Parra-Tabla, V., Ollerton, J., & Carlos Cervera, J. (2012). Environmental control of reproductive phenology and the effect of pollen supplementation on resource allocation in the cleistogamous weed, Ruellia nudiflora (Acanthaceae). Annals of Botany, 109(2), 343-350. doi: 10.1093/aob/mcr284
Murillo-Ortiz, M., Mellado-Bosque, M., Herrera-Torres, E., Reyes-Estrada, O., & Carrete-Carreon, F. O. (2014). Seasonal diet quality and metabolic profiles of steers grazing on Chihuahuan desert rangeland. Livestock Science, 165, 61-65. doi: 10.1016/j.livsci.2014.03.023
Muro Castrejón, F. J., Cruz-Vázquez, C., Fernández-Ruvalcaba, M., & Torres, J. M. (2004). Repellent effect of Melinis minutiflora extract on Boophilus microplus tick larvae. Veterinaria Mexico, 35(2), 153-159.
Niño-Sulkowska, M. S., & Lot, A. (1983). Estudio demográfico del lirio acuático Eichhornia crassipes (Mart) Solms: dinámica de crecimiento en dos localidades selectas de México. . Boletin de la Sociedad Botanica de Mexico, 45, 71-84.
Ortega-León, G., Thomas, D. B., & Soriano, E. G. (2006). A description of the nymphal stages of the African cluster bug Agonoscelis puberula Stal. Southwestern Entomologist, 31(3), 245-249.
Ortega-Pieck, A., López-Barrera, F., Ramírez-Marcial, N., & García-Franco, J. G. (2011). Early seedling establishment of two tropical montane cloud forest tree species: The role of native and exotic grasses. Forest Ecology and Management, 261(7), 1336-1343. doi: 10.1016/j.foreco.2011.01.013
Palma-Ordaz, S., & Delgadillo-Rodríguez, J. (2014). Potential distribution to eight alien species with invasive nature in the state of Baja California, Mexico. Botanical Sciences, 92(4), 587-597.
Panetta, F. (1993). A system of assessing proposed plant introductions for weed potential. Plant Protection Quarterly, 8(1), 10-14.
Panetta, F. D. (2015). Weed eradication feasibility: lessons of the 21st century. Weed Research, 55(3), 226-238. doi: 10.1111/wre.12136
Parsons, J. J. (1972). Spread of African pasture grasses to America tropics. Journal of Range Management, 25(1), 12-17. doi: 10.2307/3896654
Peña-Jiménez, A., & Neyra-González, L. (1998). Amenazas a la biodiversidad. In CONABIO (Ed.), La diversidad biológica de México: Estudio de País (pp. 157-182). México, D.F.: Comisión para el Conocimiento y uso de la Biodiversidad.
Peralta Pelaez, L. A., Moreno-Casasola, P., & López Rosas, H. (2014). Hydrophyte composition of dune lakes and its relationship to land-use and water physicochemistry in Veracruz, Mexico. Marine and Freshwater Research, 65(4), 312-326. doi: 10.1071/mf12295
Pérez-Panduro, A. (1998). Primera experiencia exitosa de control biológico de lirio acuático en México. 8:3-4. . El Entomófago, 8, 3-4.
Pheloung, P., Williams, P., & Halloy, S. (1999). A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. Journal of Environmental Management, 57(4), 239-251.
Piedra-Ibarra, E., De La Torre-Almaraz, R., Zuniga, G., Xoconostle-Cazares, B., & Ruiz-Medrano, R. (2005). Leonotis nepetaefolia: An important plant virus reservoir in central Mexico. Phytoparasitica, 33(5), 480-494. doi: 10.1007/bf02981397
Pimentel, D., McNair, S., Janecka, J., Wightman, J., Simmonds, C., O’Connell, C., . . . Tsmondo, T. (2002). Economic and environmental threats of alien plant, animal, and microbe invasions. In D. Pimentel (Ed.), Biological Invasions. Economic and Environmental Costs of Alien Plant, Aanimal, and Microbe Species (pp. 307-329). Boca Raton, Florida, U.S.A.: CRC Press.
Pimentel, D., Zuñiga, R., & Morrison, D. (2005). Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics, 52(3), 273-288. doi: 10.1016/j.ecolecon.2004.10.002
Powell, K. I., Chase, J. M., & Knight, T. M. (2011). A synthesis of plant invasion effects on biodiversity across spatial scales. American Journal of Botany, 98(3), 539-548. doi: 10.3732/ajb.1000402
Qin, R.-M., Zheng, Y.-L., Valiente-Banuet, A., Callaway, R. M., Barclay, G. F., Silva Pereyra, C., & Feng, Y.-L. (2013). The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytologist, 197(3), 979-988. doi: 10.1111/nph.12071
Quero Carrillo, A. R., Enríquez Quiroz, J. F., Morales Nieto, C. R., & Miranda Jiménez, L. (2010). Apomixis y su importancia en la selección y mejoramiento de gramineas forrajeras tropicales: Revisión. [Apomixis importance for tropical forage grass selection and breeding: Review]. Revista Mexicana De Ciencias Pecuarias, 1(1), 25-42.
Ramírez-Albores, J. E., & Badano, E. I. (2013). Perspectiva histórica, sociocultural y ecológica de una invasión biológica: el caso del Pirúl (Schinus molle L., Anacardiaceae) en México. Boletín de la Red Latinoamericana para el Estudio de Especies Invasoras Volumen 3, número 1 Noviembre 2013, 3(1), 5-15.
Ramírez-Albores, J. E., Bustamante, R. O., & Badano, E. I. (2016). Improved Predictions of the Geographic Distribution of Invasive Plants Using Climatic Niche Models. Plos One, 11(5), e0156029. doi: 10.1371/journal.pone.0156029
Ramírez-Vera, S., Terrazas, A., Delgadillo, J. A., Serafin, N., Flores, J. A., Elizundia, J. M., & Hernández, H. (2012). Feeding corn during the last 12 days of gestation improved colostrum production and neonatal activity in goats grazing subtropical semi-arid rangeland. Journal of Animal Science, 90(7), 2362-2370. doi: 10.2527/jas.2011-4306
Ramírez, D. W., Aponte, H., & Cano, A. (2010). Vascular flora and vegetation of Santa Rosa wetland (Chancay, Lima). [Flora vascular y vegetacion del humedal de Santa Rosa (Chancay, Lima)]. Revista Peruana de Biologia, 17(1), 105-110.
Ramírez Galindo, J., Barbosa Martínez, C., & Ponce de Leon, L. (2011). Study of In Situ Germination of Escontria chiotilla (Weber) Rose and Stenocereus griseus Haworth, Cacti from the Arid Tropical Scrub in Mexico. In J. A. Pascual & F. P. Alfocea (Eds.), V International Symposium on Seed, Transplant and Stand Establishment of Horticultural Crops (Vol. 898, pp. 113-121).
Ramírez, R. G., González-Rodríguez, H., Morales-Rodríguez, R., Cerrillo-Soto, A., Juárez-Reyes, A., García-Dessommes, G. J., & Guerrero-Cervantesi, M. (2009). Chemical composition and dry matter digestion of some native and cultivated grasses in Mexico. Czech Journal of Animal Science, 54(4), 150-162.
Ramírez, R. G., Haenlein, G. F. W., García-Castillo, C. G., & Nunez-González, M. A. (2004). Protein, lignin and mineral contents and in situ dry matter digestibility of native Mexican grasses consumed by range goats. Small Ruminant Research, 52(3), 261-269. doi: 10.1016/s0921-4488(03)00257-8
Rapoport, E., Díaz-Betancourt, M., & López-Moreno, I. (1983). Aspectos de la ecología urbana en la ciudad de México: flora de las calles y baldíos (E. Limusa Ed.). México, D.F.
Reiche, C. (1926). Flora Excursoria en el Valle Central de México. México, D.F.: Manuel Porrúa, S.A.
Reid, A. M., Morin, L., Downey, P. O., French, K., & Virtue, J. G. (2009). Does invasive plant management aid the restoration of natural ecosystems? Biological Conservation, 142(10), 2342-2349. doi: 10.1016/j.biocon.2009.05.011
Rejmánek, M., & Pitcairn, M. (2002). When is eradication of exotic pest plants a realistic goal? In C. R. Veitch & M. N. Clout (Eds.), Turning the tide: the eradication of invasive species (pp. 249-253). Switzerland and Cambridge, U.K.: IUCN SSC Invasive Species Group. IUCN Gland.
Rejmánek, M., & Randall, J. M. (2004). The total number of naturalized species can be a reliable predictor of the number of alien pest species. Diversity and Distributions, 10(5-6), 367-369. doi: 10.1111/j.1366-9516.2004.00107.x
Reynoso-Cuevas, L., Gallegos-Martínez, M. E., Cruz-Sosa, F., & Gutiérrez-Rojas, M. (2008). In vitro evaluation of germination and growth of five plant species on medium supplemented with hydrocarbons associated with contaminated soils. Bioresource Technology, 99(14), 6379-6385. doi: 10.1016/j.biortech.2007.11.074
Richardson, D. M., Pysek, P., Rejmánek, M., Barbour, M. G., Panetta, F. D., & West, C. J. (2000). Naturalization and invasion of alien plants: Concepts and definitions. Diversity and Distributions, 6(2), 93-107. doi: 10.1046/j.1472-4642.2000.00083.x
Robles, E. R., de la Cruz, R. S., Salinas García, J. R., Pecina Quintero, V., Loera Gallardo, J., & Esqueda Esquivel, V. A. (2006). Critical period of competition of field bindweed (Convolvulus arvensis L.) in grain sorghum. Revista Fitotecnia Mexicana, 29(1), 47-53.
Rocha-Ramírez, A., Robles-Valderrama, E., & Ramírez-Flores, E. (2014). Invasive alien species water hyacinth Eichhornia crassipes as abode for macroinvertebrates in hypertrophic Ramsar Site, Lake Xochimilco, Mexico. Journal of Environmental Biology, 35(6), 1071-1080.
Rodríguez-Estrella, R., Pérez Navarro, J. J., Granados, B., & Rivera, L. (2010). The distribution of an invasive plant in a fragile ecosystem: the rubber vine (Cryptostegia grandiflora) in oases of the Baja California penninsula. Biological Invasions, 12(10), 3389-3393. doi: 10.1007/s10530-010-9758-z
Rodríguez-Navarro, S., Flores-Macías, A., & Torres-Martínez, G. (2008). Evaluation of infesting field bindweed (Convolvulus arvensis L.) with Aceria malherbae Nuzzaci (Acari : Eriophyidae) under glasshouse conditions. International Journal of Acarology, 34(2), 151-154.
Rodríguez-Navarro, S., Rodríguez Morell, H., Alemán Martínez, J. A., Flores-Macías, A., & G. Torres-Martínez, J. (2011). Valuation of quality parameters for rearing Aceria malherbae Nuzzaci (Acari: Eriophyidae), a biological control agent of field bindweed, Convolvulus arvensis L. International Journal of Acarology, 37(3), 235-243. doi: 10.1080/01647954.2010.514287
Rodríguez-Navarro, S., Torres-Martínez, G., & Olivares-Orozco, J. (2004). Biological control of field bindweed (Convolvulus arvensis L.) using Aceria malherbae (Acari : Eriophyidae) in Mexico. International Journal of Acarology, 30(2), 153-155.
Rodríguez, A., Avila-Pérez, P., & Barceló-Quintal, I. D. (1998). Bioaccumulation of chemical elements by water hyacinth (Eichhornia crassipes) found in “Jose Antonio Alzate” dam samples in the State of Mexico, Mexico. Journal of Radioanalytical and Nuclear Chemistry, 238(1-2), 91-95. doi: 10.1007/bf02385360
Román-Contreras, R., Rocha-Ramírez, A., & Chazaro-Olvera, S. (2008). Effects of hurricane “Pauline” (1997) on the fauna associated with the plant Eichhornia crassipes in Laguna Coyuca, South Pacific of Mexico. Revista De Biologia Tropical, 56(2), 603-611.
Rzedowski, G. C. d., Rzedowski, J., & Colaboradores. (2001). Flora fanerogámica del Valle de México (Second ed.). Pátzcuaro, Michoacán, México.: Instituto de Ecología, A.C., Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Rzedowski, J. (1959). Salsola kali var. tenuifolia: una peligrosa maleza exótica que está extendiendose hacia el centro de México. Boletín de la Socidad Botánica de México, 24, 53-59.
Rzedowski, J. (1993). El papel de la familia Compositae en la flora sinantrópica de México. Fragmenta Floristica et Geobotanica. Supplementum, 2, 123-138.
Rzedowski, J., & Calderón de Rzedowski, G. (1985) Flora del Bajío y regiones adyacentes. In J. Rzedowski & G. Calderón de Rzedowski (Series Ed.). Pátzcuaro, Michoacán, México: Instituto de Ecología, A.C.
Rzedowski, J., & Calderón de Rzedowski, G. (1990). Nota sobre el elemento africano en la flora adventicia de México. Acta Botanica Mexicana(12), 21-24.
Rzedowski, J., & De Rzedowski, G. C. (2005). Crepis capillaris Wallr.(Compositae, Lactuceae), una adición a la flora adventicia de México. Acta Botanica Mexicana(73), 69-73.
Rzedowski, J., Vibrans, H., & de Rzedowski, G. C. (2003). Senecio inaequidens DC.(Compositae, Senecioneae), una maleza perjudicial introducida en México. Acta Botanica Mexicana(63), 83-96.
Sánchez-Blanco, J., Sánchez-Blanco, C., Mario Sousa, S., & Espinosa-García, F. J. (2012). Assessing introduced leguminosae in Mexico to identify potentially high-impact invasive species. Acta Botanica Mexicana, 100, 41-77.
Sánchez-Flores, E. (2007). GARP modeling of natural and human factors affecting the potential distribution of the invasives Schismus arabicus and Brassica tournefortii in ‘El Pinacate y Gran Desierto de Altar’ Biosphere Reserve. Ecological Modelling, 204(3-4), 457-474. doi: 10.1016/j.ecolmodel.2007.02.002
Sánchez-Flores, E., Rodríguez-Gallegos, H., & Yool, S. R. (2008). Plant invasions in dynamic desert landscapes. A field and remote sensing assessment of predictive and change modeling. Journal of Arid Environments, 72(3), 189-206. doi: 10.1016/j.jaridenv.2007.05.013
Sánchez-González, A., Granados-Sánchez, D., & Simón-Nabor, R. (2008). Uso medicinal de las plantas por los otomíes del municipio de Nicolás Flores, Hidalgo, México. Revista Chapingo. Serie horticultura, 14(3), 271-279.
Sánchez-González, A., & López-Mata, L. (2005). Plant species richness and diversity along an altitudinal gradient in the Sierra Nevada, Mexico. Diversity and Distributions, 11(6), 567-575. doi: 10.1111/j.1366-9516.2005.00186.x
Sánchez-Ken, J. G., Cerros-Tlatilpa, R., & Vibrans, H. (2013). Themeda quadrivalvis (Sacchareae, Panicoideae, Poaceae), una maleza reglamentada presente y establecida en el estado de Morelos, México. Botanical Sciences, 91(4), 531-536.
Sánchez-Ken, J. G., Zita Padilla, G., & Mendoza Cruz, M. (2012). Catálogo de las gramíneas malezas nativas e introducidas de México. México, D.F.: Consejo Nacional Consultivo Fitosanitario, CONACOFI-SAGARPA, ASOMECIMA, UNAM.
Santibañez-Aguilar, J. E., Ponce-Ortega, J. M., González-Campos, J. B., Serna-González, M., & El-Hawagi, M. M. (2013). Synthesis of Distributed Biorefining Networks for the Value-Added Processing of Water Hyacinth. Acs Sustainable Chemistry & Engineering, 1(2), 284-305. doi: 10.1021/sc300137a
Savage, H. M., Rejmankova, E., ArredondoJiménez, J. I., Roberts, D. R., & Rodríguez, M. H. (1990). Limnological and botanical characterization of larval habitats for 2 primary malarial vectors, Anopheles-albimanus and Anopheles-pseudopunctipennis, in coastal areas of Chiapas State, Mexico. Journal of the American Mosquito Control Association, 6(4), 612-620.
Serrano-Cárdenas, V., P. Balderas-Aguilar, and R. Pelz-Marín. 2009. Invasive Weeds of Wild Areas in the Semidesert of Querétaro, Mexico. in: Van Devender T., F.J. Espinosa-García, B.L. Harper-Lore and T. Hubbard. (Eds.) Invasive Plants on the Move. Controlling them in North America. University of Arizona Press, Tucson,
Silva, C., Kan, F. L., & Martínez-Romero, E. (2007). Population genetic structure of Sinorhizobium meliloti and S. medicae isolated from nodules of Medicago spp. in Mexico. Fems Microbiology Ecology, 60(3), 477-489. doi: 10.1111/j.1574-6941.2007.00301.x
Stohlgren, T. J., Barnett, D. T., Jarnevich, C. S., Flather, C., & Kartesz, J. (2008). The myth of plant species saturation. Ecology Letters, 11(4), 313-322.
Stohlgren, T. J., Barnett, D. T., & Kartesz, J. T. (2003). The rich get richer: patterns of plant invasions in the United States. Frontiers in Ecology and the Environment, 1(1), 11-14.
Tamayo Esquer, L. M., & Gaillardon, P. (1989). Relationships between plant growth stage and 2, 4-D and glyphosate behaviour in field bindweed (Convolvulus arvensis L.). Agronomie, 9(1), 91-100.
Tarin, D., Pepper, A. E., Goolsby, J. A., Moran, P. J., Contreras Arquieta, A., Kirk, A. E., & Manhart, J. R. (2013). Microsatellites Uncover Multiple Introductions of Clonal Giant Reed (Arundo donax). Invasive Plant Science and Management, 6(3), 328-338. doi: 10.1614/ipsm-d-12-00085.1
Tejeda, S., Zarazua, G., Avila-Pérez, P., Carapia-Morales, L., & Martínez, T. (2010). Total reflection X-ray fluorescence spectrometric determination of elements in water hyacinth from the Lerma River. Spectrochimica Acta Part B-Atomic Spectroscopy, 65(6), 483-488. doi: 10.1016/j.sab.2010.04.005
Torres-García, J. R., Núñez-Farfan, J., Uscanga-Mortera, E., Trejo, C., Conde-Martínez, V., Kohashi-Shibata, J., . . . Velázquez-Márquez, S. (2015). Competition for canopy cover between accessions of Phalaris minor that are susceptible and resistant to ACCase inhibiting herbicides. Nordic Journal of Botany, 33(5), 615-623. doi: 10.1111/njb.00764
Tovar-Sánchez, E., Rodríguez-Carmona, F., Aguilar-Mendiola, V., Mussali-Galante, P., López-Caamal, A., & Valencia-Cuevas, L. (2012). Molecular evidence of hybridization in two native invasive species: Tithonia tubaeformis and T. rotundifolia (Asteraceae) in Mexico. Plant Systematics and Evolution, 298(10), 1947-1959. doi: 10.1007/s00606-012-0693-6
Travieso-Bello, A. C., Moreno-Casasola, P., & Campos, A. (2005). Impact Produced by Different Cattle Ranching Practices on Soil and Vegetation from Wetlands Turned Into Pastures. Interciencia, 30(1), 12-18.
Tucuch-Cauich, F. M., Orona-Castro, F., Almeyda-Leon, I. H., & Aguirre-Uribe, L. A. (2013). Ecological indicators of the weed community in the cultivation of mango Mangifera indica L. in Campeche State, Mexico. Phyton-International Journal of Experimental Botany, 82, 145-149.
Van Devender, T., Espinosa-García, F. J., Harper-Lore, B. L., & Hubbard, T. (2009). Invasive Plants on the Move. Controlling them in North America. . Tucson, Arizona, U.S.A. : Arizona-Sonora Desert Museum Press.
Van Devender, T.R., R.S. Felger and A. Búrquez M. 1997. Exotic Plants in the Sonoran Desert Region Arizona and Sonora. 1997 Symposium Proceedings of the California Exotic Pest Plant Council. Pp. 1-6.
Van Devender, T. R., & Reina, A. L. (2007). Sonoran Noteworthy Records. Madroño, 54, 102-104.
van Kleunen, M., Dawson, W., Essl, F., Pergl, J., Winter, M., Weber, E., . . . Pysek, P. (2015). Global exchange and accumulation of non-native plants. Nature, 525(7567), 100-+. doi: 10.1038/nature14910
Vargas-Mendoza, C. F., Ortegon-Campos, I., Manufo-Zapata, D., Herrera, C. M., & Parra-Tabla, V. (2015). Genetic diversity, outcrossing rate, and demographic history along a climatic gradient in the ruderal plant Ruellia nudiflora (Acanthaceae). Revista Mexicana De Biodiversidad, 86(2), 508-520. doi: 10.1016/j.rmb.2015.04.034
Vera-Núñez, J. A., Grageda-Cabrera, O. A., Altamirano Hernández, J., & Peña-Cabriales, J. J. (2010). Efecto de los surfactantes sobre la absorción de agroquímicos en plantas. Nova scientia, 2(3), 14-36.
Vibrans, H. 1995. Notas sobre neófitas I. Silene noctiflora L. (Caryophyllaceae) registrada para México. Acta Botánica Mexicana 31: 79-83.
Vibrans, H. 1996. Notes on Neophytes 2. New records for Asteraceae from the center of Mexico. Phytologia 81(5): 369-381.
Vibrans, H. 2003. Notas sobre neófitas 3. Distribución de algunas Brassicaceae de reciente introducción en el centro de México. Acta Botánica Mexicana 63: 83-96.
Vibrans H. 2010. Malezas de México. http://www.conabio.gob.mx/malezasdemexico/2inicio/home-malezas-mexico.htm
Vibrans, H. 2016. Un listado oficial de plantas invasoras – ¡favor de checarlo y comentar! jueves, 24 de noviembre de 2016 http://jehuite.blogspot.mx/2016/11/un-listado-oficial-de-plantas-invasoras.html
Vibrans, H., & Alipi, A. M. H. (2008). Notes on neophtyes: 4. Polygonum nepalense (Polygonaceae), an invasive plant new for Mexico. Acta Botanica Mexicana, 82, 1-6.
Vibrans, H. & Delgado, J. C. 2010. A First Attempt to Eradicate a Quarantined Weed in Mexico: The Example of Polygonum convolvulus in Guanajuato. In, Rindos, E. (Ed.) Plant Invasions: Politics, Policies and Practices. Proceedings of the 5th Biennial Weeds Across Borders Conference, pp. 53-5. Shepherdstown, West Virginia, USA.
Vibrans, H., García-Moya, E., Clayton, D., & Sánchez-Ken, J. G. (2014). Hyparrhenia variabilis and Hyparrhenia cymbaria (Poaceae): New for the Americas, Successful in Mexico. Invasive Plant Science and Management, 7(2), 222-228. doi: 10.1614/ipsm-d-13-00107.1
Villamagna, A. M., Murphy, B. R., & Karpanty, S. M. (2012). Community-Level Waterbird Responses to Water Hyacinth (Eichhornia crassipes). Invasive Plant Science and Management, 5(3), 353-362. doi: 10.1614/ipsm-d-11-00085.1
Villamagna, A. M., Murphy, B. R., & Trauger, D. L. (2010). Behavioral Response of American Coots (Fulica americana) to Water Hyacinth (Eichhornia crassipes) in Lake Chapala, Mexico. Waterbirds, 33(4), 550-555. doi: 10.1675/063.033.0416
Villaseñor, J., Ortiz, E., Hinojosa-Espinosa, O., & Segura-Hernández, G. (2012). Especies de la familia Asteraceae exóticas a la flora de México. México, D.F., México: SAGARPA, SENASICA, CONACOFI, Instituto de Biología, UNAM, ASOMECIMA.
Villaseñor, J. L. (2013). Are the Hotspots of the Floristic Richness of Mexico Also the Hotspots for the Synanthropic Richness? Paper presented at the Proceedings of the 2012 Weeds Across Borders Conference, Meeting the Challenges of the Future April,, Cancún, Quintana Roo, México.
Villaseñor, J. L. (2016). Checklist of the native vascular plants of México. Revista Mexicana De Biodiversidad, 87(3), 559-902.
Villaseñor, J. L., & Espinosa-García, F. J. (2004). The alien flowering plants of Mexico. Diversity and Distributions, 10(2), 113-123. doi: 10.1111/j.1366-9516.2004.00059.x
Villaseñor, J. L., & Espinosa-García, F. J. (1998). Catálogo de Malezas de México. México, D.F.: Universidad Nacional Autónoma de México, Consejo Consultivo Fitosanitario, Fondo de Cultura Económica.
Villaseñor, J. L., Ortiz, E., Hinojosa-Espinosa, O., & Segura-Hernández, G. (2012). Especies de la familia Asteraceae exóticas a la flora de México. México, D.F.: SAGARPA.
Westbrooks, R. D. (1998). Invasive plants. Changing the landscape of America. Fact Book. Washington, D.C.: Federal Iteragency Committee for the Management of Noxious and Exotic Weeds. .
Williams-Linera, G., & Álvarez-Aquino, C. (2016). Evaluación del éxito de la restauración del bosque nublado en la región de Xalapa, Veracruz. In E. Ceccon & C. Martínez-Garza (Eds.), Experiencias mexicanas en la restauración de ecosistemas (Vol. 1, pp. 81-101). Cuernavaca, México: Universidad Nacional Autónoma de México, Centro Regional de Investigaciones Multidisciplinarias; Universidad Autónoma del Estado de Morelos; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Williams, J. K. (2010). Additions to the alien vascular flora of Mexico, with comments on the shared species of Texas, Mexico, and Belize. Phytoneuron, 3, 1-7.
Williamson, M., & Fitter, A. (1996). The varying success of invaders. Ecology, 77(6), 1661-1666. doi: 10.2307/2265769
Zavala-Hurtado, J., Portilla-Gutiérrez, E., Ayala-Fernández, Y., & Bravo-Rivera, M. (2003). Mala, mala, no tan mala maleza. Patrones de distribución espacial de las malezas en el campus Iztapalapa de la UAM. ContactoS, 49, 5-14.
Zheng, Y.-L., Feng, Y.-L., Zhang, L.-K., Callaway, R. M., Valiente-Banuet, A., Luo, D.-Q., . . . Silva-Pereyra, C. (2015). Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytologist, 205(3), 1350-1359. doi: 10.1111/nph.13135