José Antonio Gómez-Anaya a, Brenda Brug-Aguilar b, Gabriela Vázquez-Hurtado c, Rodolfo Novelo-Gutiérrez a, *
a Instituto de Ecología, A. C., Red de Biodiversidad y Sistemática, Carretera Antigua a Coatepec 351, Col. El Haya, 91073 Xalapa, Veracruz, Mexico
b Universidad Autónoma del Estado de Morelos, Facultad de Ciencias Agropecuarias, Av. Universidad No. 1001, Chamilpa, 62209 Cuernavaca, Morelos, Mexico
c Instituto de Ecología, A. C., Red de Ecología Funcional, Carretera Antigua a Coatepec 351, Congregación El Haya, 91070 Xalapa, Veracruz, Mexico
*Corresponding author: rodolfo.novelo@inecol.mx (R. Novelo-Gutiérrez)
Received: 24 October 2022; accepted: 9 May 2023
Abstract
Impacts caused to freshwater reservoirs by human activities have increased in tropical and subtropical regions in the last decades. We studied the effects of land use on the physicochemical properties of water and their effects on larval Odonata diversity in a subtropical lagoon. During 1 year, physicochemical variables were measured, and Odonata larvae were collected in 8 sites (4 in the urbane zone and 4 in the more conserved rural zone) with different land uses that cover the entire periphery of the lagoon. Physicochemically, no clustering of samples from urban and rural zones or by site was observed, rather clustering reflected temporal patterns. A total of 28 species were found and some of them showed a differential distribution between both zones, and between the 8 sites and samplings. The highest diversity was recorded in the rural zone. The site with domestic waste discharges had the lowest diversity and it showed high concentrations of nitrates and ammonium. We conclude that the effect of land use in this lagoon is still incipient on Odonata diversity. The diversion and treatment of urban waters and proper land management are recommended to ensure the maintenance of Odonata diversity.
Keywords: Dragonflies; Immature stages; Assemblages; Urban zone; Rural zone; Water quality
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Diversidad y distribución de larvas de Odonata (Insecta) en una laguna subtropical con diferentes usos de suelo en Veracruz, México
Resumen
Los daños por actividades humanas a los cuerpos de agua dulce tropicales y subtropicales se han incrementado en las últimas décadas. Aquí estudiamos los efectos del uso del suelo sobre la fisicoquímica del agua y en la diversidad de larvas de Odonata en una laguna subtropical. Durante 1 año se midieron variables fisicoquímicas y se colectaron larvas de Odonata en 8 sitios (4 en la zona urbana y 4 en la rural más conservada) con diferente uso de suelo en la periferia de la laguna. Fisicoquímicamente, no se agruparon las muestras de ambas zonas y sitios, la agrupación atendió a la temporalidad. Se colectaron 28 especies, algunas de ellas mostraron una distribución diferencial entre ambas zonas, y entre los 8 sitios y las colectas. La mayor diversidad se registró en la zona rural. El sitio con descargas domésticas fue el más pobre en diversidad y mostró mayores concentraciones de nitratos y amonio. Creemos que el efecto de los usos del suelo sobre la diversidad de odonatos en la laguna es aún incipiente. Se recomienda el desvío y tratamiento de las aguas urbanas y el uso adecuado del suelo para mantener la diversidad de Odonata.
Palabras clave: Libélulas; Estados inmaduros; Ensamblajes; Zona urbana; Zona rural; Calidad del agua
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Many tropical and subtropical freshwater ecosystems around the world are severely threatened by anthropogenic activities. The most harmful impacts are related to changes in riparian vegetation structure, habitat fragmentation, introduction of non-native organisms, dams’construction, dredging, modification of in-stream habitat, alteration of water chemistry, agricultural practices, housing, including stables for livestock, and inappropriate management (De la Torre et al., 2004; Tafangenyasha & Dube, 2008; Zedler & Kercher, 2005). The rapid increase of human activities has increased nutrient transport from land to water bodies in the past decades, resulting in environmental deterioration and changes in biogeochemical processes (Seitzinger et al., 2005). Studies assessing the effects of land use on lentic water bodies in the tropics are scarce and most available information is focused on changes in physicochemical aspects (Santos et al., 2014). In some Latin American countries, studies have been carried out on the effects of environmental deterioration on odonate diversity. In general, it is well agreed that natural and man-made disturbances do not present the same recovery patterns, being in the first case favorable after some time, but not in the second, where man-made deterioration leads to gradual habitat destruction and local disappearance of species (Tognelli et al., 2016).

Aquatic insects have been widely used as indicators for monitoring water quality due to their prompt response to a variety of perturbations and their wide distribution in aquatic habitats (Al-Shami et al., 2011; Whiles et al., 2000). Among insects, Odonata has been one of the most used groups for this purpose (Catling, 2005; Corbet, 1999; Dolný et al., 2012; Lee-Foote & Rice-Hornung, 2005; Subramanian & Ramachandra, 2008), because they are a constant component of the invertebrate aquatic fauna, and the adult and larval taxonomy is relatively well known. Many odonate species, especially those which are endo- and epiphytic ovipositors, are strongly dependent on the abundance and diversity of floating and submerged vegetation (Butler & de Maynadier, 2008; Westfall & May, 1996), suggesting they may be good bioindicators of the conservation status of aquatic vegetation structure. The diversity and abundance of both larvae and adults of Odonata can be adversely affected by abiotic factors as well as by the removal or disturbance of riparian vegetation used for perching, refuge, emergence, foraging, and reproduction (Bulánková, 1997; Corbet, 1999; Lee-Foote & Rice-Hornung, 2005). It is known that Odonata diversity patterns are closely related to watersheds, land cover, and management practices (Astudillo et al., 2016; Balzan, 2012; Hernández et al., 2006). However, how these practices affect the spatial distribution of Odonata larvae along managed riparian zones in tropical and subtropical lentic water bodies remains unknown. Most studies on the effects of land use on Odonata in the Neotropical region are focused on lotic environments (Bota-Sierra et al., 2021; De Paiva-Silva et al., 2010; García-García et al., 2017; Oliveira-Junior et al., 2015, 1917; Silva et al., 2021) and those on lentic tropical environments are scarce (Pires et al., 2022). These types of studies are of great importance because understanding the effects of certain urban stressors is crucial to properly managing the development of urban areas and conserving their biodiversity. This study reports the physicochemical properties of the water and relates changes in the structure of aquatic vegetation and diversity of odonate larval assemblages inhabiting the urban and rural zones of the Laguna Miradores del Mar based on 8 study sites, 4 located in the urban zone and 4 in the rural one (more conserved zone). These sites are subject to different types of land use that show a clear urban settlement in the urban zone and therefore a severe impact on it. The urban zone (the town of Miradores del Mar and its recreative area) is highly influenced by diverse human activities (tomato-corn crops, domestic discharges, small jetty, grazing goats) while the rural zone is better conserved (thorny forest used as paddock and thorny forest patches with some grazing). The temporal variation of richness and diversity of Odonata larvae throughout the collecting also was analyzed. We hypothesize that different land-use practices occurring on the periphery of LMM that promote unstable conditions for vegetation integrity (vegetation on which many odonate species depend for oviposition), the richness and composition of odonate assemblages should also change. Thus, the odonate larval assemblage would respond more strongly to urban conditions than the assemblage from the more preserved (rural) zone. In addition, we predict that assemblages from urban and tomato-corn cultivation sites with higher land-use intensity would respond more strongly to anthropogenic influence. In this manner, disturbed odonate assemblages should have less diversity with more taxonomically related species coexisting in them, while assemblages experiencing less disturbance should have higher diversity with less species taxonomically related. Also, we would expect to find species exclusive of one of the 2 zones. Because the lagoon is strongly insolated, we would expect that Anisoptera will dominate in both zones.
Materials and methods
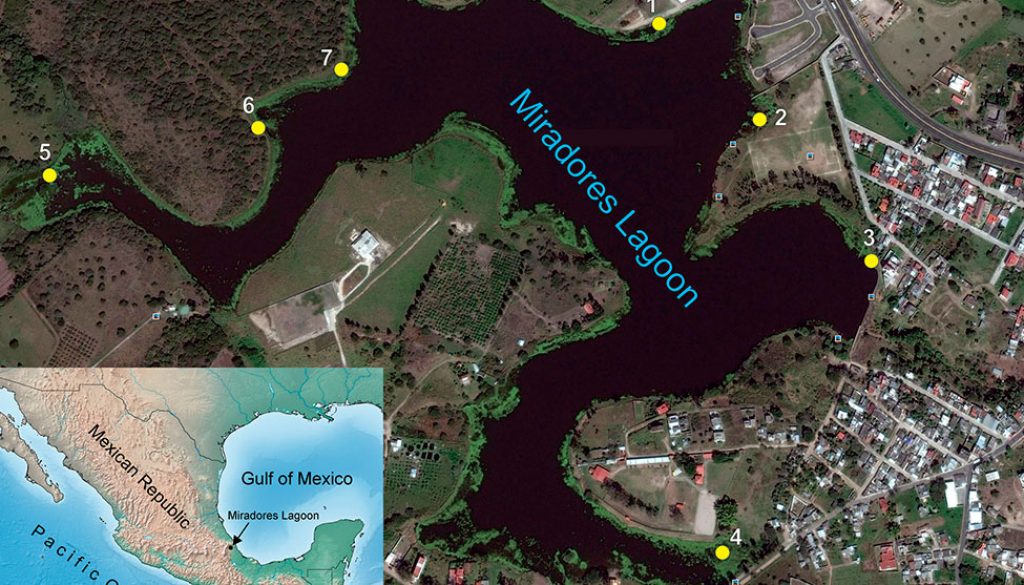
The Laguna Miradores del Mar (LMM) is located in central Veracruz in the municipality of Emiliano Zapata (19°27’53.34”-19°28’24” N, 96°47’6.3”-96°47’48.18” W), at an elevation between 850 and 900 m asl (Fig. 1).
LMM is an artificial water body supported by direct precipitation and, to a lesser extent, by the flow of a small streamlet. The climate is semi-warm with rain all year round (García, 2004). The annual average temperature is 26.5 °C and the average annual rainfall is 1,111.8 mm (Conagua, 2022). The regional climate includes 3 main seasons throughout the year: a humid-cold season (locally known as ‘nortes,’November-March), a dry season (April-June), and a rainy season (July-October) (Williams-Linera, 2007). The surrounding vegetation is made up of some sparse individuals of Casuarina L. (Casuarinaceae), lowland thorny forests, and wild grasses. Additionally, the main aquatic vegetation is composed of Nymphoides indica (Menyanthaceae) and some Pistia stratiotes (Araceae). The southeast part of LMM is more degraded by agriculture, grazing, and urbanization. Some recreational activities such as fishing are also practiced. Obregón-Barbosa (1990) cited the omnivore fishes Astyanax aeneus (Günther, 1860), Heterandria bimaculata (Heckel, 1848), Poecilia sphenops (Valenciennes, 1846), Poeciliopsis gracilis (Heckel, 1848), Cichlasoma fenestratum (Günther, 1860) and Tilapia aurea (Steindachner, 1864) from LMM.
Temperature (Temp, °C), electric conductivity (Cond, µS/cm), and dissolved oxygen (O2, mg/L) were measured in situ in 8 sites at 1 m from the water’s edge and 20-30 cm depth (coded from S1 to S8, Table 1) in 4 occasions, in May, 2008 (dry), August, 2008 (warm early rainy = rainy 1), November, 2008 (warm late rainy = rainy 2) and February, 2009 (humid-cold = cold) using a multiparametric probe (YSI 85). pH was measured with a potentiometer (Oakton, pH 11 Series). Water samples were collected with a Van Dorn-type bottle for nutrient analyses and stored at 4 °C until analysis in the laboratory. Total alkalinity (Alk) as CaCO3 (titration with phenolphthalein), ammonium (NH4+, Nessler method), nitrite (NO2–, diazotization method), nitrate (NO3–, brucine method), total phosphorus (TP, by acid persulfate digestion and the colorimetric ascorbic acid) reactive phosphorus (RP, by colorimetric ascorbic acid) and turbidity (Tur, NTU, nephelometric method) were measured following standard spectrophotometric techniques (APHA, 1998) excepting during rainy 2, and TP in the cold season. Finally, chlorophyll a (Chl-a) was measured in dry, rainy 1, and rainy 2 to classify the trophic state. Chl-a samples were filtered with Whatman GF/C filters. For Chl-a determinations, the filtered material was kept refrigerated in darkness and analyzed within the next 24 hours in the laboratory. Chl-a was extracted with 90% methanol and measured spectrophotometrically; its concentration was measured through Holden’s equations (Meeks, 1974).
Table 1
Sites, land use, and main features of the studied sites in the Laguna Miradores del Mar. Land use was assigned based on the main observed activity(s) practiced at each site.
| Sites, land use | Coordinates (N, W) | Main features |
| S1, tomato-corn crops.
Urban |
19°28’20.10”, 96°47’18.02” | Riparian vegetation composed of tall grasses. Water level and aquatic vegetation are reduced in the dry season. Rooted aquatic floating vegetation is composed mainly of Nymphoides indica and emerging grasses. Sand and clay as the main bottom substrate |
| S2, recreational area. A small jetty.
Urban |
19°’15.11”, 96°47’11.64” | No riparian vegetation at all, aquatic vegetation missing during the dry season when the water level decreases, leaving a wide beach extension. In the rainy season, only emerging grasses and scattered patches of N. indica are present |
| S3, recreational area. Grazing goats.
Urban |
19°28’10.02”, 96°47’07.19” | The aquatic vegetation and riparian grasses missing completely during the dry season. N. indica and emerging grasses are very abundant during the rainy season. A small patch of riparian Casuarina sp. trees is present. The substrate is composed of clay and rocks |
| S4, urban, domestic discharges.
Urban |
19°28’51.17”, 96°47’13.38” | It is the most disturbed and contaminated site. The level of water decreases drastically in the dry season and the vegetation disappears leaving only the clay substrate. During rains, it is covered with riparian grasses and small patches of N. indica |
| S5, conserved site used as Paddock with a small stream.
Rural |
19°28’12.79”, 96°47’47.49” | This site has the entrance of a small stream that fills the lagoon. It is used as a stable for cattle and horses. It presents a thorny forest and grasses cover the shoreline, while the aquatic vegetation is composed of abundant N. indica and emerging grasses |
| S6, Thorny Forest with some grazing.
Rural |
19°28’15.75”, 96°47’37.64” | In this site exists a remnant of thorny forest, and some grasses grow on the surface between thorny forest and shoreline that are used as forage for livestock. Scarce N. indica during rainy and cold seasons disappear in the dry season when a large beach area of clay predominates |
| S7, Thorny Forest with some grazing.
Rural |
19°28’17.98”, 96°47’33.21” | Similar to site 6 |
| S8, Thorny Forest close to El Lencero Airport. Some grazing.
Rural |
19°28’24.38”, 96°47’31.65” | The thorny forest is riparian vegetation and grasses that serve as fodder for livestock. Aquatic vegetation is formed by emerging grasses, N. indica, and Pistia stratiotes which are very abundant during the rainy and cold seasons. During the dry season, the water table is covered totally by Pistia stratiotes as the predominant substrate |
We sampled Odonata larvae in the 8 sites along the shoreline of LMM throughout different land uses (Table 1), 4 of these sites are in the southeast area (urban zone) and 4 are in the more conserved northwestern area (rural zone), and during the 4 occasions (dry, rainy 1, rainy 2, and cold). For larval sampling, a D-net (mesh 0.02mm) was used on 50m longitudinal transects on the shoreline. All Odonata larvae were preserved in the field in 96% ethanol. They were brought to the laboratory and transferred to vials containing 80% ethanol; then they were classified and quantified to species level under a stereomicroscope.
A non-parametric Kruskal-Wallis test was used to compare all physicochemical variables between the zones (urban and rural), among the 8 sites, and among the 4 samplings. Similarly, a separated Kruskal-Wallis test was used to compare Chl-a between zones, sites, and samplings using Statistica 5.0 (Statistica, 2006). To identify physicochemical patterns a Principal Component Analysis (PCA) was carried out using the CANOCO program 4.5 (ter Braak & Smilauer, 2002). This analysis was performed on centered and standardized physicochemical data and the correlation matrix was used. The number of species and composition, Shannon´s diversity index (H´), and Pielou´s equitability (J) were used to describe and compare the zones, sites, and samplings. Both diversity indexes were calculated and tested pairwise using the permutation and bootstrapping tests implemented in the PAST program (Hammer et al., 2001). Similarly, a Kruskal-Wallis test was used to compare larval Odonata abundance between zones, sites, and samplings. Species were divided into 2 groups for functional diversity (FD) analysis: those dependent on vascular hydrophytes as substrate for oviposition (endophitic and ectophitic species) and those whose larvae are strongly associated to the roots (P), and those not dependent (exophitic species) (NP) (Corbet, 1999). Then, using the P/NP ratios, the FD was calculated using Shannon´s diversity index according to Campbell et al. (2010). In this manner, FD varies according to the ratios of plant-dependent and non-dependent species so that, when both proportions are equal the FD is maximum, and when either one of these 2 categories dominates, the FD decreases. FD in the broadest sense includes a variety of functional traits that describe an assemblage of organisms, and the index (calculated across all trait-states within a functional trait) has been better linked to the maintenance of ecosystem performance than by using species numbers alone. We examined distributions of odonate FD based on riparian and aquatic plant dependence or non-dependence for species’life cycles relative to oviposition life habits. P/NP ratios were calculated in Excel and FD was calculated in the PAST program (Hammer et al., 2001) for zones and sites. To compare species composition between zones, sites, and samplings a two-way hierarchical clustering analysis (CA) based on a Bray-Curtis similarity matrix and the unweighted pair group average (UPGMA) grouping method was performed using PC ORD software (McCune & Mefford, 2011). The singletons and doubletons species were excluded in this analysis so that they did not prevent the detection of significant ecological gradients in the assemblage structure. Finally, to elucidate the relationships between physicochemical variables and Odonata species abundance, a Canonical Correspondence Analysis (CCA) using the CANOCO program 4.5 (ter Braak & Smilauer, 2002) was performed. Species abundance was log10 (x+1) transformed and rare species were unweighted. Nine physicochemical variables and twenty species were included in the CCA. The variables with a p < 0.05 of the Monte Carlo permutation test and variance inflation factors (VIF) values lower than 20 were retained.
Results
Physicochemical variables
Zones. There was no significant effect of the zones on temperature, conductivity, and oxygen (p > 0.05, Table 2a), except for pH, which was different between urban (pH = 7.44) and rural zones (pH = 7.09) (H1,64 = 5.27, p = 0.02). Regarding other physicochemical variables, the Kruskal-Wallis test showed a significant difference only in ammonium (H1,80 = 4.65, p = 0.03) and nitrites (H1,80 = 9.15, p = 0.002, Table 2a) between urban and rural zones, with a greater concentration of both in the urban zone. There was no significant difference in Chl-a (H1,38 = 0.41, p = 0.52) between the urban (mean = 28.19 mg/L) and the rural zones (mean = 21.21 mg/L). However, our study revealed that LMM changes its trophic status from mesotrophic in the rainy season (Chl-a= 16.43 mg/L) to eutrophic in the dry season (47.37 mg/L).
Sites. Physicochemical comparisons among sites showed alkalinity was significantly higher in site 1 (tomato-corn crops, Table 2b) when compared to site 3 (recreational area with grazing goats). Alkalinity was the only physicochemical variable that was significantly different among sites.
Samplings. Water temperature and conductivity showed a similar pattern throughout the samplings. Both variables were significantly higher in dry and rainy 1 than in rainy 2 and cold samplings (Table 2c). Oxygen was low in all samplings (< 5 mg/L), but significantly lower concentrations were recorded in rainy 1 and rainy 2 than in the dry and cold. The remaining comparisons of oxygen were not significantly different (p > 0.05). The lowest pH was recorded in rainy 1, being different from rainy 2 and cold (p < 0.05). Alkalinity was higher in dry collecting and statistically different when compared to rainy 1 and cold. Ammonium and nitrite concentrations were significantly high in dry, while in rainy 1 and cold were lower and similar. Nitrate and turbidity were highest in dry and lowest in rainy 1 and cold; however, the 3 samplings were statistically different. Reactive phosphorus was higher and statistically equal in rainy 1 and cold. Total phosphorus was higher in rainy 1 and statistically different to dry.
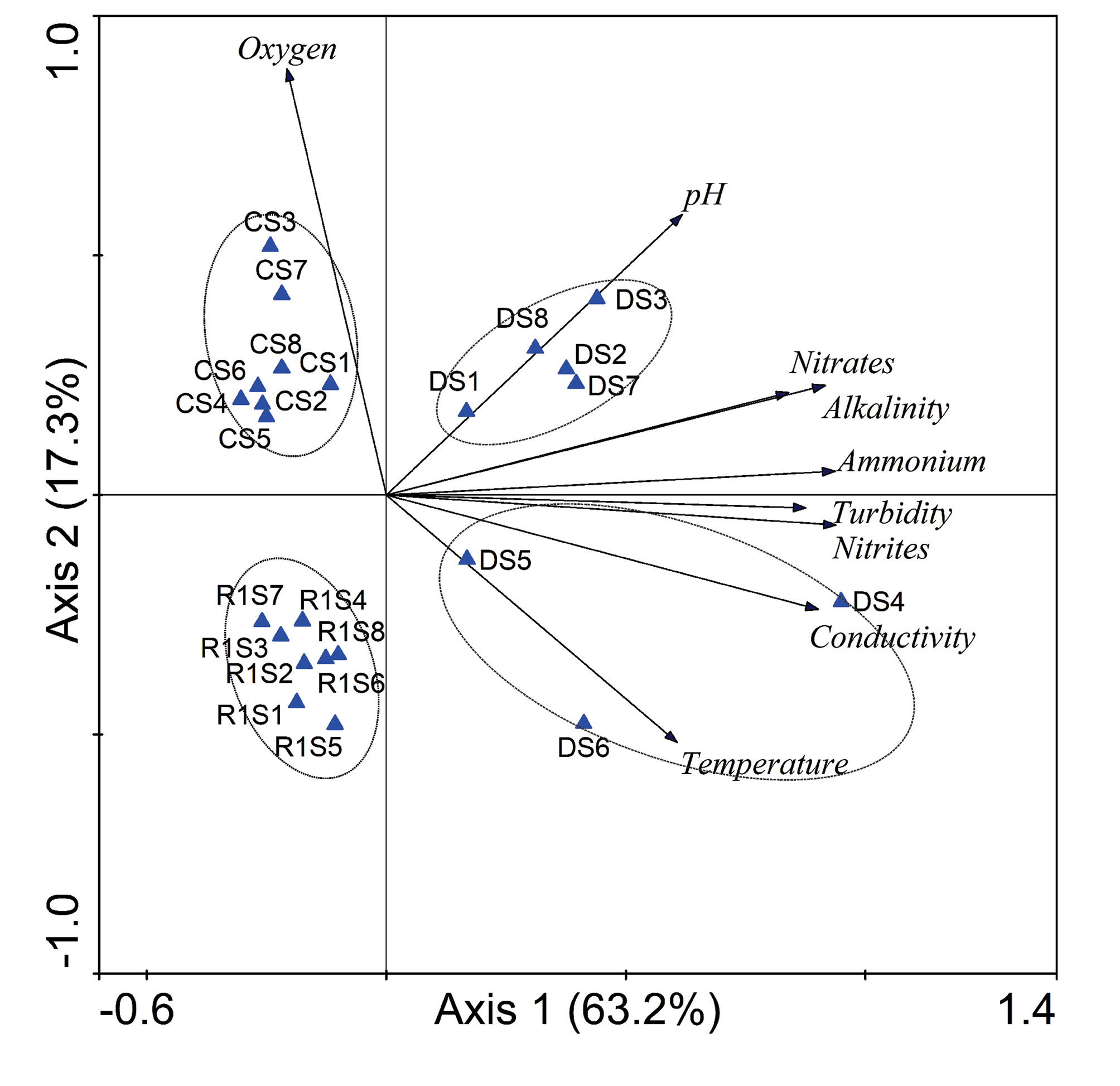
The first 2 PCA axes explained 80.5% of the total variation in the data (Fig. 2). The first axis explained 63.2% and the second explained 17.3%. On axis 1, the most important variables were nutrients (ammonium, nitrates, and nitrites), turbidity, alkalinity, and conductivity, while on axis 2 the most important variables were oxygen, temperature, and pH. According to the PCA, most samples of the dry collecting were defined by pH and temperature as shown on the right side of the PCA biplot in Figure 2. In particular, the samples DS4, DS5, and DS6 were defined by their higher conductivity and temperature while samples DS1, DS2, DS3, DS7, and DS8 were defined by higher pH. On the left side of the biplot, the rainy 1 (R1, down) and cold (C, above) samples were separated by oxygen (higher in cold and lower in rainy 1). There was no clear clustering of samples from urban and rural zones or the sites, rather the clustering of groups mainly responded to seasonality (see dry samples on the right side of the biplot).
Odonata diversity. Zones. A total of 929 larvae belonging to 4 families, 19 genera, and 28 species were collected (Table 3). In the urban zone, 413 larvae belonging to 4 families, 18 genera (7 Zygoptera and 11 Anisoptera), and 22 species were collected, while in the rural zone, 516 larvae were collected from 4 families, 18 genera (6 Zygoptera and 12 Anisoptera, and 24 species. Differences among zones were found at species and genera levels. Six species were present only in the urban zone: Ischnura capreolus, I. hastata, Leptobasis vacillans, Coryphaeschna adnexa, C. viriditas, and Pantala flavescens, while 4 species were only present in the rural zone: Nehalennia minuta, Neoerytrhomma cultellatum, Pantala hymenaea, and Perithemis domitia. Of these 4 species, it is worth mentioning that N. minuta was only present with a single specimen from site 1 (tomato-corn crop) collected in rainy 1. Enallagma novaehispaniae was noticeably more abundant in the rural zone and exclusively collected in rainy 1 and rainy 2 from sites 6 and 7. Telebasis salva was more abundant in the rural zone and exclusively collected in sites 1 and 8 during rainy 1 and rainy 2. There was no significant effect of the zone on larval abundance according to the Kruskal-Wallis test (H1, 80 = 0.04, p = 0.82) being the means very similar (urban =11.47 individuals/sample, rural = 11.72). However, the abundance of some species was significantly higher in the rural zone, as was the case of Acanthagrion quadratum (urban = 92 specimens, rural = 161), Enallagma novaehispaniae (urban = 2, rural = 65), Aphylla sp. (urban = 3, rural = 13), and Leptobasis vacillans (urban = 0, rural = 14), while Ischnura ramburi was noticeably more abundant in the urban zone (116) than in the rural zone (61), similarly, Enallagma praevarum was also more abundant in the urban zone (61) than in rural one (45). There was a significant difference in Shannon´s diversity between urban (H´ = 2.02) and rural (H´ = 2.37) zones according to the bootstrapping (p = 0.001) and permutation (p = 0.001) tests. The same difference was observed in Pielou´s equitability, which was higher in the rural zone (J = 0.755) than in the urban zone (J = 0.65) according to the same statistical tests. These results suggest a greater diversity of odonates in the rural zone and a slightly more homogeneous establishment in terms of the distribution of the larvae in the periphery of the LMM. There was no difference in the P/NP ratio between both zones according to the Kruskal-Wallis test (H1,8 = 0.083, p = 0.77). The FD was 1.15 for the urban zone and 1.07 for the rural zone. There was no statistical difference in FD between both zones according to the permutation (p = 0.65) and bootstrapping (p = 0.71) tests.
Sites. The number of families was almost constant, 3 or 4, in all the sites (Table 3). However, the number of genera and species was highest in sites 1 (tomato-corn crops) and 5 (thorny forest used as paddock) and lowest in sites 4 (domestic discharges) and 7 (thorny forest with some grazing), although the specific composition of Gomphidae and Aeshnidae varied among sites. The only species of Gomphidae, Aphylla sp., was recorded in sites 2, 3, 5, 6, and 7, while the aeshnids were recorded in sites 1, 2, 4, 5, and 8, although not the same species. The 4 species of Aeshnidae were recorded in site 5 and only 1 species in sites 1, 2, 4, and 8. Among Coenagrionidae, Argia was recorded in sites 1, 5, 6, and 7, while Nehalennia was only recorded in site 1. In Libellulidae, Perithemis was only recorded in site 1, while Pantala was only recorded in sites 2 and 6. The Aeshnidae and Gomphidae appear to have a wide distribution in the periphery of LM. Of the 929 larvae collected in total, 19% were collected in site 5, with most of the specimens being Acanthagrion quadratum, followed by site 1 (18%) with the majority being A. quadratum and Ischnura ramburii, and site 7 (15%) being most of the specimens Enallagma novaehispaniae. A. quadratum was the dominant species in site 6 (11%). In site 4 (10%) the dominant species were A. quadratum and I. ramburii. In site 8 (10%) were A. quadratum and I. denticollis. In site 2 (9.3%) the dominant species were I. ramburii followed by I. denticollis and E. praevarum. Site 3 had the lowest relative abundance (7.4%) being most of the specimens E. praevarum and I. ramburii. Sites with statistically high diversity were 1, 5, and 6 (H´ varying from 2.06 to 2.13) while sites 2, 3, 4, 7, and 8 had lower diversity (H´ varying from 1.59 to 1.86) (Fig. 3a). In site 4 (urban), we registered the lowest diversity of all sites. The site 6 had the highest equitability (Fig. 3b) and was statistically different from sites 2, 4, and 5, which showed the lowest equitability. There was no significant difference in abundance among the sites (H7, 80 = 6.26, p = 0.51) (Fig. 4a). FD was higher in sites 2, 3, 6, and 7 where similar proportions of P/NP species were recorded (Fig. 5a). In contrast, FD was lower in sites 1, 4, 5, and site 8 where higher proportions of P (plant-dependent) species were recorded. Particularly sites 4 and 8 had the highest proportions of plant-dependent species. In site 4 (urban), 9 out of the 10 registered species were plant-dependent, 7 were strictly endophytic (6 Zygoptera and Remartinia secreta), 1 ectophitic (Micrathyria aequalis), and 1 exophytic (Erythrodiplax fusca). In site 8, 11 out of the 12 species were strictly endophytic (7 Zygoptera and Coryphaeschna adnexa), 1 ectophitic (Micrathyria aequalis), and 2 exophytic (E. fusca and Miathyria marcella).
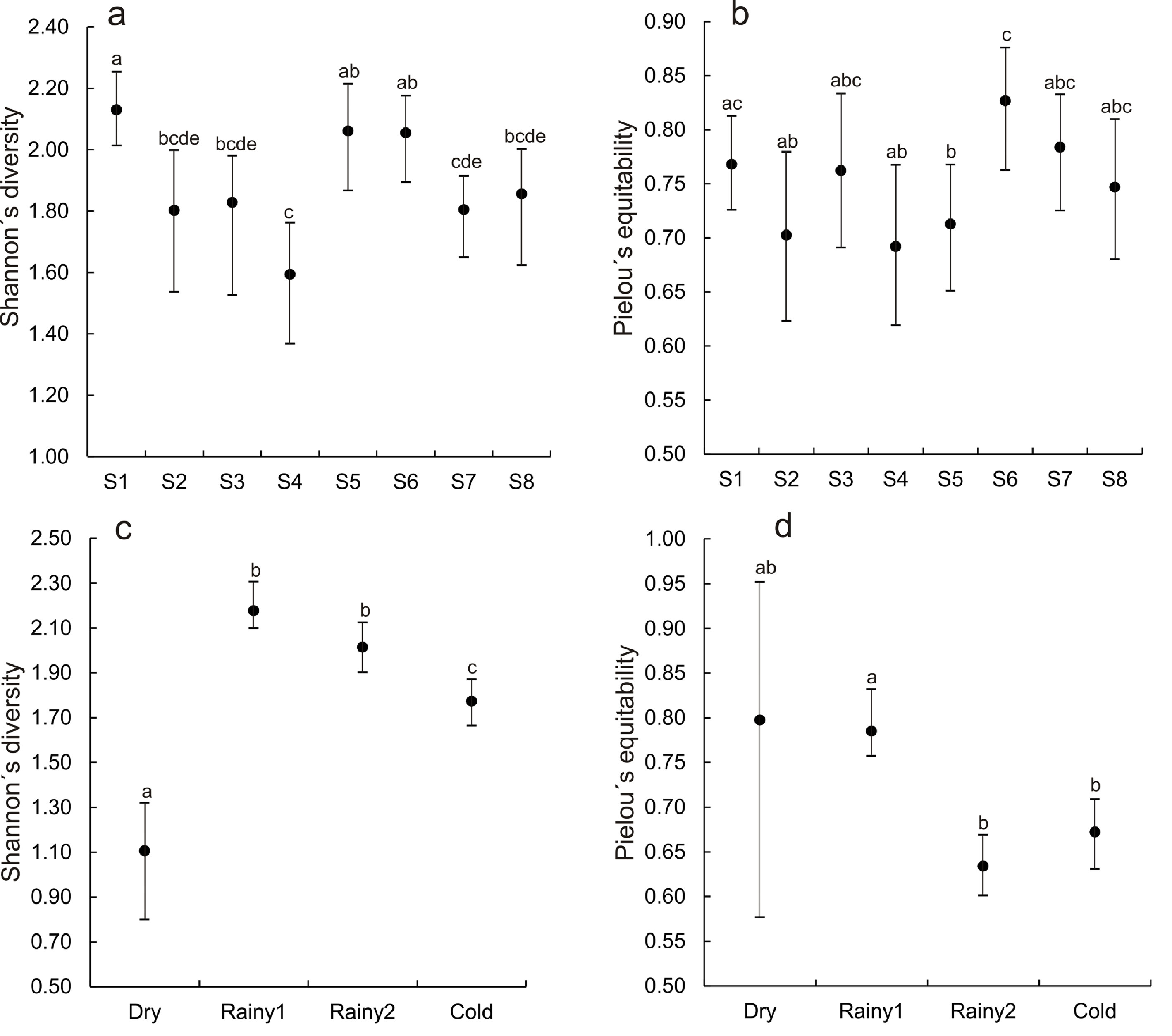
Samplings. More species were recorded in warm late rainy (rainy 2, 24 species) and only 4 in the dry collecting (Table 3). The number of families was 4 in rainy 2 and cold samplings, while 3 families were recorded in dry and rainy 1 samplings. The number of genera was higher in rainy 2 (17) and lower in dry (4). The diversity of the dry collecting was statistically different from the others (Fig. 3c). Rainy1 and rainy 2 samplings had higher diversity and were statistically equal, but different from the cold one. There was no difference in equitability between the dry and the other 3 samplings (Fig. 3d); however, there was a difference between rainy 1 and rainy 2. Temporarily, relative abundance was highest in rainy 2 (43.7%) followed by cold (36.3%) and rainy 1 (17.9%) and it was notably lower in dry (2.2%). Dominant species in rainy 2 were A. quadratum, in cold A. quadratum and I. denticollis, in rainy 1 A. quadratum and Perithemis tenera (with equal relative abundance), and finally in dry Aphylla sp dominates. There was a significant effect of collecting on larval abundance (H3,80 = 25.99, p = 0.00) (Fig. 4b). Larval abundance was higher in rainy 2 and cold, being these 2 samplings equal statistically. In the same way, there was no difference between rainy 1 and dry. The remaining pairwise comparisons were different statistically. Seasonally, FD was higher in dry and lower in cold, and equal between rainy 1 and rainy 2 (Fig. 5b). The results in FD showed an almost equal number of plant-dependent and not plant-dependent odonate species in dry, a higher number of plant-dependent species in cold, while in rainy 1 and rainy 2 almost 60% of species were plant-dependent. In Figure 5b, samplings have been ordered seasonally from left to right, so there is an evident decrease in FD throughout the time, from dry to cold. This means a temporal increase in the number of plant-dependent species from dry to cold.

Table 2
Average values of physicochemical variables (± SE) in zones (urban and rural), the 8 sites and samplings (a, b, c, respectively)
from the Laguna Miradores del Mar, Veracruz.
| Temp | Cond | O2 | pH | Alk | NH+4 | NO–2 | NO–3 | Tur | PR | PT | |
| °C | (µS/cm) | (mg/l) | (mg/l) | (mg/l) | (mg/l) | (mg/l) | NTU | (mg/l) | (mg/l) | ||
| a | |||||||||||
| H | 0.76 NS | 0.01 NS | 0.09 NS | 5.28* | 0.14 NS | 4.65* | 9.15* | 0.23 NS | 0.80 NS | 0.09 NS | 0.73 NS |
| Urban | 24.51 | 119.05 | 3.77 | 7.44 | 46.18 | 0.48 | 0.01 | 0.87 | 56.03 | 0.07 | 0.13 |
| -1.02 | -4.79 | -0.26 | -0.1 | -0.735 | -0.042 | -0.002 | -0.247 | -13.127 | -0.022 | -0.016 | |
| Rural | 26.02 | 114.24 | 3.61 | 7.09 | 46.15 | 0.47 | 0.01 | 0.64 | 54.32 | 0.03 | 0.17 |
| -0.98 | -5.78 | -0.32 | -0.07 | -0.567 | -0.024 | -0.001 | -0.143 | -10.322 | -0.003 | -0.024 | |
| b | |||||||||||
| H | 2.84 NS | 1.35 NS | 9.60 NS | 14.61 NS | 12.71* | 11.71 NS | 12.98 NS | 1.01 NS | 5.30 NS | 5.54 NS | 3.54 NS |
| Site 1 | 25.01 | 114.27 | 3.61 | 7.11 | 48.78a | 0.52 | 0.008 | 0.47 | 60.69 | 0.06 | 0.17 |
| -1.58 | -6.28 | -0.52 | -0.22 | -0.99 | -0.06 | -0.002 | -0.19 | -17.82 | -0.03 | -0.03 | |
| Site 2 | 24.99 | 117.6 | 3.83 | 7.51 | 45.70ab | 0.46 | 0.01 | 0.85 | 37.87 | 0.03 | 0.14 |
| -1.64 | -7.03 | -0.34 | -0.24 | -1.29 | -0.08 | -0.003 | -0.45 | -9.05 | -0.01 | -0.04 | |
| Site 3 | 25.7 | 120.65 | 4.65 | 7.65 | 44.34b | 0.44 | 0.006 | 1.24 | 33.94 | 0.03 | 0.12 |
| -1.65 | -8.65 | -0.37 | -0.18 | -1.52 | -0.07 | -0.002 | -0.72 | -10.4 | -0.01 | -0.03 | |
| Site 4 | 24.96 | 123.69 | 3.15 | 7.49 | 45.88ab | 0.5 | 0.014 | 0.96 | 91.63 | 0.15 | 0.08 |
| -1.81 | -15.35 | -0.64 | -0.17 | -1.79 | -0.12 | -0.006 | -0.55 | -47.37 | -0.08 | -0.01 | |
| Site 5 | 25.36 | 113.66 | 3.15 | 6.78 | 45.92ab | 0.51 | 0.009 | 0.65 | 27.44 | 0.03 | 0.21 |
| -1.68 | -3.9 | -0.64 | -0.09 | -1.57 | -0.04 | -0.002 | -0.29 | -5.64 | -0.01 | -0.06 | |
| Site 6 | 27.1 | 120.22 | 2.94 | 7.05 | 46.25ab | 0.44 | 0.009 | 0.57 | 49.86 | 0.03 | 0.14 |
| -2.17 | -12.55 | -0.55 | -0.09 | -1.14 | -0.05 | -0.003 | -0.22 | -29.57 | -0.01 | -0.04 | |
| Site 7 | 26.06 | 113.81 | 4.64 | 7.28 | 46.18ab | 0.45 | 0.008 | 0.74 | 76.37 | 0.03 | 0.18 |
| -2.07 | -12.11 | -0.61 | -0.19 | -0.81 | -0.06 | -0.002 | -0.38 | -24.09 | -0.01 | -0.05 | |
| Site 8 | 25.56 | 121.36 | 3.71 | 7.25 | 46.24ab | 0.49 | 0.009 | 0.59 | 63.06 | 0.03 | 0.15 |
| -2.21 | -12.46 | -0.68 | -0.15 | -1.05 | -0.04 | -0.001 | -0.3 | -17.74 | -0.01 | -0.04 | |
| c | |||||||||||
| H | 47.34* | 45.33* | 22.79* | 22.81* | 47.47* | 33.97* | 38.61* | 47.54* | 40.54* | 44.45* | 29.19* |
| Dry | 31.14a | 153.79a | 3.75ac | 7.68a | 52.62a | 0.82a | 0.023a | 2.34a | 134a | 0.04a | 0.05a |
| -0.65 | -5.62 | -0.55 | -0.15 | -0.56 | -0.05 | -0.003 | -0.27 | -22.76 | -0.02 | -0.02 | |
| Rainy 1 | 29.28a | 121.41a | 2.42b | 6.82b | 43.25b | 0.39b | 0.006b | 0.06b | 18.25b | 0.05b | 0.20b |
| -0.23 | -3.33 | -0.25 | -0.07 | -0.35 | -0.02 | -0.001 | -0.01 | -4.47 | -0.001 | -0.08 | |
| Rainy 2 | 20.83b | 98.11b | 3.64ab | 7.45a | ND | ND | ND | ND | ND | ND | ND |
| -0.17 | -2.41 | -0.26 | -0.1 | ND | ND | ND | ND | ND | ND | ND | |
| Cold | 20.86b | 97.86b | 4.96c | 7.10ab | 45.84c | 0.39b | 0.005b | 0.22c | 36.72c | 0.06b | ND |
| -0.33 | -3.14 | -0.19 | -0.09 | -0.45 | -0.02 | -0.001 | -0.01 | -5.56 | -0.03 | ND |
H, Kruskal-Wallis values; *, p < 0.05; NS, not significant; ND, not available data. The averages that share at least one letter are statistically equal.
The two-way hierarchical clustering analysis (CA) showed the formation of 6 groups more influenced by aspects of temporality than by land use (Fig. 6). Most samples of rainy 1 and rainy 2 samplings formed a group (G1+G2+G3); samples of the cold season formed G4 group and dry samples were included in G5 and G6 groups. The samples from the urban and rural zones were not separated by the cluster analysis, except for G2, which was formed only by samples from the urban zone, and G6 only formed by 2 samples from the dry season of the rural zone. The CA of sampling-sites indicated that Odonata larval assemblages could be divided into 6 groups (Fig. 6). The first group (group 1) was formed by the samples R1S1 (rainy 1 site 1) and R1S7 which shared Enallagma praevarum and Ischnura ramburii in low abundance and Perithemis tenera with higher abundance in R1S1. Group 2 was formed by samples of rainy 1 and rainy 2 sharing moderate abundance of E. praevarum and I. ramburii. Group 3 was also formed by samples of rainy 1 and rainy 2 but share high abundance of Acanthagrion quadratum and moderate to high abundance of E. praevarum, I. ramburii, and Micrathyria aequalis; Remartinia secreta was only present in this group. Group 4 was formed almost completely by samples of the cold season sharing the higher abundance of I. denticollis and moderate to low abundance of A. quadraturm and I. ramburii. Group 5 was a heterogeneous group formed by 2 samples of rainy 1, 1 dry and 1 cold, all with very few species and abundance. Finally, group 6 was formed by 2 samples of the dry season sharing Aphylla sp.
Table 3
Distribution of richness and composition of Odonata larval species in the zones, the 8 sites, and samplings in the Laguna Miradores del Mar. P = Plant-dependent species, NP = not plant-dependent species, U = urban, R = rural, R1 = early rainy, R2 = late rainy, D = dry and C = cold rainy.
| U | R | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | D | R1 | R2 | C | ||
| Number of individuals | 413 | 516 | 165 | 86 | 69 | 93 | 176 | 104 | 140 | 96 | 20 | 166 | 406 | 337 | |
| Number of families | 4 | 4 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | |
| Number of genera | 18 | 18 | 13 | 12 | 10 | 8 | 13 | 10 | 8 | 10 | 4 | 14 | 17 | 12 | |
| Number of species (S) | 22 | 24 | 16 | 13 | 11 | 10 | 18 | 12 | 10 | 12 | 4 | 16 | 24 | 14 | |
| Shannon diversity index (H’) | 2.06 | 2.29 | 2.1 | 1.8 | 1.8 | 1.6 | 2.1 | 2.1 | 1.8 | 1.9 | 1.11 | 2.18 | 2.02 | 1.77 | |
| Pielou equitavility (J) | 0.67 | 0.68 | 0.8 | 0.7 | 0.8 | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | 0.79 | 0.78 | 0.63 | 0.67 | |
| Functional diversity (FD) | 1.15 | 1.07 | 0.4 | 0.7 | 0.7 | 0.3 | 0.4 | 0.7 | 0.7 | 0.3 | 0.69 | 0.56 | 0.56 | 0.41 | |
| Zygoptera | |||||||||||||||
| Coenagrionidae | |||||||||||||||
| Acanthagrion quadratum P | Acaqua | 92 | 161 | 39 | 4 | 10 | 39 | 76 | 31 | 23 | 31 | 3 | 32 | 137 | 81 |
| Argia pulla P | Argpul | 2 | 6 | 2 | – | – | – | 3 | 2 | 1 | – | 3 | – | 5 | – |
| Enallagma novaehispaniae P | Enanov | 2 | 65 | 1 | – | – | 1 | – | 14 | 51 | – | – | 22 | 45 | – |
| Enallagma praevarum P | Enapra | 61 | 45 | 19 | 13 | 21 | 8 | 21 | 1 | 19 | 4 | – | 27 | 50 | 29 |
| Ischnura capreolus P | Isccap | – | 1 | – | – | – | – | 1 | – | – | – | – | – | 1 | – |
| Ischnura denticollis P | Iscden | 62 | 63 | 30 | 15 | 7 | 10 | 9 | 11 | 18 | 25 | – | 3 | 3 | 119 |
| Ischnura hastata P | Ischas | – | 2 | – | – | – | – | 2 | – | – | – | – | – | 2 | – |
| Ischnura ramburii P | Iscram | 116 | 61 | 33 | 37 | 20 | 26 | 13 | 17 | 16 | 15 | – | 20 | 96 | 61 |
| Leptobasis vacillans P | Lepvac | – | 14 | 0 | – | – | – | 10 | – | – | 4 | – | – | – | 14 |
| Nehalennia minuta P | Nahmin | 1 | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – |
| Neoerythromma cultellatum P | Neocul | 6 | – | 4 | – | 1 | 1 | – | – | – | – | – | – | 2 | 4 |
| Telebasis digiticollis P | Teldig | 1 | 5 | 1 | – | – | – | 4 | – | – | 1 | – | – | 2 | 4 |
| Telebasis salva P | Telsal | 2 | 10 | 2 | – | – | – | 2 | – | – | 8 | – | 1 | 3 | 8 |
| Anisoptera | |||||||||||||||
| Aeshnidae | |||||||||||||||
| Anax amazili P | Anaama | 1 | 7 | – | 1 | 0 | 0 | 7 | 0 | 0 | 0 | – | – | 7 | 1 |
| Coryphaeschna adnexa P | Coradn | – | 2 | – | – | – | – | 1 | 0 | 0 | 1 | – | 1 | 1 | – |
| Coryphaeschna viriditas P | Corvir | 9 | 1 | – | – | – | – | 1 | 0 | 0 | 0 | – | – | 1 | – |
| Remartinia secreta P | Remsec | – | 5 | 7 | – | – | 2 | 5 | 0 | 0 | 0 | – | 1 | 13 | – |
| Gomphidae | |||||||||||||||
| Aphylla sp. NP | Aphsp | 3 | 13 | – | 1 | 2 | – | 2 | 9 | 2 | – | 12 | – | 2 | 2 |
| Libellulidae | |||||||||||||||
| Erythrodiplax fusca P | Eryfus | 3 | 1 | – | 2 | – | 1 | – | – | – | 1 | – | 3 | – | 1 |
| Macrothemis inacuta NP | Macina | 6 | 10 | – | 4 | 2 | – | – | 5 | 5 | 0 | – | 9 | 5 | 2 |
| Miathyria marcella P | Miamar | 1 | 1 | 1 | – | – | – | – | – | – | 1 | – | 1 | – | 1 |
| Micrathyria aequalis P | Micaeq | 14 | 19 | 9 | 1 | 1 | 3 | 15 | 1 | – | 3 | – | 4 | 19 | 10 |
| Table 3. Continued | |||||||||||||||
| U | R | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | D | R1 | R2 | C | ||
| Orthemis ferruginea NP | Ortfer | 3 | 8 | – | 1 | 2 | – | 2 | 4 | 2 | – | 2 | 8 | 1 | – |
| Pantala flavescens NP | Panfla | – | 1 | – | – | – | – | – | 1 | – | – | – | – | 1 | – |
| Pantala hymenaea NP | Panhym | 1 | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – |
| Perithemis domitia NP | Perdom | 3 | – | 3 | – | – | – | – | – | – | – | – | – | 3 | – |
| Perithemis tenera NP | Perten | 21 | 13 | 12 | 5 | 2 | 2 | – | 8 | 3 | 2 | – | 32 | 2 | – |
| Planiplax sanguiniventris NP | Plasan | 3 | 2 | 1 | 1 | 1 | – | 2 | – | – | – | – | 1 | 4 | – |

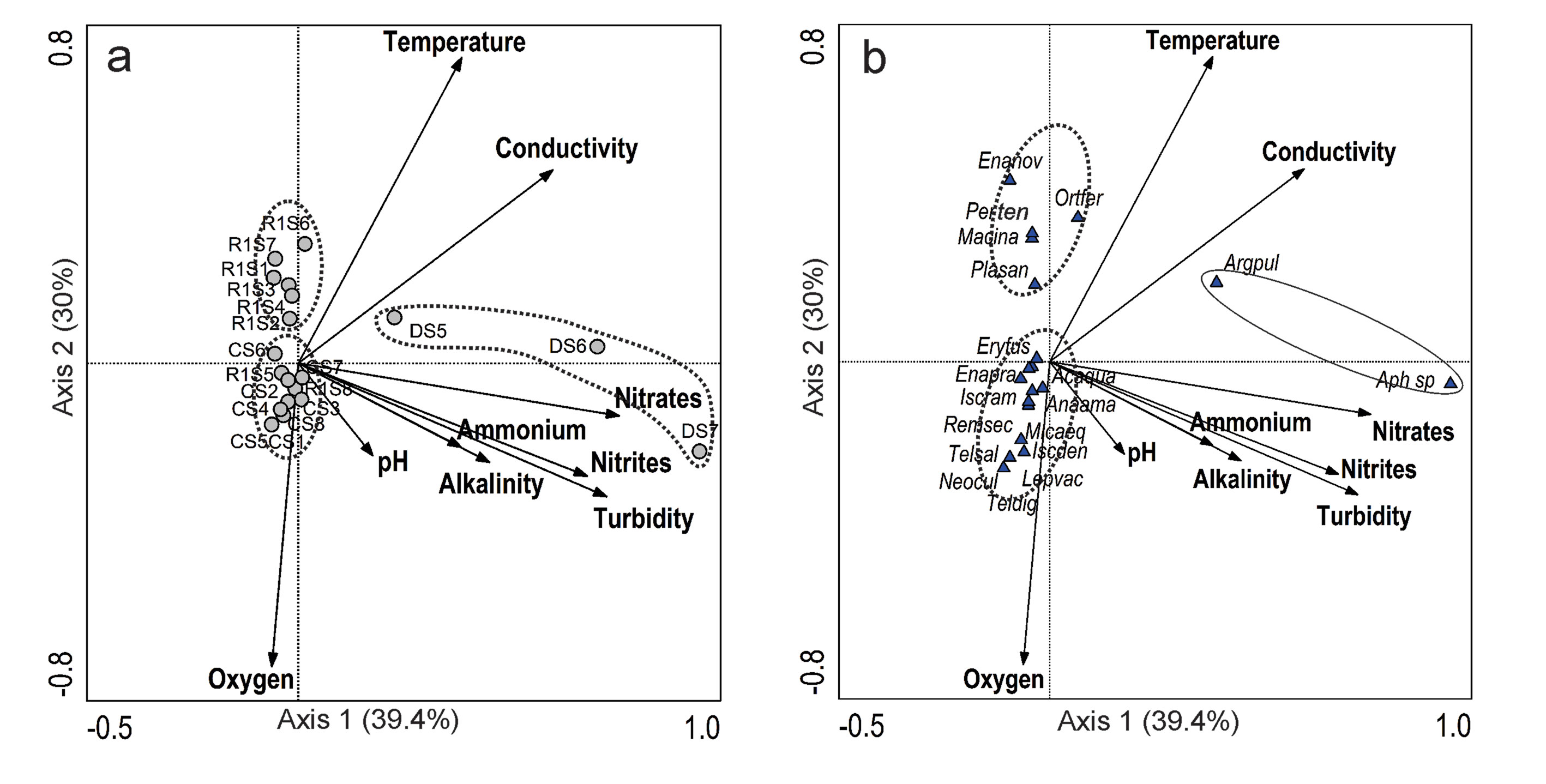
CCA analysis summarizes the main trends in the relationship between Odonata and environmental conditions (Fig. 7). The CCA showed that the first axis was significant (Monte Carlo test with 499 permutations, eigenvalue = 0.672, F = 3.48, p = 0.002). The first 2 axes explained 69.4% of the total variation in the dataset. Axis 1 explained 39.4% and axis 2 explained 30%. Samples of the dry season were well characterized by a higher content of nutrients, turbidity, conductivity, and alkalinity (Fig. 7a), and the species Aphyla sp. and Argia pulla appear to be associated with the spatial and temporal conditions of the dry season (Fig. 7b). Particularly Aphylla sp. was more abundant during the dry season. Similarly, almost all the samples from the cold season were well characterized by a higher oxygen content, and the set of species I. denticollis, I. ramburii, E. praevarum, A. quadratum, L. vacillans, and M. aequalis were well associated with the conditions of this season. Samples of the early rainy season (rainy 1) were characterized by lower oxygen, pH, turbidity, ammonium, and nitrates, and species associated with this season were mainly the libelulids P. tenera, O. ferruginea, Macrothemis inacuta, Planiplax sanguiniventris, and the zygopteran Enallagma novaehispaniae.

Discussion
In LMM, there were no significant physicochemical changes associated with the surrounding land use; however, there were some significant changes throughout the year. The data obtained from these variables agree well with the concentrations of Chl-a recorded in LMM since it was notably higher during the dry season and lower during both rainy season samplings, which shows remarkable changes from a eutrophic condition during the dry season to a mesotrophic during the rainy one. In LMM, a significant change from mesotrophic (annual maximum chlorophyll ≥ 8 < 25 mg/L) to eutrophic (≥ 25 < 75 mg/L) state could suppose a temporary phenomenon of dilution-concentration of nutrients throughout the year similar to that reported in Korean wetlands (Mamun et al., 2018). According to the classification of the lakes by the Environmental Protection Agency (EPA), the fluctuation in the levels of Chl-a recorded in LMM showed a probable low contamination in the mesotrophic state to a probable strong contamination in the eutrophic state (Toner et al., 2005), probably caused in part by the empiric observation of a gradual increase in anthropogenic occupation and its different land practices exercised in its periphery (RN pers. obs). Furthermore, the higher amount of total phosphorus in rainy 1 compared to dry in LMM also classifies the system from mesotrophic to eutrophic according to Brylinsky (2004). Our results suggest that eutrophication could be an important aspect that has caused the alteration of the natural environment in LMM. This process (eutrophication) that produces environmental degradation can be induced by the different activities performed around LMM (human influence exists on the southeast side of LMM, here called the urban zone, Fig. 1) or by agents of the same nature as climatic condition (Pathak & Pathak, 2012). In LMM, factors that produce eutrophication could come from solid and liquid wastes produced mostly on the southeast side (sites 1, 2, 3, and 4), associated with human settlements that discharge waste directly into the lagoon or by using fertilizers and their runoff to the water (Johannsen & Armitage, 2010).
The Odonata larval assemblage from LMM is composed mainly of a wide-ranging and common species, most of them belonging to neotropical Libellulidae and Coenagrionidae. The number of species in the larval stage reported in this work (28 species) is higher than those from sewage ponds from eastern Ontario (12 larvae species, 4 Zygoptera, and 8 Anisoptera) (Catling, 2005); from ponds of natural reserves in Central Italy (16 larvae species, 7 Zygoptera, and 9 Anisoptera) (Carchini et al., 2007); from Ciénega de San Juan Tocagua (Colombia) (14 larvae species, 4 Zygoptera, and 10 Anisoptera) (Altamiranda et al., 2010); from boreal ponds in Central Finland (21 larvae species, 8 Zygoptera, and 13 Anisoptera ) (Honkanen et al., 2011); and from impacted reservoirs, drainage ditches, tailrace canals, and old river channels from Drava Rivers, Croatia (12 species, 8 Zygoptera, and 4 Anisoptera) (Vilenica & Mihaljevic, 2022). Almost in all these works is notable the biased disproportion to the number of Anisoptera species. In LMM this proportion is almost equal (13 Zygoptera and 15 Anisoptera), which indicates that despite the moderate level of impact in LMM occurring mainly on the southeast side (urban zone) of the lagoon, the whole Odonata assemblage is still highly diverse and, in general, the aquatic system must provide good conditions for Odonata. The latter is according to Oliveira and Juen (2019) who proposed using the proportion of Zygoptera and Anisoptera as an indicator of alteration in the following way: Zygoptera diversity decreased with the loss of habitat integrity, whereas Anisoptera diversity increased with habitat disturbance. This is the result of finding almost half of the Odonata species recorded in LMM using a variety of available aquatic plants to lay their eggs within the stems and leaves depending on the diversity and abundance of these substrates. Plant-dependent odonate species are a cohesive functional group requiring aquatic vegetation to complete their life cycle (Guillermo-Ferreira & Del-Claro, 2011; Verdonschot & Peeters, 2012). Differences in the number of Odonata species that are highly dependent on the availability of aquatic vegetation have led some authors to suggest that the damselflies assemblage could be used itself as a good indicator of the state of conservation of plant freshwater system integrity because they are obligate endophytic ovipositors (Butler & de Maynadier, 2008). In LMM we recorded 8 genera and 13 plant-dependent species of Zygoptera which can support this point of view.
Although there were no significant differences in larval abundance related to the land uses, the diversity and composition of Odonata larval assemblages of the 8 sites at LMM showed important changes that could be the result of land use. Site 4 had the lowest Shannon´s diversity, agreeing with our prediction of a reduction in diversity in impacted sites. With 93 larvae belonging to 8 species and dominated by A. quadratum and I. ramburii, this site also had a very low functional diversity because the proportion and abundance of plant-dependent species were higher. Sites 1, 4, 5, and 8 presented a high number of plant-dependent species, that is, a high number of species that depend on vegetation to complete their life cycle. This fact could be related to the greater input of nutrients mainly in sites 1 and 4 on the southeast side of LMM, mostly due to the use of fertilizers and human wastes that can play an important role in the proliferation of floating and submerged aquatic vegetation in these sites. Changes in FD throughout sites could be explained by suitable substrate availability on the shoreline, which in turn can be related to the different land-use practices. For instance, although site 1 is a tomato-corn crop, the number of plant-dependent species was higher perhaps due to the high proliferation of aquatic plants, which in time was favored by the runoff of fertilizers used in crops (Arbuckle & Downing, 2001). This is an indirect way of “nourishing” the aquatic environment at this site, promoting further development of the first trophic basis of subsequent links such as filter feeders and predators (such as dragonflies). In this way, the site 1 that recorded the greatest Shannon diversity is a clear example of urbanized sites with a variety of vegetation compositions hosting a more diverse community of Odonata than site 4 with limited vegetation diversity and increased water pollution (Colding et al., 2009; Goertzen & Suhling, 2013).
On the other hand, the fact that site 5 is used as a paddock, also had a high ratio of plant-dependent species (and therefore lower FD) which can be explained by the “fertilizer” effect of manure and cattle urine being washed and hauled up to water, which nourishes and promote the proliferation of aquatic vegetation used by endophytic species. This site, located on the northwest side of LMM and away from the human impacts, represents one of the sites with the greatest abundance of aquatic plants having a lot of patches of Nymphoides indica and emerging grasses, which represent suitable conditions for the endophytic Ischnura capreolus, I. hastata, I. ramburii, Leptobasis vacillans, Coryphaeschna viriditas, C. adnexa, and the ectophytic Micrathyria aequalis.
Seasonally, endophytic species dominate throughout the annual cycle in LMM. They increased in number from the dry to the cold season. The phenological changes observed during the study indicate that the availability of floating plant patches of N. indica and some Pistia stratiotes during the dry season is reduced, increasing during the rainy season, and sustained so until the cold season. The availability of these macrophytes is crucial because they play an important functional role for Odonata by providing critical habitat (e.g., refuge) during the larval and emergence phases of their life cycle (Corbet, 1999; Hornung & Rice, 2002; Thomaz & Cunha, 2010). Likewise, the aquatic macrophytes reduce the risk of predation for larvae and decrease their detection by potential predators (Andersson, 2006) such as the omnivore fish species Astyanax aeneus, Heterandria bimaculata, Poecilia sphenops, Poeciliopsis gracilis, Cichlasoma fenestratum, and Tilapia aurea that inhabit LMM (Obregón-Barbosa, 1990), which diets include invertebrates.
In LMM, Odonata diversity and abundance are more related to seasonality than to land use (Fig. 6). As it is well known, water-level fluctuations in lakes and ponds are dominant forces controlling the functioning and play an important role in the water bodies´ physical processes. Biota living in vegetated riparian areas, respond differentially to water-level dynamics, either directly or indirectly. Direct effects on the biological communities include physical disturbance by wave activity. Indirect effects include the reworking of substrates (which can enhance or restrict colonization by vegetation, and which in turn depends on silt accumulation to establish roots), and alteration of habitats suitable for aquatic flora and fauna (Brauns et al., 2008; Hann, 1995). We propose that seasonal variation in the water level affects indirectly the Odonata larval assemblages by depleting the growth of aquatic vegetation, and as we did observe, the low water level during the dry season had negative effects on the odonate richness and abundance. The main effects of the water level fluctuation occurred seasonally in the drift line where the Odonata larvae were sampled. This is the most important reason why diversity was lowest during the dry season in LMM.
Our results show that site 4 urban was the site with the highest concentration of phosphorus during the dry season and the greatest human impact. It was also the site with the least structural diversity (Shannon) and FD. Likewise, it had the highest proportion of PD species, which in turn implies a greater availability of plant substrates to be used by endophytic and ectophitic species. The greater proportion of PD species results in a small FD since the greatest FD would be found when both proportions of P and NP species are equal. A low FD found in the periphery of LMM would show a possible important disturbance that should be investigated particularly as occurred in site 4 where domestic discharges are evident. In this work, both the structural and functional measures of Odonata larval assemblages agree well in pointing out that site 4 is where a greater effect is registered due to land use. We do not know if endophytic species at LMM are selective of specific plants, but it seems that if aquatic vegetation is abundant, as was water lily, many endophytic species will use it. Water lily is an abundant hydrophyte in LMM, and its abundance is a clear indicator of system alteration. Its production can be high even in heavily contaminated sites, but it is a potential substrate for Zygoptera and some Anisoptera (aeshnids). Thus, the abundance of water lily would predict that the proportion of endophytic species will be high at any site and that FD will be therefore low, showing the system is altered.
It is possible that the impact caused to the odonate fauna in LMM by land use is still incipient, however, our study shows a clear impact on site 4 (located in the urban zone), which has inputs of domestic waters. On this site, the lowest diversity and highest nutrient concentrations (ammonium and nitrites) were registered. The early diversion and treatment of wastewater is the best alternative to maintain urban water bodies in the best conditions and prevent them from becoming potential sites of diseases, unsightly, and lacking cultural and recreational value.
Acknowledgments
We thank Ariadna Martínez for the technical support in laboratory analyses. Javier Tolome supplied invaluable help in the field. Also, thanks to Dr. Alonso Ramírez (North Carolina University) for his criticism to the final manuscript and corrections to English syntax, as well as two anonymous reviewers.
References
Al-Shami, S. A., Rawi, C. S. M., Ahmad, A. H., Hamid, S. A., & Nor, S. A. M. (2011). Influence of agriculture, industrial and anthropogenic stresses on the distribution and diversity of macroinvertebrates in Juru River Basin, Penang, Malaysia. Ecotoxicology and Environmental Safety, 74, 1195–1202. https://doi.org/10.1016/j.ecoenv.2011.02.022
Altamiranda-S, M., Pérez, G. L. A., & Gutiérrez, M. L. C. (2010). Composición y preferencia de microhábitat de larvas de Odonata (Insecta), en la ciénaga de San Juan de Tocagua (Atlántico, Colombia). Caldasia, 32, 399–410.
Andersson, D. (2006). Effect of the diversity, ecology and composition of species of fish on the odonate community (M. Sc. Thesis). Halmstad University, Sweden.
APHA, (1998). Standard methods for the examination of water and wastewater. Washington, DC.: American Public Health Association.
Arbuckle, K. E., & Downing, J. A. (2001). The influence of watershed land use on lake N:P in a predominantly agricultural landscape. Limnology and Oceanography, 46, 970–975. https://doi.org/10.4319/lo.2001.46.4.0970
Astudillo, M. R., Novelo-Gutiérrez, R., Vázquez, G., García-Franco, J. G., & Ramírez, A. (2016). Relationships between land cover, riparian vegetation, stream characteristics, and aquatic insects in cloud forest streams. Mexico. Hydrobiologia, 768, 167–181. https://doi.org/10.1007/s10750-015-2545-1
Balzan, M. V. (2012). Associations of dragonflies (Odonata) to habitat variables within the Maltese Islands: a spatiotemporal approach. Journal of Insect Science, 12, 1-18. https://doi.org/10.1673/031.012.8701
Brauns, M., García, X. F., & Pusch, M. T. (2008). Potential effects of water-level fluctuations on littoral invertebrates in lowland lakes. Hydrobiologia, 613, 5–12. https://doi.org/10.1007/978-1-4020-9192-6_2
Brylinsky, M. (2004). User’s manual for prediction of Phosphorus concentration in Nova Scotia lakes: a tool for decision making. Nova Scotia: Nova Scotia Department of Environment and Labour.
Bota-Sierra, C. A., Flórez, V. C., Escobar, F., Sandoval, H. J., Novelo-Gutiérrez, R., Londoño, G. A. et al. (2021). The importance of tropical mountain forests for the conservation of dragonfly biodiversity: a case from the Colombian Western Andes. International Journal of Odonatology, 24, 233–247. https://doi.org/10.23797/2159-6719_24_18
Bulánková, E. (1997). Dragonflies (Odonata) as bioindicators of environment quality. Biologia, Bratislava, 52, 177–180.
Butler, R. G., & de Maynadier, P. G. (2008). The significance of littoral and shoreline habitat integrity to the conservation of lacustrine damselflies (Odonata). Journal of Insect Conservation, 12, 23–36. https://doi.org/10.1007/s10841-006-9059-0
Campbell, W. B., Novelo-Gutiérrez, R., & Gómez-Anaya, J. A. (2010). Distributions of odonate richness and diversity with elevation depend on windward or leeward aspect: implications for research and conservation planning. Insect Conservation and Diversity, 3, 302–312. https://doi.org/10.1111/j.1752-4598.2010.00108.x
Carchini, G., Della Bella, V., Solimini, A. G., & Bazzanti, M. (2007). Relationships between the presence of odonate species and environmental characteristics in lowland ponds of central Italy. Annales de Limnologie – International Journal of Limnology, 43, 81–87. https://doi.org/10.1051/limn/2007020
Catling, P. M., (2005). A potential for the use of dragonfly (Odonata) diversity as a bioindicator of the efficiency of sewage lagoons. The Canadian Field Naturalist, 119, 233–237. https://doi.org/10.22621/cfn.v119i2.111
Colding J., Lundberg, J., Lundberg, S., & Andersson, E. (2009). Golf courses and wetland fauna. Ecological Applications, 19, 1481–1491. https://doi.org/10.1890/07-2092.1
Conagua (Comisión Nacional del Agua). (2022). Normales Climatológica por Estado [en línea]. Disponible en: https://smn.conagua.gob.mx/es/informacion-climatologica-por-estado?estado=ver
Corbet, P. S. (1999). Dragonflies: behavior and ecology of Odonata. Ithaca, N.Y.: Cornell University Press. https://doi.org/10.1016/s0006-3207(99)00178-0
De la Torre, A., Domínguez, L., González, M., Aguayo, S., Carballo, M., & Muñoz, M. J. (2004). Impact from a cattle waste lagoon rupture on a downstream fish farm: a case study. Ecología Austral, 14, 135–139.
De Paiva-Silva, D., de Marco, P., & Chaves-Resende, D. 2010. Adult odonate abundance and community assemblage measures as indicators of stream ecological integrity: A case study. Ecological Indicators, 10, 744–752. https://doi.org/10.1016/j.ecolind.2009.12.004
Dolný A., Harabiš, F., Bárta, D., & Lhota, S. (2012). Aquatic insects indicate terrestrial habitat degradation: changes in taxonomical structure and functional diversity of dragonflies in the tropical rainforest of East Kalimantan. Tropical Zoology, 25, 37–41. https://doi.org/10.1080/03946975.2012.717480
García, E. (2004). Modificaciones al sistema de clasificación climática de Köppen. México D.F.: Instituto de Geografía, UNAM.
García-García, P. L., Vázquez, G., Novelo-Gutiérrez, R., & Favila, M. E. (2017). Effects of land use on larval Odonata assemblages in cloud forest streams in central Veracruz, Mexico. Hydrobiologia, 785, 19–33. https://doi.org/10.1007/s10750-016-2900-x
Goertzen D., & Suhling, F. (2013). Promoting dragonfly diversity in cities: major determinants and implications for urban pond design. Journal of Insect Conservation, 17, 399–409. https://doi.org/10.1007/s10841-012-9522-z
Guillermo-Ferreira, R., & Del-Claro, K. (2011). Oviposition site selection in Oxyagrion microstigma Selys, 1876 (Odonata: Coenagrionidae) is related to aquatic vegetation structure. International Journal of Odonatology, 14, 275–279. https://doi.org/10.1080/13887890.2011.621109
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9.
Hann, B. (1995). Invertebrate associations with submersed aquatic plants in a prairie wetland. UFS (Delta Marsh) Annual Report, 30, 78–84.
Hernández, K. M., Reece, B. A., & McIntyre, N. E. (2006). Effects of anthropogenic land use on Odonata in playas of the Southern High Plains. Western North American Naturalist, 66, 273–278. https://doi.org/10.3398/1527-0904(2006)66[273:eoaluo]2.0.co;2
Honkanen, M., Sorjanen, A., & Monkkonen, M. (2011). Deconstructing responses of dragonfly species richness to area, nutrient, water plant diversity and forestry. Oecologia, 66, 457–467. https://doi.org/10.1007/s00442-010-1846-3
Hornung, J. P., & Rice, C. L. (2002). Odonata and wetland quality in southern Alberta, Canada: a preliminary study. Odonatologica, 32, 119–129.
Johannsen, S. S., & Armitage, P. (2010). Agricultural practice and the effects of agricultural land-use on water quality. Freshwater Biological Association. Freshwater Forum, 28, 45–59.
Lee-Foote, A., & Rice-Hornung, C. L. (2005). Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecological Entomology, 30, 273–283. https://doi.org/10.1111/j.0307-6946.2005.00701.x
Mamun, Md., Sang-Jae, L., & Kwang-Guk, A. (2018). Temporal and spatial variation of nutrients, suspended solids, and chlorophyll in Yeongsan watershed. Journal of Asia-Pacific Biodiversity, 11, 206–216. https://doi.org/10.1016/j.japb.2018.02.006
McCune, B., & Mefford, M. J. (2011). PC-ORD. Multivariate analysis of ecological data. Version 6. MjM Software, Gleneden Beach, Oregon, U.S.A.
Meeks, J. (1974). Chlorophylls. In P. Stewart (Ed.), Algal physiology and biochemistry. Oxford: Blackwell.
Obregón-Barbosa, H. (1990). Análisis taxonómico y zoogeográfico de los peces de la zona norte y centro del estado de Veracruz, México (M. Sc. Thesis). Facultad de Ciencias Biológicas., Universidad Autónoma de Nuevo León. México.
Oliveira-Junior, J. M. B., De Marco J. P., Dias-Silva, K., Pereira-Leitão, R., Gontijo-Leal, C., Santos-Pompeu, P. et al. (2017). Effects of human disturbance and riparian conditions on Odonata (Insecta) assemblages in eastern Amazon basin streams. Limnologica, 66, 31–39. https://doi.org/10.1016/j.limno.2017.04.007
Oliveira-Junior, J. M. B., & Juen, L. (2019). The Zygoptera/Anisoptera ratio (Insecta: Odonata): a new tool for hábitat alterations assessment in Amazonian streams. Neotropical Entomology, 48, 552–560. https://doi.org/10.1007/s13744-019-00672-x
Oliveira-Junior, J. M. B., Shimano, Y., Gardner, T. A., Hughes, R. M., De Marco-Júnior, P., & Juen, L. (2015). Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral Ecology, 40, 733–744. https://doi.org/10.1111/aec.12242
Pathak, H., & Pathak, D. (2012). Eutrophication: Impact of Excess Nutrient Status in Lake Water Ecosystem. Journal of Environmental & Analytical Toxicology, 2, 1–5. https://doi.org/10.4172/2161-0525.1000148
Pires, M. M., Sahlén, G., & Périco, E. (2022). Agricultural land use affects the heterogeneity of Odonata communities in the Brazilian Pampa, Journal of Insect Conservation, 26, 503–514. https://doi.org/10.1007/s10841-021-00349-0
Santos, J. C. N., Andrade, E. M., Araújo, E. M., Meireles, J. R. N. C. M., & Palacio, H. A. Q. (2014). Land use and trophic state dynamics in a tropical semi-arid reservoir. Revista Ciência Agronômica, 45, 35–44. https://doi.org/10.1590/s1806-66902014000100005
Seitzinger, S. P., Harrison, E., Dumont, J. A., Beusen, A. H. W., & Bouwman, A. F. (2005). Sources and delivery of carbon, nitrogen, and phosphorus to the coastal zone: an overview of global nutrient export from watersheds (NEWS) models and their application. Global Biogeochemical Cycles, 19, 1–11. https://doi.org/10.1029/2005gb002606
Silva, L. F. R., Castro, D. M. P., Juen, L., Callisto, M., Hughes, R. M. & Hermes, M. G. (2021). Functional responses of Odonata larvae to human disturbances in neotropical savanna headwater streams. Ecological Indicators, 133, 108367. https://doi.org/10.1016/j.ecolind.2021.108367
StatSoft. (2006). STATISTICA (data analysis software system and computer program manual) Version 7.1. StatSoft Inc., Tulsa.
Subramanian, K. A., Ali, S., & Ramachandra, T. V. (2008). Odonata as indicators of riparian ecosystem health a case study from southwestern Karnataka, India. Fraseria (N.S.), 7, 83–95.
Tafangenyasha, C., & Dube, L. T. (2008). An Investigation of the Impacts of Agricultural Runoff on the Water Quality and Aquatic Organisms in a Lowveld Sand River System in Southeast Zimbabwe. Water Resources Management, 22, 119–130. https://doi.org/10.1007/s11269-006-9147-7
Ter Braak, C. J. F., & Smilauer, P. (2002). CANOCO Reference manual and CanoDraw for Windows user’s guide: Software for Canonical Community Ordination (version 4.5). Ithaca, Microcomputer Power.
Thomaz, S. M., & Cunha, E. R. (2010). The role of macrophytes in habitat structuring in aquatic ecosystems: Mmethods of measurement, causes and consequences on animal assemblages, composition, and biodiversity. Acta Limnologica Brasiliensia, 22, 218–236. https://doi.org/10.4322/actalb.02202011
Tognelli, M. F., Lasso, C. A., Bota-Sierra, C. A., Jimenez-Segura, L. F., & Cox, N. A. (Editores). (2016). Estado de conservación y distribución de la biodiversidad de agua dulce en los Andes Tropicales. Gland, Suiza, Cambridge, UK y Arlington, USA: UICN. https://doi.org/10.2305/iucn.ch.2016.02.en
Toner, P., Bowman, J., Clabby, K., Lucey, J., McGarrigle, M., Concannon, C. et al. (2005). Water quality in Ireland 2001-2003. Johnstown Castle: Environment Protection Agency.
Verdonschot, R. C. M., & Peeters, E. T. H. M. (2012). Preference of larvae of Enallagma cyathigerum (Odonata: Coenagrionidae) for habitats of varying structural complexity. European Journal of Entomology, 109, 229–234. https://doi.org/10.14411/eje.2012.030
Vilenica, M., & Mihaljevic, Z. (2022). Odonata assemblages in anthropogenically impacted habitats in the Drava River —a long-term study. Water, 14, 3119. https://doi.org/10.3390/w14193119
Westfall, M. J., & May, M. L. (1996). Damselflies of North America. Gainesville, Florida: Scientific Publishers.
Whiles, M. R., Brock, B. L., Franzen, A. C., & Dinsmore II, S. C. (2000). Stream invertebrate communities, water quality, and land-use patterns in an Agricultural Drainage Basin of Northeastern Nebraska, USA. Environmental Management, 26, 563–576. https://doi.org/10.1007/s002670010113
Williams-Linera, G. (2007). El bosque de niebla del centro de Veracruz: ecología, historia y destino en tiempos de fragmentación y cambio climático. Xalapa: Instituto de Ecología, A.C./ Conabio.
Zedler, J. B., & Kercher, S. (2005). Wetland resources: status, trends, ecosystem services, and restorability. Annual Review of Environment and Resources, 30, 39–74. https://doi.org/10.1146/annurev.energy.30.050504.144248