Fernando M. Contreras-Moreno a, Mircea G. Hidalgo-Mihart b, *, Rafael Reyna-Hurtado c, Carlos A. López-González d, Alejandro Jesús-de la Cruz b
a Comisión Nacional de Áreas Naturales Protegidas, Reserva de la Biósfera de Calakmul, Calakmul, Calle Puerto Rico s/n, Colonia Fundadores, 24640 Xpujil, Campeche, Mexico
b Universidad Juárez Autónoma de Tabasco, División Académica de Ciencias Biológicas, Carretera Villahermosa-Cárdenas Km. 0.5 s/n, entronque a Bosques de Saloya, 86150 Villahermosa, Tabasco, Mexico
c El Colegio de la Frontera Sur, Unidad Campeche, Departamento de Conservación de la Biodiversidad, Av. Rancho, Polígono 2A, 24500 Ciudad Industrial Lerma, Campeche, Mexico
d Universidad Autónoma de Querétaro, Facultad de Ciencias Naturales, Av. de las Ciencias s/n, 76230 Juriquilla, Querétaro, Mexico
*Corresponding author: mhidalgo@yahoo.com (M.G. Hidalgo-Mihart)
Received: 22 July 2020; accepted: 9 October 2020
Abstract
White-tailed deer, Odocoileus virginianus, home range size (HR) is one of the most studied aspects of its biology. However, in the southern portion of its distribution, information about its HR is scarce, limiting the capacity for management of the species. To understand the effect of sex and seasonality on the HR of white-tailed deer in a tropical area, we radiotracked 11 adult white-tailed deer (7 females and 4 males) from 2016 to 2019 in a tropical wetland of western Campeche, Mexico. We found female HR to vary from 12.67 ha (±3.52) in the early dry season to 21.57 ha (±18.14) during the late dry season, and from 37.31 ha (±16.93) for the late dry season to 90.16 ha (±72.64) during the rainy season in males. We did not find differences of HR among seasons. However, we found seasonal differences when we tested separately the HR for females and males, showing that female HR is similar among seasons, whereas males had smaller HR during the dry seasons than during the rainy season. Our results indicate that water availability and flooding levels could affect the HR and configuration of the white-tailed deer in the study area.
Keywords: Laguna de Términos; Core area size; Home-range overlap; Campeche
Tamaño del ámbito hogareño estacional del venado de cola blanca, Odocoileus virginianus thomasi, en un humedal tropical del sureste de México
Resumen
El tamaño del ámbito hogareño (AH) del venado cola blanca (Odocoileus virginianus) es uno de los temas más estudiados de su biología. Sin embargo, en la porción sur de la distribución de la especie, la información sobre AH es escasa. Para entender el efecto del sexo y la estacionalidad en el AH del venado cola blanca en un área tropical, realizamos radioseguimiento a 11 venados cola blanca (7 hembras y 4 machos) de 2016 a 2019 en un humedal de Campeche, México. Encontramos que el AH de las hembras varió de 12.67 ha (± 3.52) en la estación seca temprana a 21.57 ha (± 18.14) durante la estación seca tardía, y de 37.31 ha (± 16.93) para la estación seca tardía a 90.16 ha (± 72.64) en la temporada de lluvias en el caso de los machos. Encontramos diferencias estacionales en el tamaño del AH de machos y hembras, de forma que el tamaño del AH de las hembras es similar entre las estaciones, mientras que los machos presentan AH más pequeños durante las temporadas secas. Nuestros resultados indican que la disponibilidad de agua y los niveles de inundación tienen efecto en el AH de venados de cola blanca en el área de estudio.
Palabras clave: Laguna de Términos; Tamaño del centro de actividad; Sobreposición de ámbito hogareño; Campeche
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Home-range —the area used by an animal to meet its needs of food, water, cover, social interactions, and caring for young— serves as the most fundamental index of space use by wildlife (Burt, 1943; Hemson et al., 2005). Determining home-range size of a species is an important prerequisite to understand species behavior, ecology, and management (Sanderson, 1966). In most of North America (United States, Canada, and northern Mexico) the home-range size and configuration of white-tailed deer (Odocoileus virginianus), including the effect that numerous environmental (e.g., seasonality, landscape configuration, etc.) and/or anthropogenic factors (e.g., hunting pressure, urbanization, etc.) have on these ecological characteristics, is one of the most studied aspects of this species’ ecology (see Stewart et al., 2011, for a review). However, in the southern portion of its distribution (Mesoamerican Mexico, Central America, and northern South America), information about the home-range of this species is scarce; especially in tropical areas (Table 1).
Home-range size determination of white-tailed deer has been identified as one of the research priorities for the species in Mexico, mostly because it will help to improve the conservation and management programs for the species (Ortega et al., 2011). Such programs are crucial for white-tailed deer populations as it is the most hunted species in Mexico (Mandujano et al., 2014). White tailed deer hunting is commonly practiced for sport, subsistence, handcraft carving, or as a part of the religious ceremonies of native cultures (Ortega et al., 2011). Hunting also represents an important activity, particularly in the northern part of the country where hunters provide significant economic benefits to local inhabitants (Contreras-Gil et al., 2010; Guajardo-Quiroga & Martínez-Muñoz, 2004; Villarreal, 2013). In some southern states of Mexico there has been a significant development of white tailed-deer sport hunting activities (Conabio, 2012). However, on few occasions this commercial activity has allowed local inhabitants to obtain economic benefits, mostly due to difficulties related to wildlife management and community organization (García-Marmolejo et al., 2008; Weber et al., 2006).
Resource (i.e., food, water, and cover) dispersion and seasonal availability are some of the most important environmental variables that influence the home-range size of white-tailed deer (Stewart et al., 2011). White-tailed deer living in areas where higher quality resources are abundant and well distributed tend to have smaller home-ranges than deer that occupy less productive areas with unevenly distributed resources (Lesage et al., 2000; Marchinton & Hirth 1984; Stewart et al., 2011). Nevertheless, home-ranges may decrease or expand as food or water availability changes (Hellickson et al., 2008; Stewart et al., 2011). In the case of white-tailed deer populations in the southern portion of the range, it has been observed that areas with extreme variations in temperature and precipitation found in the scrub lands in the northwest of Mexico or in the tropical dry forests of the western portion of the country (Bello et al., 2001; Sánchez-Rojas et al., 1997), had strong
effects on the home-range size. However, tropical climates are highly variable, especially in Mexico, making it difficult to predict the effects of other seasonal tropical and subtropical climates and their associated environments on the home-range of white-tailed deer.
An example of a unique, highly seasonal tropical region is the extensive wetland system located in the western portion of Campeche, Mexico, subject to severe seasonal changes, with a marked dry season with temperatures above 40 °C (INEGI, 2013), contrasting with an extended flooding season that can last for more than 8 months (Rivera-Arriaga & Villalobos-Zapata, 2005). These climatic conditions have a strong effect on the resource availability for the wildlife inhabiting the area (Hidalgo-Mihart et al., 2017).
Western Campeche maintains an important population of white-tailed deer. Previous studies report that the species responds to contrasting environmental conditions by increasing the daily distances traveled during the harshest portion of the dry season compared to the rainy season (Contreras-Moreno, Hidalgo-Mihart, & Contreras-Sánchez, 2019). Additionally, it has been found that fawning synchronizes with the dry months (February-June) of the year and the seasonality of the area is one of the possible causes of it (Contreras-Moreno, Hidalgo-Mihart, Jesús-de la Cruz et al., 2019). Comprehending how white-tailed deer home-range size and configuration respond to the seasonality of the area will help understand how this species could be affected by climate change in a region that has experimented a general decrease in mean precipitation and an increase in drought in the last years (Chiabai, 2015; Imbach et al., 2012).
Understanding the effect of environmental conditions on individual home-ranges of white-tailed deer in the Laguna de Términos Area is of great importance because the white tailed-deer population found in Campeche and Tabasco is a different subspecies (O. virginianus thomasi) which is included in the Hubert Thummler Award for the Mexican deer slam (SCI, 2014a, b; Villarreal, 2008) and should encourage the development of sustainable sport hunting initiatives in the region. Thus, the objective of this study was to determine the effect of seasonality on the home-range size of white-tailed deer in the wetlands of Laguna de Términos Area, in southeastern Mexico.
Materials and methods
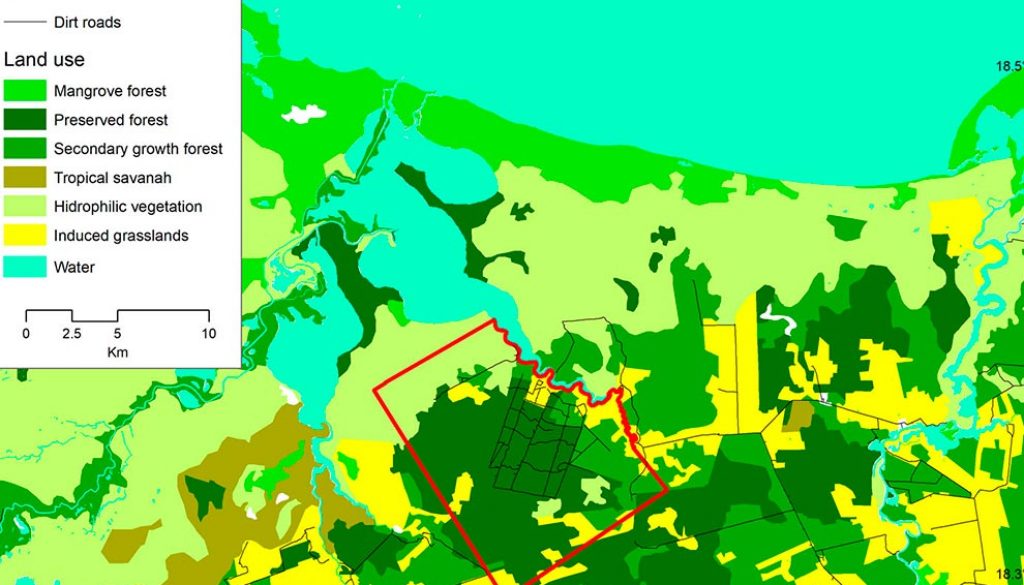
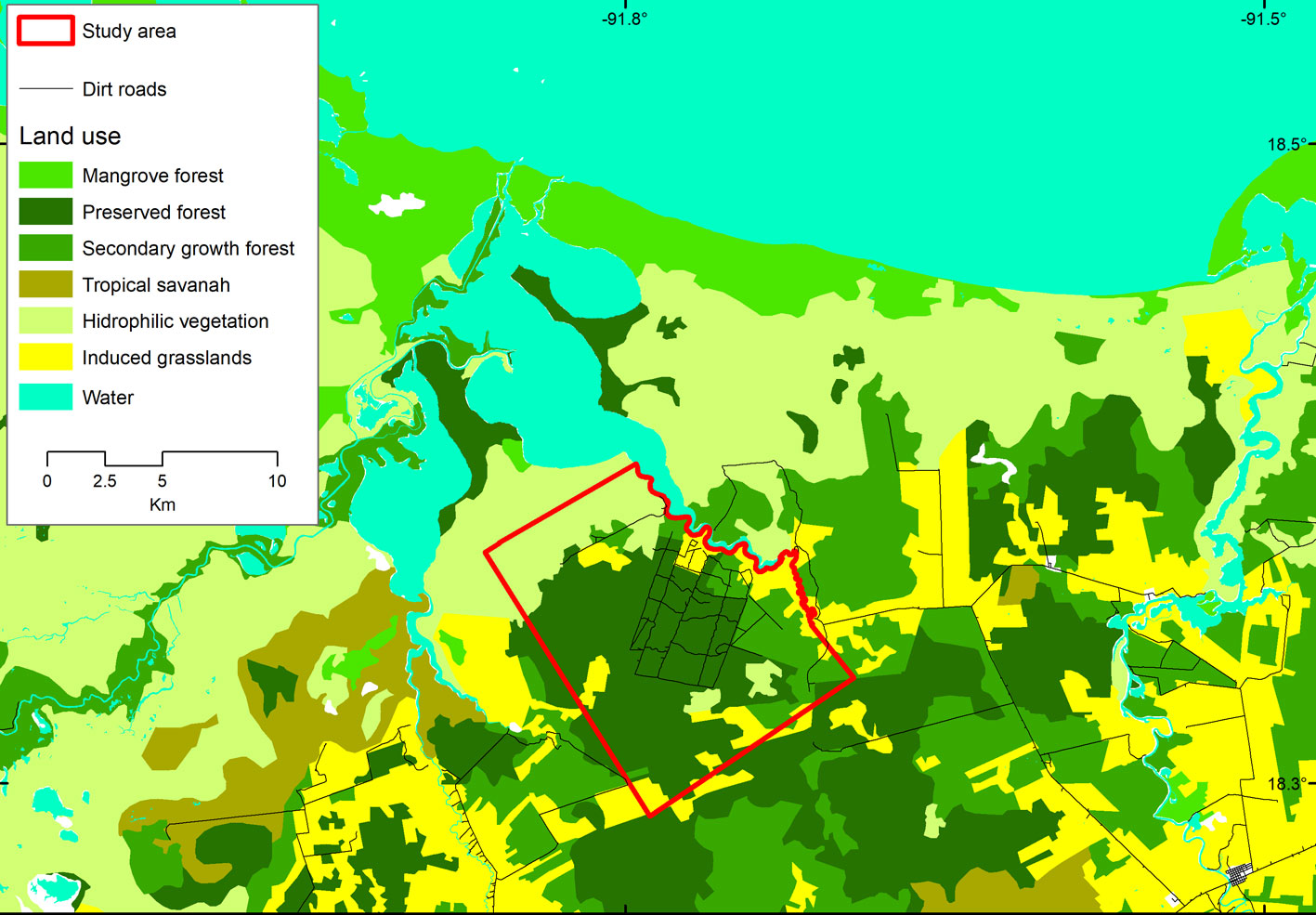
Our research was conducted at the Nicte-Ha UMA (Wildlife Management and Conservation Unit per it Spanish initials; hereafter called Nicte-Ha), located in southwestern Campeche, Mexico (91°43’56” W, 18°19’56” N) and adjacent to the Laguna de Términos Flora and Fauna Protected Area (Fig. 1). The area is a 2,300 ha tract of tropical lower coastal plain habitat situated between -1 and 3 m asl. The climate in the region is warm-humid, with a mean temperature of 27 °C and up to 2,000 mm of precipitation per year (INEGI, 2013). The vegetation types of the area vary from hydrophilic vegetation, flooded savannas, mangroves, sub-evergreen flooded rain forests, tropical deciduous flooded forests, secondary growth forests, agricultural areas, and induced grasslands for cattle grazing (Ocaña & Lot, 1996). In 2010, the owners of Nicte-Ha removed all cattle from the ranch, and since then the area has been used exclusively for sustainable white-tailed deer hunting.
Table 1
Seasonal home range size reported for white-tailed deer along the southern range of the species. N, Number of followed individuals; NA, not specified.
|
Country |
Vegetation type |
N |
Seasonal home range size (ha) |
Source |
|
|
Females |
Males |
||||
|
Mexico |
Chihuahuan desert |
18 |
100-193 |
234-300 |
Bello et al. (2004) |
|
Mexico |
Tropical dry forest |
2 |
11-44 |
26 |
Sánchez-Rojas et al. (1997) |
|
Mexico |
Pine-oak forest |
6 |
14.5-16.9 |
|
González-Pérez (2003) |
|
Costa Rica |
Tropical dry forest |
41 |
334.4-754.3 |
|
Sáenz-Méndez and Vaughan-Dickhaut (1998) |
|
Costa Rica |
Tropical dry forest |
42 |
159.4-510.63 |
Sáenz-Méndez and Vaughan-Dickhaut (1998) |
|
|
Costa Rica |
Tropical dry forest |
|
323.23 |
Calvopiña (1994 [in Sáenz-Méndez and Vaughan-Dickhaut, 1998 ]) |
|
|
Colombia |
Tropical dry forest |
24 |
17-45 |
26-96 |
Camargo-Sanabria (2005) |
|
Colombia |
NA |
31 |
114 |
378 |
Gómez-Giraldo (2005) |
|
Venezuela |
Llanos |
2 |
|
80 |
Correa-Viana (2000) |
|
Mexico |
Tropical wetlands |
11 |
6.12-83.33 |
21.28-195.63 |
This study |
1 Hand-raised individuals released as adults in wild conditions.
2 Relocated individuals.
3 Sex not specified.
4 Semi-captivity conditions
Water is probably the most limiting factor for wild ungulates during the dry season in the Yucatán Peninsula (Reyna-Hurtado et al., 2010). In Nicte-Ha, rain is concentrated in a single season that usually begins in June and ends by early November. This is followed by a dry season that lasts around 6 months (December-May). The intensity and seasonality of the rains in combination with flat terrain, produces seasonal floods that can reach 0.5 m. The flood lasts for the entire rainy season, but once it is over, water for ungulates remains available but concentrated in numerous puddles within the area that usually dry out by mid-February. After this date, the only available free-standing water in Nicte-Ha is the Rio del Este and artificial water holes that owners have built along Nicte-Ha area to improve the habitat quality for white-tailed deer. However, on occasions due to a very extended drought such as the one that occurred at the end of the dry season in 2017, even the artificial water holes dry out and the only water source for the wildlife on Nicte-Ha is the Río del Este. During May of 2016 and May of 2018, 11 adult white-tailed deer (5 females and 1 male in 2016, and 2 females and 3 males in 2018) were captured using a 20 × 20 m drop net (Ramsey, 1968). Once captured, the deer were physically restrained, and conventional measurements and weight were obtained. Each captured deer during 2016 was fitted with a VERTEX Survey Iridium Collar (Vectronic Aerospace GmbH, Berlin, Germany), and those captured in 2018 with a VERTEX Survey Globalstar Collar (Vectronic Aerospace GmbH, Berlin, Germany). Once equipped, all the captured deer were released immediately on the capture site. The capture, management, and collaring of the white-tailed deer was carried out under the collection permits SGPA/DGVS/01097/16 and SGPA/DGVS/003244/18 by the Dirección General de Vida Silvestre-SEMARNAT-México, and we followed the capture and management guidelines of the American Society of Mammalogists (Sikes et al., 2016). Iridium radio collars were programmed to take fixes every 6 h and the Globalstar every 12 h.
Although we captured and followed white-tailed deer in 2 different years (2016-2017 and 2018-2019), the small sample size precluded us from combining locations of individuals obtained in the different years of the study. We divided the location data into 3 seasons according to the availability of water for the white-tailed deer in Nicte-Ha: 1) rainy season (June-November) when most of the area is flooded and water is freely available along Nicte-Ha; 2) early dry season (December-February) in which eventual rains could occur and free water is available in puddles and water holes distributed along Nicte-Ha; 3) late dry season (March-May) in which rain is extremely rare and free standing water is only available in artificial water holes and in the Río del Este. These seasons partially coincide with the reproductive cycle of the white-tailed deer in the study, where the antlered males are found from June-November, the antlerless males from November to February, and the fawning season begins in March and extends until June (Contreras-Moreno, Hidalgo-Mihart, Jesús-de la Cruz et al., 2019).
Home-range was estimated for each deer for each season (rainy season, early dry season, and late dry season). We used HRT Home-range Tools in Arc GIS 9 (Rodgers et al., 2007) to estimate home-range size and the core area with the Fixed Kernel method using 95% and 50% of the locations, respectively (Kernohan et al., 2001). The smoothing parameter (h) for each estimate was obtained using the least squares cross-validation method (Kernohan et al., 2001). To compare the white-tailed deer home-range with other studies (Gula & Theuerkauf, 2013), we also calculated seasonal home-range size with the Minimum Convex Polygon (MCP) with 100% of the locations using The Animal Movement extension (Hooge et al., 2001; Table 2).
To determine if there were differences in home-range sizes and core areas due to the seasonality and sex, we combined data from individuals of the different studied years. Temporal (rainy season, early dry season, and late dry season) and sex (female vs. male) differences in home-range size and core area, were analyzed using a general linear mixed model (GLMM; repeated measures analysis with random effects), with the seasonal home-range size/seasonal core area size, and the sex as the explanatory variables using nlme in R (Pinheiro et al., 2019). Even though these analyses allowed us to determine if the explanatory variables had a significant influence on the home-range size and the core area size of the white-tailed deer, because we combined the data of individuals from the different studied years, the differences resulting from inter-annual variations could not be tested and remained in the analysis as a source of error.
To quantify the degree of seasonal fidelity of the white-tailed deer to their home-range, we calculated percent overlap of seasonal home-ranges and core areas during consecutive seasons, i.e., the percentage of the home-range area utilized in a given season that was used again in the following season. We averaged percent overlap across seasons to determine average seasonal male and female percent overlap.
Table 2
Home range size and core area size of the white-tailed deer followed in the Campeche wetlands in southeastern Mexico obtained using the Minimum convex polygon method. Rainy season goes from June-November. Transition season goes from December to February. Dry season goes to March to May. HR, Seasonal home range size (ha); CA, seasonal core area size (ha); N, seasonal number of locations used to determine the home range and core area size.
|
Sex |
Id |
Year |
Rainy season |
Transition season |
Dry season |
|||
|
HR |
N |
HR |
N |
HR |
N |
|||
|
Female |
A |
2016-2017 |
15.09 |
240 |
15.16 |
169 |
89.01 |
269 |
|
Female |
B |
14.54 |
303 |
9.27 |
203 |
96.06 |
253 |
|
|
Female |
C |
8.22 |
333 |
47.00 |
258 |
45.19 |
156 |
|
|
Female |
D |
22.24 |
419 |
16.15 |
228 |
90.82 |
275 |
|
|
Female |
E |
108.81 |
263 |
30.20 |
136 |
12.19 |
51 |
|
|
Male |
A |
41.42 |
129 |
32.77 |
96 |
67.45 |
61 |
|
|
Female |
F |
2018-2019 |
44.78 |
134 |
22.82 |
94 |
153.30 |
76 |
|
Female |
G |
233.97 |
145 |
7.48 |
101 |
10.39 |
98 |
|
|
Male |
B |
87.43 |
115 |
65.06 |
98 |
201.75 |
78 |
|
|
Male |
C |
671.65 |
115 |
167.87 |
101 |
231.15 |
90 |
|
|
Male |
D |
207.20 |
165 |
116.46 |
123 |
32.79 |
117 |
Results
All 11 captured deer were alive at the end of the one-year cycle, and their collars functioned properly during the 3 studied seasons. We obtained 5,492 locations from all radio-collared deer during the study (range = 291-922; Table 3), distributed at 2,361 locations during the rainy season (range 115-419 locations/individual), 1,607 during the early dry season (range = 94-258) and 1,524 during the late dry season (range = 78-275).
The mean (± SD) home-range size for females during the study was from 12.67 (± 3.52) during the early dry season to 21.57 (± 27.44) in the dry season (Table 3). For males, the mean home-range size was from 37.31 (± 16.93) during the late dry season to 90.16 (± 72.64) during the rainy season (Table 3). We found white-tailed deer home-range size larger for males than for females (χ2 = 13.043, g. l. = 1, p < 0.001; Fig. 2). Although we did not find differences between seasonal home-range sizes (χ2 = 1.43, g. l. = 1, p = 0.23), we found that differences exist when we compared all individuals (males and females) with seasonality of the area (χ2 = 5.69, g. l. = 1, p = 0.0171), showing that female home-range size is similar throughout the year, and that males, had smaller home-range size during the early and late dry seasons, than during the rainy season (Fig. 2).
We found that the mean core area size (± SD) for females varied from 3.52 ha (± 1.58 ha) during the early dry season to 5.15 ha (± 6.96 ha) during the late dry season (Table 3). In the case of the males, mean core area size varied from 20.45 (± 18.14 ha) during the rainy season to 7.91 ha (± 5.52 ha) during the dry season (Table 3). When we compared the seasonal core area size between males and females, we found similar results to those obtained with home-range size, with larger core area size for males than for females (χ2 = 10.00, g. l. = 1, p = 0.002; Fig. 2), and no differences in the core area size (χ2 = 1.70, g. l. = 1, p = 0.19) among seasons. However, we found differences in the core area size when we compared seasons using combined all individuals regardless of sex (χ2 = 4.41, g. l. = 1, p = 0.03), showing that the core area size for females were similar among the year, and males with larger core area size during the rainy season, compared with the early dry and late dry season, which is similar to our home-range size results (Fig. 2).
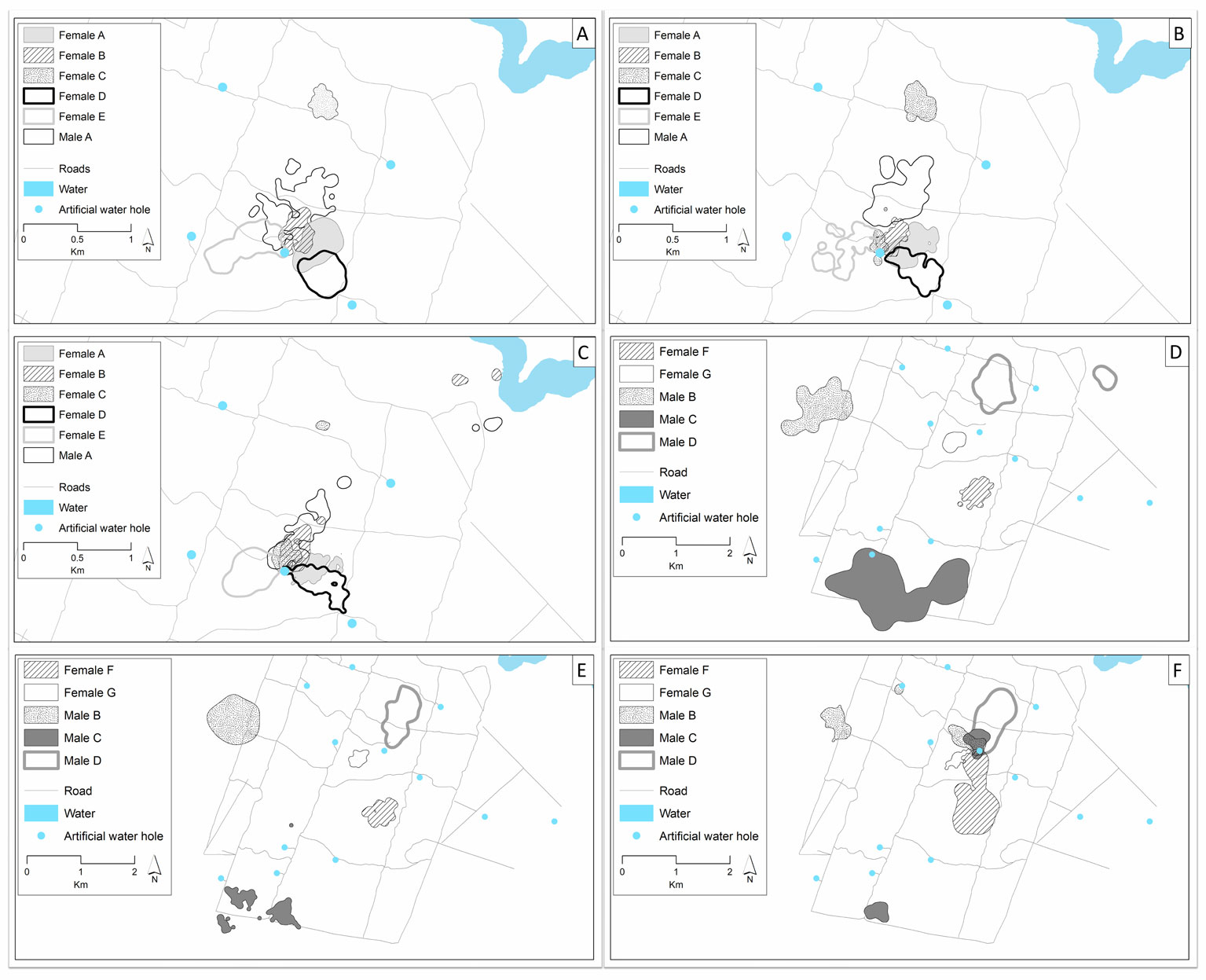
We found that the home-range and core area overlap for females was slightly larger between the rainy season and the early dry season than between the early dry season and the late dry season (Table 4). However, even some seasonal changes occur (e.g., home-range overlap of the male C during the rainy season to early dry season). For most of the radiotracked white-tailed deer in Nicte-Ha, both females and males remain in the same areas and maintain most of their home-ranges and core areas during the year. Also, even during the rainy season and the early dry season some individuals (e.g., female C and males B, C, and D) had separate home-ranges from each other (Fig. 3), while during the late dry season, even when they maintain portions of their home-ranges separate, all had portions of their home-ranges in a single area around an artificial water hole.
Table 3
Home-range size and core area size of the white-tailed deer followed in the Campeche wetlands in southeastern Mexico obtained using the Adaptive Kernel Method. Rainy season occurs from June-November. Early dry season occurs from December to February and late dry season occurs from March to May. HR, Seasonal home-range size (ha); CA, seasonal core area size (ha); N, seasonal number of locations used to determine the home-range and core area size; SD, standard deviation; NA, not available.
|
Sex |
Id |
Year |
Weight on capture (kg) |
Rainy season |
Early dry season |
Late dry season |
||||||
|
HR |
CA |
N |
HR |
CA |
N |
HR |
CA |
N |
||||
|
Female |
A |
2016-2017 |
28.5 |
15.85 |
4.41 |
240 |
13.00 |
3.35 |
169 |
9.39 |
2.46 |
269 |
|
Female |
B |
32.6 |
9.98 |
2.61 |
303 |
6.35 |
1.91 |
203 |
12.55 |
2.56 |
253 |
|
|
Female |
C |
24.3 |
6.12 |
1.89 |
333 |
11.41 |
2.93 |
258 |
6.70 |
1.06 |
156 |
|
|
Female |
D |
28.8 |
13.88 |
5.05 |
419 |
12.65 |
3.48 |
228 |
12.87 |
2.70 |
275 |
|
|
Female |
E |
34.0 |
20.47 |
6.82 |
263 |
15.33 |
3.82 |
136 |
17.21 |
3.90 |
51 |
|
|
Male |
A |
31.0 |
29.58 |
6.91 |
129 |
26.45 |
6.85 |
96 |
21.28 |
5.23 |
61 |
|
|
Female |
F |
2018-2019 |
NA |
21.43 |
6.08 |
134 |
21.86 |
6.80 |
94 |
83.33 |
20.83 |
76 |
|
Female |
G |
31.2 |
11.71 |
3.14 |
145 |
8.09 |
2.37 |
101 |
8.92 |
2.55 |
98 |
|
|
Male |
B |
31.7 |
71.47 |
14.18 |
115 |
64.11 |
16.40 |
98 |
41.15 |
5.44 |
78 |
|
|
Male |
C |
30.6 |
195.63 |
47.21 |
115 |
36.46 |
7.46 |
101 |
27.39 |
4.79 |
90 |
|
|
Male |
D |
31.2 |
63.96 |
13.50 |
165 |
45.24 |
12.85 |
123 |
59.44 |
16.18 |
117 |
|
|
Mean females |
14.20 (±5.53) |
4.28 (±1.82) |
12.67 (±3.52) |
3.52 (±1.58) |
21.57 (±27.44) |
5.15 (±6.96) |
||||||
|
Mean males (± SD) |
90.16 (±72.64) |
20.45 (±18.14) |
43.07 (±15.99) |
10.89 (±4.55) |
37.31 (±16.93) |
7.91 (±5.52) |
Table 4
Percentage of home-range (HR) and core area (CA) seasonal overlap in white-tailed deer followed in the Campeche wetlands in southeastern Mexico. Rainy season goes from June-November. Early dry season goes from December to February. Dry season goes from March to May. HR, Home-range; CA, core area; SD, standard deviation.
|
Sex |
Id |
Year |
Seasonal overlap |
|||
|
Percent overlap rainy season-early dry season |
Percent overlap early dry season- late dry season |
|||||
|
HR |
CA |
HR |
CA |
|||
|
Female |
A |
2016-2017 |
62.55 |
27.96 |
38.67 |
17.01 |
|
Female |
B |
75.14 |
37.95 |
65.24 |
38.08 |
|
|
Female |
C |
54.91 |
18.69 |
88.93 |
32.20 |
|
|
Female |
D |
98.11 |
65.98 |
27.99 |
13.23 |
|
|
Female |
E |
74.57 |
42.36 |
77.11 |
39.66 |
|
|
Male |
A |
55.24 |
15.70 |
69.08 |
26.11 |
|
|
Female |
F |
2018-2019 |
85.91 |
55.95 |
26.23 |
38.28 |
|
Female |
G |
66.48 |
42.80 |
64.04 |
34.43 |
|
|
Male |
B |
67.82 |
52.63 |
30.87 |
0.00 |
|
|
Male |
C |
13.81 |
10.23 |
30.03 |
0.00 |
|
|
Male |
D |
49.47 |
31.71 |
95.32 |
61.07 |
|
|
Mean females (± SD) |
73.95 (±14.58) |
41.67 (±15.95) |
55.46 (±24.66) |
30.41 (±10.80) |
||
|
Mean males (± SD) |
46.59 (±23.16) |
27.57 (±19.03) |
56.32 (±31.74) |
21.79 (±28.93) |

Discussion
White tailed deer in areas with abundant, well distributed resources tend to have smaller home-ranges than deer that occupy less productive areas (Lesage et al., 2000; Marchinton & Hirth 1984; Stewart et al., 2011). Productivity in tropical areas like Nicte-Ha is greater than in subtropical and temperate areas (Gillman et al., 2015). Higher productivity has a direct impact in the available food resources for ungulates (Mandujano & Naranjo, 2010). This is the case in Nicte-Ha, as white-tailed deer apparently were able to obtain all their basic needs in smaller home-ranges compared to white-tailed deer home-ranges in subtropical and temperate ecosystems (Table 1).
The mean home-range size for the female white-tailed deer in Nicte-Ha was similar to the home-range areas observed in other tropical and subtropical areas in Mexico (González-Pérez, 2003; Sánchez-Rojas et al., 1997; Table 3), but smaller than home-ranges observed in Costa Rica, Colombia, and Venezuela. However, the animals monitored in Central and South America in all cases were hand-raised individuals released as adults or relocated individuals (Camargo-Sanabria, 2005; Sáenz-Méndez & Vaughan-Dickhaut, 1998; Table 3) and the large home-ranges could have resulted from the animals dispersing from the releasing area before establishing a more stable home-range (Nelson, 2015). In the case of males, the observed home-range in Nicte-Ha is similar to the home-ranges of deer in the Venezuela llanos (Correa-Viana, 2000; Table 3) and tropical dry forests of Mexico (Sánchez-Rojas et al., 1997) and Colombia (Camargo-Sanabria, 2005), but smaller than other areas of Colombia (Correa-Viana, 2000). However, as with the females, most of the studies of white-tailed deer in South America have been made with hand-raised or relocated individuals that probably were exploring the new habitat.
Forage quality and quantity for deer in the Campeche area had significant variations throughout the year in response to the seasonality of the region, with a drastic reduction in the available forage during the dry season compared with the rainy season (Granados et al., 2014). We found that home-range size and core area size were similar throughout the year for the studied females, even during the fawning months (February to June; Contreras-Moreno, Hidalgo-Mihart, Jesús-de la Cruz et al., 2019), when the forage availability was reduced. These observations probably indicate that the forage availability and fawning period are not the main factors that influenced the home-range size of female white-tailed deer in Nicte-Ha. Although there were no changes in the home-range size, the daily traveled distances for female deer were significantly greater during the dry season (Contreras-Moreno, Hidalgo-Mihart, & Contreras-Sánchez, 2019), mostly driven by the search of water (including roundabout exploration movements to the Río del Este longer than 2 km, followed by a return to their home-range when the artificial water holes dried during the 2016-2017 dry season). Also, it is notable the fidelity of those females that occupied areas close to an artificial water hole during all 3 seasons. They presented very stable home-ranges during the year, with a high home-range overlap between seasons. The exception of this observation were the females C and F, that spent the rainy and the early dry seasons distant from a water hole, but during the late dry season their home-range were close to a permanent water hole.
We found that during the late dry season in Nicte-Ha, the home-range sizes of males were smaller than the other 2 seasons. Rut season in the area is coincident with the rainy season (June to October; Contreras-Moreno, Hidalgo-Mihart, Jesús-de la Cruz et al., 2019), and it is common that males during this time tend to have larger home-ranges (Hellickson et al., 2008). The velvet season (February to June) is coincident with the last part of the early dry season and beginning of the late dry season. Male nutrient requirements during the velvet season increases in order to gain weight and cover the energy requirements for antler development (Dryden, 2016). As a consequence of the dry season, the forage quality and quantity for deer in the study area is reduced (Granados et al., 2014). However, we observed that males reduced the home-range during this time and relocated their home-ranges and reduced the seasonal and core area overlap using areas close to the artificial water holes. Restriction in water availability causes important reductions in food intake by deer and can generate severe weight loss in deer (Jenks et al., 1990; Lautier et al., 1988). It is possible that, like females, males in the intense dry season in Nicte-Ha reduced their movements to areas close to the main water sources, instead of using larger areas to search for the available food. Thus, the dry season appeared to force white tailed deer to concentrate their activities around the water sources, as has been found for other large sized herbivores in the region such as the white-lipped peccary (Tayassu pecari) and the tapir (Tapirus bairdii; Pérez-Cortez et al., 2012; Reyna-Hurtado et al., 2009), indicating that water is the most limiting resource for this mammal guild during the dry season.
We observed that during the rainy season and the consequent flooding of the area, white-tailed deer did not search for higher ground. An increment in home-range and core area overlap between the rainy and the early dry seasons also indicated that deer inhabited the same areas after the flooding that occurred during the rainy season. Increasing flooding levels reduce the mobility of the deer and individuals probably concentrate their activities in areas of their home-ranges where the flooding level is lower, as occurs in other wetland areas such as in Florida (Contreras-Moreno, Hidalgo-Mihart, & Contreras-Sánchez, 2019; Fleming et al., 1994; López et al., 2004).
Our results showed that water availability during the dry season, and flooding levels during the rainy season may have significant effects on the home-range size and spatial configuration of the white-tailed deer in Nicte-Ha. It is important to understand how the white-tailed deer could be affected by changes that are already occurring the region. It is anticipated that the Nicte-Ha area will suffer a general decrease in mean precipitation and an increase in drought (Chiabai, 2015; Imbach et al., 2012). In contrast, sea level rise due to global warming will produce longer periods of flooding in the costal low terrain of the Nicte-Ha region (Yáñez-Arancibia et al., 2014). This will probably have a severe impact on the white-tailed deer, as has already been detected in ungulates from the Amazon basin where extreme drought and flooding is already occurring (Bodmer et al., 2014).
White tailed deer populations in southeastern Mexico are subject to intense subsistence hunting (e.g., Santos-Fita et al., 2012; Weber, 2014), which has produced the local extinction of the species in several areas (Ortiz-Lozada, et al. 2017; Weber, 2014). Land management in Nicte-Ha is under the scheme of “Unidad para el Manejo Sustentable de la Vida Silvestre” (UMA; i.e., private or social property that operates as a wildlife and conservation area that has a specific wildlife management plan authorized by the federal government; Valdez et al., 2006) that is strictly enforced by the owners and managers of the property, with no subsistence hunting allowed. This has resulted in a reduction in poaching, and although sport hunting is permitted, white tailed-deer abundance in the Nicte-Ha
area is higher than that observed in the surrounding areas, where due to extensive subsistence hunting white tailed deer density has been estimated at 0.63 ±0.43 to 1.13 ± 0.44 deer/km2 (Castro-Fócil, 2012; Contreras-Moreno et al., 2015), one of the lowest densities observed in Mexico (Gallina et al., 2019). The higher abundance and strict management of white-tailed deer in Nicte-Ha compared to other areas of Campeche, probably had important effects on the home-range size of the Nicte-Ha white tailed-deer. Thus, the results of this study should be weighed cautiously when extrapolated to other areas where subsistence hunting is allowed and not regulated. In order to understand the effect of subsistence hunting on the spatial ecology of the species in southeastern Mexico, and especially in Campeche, future studies should focus in areas where this activity is allowed.
The observed effect of permanent water sources during the late dry season on the spatial ecology of the white-tailed deer in Nicte-Ha should be considered as one of the key findings of our study. Our results showed the importance of water availability for both males and females during this season and should be considered for the regional current and future management initiatives, especially due to the inclusion of O. virginianus thomasi subspecies in the Hubert Thummler Award for the Mexican deer slam and under an scenario where the climatic projections of the region suggest that extreme droughts will occur in the near future reducing the water availability and affecting the movements of wildlife (IPCC, 2007; Mardero et al., 2012; O’Farrill et al., 2014; Reyna-Hurtado et al., 2009).
Acknowledgments
The UMA Nicte-Ha, Conservación Panthera Mexico A.C., and the División Académica de Ciencias Biológicas-UJAT (DACBiol-UJAT) provided logistic support for the project. We specially thank Yaribeth Bravata, Adrían Chahín, Ramón Sanz-Freeman, Martín Moreno, Ismael Sánchez, Diana Friedeberg, and Raul Valdez for their help with the fieldwork and their continuous support. We thank Robert W. Jones for his help to improve the final version of the manuscript. The PFCE 2018 (Programa para el Mejoramiento de la Calidad Educativa) partially sponsored the acquisition of the radio collars used in this project through the DACBiol-UJAT. FCM received support from CONACYT (scholarship 271627) for his Ph. D. studies in Ecology and Management of Tropical Systems at the DACBiol-UJAT.
References
Bello, J., Gallina, S., & Equihua, M. (2004). Movements of white-tailed deer and their relationship with precipitation in the northeastern of Mexico. Interciencia, 29, 357–361.
Bello, J., Gallina, S., Equihua, M., Mandujano, M., & Delfín, C. (2001). Home-range, core area and distance to water sources by white tailed deer in northeastern Mexico. Vida Silvestre Neotropical, 10, 30–37.
Bodmer, R. E., Fang, T. G., Puertas, P. E., Antúnez, M., Chota, K., & Bodmer, W. E. (2014). Cambio climático y fauna silvestre en la Amazonia peruana: impacto de la sequía e inundaciones intensas en la Reserva Nacional Pacaya Samiria. Iquitos, Perú: Fundación Latinoamericana para el Trópico Amazonico-Fundamazonia.
Burt, W. (1943). Territoriality and home-range concepts as applied to mammals. Journal of Mammalogy, 24, 346–352. https://doi.org/10.2307/1374834
Camargo-Sanabria, A. A. (2005). Evaluación preliminar del área de acción y patrón de actividad del venado cola blanca (Odocoileus virginianus), como parte de una alternativa de manejo ex situ en un bosque seco tropical (Bachelor Thesis). Universidad Nacional de Colombia. Bogotá, Colombia.
Castro-Fócil, G. (2012). Abundancia del venado cola blanca y pecarí de collar en tres sitios con diferente tipo de tenencia de la tierra en Laguna de Términos, Campeche (Bachelor Thesis). Universidad Juárez Autónoma de Tabasco. Villahermosa, México.
Chiabai, A. (2015). Climate change impacts on tropical forests in Central America: an ecosystem service perspective. Routledge, London: The Earthscan Forest Library,
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2012). Proyecto de Evaluación de las Unidades de Manejo para la Conservación de la Vida Silvestre (UMA) (1997-2008). Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Resultados de la fase I: gestión y administración. Proyectos Conabio, México. Recuperado el 15 de junio de 2020 de http://www.biodiversidad.gob.mx/usos/UMAs_pdf/Informe_CONABIO_Proyecto_UMA_FASE_I.pdf
Contreras-Gil, J. M., Alcalá-Álvarez, D., Mellink, E., Martínez-Gallardo, R., & Ojinaga, M. D. (2010). Estado de la cacería deportiva como una alternativa económica de diversificación productiva para el municipio de Ensenada, Baja California. Investigación Ciencia y Política Pública, 2, 65–74.
Contreras-Moreno, F. M., Hidalgo-Mihart, M. G., & Contreras-Sánchez, W. M. (2019). Daily traveled distances by the white-tailed deer in relation to seasonality and reproductive phenology in a tropical lowland of southeastern Mexico. In R. Reyna-Hurtado, & C. Chapman (Eds.), Movement ecology of neotropical forest mammals (pp.111–123). Cham: Springer. https://doi.org/10.1007/978-3-030-03463-4_8
Contreras-Moreno, F. M., Hidalgo-Mihart, M. G., Jesús-de la Cruz, A., Juárez-López, R., Bravata-de la Cruz, Y., & Chahín-Perdomo, A. (2019). Seasonal antler cycle in white-tailed deer in Campeche wetlands in Southeastern Mexico. European Journal of Wildlife Research, 65, 53. https://doi.org/10.1007/s10344-019-1291-5
Contreras-Moreno, F. M., Zúñiga-Sánchez, J. S., & Bello-Gutiérrez, J. (2015). Parámetros poblacionales de Odocoileus virginianus (Cervidae) en dos comunidades de Tabasco, México. Revista Latinoamericana de Conservación, 4, 7–13.
Correa-Viana, M. (2000). Movimientos, actividad y uso de hábitat de venados liberados en la finca el Jaimero, Portuguesa, Venezuela (Bachelor Thesis). Universidad Central de Venezuela. Caracas, Venezuela.
Dryden, G. M. (2016). Nutrition of antler growth in deer. Animal Production Science, 56, 962–970. https://doi.org/10.1071/AN15051
Fleming, M., Schortemeyer, J., & Ault, J. (1994). Distribution, abundance, and demography of white-tailed deer in the Everglades. In J. Dennis (Ed.), Proceedings of the Florida Panther Conference (pp. 247–274). University of Florida, Gainsville, USA.
Gallina-Tessaro, S., López-Tello, E., & Mandujano, S. (2019). Recent studies of white-tailed deer in the Neotropics. In Ecology and Conservation of Tropical Ungulates in Latin America (pp. 371-393). Cham: Springer. https://doi.org/10.1007/978-3-030-28868-6_15
García-Marmolejo, G., Escalona-Segura, G., & Van Der Wal, H. (2008). Multicriteria evaluation of wildlife management units in Campeche, Mexico. The Journal of Wildlife Management, 72, 1194–1202. https://doi.org/10.2193/2006-050
Gillman, L. N., Wright, S. D., Cusens, J., McBride, P. D., Malhi, Y., & Whittaker, R. J. (2015). Latitude and productivity. Global Ecology and Biogeography, 24, 107–117 https://doi.org/10.1111/geb.12245
Gómez-Giraldo, C. (2005). Radio-telemetría aplicada a la reintroducción del venado cola blanca (Odocoileus virginianus) (Master Thesis). Universidad Nacional de Colombia, Medellín, Colombia.
González-Pérez, G. E. (2003). Uso del hábitat y área de actividad del venado cola blanca (Odocoileus virginianus sinaloae J. Allen) en la Estación Científica Las Joyas, Reserva de la Biosfera de Manantlán, Jalisco (Bachelor Thesis). Universidad Nacional Autónoma de México. Ciudad de México, Mexico.
Granados, D., Tarango, L., Olmos, G., Palacio, J., Clemente, F., & Mendoza, G. (2014). Dieta y disponibilidad de forraje del venado cola blanca Odocoileus virginianus thomasi (Artiodactyla: Cervidae) en un campo experimental de Campeche, México. Revista de Biología Tropical, 62, 699–710. https://doi.org/ 10.15517/RBT.V62I2.9853
Guajardo-Quiroga, R. G., & Martínez-Muñoz, A. (2004). Cuantificación del impacto económico de la caza deportiva en el norte de México y perspectivas de su desarrollo. Entorno Económico, 42, 1–17.
Gula, R., & Theuerkauf, J. (2013). The need for standardization in wildlife science: home-range estimators as an example. European Journal of Wildlife Resarch, 59, 713–718. https://doi.org/10.1007/s10344-013-0726-7
Hellickson, M. W., Campbell, T. A., Miller, K. V., Marchinton, R. L., & DeYoung, C. A. (2008). Seasonal ranges and site fidelity of adult male white-tailed deer (Odocoileus virginianus) in southern Texas. The Southwestern Naturalist, 53, 1–8. https://doi.org/10.1894/0038-4909(2008)53[1:SRASFO]2.0.CO;2
Hemson, G., Johnson, P., South, A., Kenward, R., Ripley, R., & Macdonald, D. (2005). Are kernels the mustard? Data from global positioning system (GPS) collars suggests problems for kernel home-range analyses with least squares cross validation. Journal of Animal Ecology, 74, 455–463. https://doi.org/10.1111/j.1365-2656.2005.00944.x
Hidalgo-Mihart, M. G., Jesús-De la Cruz, A., Juárez-López, R, Contreras-Moreno, F. M., Bravata, Y., Pérez-Solano, L. A. et al. (2017). Inventory of medium-sized and large mammals in the wetlands of Laguna de Términos and Pantanos de Centla, Mexico. Check List, 13, 711–726. https://doi.org/10.15560/13.6.711
Hooge, P. N., Eichenlaub, W. M., & Solomon, E. K. (2001). Using GIS to analyze animal movements in the marine environment. In G. H. Kruse, N. Bez, A. Booth, M. W. Dorn, S. Hills, R. Lipcius et al. (Eds.), Spatial processes and management of marine populations (pp. 37–51). Anchorage: Alaska Sea Grant College Program. https://doi.org/10.4027/spmmp.2001
Imbach, P., Molina, L., Locatelli, B., Roupsard, O., Mahé G, Neilson, R. et al. (2012). Modeling potential equilibrium states of vegetation and terrestrial water cycle of Mesoamerica under climate scenarios. Journal Hydrometeorology in Earth System Sciencie and Society, 13, 665–680. https://doi.org/10.1175/JHM-D-11-023.1
INEGI (Instituto Nacional de Estadística y Geografía). (2013). Anuario estadístico de los Estados Unidos Mexicanos 2013. Instituto Nacional de Estadística y Geografía. Aguascalientes, México. Retrieved on September 25, 2019 from: http://internet.contenidos.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/aegeum/702825063979.pdf
INEGI (Instituto Nacional de Estadística y Geografía). (2015). Conjunto de datos vectoriales de uso del suelo y vegetación escala 1:250 000, serie V. Instituto Nacional de Estadística y Geografía, Aguascalientes, México. Retrieved on September 30, 2020 from: http://www.inegi.org.mx/geo/contenidos/recnat/usosuelo/default.aspx
IPCC (Intergovernmental Panel on Climate Change). (2007). Climate change 2007: the physical science basis, Contribution on Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change.
Jenks, J. A., Soper, R. B., Lochmiller, R. L., & Leslie, D. M. Jr. (1990). Effect of exposure on nitrogen and fiber characteristics of white-tailed deer feces. The Journal of Wildlife Management, 54, 389–391. http://doi.org/10.2307/
3809644
Kernohan, B. J., Gitzen, R. A., & Millspaugh, J. J. (2001). Analysis of animal space use and movements. In J. Millspaugh, & J. M. Marzluff (Eds.), Radio tracking and animal populations (pp. 125–166), San Diego, CA: Academic Press.
Lautier, J. K., Dailey, T. V., & Brown, R. D. (1988). Effect of water restriction on feed intake of white-tailed deer. The Journal of Wildlife Management, 52, 602–606. https://doi.org/10.2307/3800915
Lesage, L., Crête, M., Huot, J., Dumont, A., & Ouellet, J. P. (2000). Seasonal home-range size and philopatry in two northern white-tailed deer populations. Canadian Journal of Zoology, 78, 1930–1940. https://doi.org/10.1139/z00-117
López, R. R., Silvy, N. J., Wilkins, R. N., Frank, P. A., Peterson, M. J., & Peterson, M. N. (2004). Habitat use patterns of Florida key deer: implications of urban development. The Journal of Wildlife Management, 68, 900 –908. https://doi.org/10.2193/0022-541X(2004)068[0900:HPOFKD]2.0.CO;2
Mandujano, S., Gallina, S., & Ortega, J. A. (2014). Venado cola blanca en México. In R. Valdez, & J. A. Ortega-S. (Eds.), Ecología y manejo de fauna silvestre en México (pp. 399–420). Texcoco, México: Colegio de Postgraduados.
Mandujano, S., & Naranjo, E. (2010). Ungulate biomass across a rainfall gradient: A comparison of data from neotropical and palaeotropical forests and local analyses in Mexico. Journal of Tropical Ecology, 26, 13–23. https://doi.org/10.1017/S0266467409990411
Marchinton, R. L., & Hirth, D. H. (1984). Behavior. In L. K. Halls (Ed.), White-tailed deer: ecology and management (pp. 129–168). Harrisburg. USA: Stackpole Books.
Mardero, S., Nickl, E., Schmook, B., Schneider, L., Rogan, J., Christman, Z. et al. (2012). Sequías en el sur de la península de Yucatán: análisis de la variabilidad anual y estacional de la precipitación. Investigaciones Geográficas, 78, 19–33. http://dx.doi.org/10.14350/rig.32466
Nelson, M. E. (2015). Experimental evidence of spatial memory and home-range affinity in white-tailed deer (Odocoileus virginianus). The Canadian Field-Nataralist, 129, 1–7. http://dx.doi.org/10.22621/cfn.v129i1.1661
Ocaña, D., & Lot, A. (1996). Estudio de la vegetación acuática vascular del sistema fluvio-lagunar-deltaico del río Palizada, en Campeche, México. Anales del Instituto de Biología,Universidad Nacional Autónoma de México, Serie Botánica, 67, 303–327.
O’Farrill, G., Gauthier-Schampaert, K., Rayfield, B., Bodin, Ö., Calmé, S., Sengupta, R. et al. (2014). The potential connectivity of waterhole networks and the effectiveness of a protected area under various drought scenarios. Plos One, 9, e95049. https://doi.org/10.1371/journal.pone.0095049
Ortega, S. J. A., Mandujano, S., Villarreal, J., Di Mare, M. I., López-Arévalo, H., & Correa, M. (2011). Management of white-tailed deer: Latin America. In D. Hewitt (Ed.), Biology and management of white-tailed deer (pp. 566–598). Boca Raton: CRC Press.
Ortíz-Lozada, L., Pelayo-Martínez, J., Mota-Vargas, C., Demeneghi-Calatayud, A. P., & Sosa, V. J. (2017). Absence of large and presence of medium-sized mammal species of conservation concern in a privately protected area of rain forest in southeastern Mexico. Tropical Conservation Science, 10, 1 –13. https://doi.org/10.1177/1940082917738093
Pérez-Cortez, S., Sima-Panti, D., Reyna-Hurtado, R., & Naranjo, E. J. (2012). Influencia de la disponibilidad de agua en la presencia y abundancia de Tapirus bairdii en la selva de Calakmul, Campeche, México. Revista Mexicana de Biodiversidad, 83, 753–761. http://dx.doi.org/10.7550/rmb.25095
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., & Team, R. C. (2019). nlme: Linear and nonlinear mixed effects models. R package version 3.1–141. Rescatado el 20 de mayo de 2020. Available from: https://CRAN.R-project.org/package = nlme
Ramsey, C. W. (1968). A drop-net deer trap. The Journal of Wildlife Management, 32, 187–90. https://doi.org/10.2307/3798257
Reyna-Hurtado, R., O’Farril, G., Sima, D., Andrade, M., Padilla, A., & Sosa, L. (2010). Las aguadas de Calakmul: Reservorios de vida silvestre y de la riqueza natural de México. Biodiversitas, 93, 1–6.
Reyna-Hurtado, R., Rojas-Flores, E., & Tanner, G. (2009). Home-range and habitat preferences of white-lipped peccaries (Tayassu pecari) in Calakmul, Campeche, Mexico. Journal of Mammalogy, 90, 1199 –1209. https://doi.org/10.1644/08-MAMM-A-246.1
Rivera-Arriaga, E., & Villalobos-Zapata, G. J. (2005). The coastal zone of Campeche, Mexico: Opportunities for implementing an integrated coastal management framework. Journal of Coastal Research, 42, 184–190. https://www.jstor.org/stable/25736983
Rodgers, A. R., Carr, A., Beyer, H., Smith, L., & Kie, J. (2007). HRT: Home-range tools for ArcGIS. Ontario Ministry of Natural Resources, Thunder Bay, Ontario. Retrieved on September 2, 2019 from: http://flash.lakeheadu.ca/~arodgers/hre/
Sáenz-Méndez, J. C., & Vaughan-Dickhaut, C. (1998). Home-range and habitat use by two groups of Odocoileus virginianus (Artiodactyla: Cervidae) relocated in a tropical environment. Revista de Biología Tropical, 46, 1185–1197.
Sánchez-Rojas, G., Gallina, S., & Mandujano, S. (1997). Área de actividad y uso del hábitat de dos venados cola blanca (Odocoileus virginianus) en un bosque tropical de la costa de Jalisco, México. Acta Zoológica Mexicana (n.s.), 72, 39–54.
Sanderson, G. C. (1966). The study of mammal movements a review. The Journal of Wildlife Management, 30, 215–235. https://doi.org/10.2307/3797914
Santos-Fita, D., Naranjo, E. J., & Rangel-Salazar, J. L. (2012). Wildlife uses and hunting patterns in rural communities of the Yucatán Peninsula, Mexico. Journal of Ethnobiology and Ethnomedicine, 8, 1–17. https://doi.org/10.1186/1746-4269-8-38
SCI (Safari Club International) (2014a). Record book. Safari Club Internacional. Retrieved on September 2, 2019 from: http://www.scirecordbook.org
SCI (Safari Club International) (2014b). Bases Premio Hubert Thummler a los Venados de México, Safari Club Internacional. Retrieved on September 2, 2019 from: http://sci-mty.com/programas/
Sikes, R. S., Gannon, W. L., & Animal Care and Use Comittee of the American Society of Mammalogists. (2016). Guidelines of the American Society of Mammalogists for the use of wildl mammals in research and education. Journal of Mammalogy, 97, 663–688. https://doi.org/10.1644/10-MAMM-F-355.1
Stewart, K. R., Bowyer, T., & Weisberg, P. (2011). Spatial use of landscapes. In D. Hewitt (Ed.), Biology and management of white-tailed deer (pp. 181–217). Boca Raton: CRC Press.
Valdez, R., Guzmán-Aranda, J. C., Abarca, F. J., Tarango-Arámbula, L. A., & Sánchez, F. C. (2006). Wildlife Conservation and Management in Mexico. Wildlife Society Bulletin, 34, 270–282. https://doi.org/10.2193/0091-7648(2006)34[270:WCAMIM]2.0.CO;2
Villarreal, J. (2013). Ganadería diversificada: Importancia ecológica, Cinegética y económica de los venados cola blanca mexicanos. SAGARPA. Ciudad de México.
Villarreal, O., Thummler, H., Hernández, J., Franco, F. J., Campos, L. R., & Reséndiz, R. (2008). Premio Thummler: el súper slam de los venados de México. En O. Villarreal, F. Franco, J. Hernández, & S. Romero (Eds.), Conservación y manejo de fauna cinegética de México (pp. 31–48). Benemérita Universidad Autónoma de Puebla/ Fundación PRODUCE. Puebla, México: Puebla, A.C./ Mazamiztli,
A. C.
Weber, M. (2014). Temazates y venados cola blanca tropicales. In R. Valdéz, & J. A. Ortega-S. (Eds.), Ecología y manejo de fauna silvestre en México (pp. 421–452). Texcoco, México: Colegio de Posgraduados.
Weber, M., García-Marmolejo, G., & R. Reyna-Hurtado. (2006). The tragedy of the commons: wildlife management units in southeastern Mexico. Wildlife Society Bulletin, 34, 1480–1488. https://doi.org/10.2193/0091-7648(2006)34[1480:TTOTCW]2.0.CO;2
Yáñez-Arancibia, A., Day, J. W., Twilley, R. R., & Day, R. H. (2014). Manglares: ecosistema centinela frente al cambio climático, Golfo de México. Madera y Bosques, 20, 39–75.