Ontogenetic changes in wild chagasic bugs (Dipetalogaster maxima): exploring morphological adaptations in pre-adult and adult stages
Rafael Bello-Bedoy a, *, Haran Peiro-Nuño a, Alex Córdoba-Aguilar b, Carlos Alberto Flores-López a, Guillermo Romero-Figueroa a, María Clara Arteaga c, Ana E. Gutiérrez-Cabrera d, Leonardo De la Rosa-Conroy c
a Facultad de Ciencias, Universidad Autónoma de Baja California, Carretera Transpeninsular, Ensenada- Tijuana, 3917, Playitas, 22860 Ensenada, Baja California, Mexico
b Departamento de Ecología Evolutiva, Instituto de Ecología, Universidad Nacional Autónoma de México, Apartado postal 70-275, Ciudad Universitaria, 04510 Ciudad de México, Mexico
c Departamento de Biología de la Conservación, Centro de Investigación Científica y de Educación Superior de Ensenada, Carretera Tijuana-Ensenada 3918, Zona Playitas, 22860 Ensenada, Baja California, Mexico
d Conacyt-Centro de Investigación Sobre Enfermedades Infecciosas, Instituto Nacional de Salud Pública, Avenida Universidad 655, Col. Santa María Ahuacatitlán, Cerrada Los Pinos y Caminera, 62100 Cuernavaca, Morelos, Mexico
*Corresponding author: rbello@uabc.edu.mx (R. Bello-Bedoy)
Abstract
Triatomine insects are vectors of Trypanosoma cruzi (Chagas, 1909), the causing agent of Chagas disease. We studied the morphological ontogenetic changes of Dipetalogaster maxima (Uhler, 1894), an endemic Chagas vector of Baja California Sur, Mexico. We measured and compared among nymphal stages and adults and, between sexes phenotypic traits linked to the following functions: a) feeding: proboscis length and width; b) vision: head length and width; c) mobility: pronotum width and length and; feeding capacity and fecundity: abdomen length in 5 nymphal stages and in adults of both sexes, respectively. We found a steady increase in proboscis and head size. The length of pronotum, and abdomen increased abruptly in adults compared to the fifth nymphal stage. Adult females were only bigger than males in total length, whereas males had larger pronotum. Our results demonstrated a stable increase along the fifth stage, but an exaggerated increase of the pronotum when insects become adults. This growth is associated with wing muscular growth, potentially used for dispersal and finding mates and preys during adult life. In turn, fecundity is operating to enlarge female’s total length and abdominal size, but male abdomen may be selected to storage large blood meals.
Keywords:
Development; Feeding; Growth; Instar; Mobility; Phenotype; Triatominae; Vision
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Cambios ontogenéticos en la chinche silvestre (Dipetalogaster maxima): explorando adaptaciones morfológicas en estados preadultos y adultos
Resumen
Los triatominos son insectos vectores de Trypanosoma cruzi (Chagas, 1909), el agente etiológico de la enfermedad de Chagas. Se estudian los cambios morfológicos- ontogenéticos de Dipetalogaster maxima (Uhler, 1894), vector chagásico y endémico de Baja California Sur, México. Se midieron los siguientes rasgos fenotípicos funcionales: a) alimentación: largo y ancho de la probóscide; b) visión: largo y ancho de la cabeza; c) movilidad: ancho y largo del pronoto, y capacidad de alimentación y fecundidad: longitud del abdomen en juveniles y en adultos de ambos sexos, respectivamente. Estos rasgos se compararon entre los 5 estadios ninfales y adultos, y entre hembras y machos en estadio adulto. Se encontró un aumento constante en la probóscide y el tamaño de la cabeza a lo largo de la ontogenia, que se interpreta como la inversión en funciones visuales y de alimentación. La longitud total del pronoto y el abdomen aumentaron abruptamente en adultos en contraste con los 5 estadios ninfales. Entre sexos, las hembras solo eran más grandes que los machos en longitud total, mientras el pronoto de los machos era significativamente más grande que en las hembras. El aumento notable del pronoto se asocia con el crecimiento de los músculos alares, potencialmente utilizados para desplazarse a grandes distancias. Esto podría ser adaptativamente ventajoso si la búsqueda de parejas y presas aumenta durante la vida adulta. A su vez, la fecundidad puede estar operando para agrandar la longitud total y el tamaño abdominal de las hembras, mientras que un abdomen masculino alargado incrementaría la capacidad alimenticia.
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Desarrollo; Alimentación; Crecimiento; Estadios; Movilidad; Fenotipo; Triatominae; Visión
Introduction
Hematophagous insect vectors such as triatomine bugs are a major issue for public health (Mehlhorn, 2015). These insects are responsible for transmitting the protozoa Trypanosoma cruzi, the etiological agent of Chagas disease that is recognized as one of the world’s 13 most neglected tropical diseases (Hotez et al., 2012). Current taxonomic knowledge recognizes 151 triatomine species which vary in their vector capacity and, hence, their importance in terms of Chagas control and/or prevention. Despite such importance, most studies of Triatominae biology have been concentrated in a handful of species, and attention to the knowledge of animals from natural populations is currently scarce. Recent research in several vector transmitting neglected diseases, including Triatominae bugs, have demonstrated that basic knowledge about vectors’ biology and zoonosis helps to find potential solutions to minimize transmission risk (De Moraes et al., 2018; Rojas-de Arias et al., 2017), and phenotypic traits can indirectly provide ecological information to understand vector-parasite ecology (Nattero et al., 2017). For example, Miura et al. (2006) found in a trematode-snail host system that infected snails were markedly larger and exhibited different shell morphology than non-infected individuals. Thus, the detailed study of phenotype is of major relevance for public health, if by means of a phenotypic analysis, it is possible to differentiate between infected and non-infected organisms, becoming a tool for vector control and prevention of tropical diseases, such as Chagas.
As part of a hematophagous life style, triatomine bugs feed on blood of a variety of vertebrate hosts (Fuentes-Vicente et al., 2018). This means that bugs must look for, detect, get attached to and escape from hosts that vary in their mobility, habits and defense ability (Barrozo et al., 2017, Noireau et al., 2009). Both, the triatomine abilities and interspecific variation of hosts’ susceptibility, have likely shaped triatomine development, morphology, physiology and behavior. For example, triatomines guide themselves towards CO2 (Barrozo et al., 2004) and heat (Lazzari & Núñez, 1989) emitted by vertebrate hosts.
Morphologically, there is interspecific variation in different body parts, which have been ecologically and evolutionarily shaped triatomines to track and feed on a large diversity of vertebrates (Gaunt & Miles, 2000). For example, 6 triatomine species consistently showed more antennal sensilia in wild sylvatic (predominantly palm-related vegetation) compared to domestic and peridomestic habitats (Carbajal-De la Fuente & Catalá, 2002). One underlying reason for this is that vertebrate prey occurring in sylvatic vs domestic and peridomestic habitats are different and this heterogeneity in feeding prey use promoted the evolution of structures with distinct sensory abilities (Catalá, 1997). Adaptive changes must occur during each developmental stage, because each stage could feed on different prey. However, it is not clear in which life-stage those critical phenotypic changes are expressed.
We know extremely little in terms of how triatomines change in different body parts due to possible changes in feeding-prey diversity as bugs grow. Indeed, morphological changes along different developmental stages have been well documented, but this information has been used mainly for taxonomic purposes (Silva et al., 2003; Villalobos et al., 2012). In this work, following an ontogenetic approach, we compared the phenotypic changes along with development in the triatomine bug Dipetalogaster maxima, a species endemic to the Cape region of Baja California Sur, Mexico. This bug is of epidemiological importance for Chagas transmission, given its ability to inhabit in peridomestic and intradomestic rural areas (Jiménez & Palacios, 1999; Marsden et al., 1979). In addition, it is a large, aggressive bug that ingests large blood meals during daylight (Mazzotti, 1970). There is evidence that this species feeds on humans, woodrats (genus Neotoma), cattle (pers. obs.) and lizards (Jiménez & Palacios, 1999; Jiménez et al., 2003; Marsden et al., 1979), although it can feed on pigeons in insectary conditions (Costa et al., 1987). Nevertheless, information on the biology of this species from wild specimens is extremely fragmented (Guzmán-Bracho, 2001; Ryckman & Ryckman, 1967). Therefore, we conducted a study to understand development phenotypic variation, sampling individuals from wild populations along the entire distribution range of this species.
We measured those diagnostic phenotypic traits that characterize D. maxima (Jurberg et al., 1993) to explore 2 issues. First, we compared growth patterns in traits related to a) feeding; b) vision, and, c) mobility. For feeding, we used proboscis length and width measured. The underlying assumption for this is that triatomines are equipped with a relatively long proboscis compared to other blood-sucking insects, given that the former employs their proboscis to puncture and suck blood directly from vessels and capillaries unlike “pool-feeders” insects which superficially pierce skin and suck directly from the bleeding wound (Krenn & Aspock, 2012). This implies that proboscis length would evolve as a response to how deep a blood source is found, a prediction that, to our knowledge, has not been put forward for triatomines, but that is well supported in other insects (flies, Pauw et al., 2009). The proboscis may be also associated to communication, as it stridulates when its tip is rubbed against the transverse ridges lying on the prosternal groove (reviewed by Schilman et al., 2001). Nevertheless, proboscis length has not been associated to sound magnitude (Roces & Manrique, 1996) so such length cannot be associated to a communication function.
For visual capacity, we used head length and width to include both eyes and associated visual capacity traits. The logic is that head size is a good indicator of vision capacity as the machinery linked to this sense occupies most of the head in triatomines (Dujardin et al., 2009). For mobility, we used pronotal dimensions as, like other insects, the pronotum contain the muscular machinery that allows mobility (Gurevitz et al., 2009). From early stages, these insects are very active walkers (Ryckman & Ryckman, 1967) and at adult stages the wings fully develop to potentially improve dispersal while seeking for prey, mates or shelter. Secondly, we compared how both sexes in the adult stage differ in their investment to traits related to the same functions indicated above, but we also included fecundity. Thus, for the fecundity function, we measured abdominal length as this is a proxy of fecundity in insects (Preziosi et al., 1996) and triatomines (Botto-Mahan et al., 2008). For comparative purposes, we also measured immature abdominal length. We do not have a priori predictions for all comparisons except for the fecundity topic, whereby females would have a larger abdomen compared to males. However, we elaborate on our results by putting forward adaptive functions underlying the observed patterns. All dimensions were taken for all 6 stages of field-collected animals.
Materials and methods
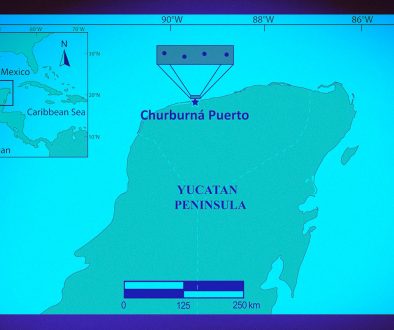
All insects were collected in rocky zones distributed in the cape region of the Sierra de la Laguna, Baja California Sur, Mexico (Fig. 1). These localities show tropical xerophytic shrub and tropical deciduous forest, where this species occurs endemically (Jiménez & Palacios, 1999; Marsden et al., 1979). Dipetalogaster maxima is well attracted by CO2 emitted by vertebrates, as it has been reported for other reduviidous insects (Barrozo et al., 2004). Thus, to collect specimens, we sat down in each locality and waited a minimum time of 15 minutes and a maximum of 2h for D. maxima bugs to come out and approach us. Each approaching bug was captured and placed in an individual plastic tube filled with 70% ethanol. Further, we moved to another location separated by 10 km. We left localities where no insect came out after 15 minutes. Using this sampling method, we captured a total of 322 animals from 18 localities (Fig. 1) of the following developmental stages: 85 first stage, 91 second stage, 80 third stage, 23 fourth stage, 26 fifth stage, and 17 sixth stage adults (10 females and 7 males).
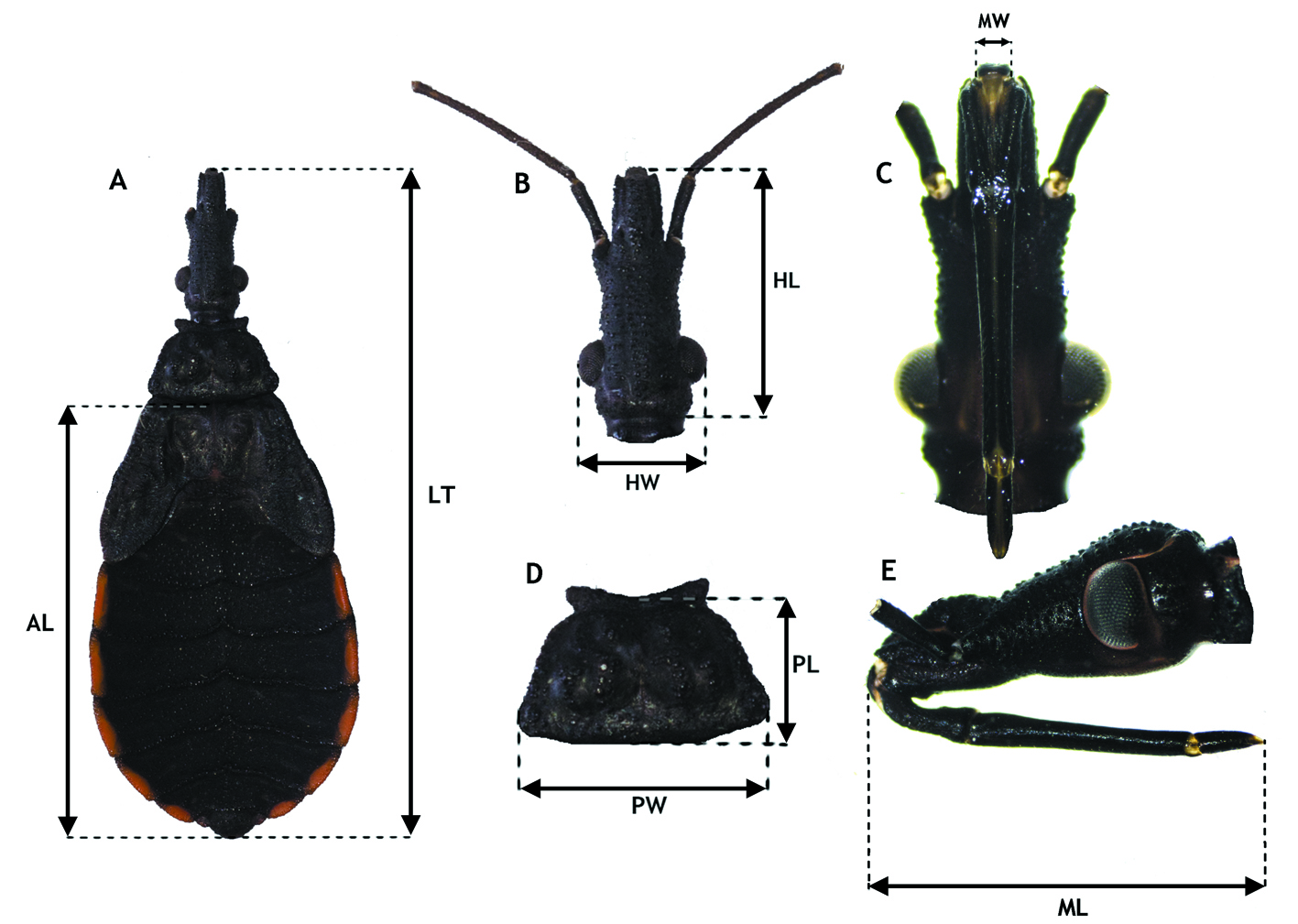
Prior to being measured, each individual was photographed using a digital camera Axiocam ICc 5s attached to a stereomicroscope STEMI DV4 (Carl Zeiss Microscopy®). From such pictures, we then measured (to the nearest 0.01 mm) the following body traits: total body length, head length, head width, proboscis length, proboscis width, pronotum length, pronotum width and abdomen length (Fig. 2). We used ZEN (software Blue Edition de 2012) software package (Carl Zeiss Microscopy®) to carry out such measurements.

We calculated mean, standard deviation and coefficient of variation using untransformed data to summarize the variation in 8 morphological traits measured: total body length, head length, head width, proboscis length, proboscis width, pronotum length, pronotum width and abdomen length in each of the 6 developmental stages (Fig. 2).
To compare variation in growth patterns that would be interpreted as differential investment to distinct functions across different development stages, we conducted a multivariate analysis of covariance (Mancova). In this model, we used stage as a fixed factor, and body length as a covariate to control the effect of total body length on growth of other traits. We performed univariate analyses of covariance (Ancova) for each trait to detect statistical difference among the 6 developmental stages and body length as a covariate.
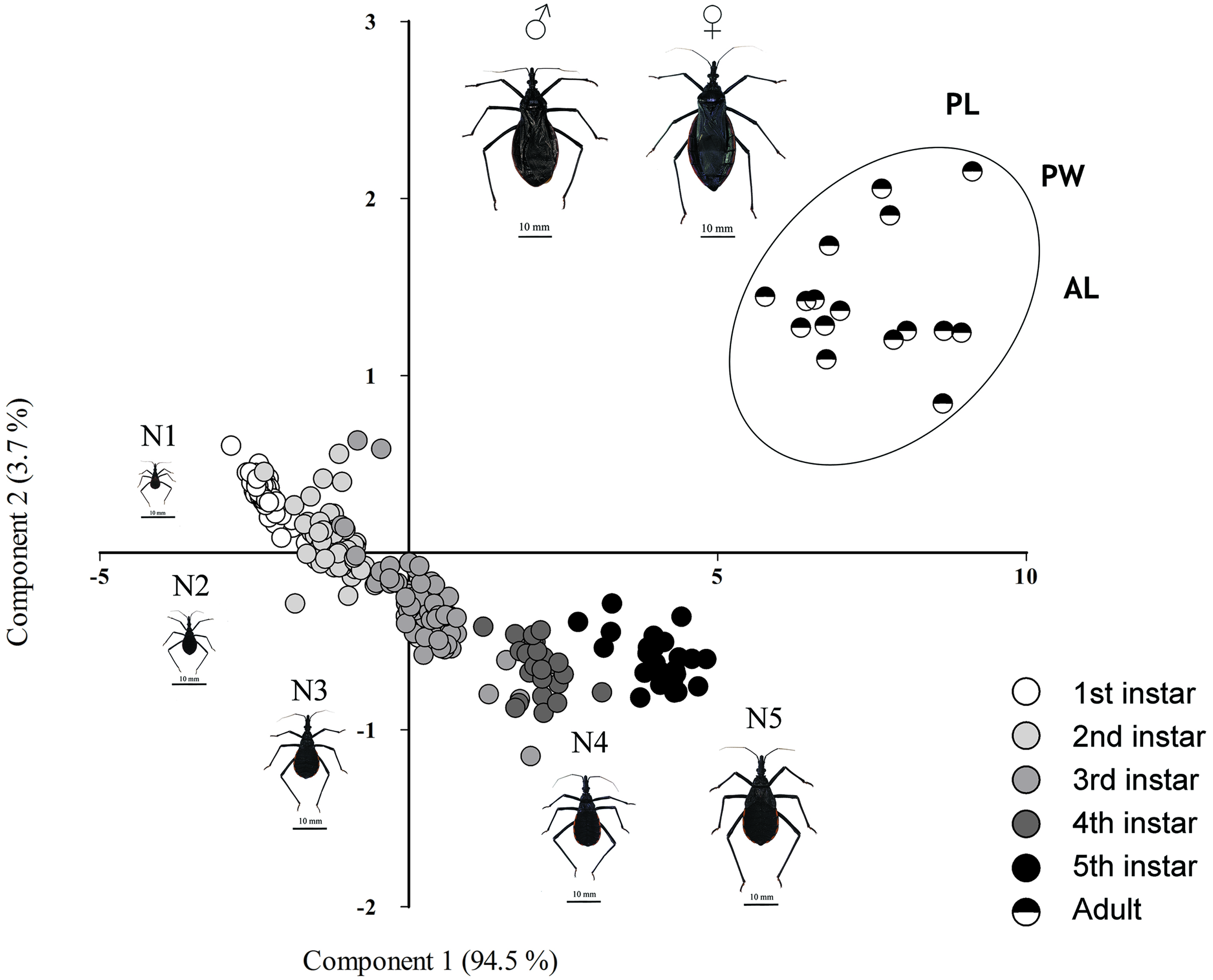
To examine which morphometric traits are influencing the variation between stages along the ontogeny, we conducted a principal component analysis (PCA) with all measures. We explored only the 2 principal components (PCs) that explained most of the variance.
To assess potential differences in total body length between males and females, we performed a t-test including body length as response variable and sex (i.e., male, female) as a fixed factor. To determine the phenotypic differences between adult males and females, we conducted an individual analysis of covariance (Ancova) of each morphometric character as a function of sex and body length as a covariate. All statistical analyses were conducted using JMP® 5.1 software (SAS CARY, NJ).
Results
Growth patterns. Overall, we detected significant variation across developmental stages in the expression of all traits (Wilks’ λ = 0.02; F = 55.135; d.f. = 35; p < 0.0001; n = 322; for inspection of means and associated variation see Table 1), even after controlling for the significant effect of body length (F = 2.28; d.f. = 7; p < 0.0001). Visual inspection of results indicated variation for pronotum traits along ontogeny (Fig. 3G-H). Univariate Ancova showed significant differences for each trait across developmental stages (Table 2), after controlling body length. In average, total body length increased 4.8 times from first to adults. Traits that showed a disproportionate increment in size were pronotum length and width, which scaled 7.7 and 6.1 times larger respectively in adults compared to first instar animals. The remaining traits showed lower scaling values such as 2.7 and 2.5 for proboscis length and width respectively.
The first 2 components significantly explained 98.2% of total variance. Axes 1 accounted for 95.1% (Eigenvalue = 6.61) of total variance while axes 2 explained 3.3% (Eigenvalue = 0.26) of total variance (Table 3). The 5 instars are continuously distributed along the first axis (Fig. 4). In contrast, at the right side of the PCA, male and female adults are well-grouped and separated from the rest of the instars. All pre-adult instars, pronotum length and width, total body length and abdomen length showed positive values along the second axis only for adult individuals, segregating adults (i.e., male and female), whereas the continuous dispersion shown by pre-adult individuals along the first axis is driven by proboscis and head trait (Fig. 3), which shows the largest dispersion.
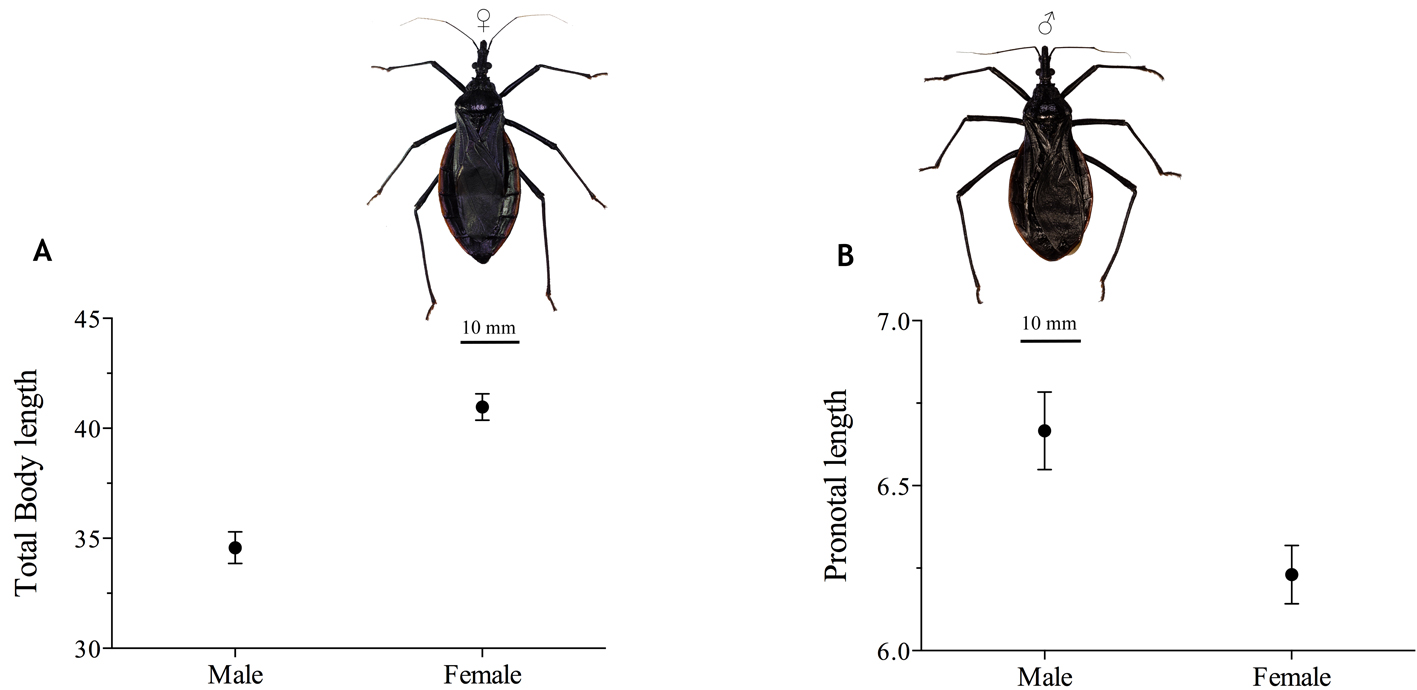
Sexual differentiation. We detected a significant difference in body length between females and males (t = 7.87; d.f. 12.7; p < 0.0001). Female body was 15% larger than male body. After correcting the effect of body length on body measures (Fig. 5), the Ancova analysis indicated significant difference in pronotal length between males and females (F = 5.5606; d.f. = 1; p = 0.0347). Pronotum was 6% larger in males than in females. No significant differences between sexes were found for the other traits (Table 4).
Table 1
Mean (µ) standard deviation (sd) and coefficient of variation (cv) for 8 traits measured in 5 stages and adults (A) of Dipetalogaster maxima. TL: Total body length, HL: head length; HW: head width; ML: proboscis length; MW; proboscis width PL: pronotum length; PW: pronotum width; AL: abdomen length.
|
Instar |
TL |
HL |
HW |
ML |
MW |
PL |
PW |
AL |
||||||||||||||||
|
|
µ |
sd |
cv |
µ |
sd |
cv |
µ |
sd |
cv |
µ |
sd |
cv |
µ |
sd |
cv |
µ |
sd |
cv |
µ |
sd |
cv |
µ |
sd |
cv |
|
1 |
8.19 |
0.54 |
6.55 |
2.50 |
0.12 |
4.88 |
1.11 |
0.06 |
5.27 |
2.70 |
0.16 |
6.02 |
0.21 |
0.02 |
8.53 |
0.83 |
0.05 |
5.73 |
1.44 |
0.07 |
4.69 |
4.85 |
0.47 |
9.71 |
|
2 |
11.55 |
0.95 |
8.25 |
3.10 |
0.22 |
6.95 |
1.43 |
0.11 |
7.37 |
3.43 |
0.28 |
8.05 |
0.27 |
0.03 |
10.72 |
1.17 |
0.17 |
14.18 |
1.89 |
0.26 |
13.89 |
7.28 |
0.77 |
10.58 |
|
3 |
15.40 |
1.50 |
9.77 |
3.81 |
0.51 |
13.45 |
1.82 |
0.15 |
8.06 |
4.24 |
0.31 |
7.27 |
0.35 |
0.04 |
11.73 |
1.55 |
0.14 |
9.13 |
2.61 |
0.29 |
11.07 |
10.04 |
1.19 |
11.86 |
|
4 |
19.54 |
1.55 |
7.93 |
4.71 |
0.18 |
3.79 |
2.33 |
0.10 |
4.49 |
5.27 |
0.21 |
4.07 |
0.44 |
0.04 |
8.76 |
2.17 |
0.15 |
6.73 |
3.69 |
0.25 |
6.71 |
12.66 |
1.36 |
10.72 |
|
5 |
25.74 |
1.53 |
5.96 |
5.39 |
0.21 |
3.97 |
2.94 |
0.15 |
5.21 |
6.08 |
0.40 |
6.52 |
0.50 |
0.03 |
6.78 |
2.97 |
0.18 |
6.01 |
5.02 |
0.32 |
6.38 |
17.39 |
1.34 |
7.68 |
|
6 |
38.33 |
3.73 |
9.72 |
5.99 |
0.41 |
6.89 |
3.38 |
0.27 |
7.97 |
6.86 |
0.46 |
6.77 |
0.61 |
0.08 |
12.38 |
6.41 |
0.49 |
7.70 |
8.79 |
0.76 |
8.62 |
25.94 |
2.98 |
11.49 |
Discussion
Given the relations among different body parts in D. maxima, the following patterns emerged: a) there is a considerable increase in proboscis, head, pronotum, total length and abdomen in the adult stage compared to the pre-adult stage; moreover, adult males have a larger pronotum than adult females, and b) at the adult stage, sexes do not differ in proboscis, abdominal and pronotum length but females have a larger body than males (this, despite the relatively small sample sizes for these 2 subgroups). Our functional explanations underlying these findings are explained below.
A massive increase in head and proboscis size as the animal ages may reflect that investment in visual and/or feeding ability may become similarly proportional as the animal grows. Perhaps this implies that finding resources such as shelter, and vertebrate preys follow a steady, upper need. For example, it may be that a larger proboscis from one developmental stage to the next one supposedly requires access to prey whose skin can be thicker and thus the need to elongate such structure.
A similar situation would explain head length increase. In relation to this, intra-specific variation among different populations in Triatoma dimidiata (Latreille,1811), have found differences in head size (a proxy of eye size), in specimens living in caves vs those living in non-caves, where cave individuals showed smaller eyes than non-cave ones (Bustamante et al., 2004). This difference suggests the need of a larger and wider head and, thus, eye, in non-cave animals to facilitate host detection in illuminated environments (Dujardin et al., 2009). Although this interpretation is exclusive for adults only that differ in habitat, a similar rationale may apply to our study for visual and feeding functions when related to finding hosts. However, we are largely unaware of whether prey diversity increases as the triatomine grows. Research related to using a different and possibly larger diversity of preys as the triatomine grows is a topic of interest not only in terms of niche breadth in these animals, but also in terms of how much T. cruzi can be potentially disseminated in an environment with a varying degree of host diversity (Roque et al., 2008).

Table 2
Analyses of covariance for each phenotypic trait measured in Dipetalogaster maxima. Sample size for each analysis was 322.
|
Trait |
Source of Variation |
d.f. |
m.s. |
F |
p |
|
Head length |
Stage |
5 |
22.59 |
65.24 |
< 0.0001 |
|
Total body length |
1 |
8.95 |
129.19 |
< 0.0001 |
|
|
Head width |
Stage |
5 |
5.50 |
153.54 |
< 0.0001 |
|
Total body length |
1 |
2.71 |
378.84 |
< 0.0001 |
|
|
Proboscis length |
Stage |
5 |
28.81 |
113.97 |
< 0.0001 |
|
Total body length |
1 |
9.04 |
178.82 |
< 0.0001 |
|
|
Proboscis width |
Stage |
5 |
0.15 |
34.85 |
< 0.0001 |
|
Total body length |
1 |
0.12 |
144.21 |
< 0.0001 |
|
|
Pronotum length |
Stage |
5 |
32.83 |
506.34 |
< 0.0001 |
|
Total body length |
1 |
5.15 |
396.98 |
< 0.0001 |
|
|
Pronotum width |
Stage |
5 |
23.13 |
125.54 |
< 0.0001 |
|
Total body length |
1 |
14.15 |
384.00 |
< 0.0001 |
|
|
Abdomen length |
Stage |
5 |
6.93 |
15.29 |
< 0.0001 |
|
Total body length |
1 |
378.25 |
4172.33 |
< 0.0001 |
The abrupt pronotal enlargement from pre-adult to adult stage likely reflects an investment in the ability to move. Such movements at the adult stage would be expected in different contexts such as finding and chasing hosts and/or mates. For the former, it may be that adults demand larger hosts as these have more blood to satisfy the energetic requirements. In addition, the non-domestic habitat (as opposed to domestic habitats) of this bug would limit food availability, favoring a disparate pronotal and muscle growth to enhance searching and finding of prey. However, although adult stages consume more blood than first to third instar, the former consume considerably less than fourth and fifth instar (Bar et al., 2003; Rubio et al., 2013). For finding and chasing mates, a large musculature in the pronotal region may be useful also. In this respect, little is known about the sexual behavior of triatomines in general and practically (Téllez-García et al., in press). However, triatomine mating is likely to take place at night when both sexes abandon their refuges (Lazzari, 1992; Pontes et al., 2014).
How males and females find each other is possibly via chemical communication (Pontes et al., 2008). In this scenario, females may emit volatile compounds to which males are attracted (Zacharias et al., 2010). However, female scents can elicit attraction by more than 1 male, so that male aggregations can be found around a mating pair (Crespo & Manrique, 2007; Manrique & Lazzari, 1995). This chemical evidence and the fact that females of most species of triatomines studied so far are polygamous (Manrique & Lorenzo, 2012), may be interpreted as males competing for females. In situations like this, males of different insect species use enlarged structures that facilitate finding females or repelling other competing males once a female mate has been secured (Arnqvist & Rowe, 2013). These structures are sexually dimorphic, for example, longer wings or legs (Bergsten et al., 2001) and/or with a larger musculature in males than in females (Anholt et al., 1991). This may be the case for D. maxima as males have a larger pronotum than females, but further observations of sexual behavior should investigate this idea.
Table 3. Loadings for each trait in each component of the PCA. This analysis included the 322 individuals entailing the sixth stages (see also Fig. 3). TL: Total body length; HL: head length; HW: head width; ML: proboscis length; MW: proboscis width; PL: pronotum length; PW: pronotum width; AL: abdomen length.
|
Trait |
PC1 |
PC2 |
|
HL |
0.374 |
-0.414 |
|
HW |
0.384 |
-0.195 |
|
ML |
0.380 |
-0.327 |
|
MW |
0.378 |
-0.282 |
|
PL |
0.367 |
0.629 |
|
PW |
0.379 |
0.417 |
|
AL |
0.383 |
0.187 |
|
%variance |
94.53 |
3.58 |
|
Eigenvalue |
6.61 |
0.25 |
Table 4. Analysis of covariance of 7 body measures for females and males of Dipetalogaster maxima to compare morphometric variation.
|
Trait |
Source of Variation |
d.f. |
m.s. |
F |
p |
|
Head length |
Sex |
1 |
0.04 |
0.52 |
0.4 |
|
Total body length |
1 |
0.18 |
2.10 |
0.1 |
|
|
Head width |
Sex |
1 |
0.04 |
2.00 |
0.1 |
|
Total length |
1 |
0.40 |
22.96 |
0.0004 |
|
|
Proboscis length |
Sex |
1 |
0.07 |
0.53 |
0.4 |
|
Total body length |
1 |
0.76 |
5.74 |
0.03 |
|
|
Proboscis width |
Sex |
1 |
0.00 |
0.67 |
0.4 |
|
Total body length |
1 |
0.00 |
0.33 |
0.5 |
|
|
Pronotum length |
Sex |
1 |
0.20 |
5.56 |
0.03 |
|
Total body length |
1 |
1.59 |
44.71 |
< 0.0001 |
|
|
Pronotum width |
Sex |
1 |
0.22 |
3.00 |
0.1 |
|
Total body length |
1 |
3.15 |
42.32 |
< 0.0001 |
|
|
Abdomen length |
Sex |
1 |
0.05 |
0.55 |
0.4 |
|
|
Total body length |
1 |
31.96 |
321.70 |
< 0.0001 |
In general, there was a massive increase in different body parts from pre-adult stages to adults. On one hand, this possibly explains why triatomines take so long during the fifth stage to develop (Aldana et al., 2017) as animals need such a long time to produce a large body. In Rhodnius prolixus (Stahl, 1859), this delay seems adaptive and has been associated to deal with environmental stochasticity that may take place at the end of the dry season (Aldana et al., 2017). At this time, triatomines may remain as fifth stage and become adults when the rainy season starts (whose occurrence is uncertain). So, such delay seems a bet-hedging strategy until better (rain) conditions prevail, which is when more vertebrate hosts can be secured (Aldana et al., 2017). The marked dry season but unpredictable onset of rainy season in our study site support the above explanation for D. maxima. Thus, the massive increase in body traits may be a consequence of a long developmental time required during the fifth stage period. One interesting pattern is that even when adult females had a larger body than males, there was no difference in abdominal length. This cannot be taken as no evidence for fecundity selection as other explanations may be operating such as selection acting also on male abdominal length, that may cancel out dimorphic patterns (Blanckenhorn, 2000). Why females become in general larger than males, even when this is not explained by abdominal length, is unclear.
Finally, our study can be used to understand phenotypic changes in D. maxima and provide further information of this species. There are few studies of these animals and the localities where we trapped them can be used as a stand point for further Chagas control/prevention research in the Cape region.
Acknowledgments
To Carlos A. González for helping in the figures design; A. Córdoba-Aguilar had a visiting grant from the Coordinación de la Investigación Científica, UNAM. This project was supported by the Conacyt Fondo Infraestructura INFR-2014-01-226239 grant; Problemas Nacionales grant 2015-01-547, and by an internal UABC grant “Caracterización de las cepas del patógeno humano Trypanosoma cruzi en la Península de Baja California”.
References
Aldana, E., Medone, P., Pineda, D., Menu, F., & Rabinovich, J. (2017). Development time and fitness: is there an adaptive development delay in the Rhodnius prolixus fifth nymphal stage? Entomologia Experimentalis et Applicata, 163, 1–8.
Anholt, B. R., Marden, J. H., & Jenkins, D. M. (1991). Patterns of mass gain and sexual dimorphism in adult dragonflies (Insecta: Odonata). Canadian Journal of Zoology, 69, 1156–1163.
Arnqvist, G., & Rowe, L. (2013). Sexual conflict. Princeton: Princeton University Press.
Bar, M. E. A., Milano, A. M. F., Damborsky, M. P., Oscherov, E. B., & Avalos, G. (2003). Patrones de alimentación y de defecación de Triatoma rubrovria (Heteroptera: Reduviidae) bajo condiciones de laboratorio. Revista de la Sociedad. Entomológica Argentina, 62, 107–113.
Barrozo, R. B., Minoli, S. A., & Lazzari, C. R. (2004). Circadian rhythm of behavioural responsiveness to carbon dioxide in the blood-sucking bug Triatoma infestans (Heteroptera: Reduviidae). Journal of Insect Physiology, 50, 249–254.
Barrozo, R. B., Reisenman, C. E., Guerenstein, P., Lazzari, C. R., & Lorenzo, M. G. (2017). An inside look at the sensory biology of triatomines. Journal of Insect Physiology, 97, 3–19.
Bergsten, J., Töyrä, A., & Nilsson, A. N. (2001). Intraspecific variation and intersexual correlation in secondary sexual characters of three diving beetles (Coleoptera: Dytiscidae). Biological Journal of the Linnean Society, 73, 221–232.
Blanckenhorn, W. U. (2000). The evolution of body size: What keeps organisms small? Quaterly Review in Biology, 75, 385–407.
Botto-Mahan, C., Ossa, C. G., & Medel, R. (2008). Direct and indirect pathways of fitness-impact in a protozoan-infected kissing bug. Physiological Entomology, 33, 25–30.
Bustamante, D. M., Monroy, C., Menes, M., Rodas, A., Salazar-Schettino, P. M., Rojas, G. et al. (2004). Metric variation among geographic populations of the Chagas vector Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) and related species. Journal of Medical Entomology, 41, 296–301.
Carbajal-De La Fuente, A. L., & Catalá, S. (2002). Relationship between antennal sensilla pattern and habitat in six species of Triatominae. Memorias do Instituto Oswaldo Cruz, 97, 1121–1125.
Catalá, S. S. (1997) Antennal sensilla of Triatominae (Hemiptera, Reduviidae): a comparative study of five genera. International Journal of Insect Morphology and Embryology, 26, 67–73.
Costa, J. M., Jurberg, J., & Almeida, J. R. (1987). Estudos bionômicos de Dipetalogaster maximus (Ulher, 1984) (Hemiptera-Triatominae). II-Influencia da dieta sobre o ciclo biológico e resistência ao jejum. Memorias do Instituto Oswaldo Cruz, 82, 111–118.
Crespo, J. G., & Manrique, G. (2007). Mating behavior of the hematophagous bug Triatoma infestans: role of Brindley’s and metasternal glands. Journal of Insect Physiology, 53, 708–714.
De-Moraes, C. M., Wanjiku, C., Stanczyk, N. M., Pulido, H., Sims, J. W. et al. (2018). Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proceedings of the National Academy of Sciences, 115, 5780–5785.
Dujardin, J. P., Costa, J., Bustamante, D., Jaramillo, N., & Catalá, S. (2009). Deciphering morphology in Triatominae: the evolutionary signals. Acta Tropica, 110, 101–111.
Fuentes-Vicente, J. A., Gutiérrez-Cabrera, A. E., Flores-Villegas, A. L., Lowenberger, C., Benelli, G., Salazar-Schettino, P. M. et al. (2018). What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Tropica, 183, 23–31.
Gaunt, M., & Miles, M. (2000). The ecotopes and evolution of triatomine bugs (Triatominae) and their associated trypanosomes. Memorias do Instituto Oswaldo Cruz, 95, 557–565.
Gurevitz, J. M., Kitron, U., & Gürtler, R. E. (2009). Temporal dynamics of flight muscle development in Triatoma infestans (Hemiptera: Reduviidae). Journal of Medical Entomology, 46, 1021–1024.
Guzmán-Bracho, C. (2001). Epidemiology of Chagas disease in Mexico: an update. Trends in Parasitology, 17, 372–376.
Hotez, P. J., Dumonteil, E., Woc-Colburn, L., Serpa, J. A., Bezek, S., Edwards, M. S. et al. (2012). Chagas disease: “The New HIV/AIDS of the Americas”. Plos Neglected Tropical Diseases, 6, e1498.
Jiménez, M. L., & Palacios, C. (1999). Incidencia de la chinche piedrera (Dipetalogaster maximus) (Hemiptera: Heteroptera: Reduviidae) vector de Trypanosoma cruzi en zonas urbanas de La Paz, Baja California Sur, México. Anales del Instituto de Biologia, Universidad Nacional Autónoma de México, Serie Zoología, 70, 215–221.
Jiménez, M. L., Llinas, J., & Palacios, C. (2003). Infection rates in Dipetalogaster maximus (Reduviidae: Triatominae) by Trypanosoma cruzi in the Cape Region, Baja California Sur, Mexico. Journal of Medical Entomology, 40, 18–21.
Jurberg, J., Fagundes, L. M., & Barth, O. M. (1993). Morphological study of ovum and nymphs of Dipetalogaster maximus (Uhler, 1894) (Hemiptera, Reduviidae, Triatominae). Revista Brasileira de Biologia, 53, 269–283.
Krenn, H. W., & Aspöck, H. (2012). Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Structure and Development, 41, 101–118.
Lazzari, C. R. (1992). Circadian organization of locomotion activity in the hematophagous bug Triatoma infestans. Journal of Insect Physiology, 38, 895–903.
Lazzari, C. R., & Núñez, J. (1989). The response to radiant heat and the estimation of the temperature of distant sources in Triatoma infestans. Journal of Insect Physiology, 35, 525–529.
Manrique, G., & Lazzari, C. R. (1995). Existence of a sex pheromone in Triatoma infestans (Hemiptera: Reduviidae). I. Behavioural evidence. Memorias do Instituto Oswaldo Cruz, 90, 645–648.
Manrique, G., & Lorenzo, M. G. (2012). The sexual behaviour of Chagas’ disease vectors: chemical signals mediating communication between male and female Triatomine bugs. Psyche, 2012, 1–8.
Marsden, P. D., Cuba, C. C., Alvarenga, N. J., & Barreto, A. C. (1979). Report on a field collection of Dipetalogaster maximus (Hemiptera; Triatominae) (Uhler, 1894). Revista Instituto Medicina Tropical de São Paulo, 21, 202–206.
Mazzotti, L. (1970). Observations on Dipetalogaster maximus. Revista Instituto Medicina Tropical de São Paulo, 12, 320–324.
Mehlhorn, H. (2015). Encyclopedia of Parasitology. New York: Springer.
Miura, O., Kuris, A. M., Torchin, M. E., Hechinger, R. F., & Chiba, S. (2006). Parasites alter host phenotype and may create a new ecological niche for snail hosts. Proceedings of the Royal Society of London B: Biological Sciences, 273, 1323–1328.
Nattero, J., Piccinali, R. V., Lopes, C. M., Hernández, M. L., Abrahan, L., Lobbia, P. A. et al. (2017). Morphometric variability among the species of the sordida subcomplex (Hemiptera: Reduviidae: Triatominae): evidence for differentiation across the distribution range of Triatoma sordida. Parasites & Vectors, 10, 412–425.
Noireau, F., Diosque, P., & Jansen, A. M. (2009). Trypanosoma cruzi: adaptation to its vectors and its hosts. Veterianrian Research, 40, 1–23.
Pauw, A., Stofberg, J., & Waterman, R. J. (2009). Flies and flowers in Darwin’s race. Evolution, 63, 268–279.
Pontes, G. B., Bohman, B., Rikard, U. C., & Lorenzo, M. G. (2008). Metasternal gland volatiles and sexual communication in the triatomine bug, Rhodnius prolixus. Journal Chemical Ecology, 34, 450–457.
Pontes, G., Minoli, S., Insaurralde, I. O., de Brito-Sánchez, M. G. & Barrozo, R. B. (2014). Bitter stimuli modulate the feeding decision of a blood-sucking insect via two sensory inputs. Journal of Experimental Biology, 15, 3708–3717.
Preziosi, R. F., Fairbairn, D. J., Roff, D. A., & Brennan, J. M. (1996). Body size and fecundity in the waterstrider Aquarius remigis: a test of Darwin’s fecundity advantage hypothesis. Oecologia, 108, 424–431.
Roces, F., & Manrique, G. (1996). Different stridulatory vibrations during sexual behaviour and disturbance in the blood-sucking bug Triatoma infestans (Hemiptera: Reduviidae). Journal Insect Physiology, 42, 231–238.
Rojas-de Arias, A., Carbajal-De la Fuente, A. L., Gómez, A., Cecere, M. C., Rolón, M., Vega-Gómez, M. C. et al. (2017). Morphometric wings similarity among sylvatic and domestic populations of Triatoma infestans (Hemiptera: Reduviidae) from the Gran Chaco Region of Paraguay. The American Journal of Tropical Medicine and Hygiene, 97, 481–488.
Roque, A. L. R., Xavier, S. C., da-Rocha, M. G., Duarte, A. C. M., D’Andrea, P. S., & Jansen, A. M. (2008). Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. American Journal of Tropical Medicine and Hygiene, 79, 742–749.
Rubio, C., Moncada, L. I., Rojas, M. A., & García, A. (2013). Feeding behavior of Rhodnius robustus Larouse, 1927 (Hemiptera, Reduviidae) under laboratory conditions. Biomédica, 33, 205–213.
Ryckman, R. E., & Ryckman, A. E. (1967). Epizootiology of Trypanosoma cruzi in Southwestern North America part x: the biosystematics of Dipetalogaster maximus in Mexico (Hemiptera: Reduviidae)(Kinetoplastida: Trypanosomidae). Journal of Medical Entomology, 4, 180–188.
Schilman, P. E., Lazzari, C. R., & Manrique, G. (2001). Comparison of disturbance stridulations in five species of triatominae bugs. Acta Tropica, 79, 171–178.
Silva, M. B. A., Barbosa, H. S., Galvão, C., Jurberg, J., & Carcavallo, R. U. (2003). Comparative study of the stridulatory sulcus, buccula and rostrum of the nymphs of Triatoma guazu Lent & Wygodzinsky, 1979 and Triatoma jurbergi Carcavallo, Galvão & Lent, 1998 by scanning electron microscopy (Hemiptera, Reduviidae). Memorias do Instituto Oswaldo Cruz, 98, 335–344.
Téllez-García, A. A., Bello-Bedoy, R., Enríquez-Vara, J. N., Córdoba-Aguilar, A., & Gutiérrez-Cabrera, A. E. (In press). Genital morphology and copulatory behavior in triatomine bugs (Reduviidae: Triatominae). Arthropod Structure & Development.
Villalobos, G., Martínez-Ibarra, J. A., Martínez-Hernández, F., López-Alcaide, S., & Alejandre-Aguilar, R. (2012). The morphological variation of the eggs and genital plates of two morphotypes of Triatoma protracta Uhler, 1894. Journal of Vector Ecology, 37, 179–186.
Zacharias, C. A., Pontes, G. B., Lorenzo, M. G., & Manrique, G. (2010). Flight initiation by male Rhodnius prolixus is promoted by female odors. Journal of Chemical Ecology, 36, 449–451.