Joxmer Scott-Frías a, *, Fernando Cervigón b,
Evelyn Zoppi de Roa a, Eduardo Suárez-Morales c
a Universidad Central de Venezuela, Facultad de Ciencias, Instituto de Ecología y Zoología Tropical, Laboratorio de Ecología de Sistemas Acuáticos, Línea de Investigación del Plancton, Los Chaguaramos, Caracas 1053, Venezuela
b Museo Marino de Margarita, Boca de Río 6304, Nueva Esparta, Venezuela
c El Colegio de la Frontera Sur-Chetumal, Apartado postal 424, 77014 Chetumal, Quintana Roo, Mexico
*Corresponding author: joxmer@gmail.com (J. Scott-Frías)
Received: 21 September 2021; accepted: 21 September 2022
Abstract
The Southern Caribbean (SCA) represents the most productive ecoregion of the Tropical Northwestern Atlantic (TNWA) province. In order to assess the diversity of pelagic copepods in the ecoregion, we present an inventory based on unpublished data obtained from several oceanographic cruises made in oceanic and neritic waters of Venezuela (1967-1968). We complement this information with previous regional surveys to obtain a revised systematic checklist of the pelagic copepod species in the SCA. We included in our list a total of 346 species and 2 subspecies; up to 11 species represent new records. This study allowed us to: 1) expand the distributional range of some species within the TNWA, 2) record the occurrence, in the SCA, of all species known to 12 of 42 families to occur in the TNWA, 3) determine that the number of species of Corycaeidae and Paracalanidae in the SCA is greater than previously documented for the TNWA. Overall, the species accumulation curve resulting from our data analysis allowed us to determine that the diversity of pelagic copepods is underestimated; our estimations suggest that the potential species number that can be recorded in the SCA is 12 to 48% above the figure herein established.
Keywords: Pelagic copepods; Diversity; Marine zooplankton; Southern Caribbean; Tropical Northwestern Atlantic
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Diversidad de copépodos pelágicos (Crustacea: Copepoda) en el Caribe sur: evidencia de una asignación pendiente
Resumen
The Southern Caribbean (SCA) represents the most productive ecoregion of the Tropical Northwestern Atlantic (TNWA) province. In order to assess the diversity of pelagic copepods in the ecoregion, we present an inventory based on unpublished data obtained from several oceanographic cruises made in oceanic and neritic waters of Venezuela (1967-1968). We complement this information with previous regional surveys to obtain a revised systematic checklist of the pelagic copepod species in the SCA. We included in our list a total of 346 species and 2 subspecies; up to 11 species represent new records. This study allowed us to: 1) expand the distributional range of some species within the TNWA, 2) record the occurrence, in the SCA, of all species known to 12 of 42 families to occur in the TNWA, 3) determine that the number of species of Corycaeidae and Paracalanidae in the SCA is greater than previously documented for the TNWA. Overall, the species accumulation curve resulting from our data analysis allowed us to determine that the diversity of pelagic copepods is underestimated; our estimations suggest that the potential species number that can be recorded in the SCA is 12 to 48% above the figure herein established.
Keywords: Pelagic copepods; Diversity; Marine zooplankton; Southern Caribbean; Tropical Northwestern Atlantic
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Copepods are a group of microcrustaceans considered the most abundant metazoans on the planet, particularly the small-sized forms (Boxshall & Halsey, 2004; Huys & Boxshall, 1991; Turner, 2004). They are among the best studied organisms of the marine zooplankton (Campos-Hernández & Suárez-Morales, 1994). This interest is due to their importance within the dynamics of the marine ecosystem where they represent between 60 and 97% of the zooplankton biomass in neritic-coastal and oceanic areas (Björnberg, 1981; Bradford-Grieve et al., 1999). They are an essential part of the biological pump, which represents a key process to understand the carbon flux in the oceans, where the microbial decomposition of their remains contributes to the biogeochemical transformation of organic matter flowing into the deep sea (Glud et al., 2015).
Their trophic role is highly relevant and broad in the pelagic community, as the group includes omnivorous, herbivorous and carnivorous forms. Most are phytoplankton-consuming herbivores, thus playing a pivotal role in transferring this energy to higher trophic levels and showing a variety of adaptations that make them a highly successful group in the pelagic environments (Chen et al., 2018; Kiørboe, 2011). Therefore, fisheries worldwide depend, at least partly, on the biomass, distribution and trophic dynamics of the zooplankton community (Saiz et al., 2007), as copepods are the main food item for larval and juvenile fish (Tilley et al., 2016). Consequently, the diversity and distribution patterns of the planktonic copepods have been extensively studied (Piontkovski & Landry, 2003). These works are a current priority, as the plankton community is sensitive to processes related to global warming (Comeau et al., 2009; Rombouts et al., 2009). Despite its ecological and economic importance, the information on the biology and diversity of pelagic copepods carried in the Southern Caribbean ecoregion is scarce.
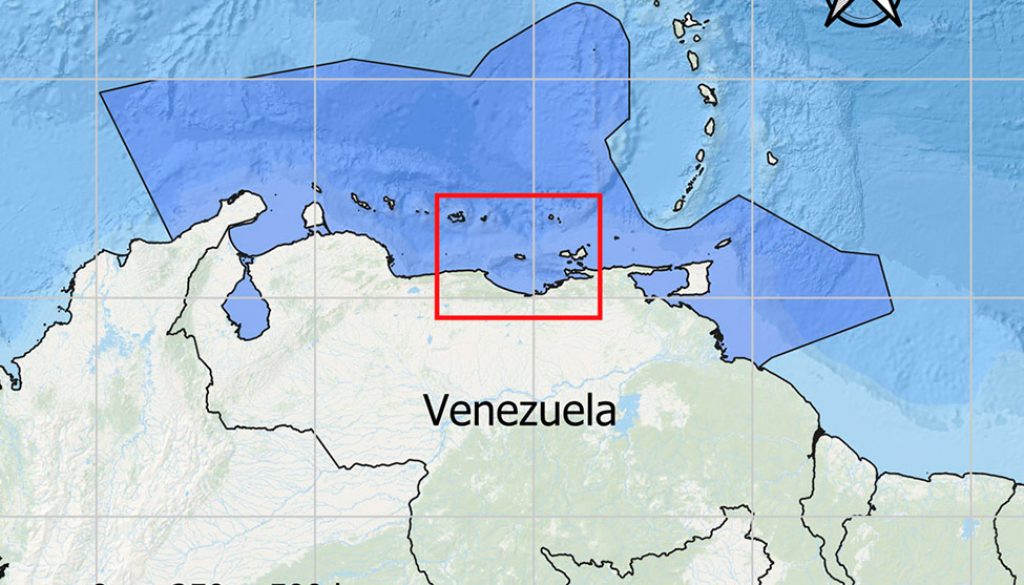
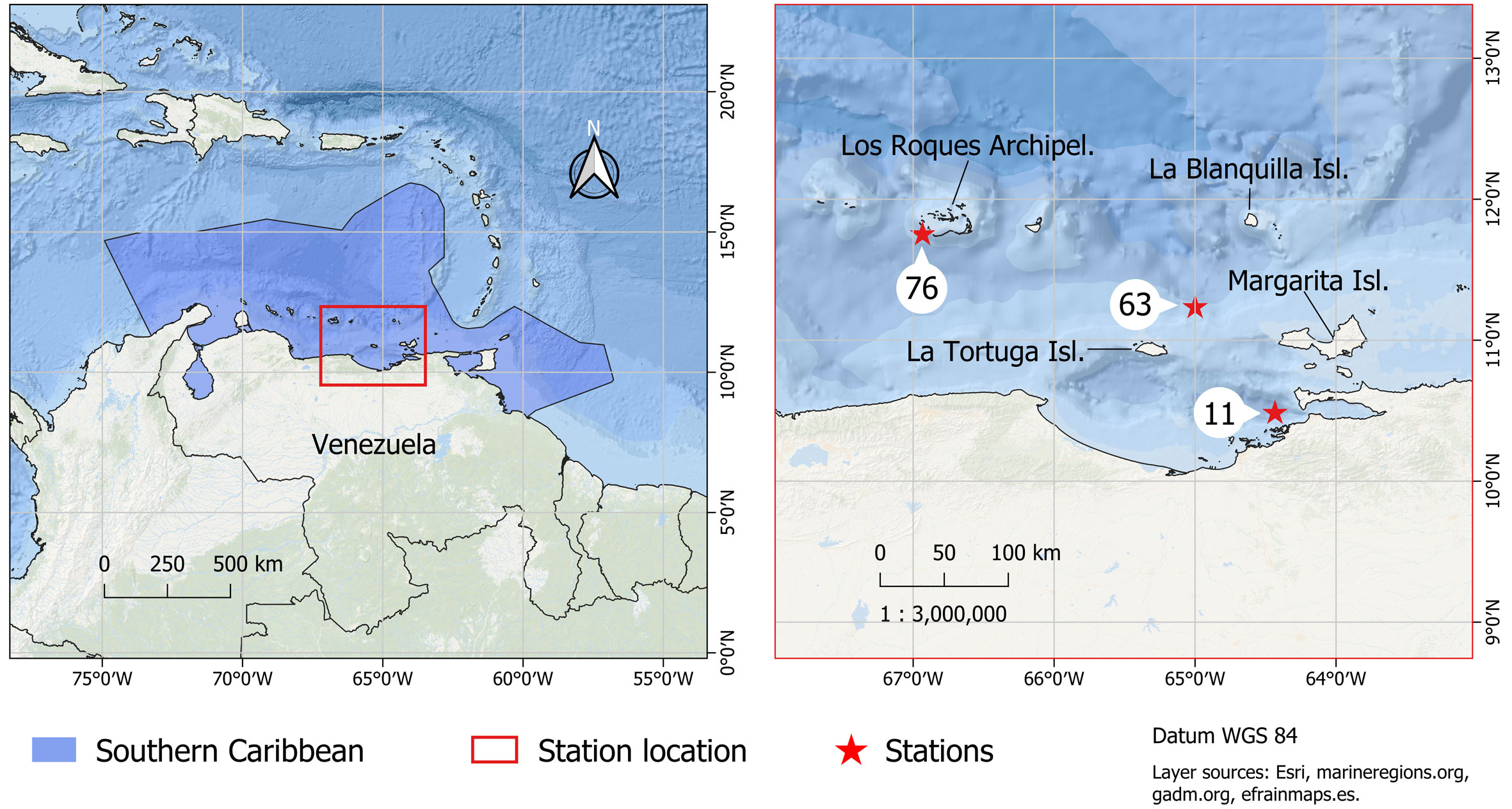
The Southern Caribbean (SCA) is a Marine Ecoregion of the World (MEOW) that belongs to the Tropical Northwestern Atlantic province (TNWA) or “Greater Caribbean” (Spalding et al., 2007, 2012). The SCA ecoregion is characterized by its high productivity, a condition resulting from prevalent coastal upwelling processes and nutrient inputs from the Orinoco and Amazon rivers (Castellanos et al., 2002; Muller-Karger & Varela, 1990; Okolodkov, 2003; Rueda-Roa et al., 2018). It includes the Exclusive Economic Zones (EEZs) of several Caribbean countries including: Aruba, Curaçao, Bonaire, Trinidad and Tobago, Venezuela and part of Colombia (La Guajira Peninsula). Venezuela’s EEZ is about 58% of the total area of the ecoregion, which extends to 16º44’49” N, because of Isla de Aves position (Flanders Marine Institute, 2021). The main physical and bioecological characteristics of the ecoregion were described by Villamizar and Cervigón (2017) and Correa-Ramírez et al. (2020).
Since its beginnings, several decades ago, research on the SCA area has focused on the neritic zone off eastern Venezuela (Cervigón, 1963; Legaré, 1961; Zoppi, 1961), and some other studies involved oceanic and mesopelagic environments of this area (i.e., MHI Archive, 1970; Owre & Foyo, 1964; Wilson, 1942), known to host an increased zooplankton biodiversity (Angel, 1993). The first diversity evaluations of the Venezuelan pelagic copepods yielded a total of 115 to 210 species (Miloslavich et al., 2005; Rodríguez & Suárez, 2003), but these data only incorporate general references. In a recent review for eastern Venezuela (Gulf of Cariaco), Márquez-Rojas et al. (2020) reported 136 copepod species, a figure that includes some parasitic forms found in the plankton. Contrastingly, the species richness of other, less abundant groups of planktonic crustaceans like amphipods and euphausiids is better documented in Venezuela (González-Cebrero et al., 2017; Miloslavich et al., 2005). This work aims to offer basic information on the diversity of the pelagic copepods from the SCA in order to expand the knowledge of the zooplankton community in this ecoregion and motivate future studies on this group of pelagic crustaceans.
Materials and methods
In this work we compiled unpublished data obtained in 6 oceanographic cruises carried out on board the M/S “La Salle” between September 1967 and July 1968. Three stations were visited (Fig. 1), station 11 near the Venezuelan coastline (10º28’60” N, 64º26’00” W), at the western part of the Cariaco Basin. Station 63 (11º13’60” N, 65º00’00” W), was located between La Tortuga and La Blanquilla islands, and station 76 (11º45’00” N, 66º56’00” W), south of Los Roques Archipelago.
During the oceanographic cruises, zooplankton samples were taken with a Clark-Bumpus collector (net No. 20, mesh size = 76 µm), performing horizontal hauls at 0, 25, 50, 100, 200, 500, and 800 m depths. In addition, vertical trawls were made from depths of 800, 1,000, or 1,200 m to the surface with a standard plankton net No. 2 (mesh size = 333 µm). All samples were obtained during the day between 09:00 and 18:00 h, preserved with 5% formaldehyde buffered with sodium borate and later processed for their taxonomic identification. The taxonomic keys, descriptions and illustrations by Rose (1933), Farran (1936), Mori (1937), Sewell (1947), Tanaka (1960), Vervoort (1963, 1965) and Owre & Foyo (1967) were used for initial taxonomic identification of the material. Additional sampling information, species composition and vertical distribution is available at https://doi.org/10.15468/jzyssw
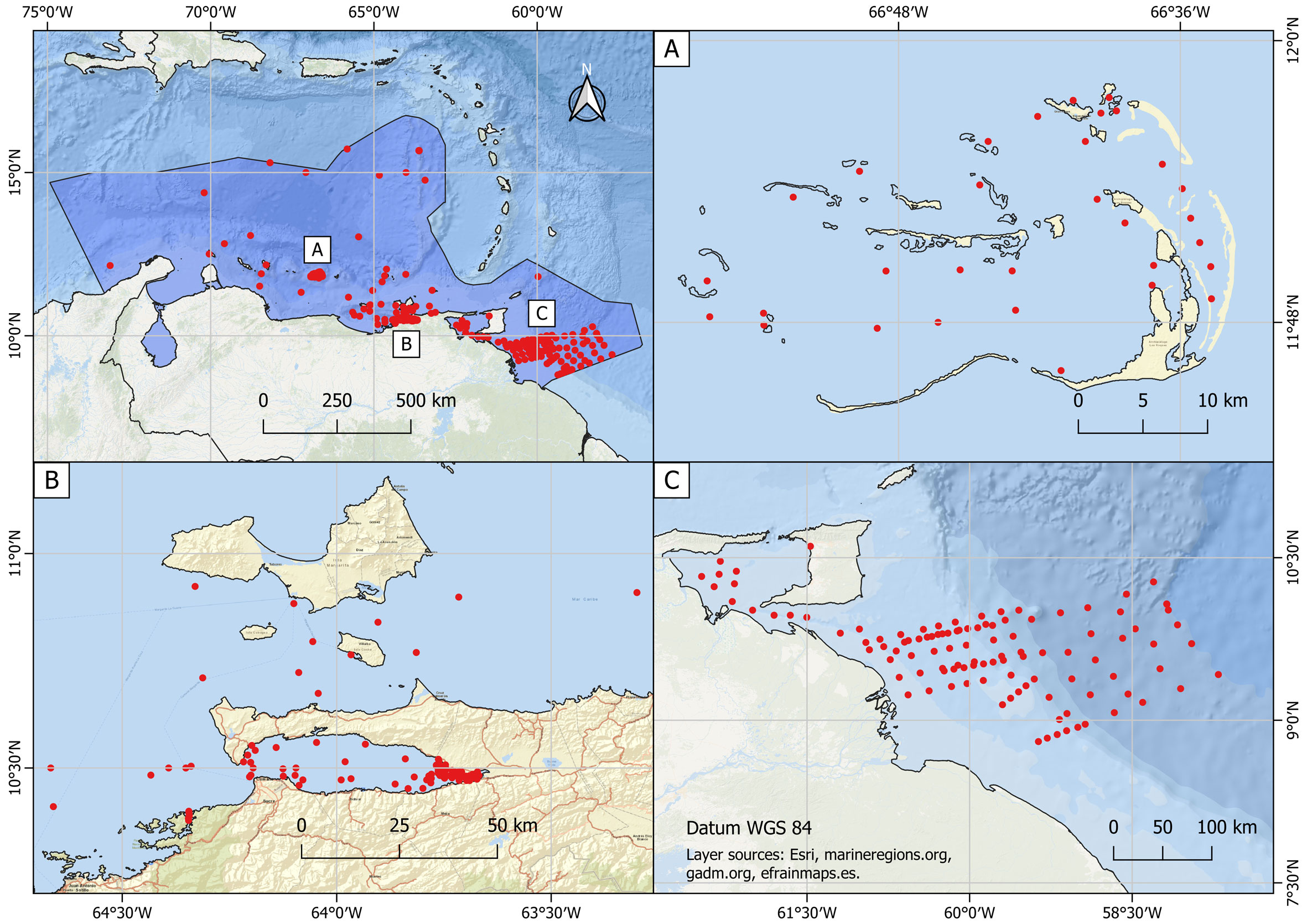
In order to compile this checklist of the pelagic copepod species from the SCA (Appendix), the information was supplemented by a review of the available regional literature, from which we selected 28 references including articles, technical reports and dissertations. References containing data already reported in the previous works selected for this review were excluded. The references are arranged chronologically, these studies were conducted under varying conditions and sampling gears (Table 1).
Taxonomic considerations
The copepod nomenclature and classification used were based on Walter and Boxshall (2021). The following considerations were taken into account during the list preparation: 1) 3 taxa with open nomenclature at the genus level were included: Saphirella sp., Oithona sp. 1 and 2, whose taxonomic status could not be established; 2) Ditrichocorycaeus affinis (McMurrich) is designated here as D. cf. affinis according to Márquez-Rojas et al. (2014b), awaiting a detailed revision of the material available in the collection; 3) the SCA records of the genus Saphirella consist of larval stages of the family Clausidiidae (Walter & Boxshall, 2021); their taxonomic position should be clarified in the future; 4) the current position of Paracalanus pygmaeus (Claus) and Oncaea gracilis (Dana) is uncertain, so they are included in the list with reservations; 5) Due to the difficulty in establishing its affinities with other species, Pachos punctatum (Claus) is provisionally included in Cyclopoida incertae sedis (Walter & Boxshall, 2021); 6) the families Clausidiidae, Corycaeidae, Lubbockiidae, Oncaeidae and Sapphirinidae belonging to the poecilostome lineage, are included in the order Cyclopoida following Khodami et al. (2017) and Walter and Boxshall (2021).
Species of the family Caligidae are not considered in this review, because it is a group with parasitic forms of fish that are often found in plankton samples (Ohtsuka et al., 2018). Additional data on the Caligidae diversity of the SCA can be obtained from the works by Ho and Bashirullah (1977), González et al. (1986), Lagarde (1989), Díaz (2000), Suárez-Morales et al. (2012), and Kim et al. (2019).
Other components of copepod diversity excluded from our analysis are the benthic and symbiotic forms. For example, this is the case of the siphonostomatoid Tychidion guyanense Humes, a symbiont associated with deep-water annelids from the Atlantic shelf (Humes, 1973) and the cyclopoid Lobosomatium enigmaticum Stock, a parasite of small polychaetes in Curaçao (Stock, 1995). Reid (1990) presented a detailed checklist that included benthic and demersal copepods present in the SCA (Aruba, Bonaire, Curaçao, Trinidad and Tobago, and coasts of South America). The benthic copepods diversity of the order Harpacticoida recorded in the Caribbean Sea was evaluated by Suárez-Morales et al. (2006).
In order to compare the number of species by family present in the SCA, which largely coincide with those documented in the TNWA (Venezuela, Caribbean Sea, Gulf of Mexico, Florida and Sargasso Sea), we used information from Razouls et al.’s (2021) online database.
Estimation of copepod diversity
Species richness is a widely used parameter to describe communities, thus providing a quick idea of the local alpha diversity in the area (Chiarucci et al., 2011; van der Spoel, 1994). In order to analyze the SCA pelagic copepod diversity, we used the program EstimateS v.9.1.0 (Colwell, 2013). From the selected references, the stations visited during each oceanographic cruise (Fig. 2) were established as the unit of sampling effort (n = 613). In this way, it was possible to prepare a dataset with the species incidence (Supplementary material) to estimate the species rarefaction curve, using 100 randomizations without replacement. We also integrate data from Wilson (1942), Bowman (1957) (Table 1, references 1, 2) and Owre and Foyo (1964, 1967) (references 8, 10), because the records come from the same samples. The Clench model (Soberón & Llorente, 1993) was used to calculate the fitted function of the accumulated curve, as:
S = (a * n) / (1 + b * n),
where S represents the species richness and n is the sampling effort. Values for a and b were obtained by non-linear estimation using the Simplex & quasi-Newton algorithm based on geometrical procedures to reduce the loss function. The values were calculated in the program STATISTICA v.10 (StatSoft, 2011). For the Clench model, the first derivative represents the slope of the curve: S’ = (a) / [(1 + b * n)]2. While the ratio (a/b) is the asymptote of the curve (Soberón & Llorente, 1993), so the species proportion recorded can be represented as:
q (%) = [(S) / (a/b) * 100)]
A complete survey of large marine areas is usually logistically infeasible, so rare species are frequently missed (Branco et al., 2018). One solution is to use species richness estimators (Chiarucci et al., 2011). Expected richness was estimated from the Bootstrap and second-order Jackknife non-parametric models, based on the rare species incidence such as “uniques” and “duplicates” (Colwell et al., 2004; Smith & van Belle, 1984). These estimators have the advantage of making no assumptions about the species abundance distribution, which reduces the bias (number of missing species) and thus allowing inferences about the least possible number of species present in the community (Branco et al., 2018).
Table 1
Selected references with information from pelagic copepods from the Southern Caribbean (SCA). Number (#) corresponds to the reference citation in the species list (Appendix). Some relevant conditions of the studies are provided, including the geographical position of the locality or sampling Station (Sta.).
| # | Reference | Studies conditions and locations |
| 1 | Wilson (1942) | Cruise VII (1928-1929), R/V Carnegie. Venezuela Basin, Sta. Nº 31, 14º46’00” N, 63º26’00” W. Sta. Nº 32, 15º18’00” N, 68º11’00” W. |
| 2 | Bowman (1957) | Wilson (1942) emendation, only new species description. Venezuela Basin, Sta. Nº 32, 15º18’00” N, 68º11’00” W. |
| 3 | Legaré (1961) | Cariaco project, Cruise 1960, R/V Guaiquerí. Gulf and Cariaco Basin, 10º27’45” to 10º33’34” N, 63º44’54” to 64º23’30” W. |
| 4 | Zoppi (1961) | Cariaco project, Cruise 1960, R/V Guaiquerí. Gulf and Cariaco Basin, 10º30’00” to 10º30’52” N, 63º58’49” to 64º21’06” W. |
| 5 | Cervigón (1963) | Cruise 1960, M/S La Salle. Margarita Island (Nueva Esparta State) and Cariaco Basin, 10º15’00” to 10º55’00” N, 63º45’00” to 65º10’00” W. |
| 6 | Cervigón (1964) | Cruises (1963-1964), M/S La Salle. Only Corycaeidae. Cariaco Basin, north of the Sucre State, Gulf of Paria (Venezuela) and north of Trinidad, 10º20’38” to 12º02’22” N, 63º13’03” to 65º26’02” W. |
| 7 | Legaré (1964) | Cruises (1960-1961), R/V Guaiquerí. Single Station. Cariaco Basin, 10º30’15” N, 64º20’22” W. |
| 8 | Owre and Foyo (1964) | Caribbean Sea Expedition “Carib”, R/V Spencer F. Baird. Venezuela Basin, Sta. CT-8, 15º00’00” N, 67º05’00” W, and Sta. CT-17, 14º55’00” N, 64º50’00” W. |
| Table 1. Continues | ||
| # | Reference | Studies conditions and locations |
| 9 | Cervigón and Marcano (1965) | Cruises (1962-1965), M/S La Salle. Cariaco Basin, north of the Sucre State (Venezuela), north of Trinidad, Gulf of Paria and Venezuelan Atlantic, 10º20’38” to 12º02’22” N, 63º13’03” to 65º26’02” W. |
| 10 | Owre and Foyo (1967) | Owre and Foyo (1964) update. Venezuela Basin, Sta. CT-8, 15º00’00” N, 67º05’00” W. Sta. CT-17, 14º55’00” N, 64º50’00” W. |
| 11 | MHI Archive (1970) | Cruise AV03, R/V Akademik Vernadskiy. Colombian Guajira, Bonaire, Cariaco and Venezuela Basin, 10º37’48” to 16º00’00” N, 65º35’60” to 73º04’48” W. |
| 12 | Owre and Foyo (1972) | Cruise P-6602, R/V John Elliott Pillsbury. Venezuela Basin, Sta. Nº 14, 15º00’00” N, 64º01’00” W. Venezuelan Atlantic, Sta. Nº 31, 10º01’00” N, 58º10’00” W. |
| 13 | Fleminger and Hulsemann (1974) | R/V Thomas Washington. Cumaná (Sucre State), Venezuela, Sta. Nº 8, 10º29’00” N, 64º12’00” W. |
| 14 | Zoppi de Roa (1977) | Cruises 1968-1970, R/V Gulf of Cariaco. Between Margarita Island, north of the Sucre State and Gulf of Paria, Venezuela, Sta. 6, 10º28’00” N, 62º18’00” W. Sta. M, 10º43’00” N, 63º07’00” W. |
| 15 | Pineda-Polo (1979) | February 1979, R/V USNS Bartlett. New species description. Cariaco Basin, Venezuela, Sta. 10º30’00” N, 64º40’00” W. |
| 16 | Ferrari and Bowman (1980) | Cruise 1977, R/V Alpha Helix. Only Oithonidae. Carupano harbor (Sucre State, Venezuela), Sta. PN-13-60, 10º41’12” N, 63º14’48” W. Netherlands Antilles, Bonaire, Sta. PN-14-60, 12º10’12” N, 68º18’12” W. Aruba, Sta. PN-18-60, 12º30’18” N, 70º02’42” W. |
| 17 | Walter (1989) | National Museum of Natural History collection, Smithsonian Institution (USNM) and others researchers. Only Pseudodiaptomidae. Trinidad and Venezuela, Sta. PP, 10º53’00” N, 64º06’00” W. Sta. TT, 10º36’19” N, 61º28’08” W. Sta. IC, 10º45’50” N, 63º58’00” W. |
| 18 | Zoppi de Roa and Palacios-Cáceres (2005) | Project “Estudio Geológico – Ambiental de la Fachada Atlántica”. Cruise October 2001, R/V ARBV “Punta Brava”. Venezuelan Atlantic, 08º48’12” to 10º16’36” N, 57º42’18” to 60º33’54” W. |
| 19 | Zoppi de Roa et al. (2007) | Project “Línea Base Ambiental Plataforma Deltana”. Cruises (2004-2005), R/V Hermano Ginés. Gulf of Paria and Venezuelan Atlantic, 09º14’03” to 10º22’29” N, 59º31’34” to 62º28’21” W. |
| 20 | Rojas-Márquez (2008) | CARIACO project, La Salle Foundation of Natural Sciences. Cruises 2006-2007, R/V Hermano Ginés. Single station, Cariaco Basin, Sta. 10º30’00” N, 64º40’00” W. |
| 21 | Morales (2014) | Campaigns 2012-2013. Single station, Guaracayal Depression, Gulf of Cariaco (Sucre State, Venezuela), Sta. 10º28’30” N, 63º58’00” W. |
| 22 | Casanova (2017) | Cruise Hydro-Oceanografic Isla de Aves I-2009, R/V ARBV “Punta Brava”. Isla de Aves, Venezuela, 15º39’50” to 15º40’29” N, 63º36’46” to 63º37’28” W. |
| 23 | Márquez-Rojas et al. (2021) | Campaigns 2003-2004. Only Corycaeidae. Gulf of Cariaco (Sucre State, Venezuela), 10º27’34” to 10º33’09” N, 64º04’44” to 64º13’02” W. |
| 24 | Márquez et al. (2021) | Campaigns 2009-2010. Gulf of Cariaco (Sucre State, Venezuela), 10º28’18” to 10º30’00”N, 63º39’50” to 63º43’42” W. |
| 25 | Casanova et al. (2021) | Campaign 2000. Los Roques Archipelago, Venezuela, 11º44’51” to 11º56’00” N, 66º35’00” to 67º54’20” W. |
| 26 | Camisotti and Pérez (2021) | Project “Estudio Geológico – Ambiental de la Fachada Atlántica”. Cruise October 2001, R/V ARBV “Punta Brava”. Venezuelan Atlantic, 08º48’12” to 10º16’36” N, 57º42’18” to 60º33’54” W. |
| 27 | Orrell and Informatics Office (2022) | May, 04, 1962. NMNH Extant Specimen Records (USNM, US). Single station, North Atlantic Ocean, Caribbean Sea. Sta. 4 BSP, 13º01’48” N, 65º28’12” W. |
| 28 | Segovia-Kancev et al. (2022) | Campaigns Mach 2016. Mochima Bay (Sucre State, Venezuela), 10º22’40” to 10º23’57” N, 64º20’38” to 64º20’45” W. |
Results
We determined that the SCA pelagic copepod community is represented by 5 orders, 42 families, 114 genera, 346 species and 2 subspecies (Appendix). The order Calanoida is the most species-rich (244 spp.), followed by Cyclopoida (88), and Harpacticoida (11). Only 3 species are reported of the orders Siphonostomatoida and Monstrilloida.
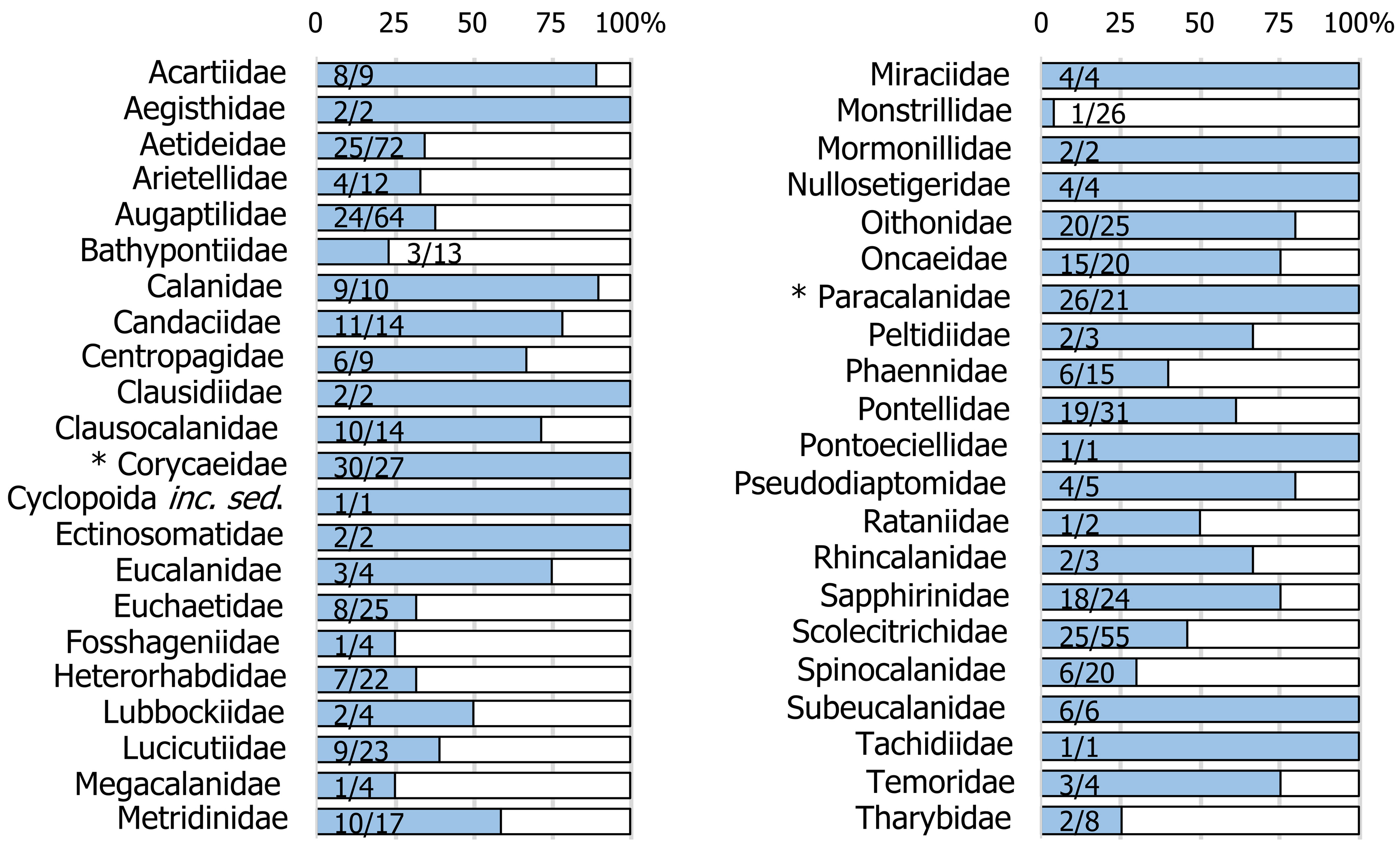
At the family level, the largest number of species found is contained in Corycaeidae (30 spp.), followed by Paracalanidae (26), Aetideidae (25), Scolecithricidae (25), and Augaptilidae (24). The most diverse genera in the SCA ecoregion are Oithona (19 spp.), Sapphirina (13), Calocalanus (12), Candacia (11), and Euaugaptilus (11). We recorded 2 subspecies: Rhincalanus cornutus atlanticus Schmaus and Oncaea venusta venella Farran, both reported in the SCA only once in different works (Owre & Foyo, 1967; MHI Archive, 1970).
From the literature data it was possible to assess the frequency in which the species were reported (Appendix). In the current inventory, only 40 species have been mentioned in more than 10 publications and can be considered frequent elements of the SCA pelagic copepod community. Eight of these species belong to the family Corycaeidae; Corycaeus speciosus is the species with the highest number of records, mentioned in 22 of the regional works consulted. It is followed by Clausocalanus arcuicornis and Temora stylifera, each with 20 reports. Up to 141 species (40.8% of our inventory) are linked to single reports.
This work includes 11 new records of copepods for the SCA ecoregion (Table 2). Most of the species not hitherto recorded were found at station 63, between La Tortuga and La Blanquilla islands. Among the new findings, the mesopelagic Mimocalanus cultrifer Farran, Arietellus setosus Giesbrecht, and Pseudoamallothrix ovata (Farran) had the highest number of specimens. The depth at which they were obtained is indicated for each record; most were found at depths greater than 200 m. Only Euchaeta pubera Sars and M. cultrifer were found above 100 m.
The comparison between the number of species by family recorded in the present study that concurs with those documented for the TNWA (i.e., Venezuela, Caribbean Sea, Gulf of Mexico, Florida, Sargasso Sea) is shown in figure 3. For 27 of the 42 families of copepods recorded in the Southern Caribbean, the number of species occurring in both areas exceeds 50%. Of these, the species of 12 families are represented by all those documented in the TNWA province. In the case of both Corycaeidae and Paracalanidae, the number of species documented in this work is greater than that reported for the TNWA province.
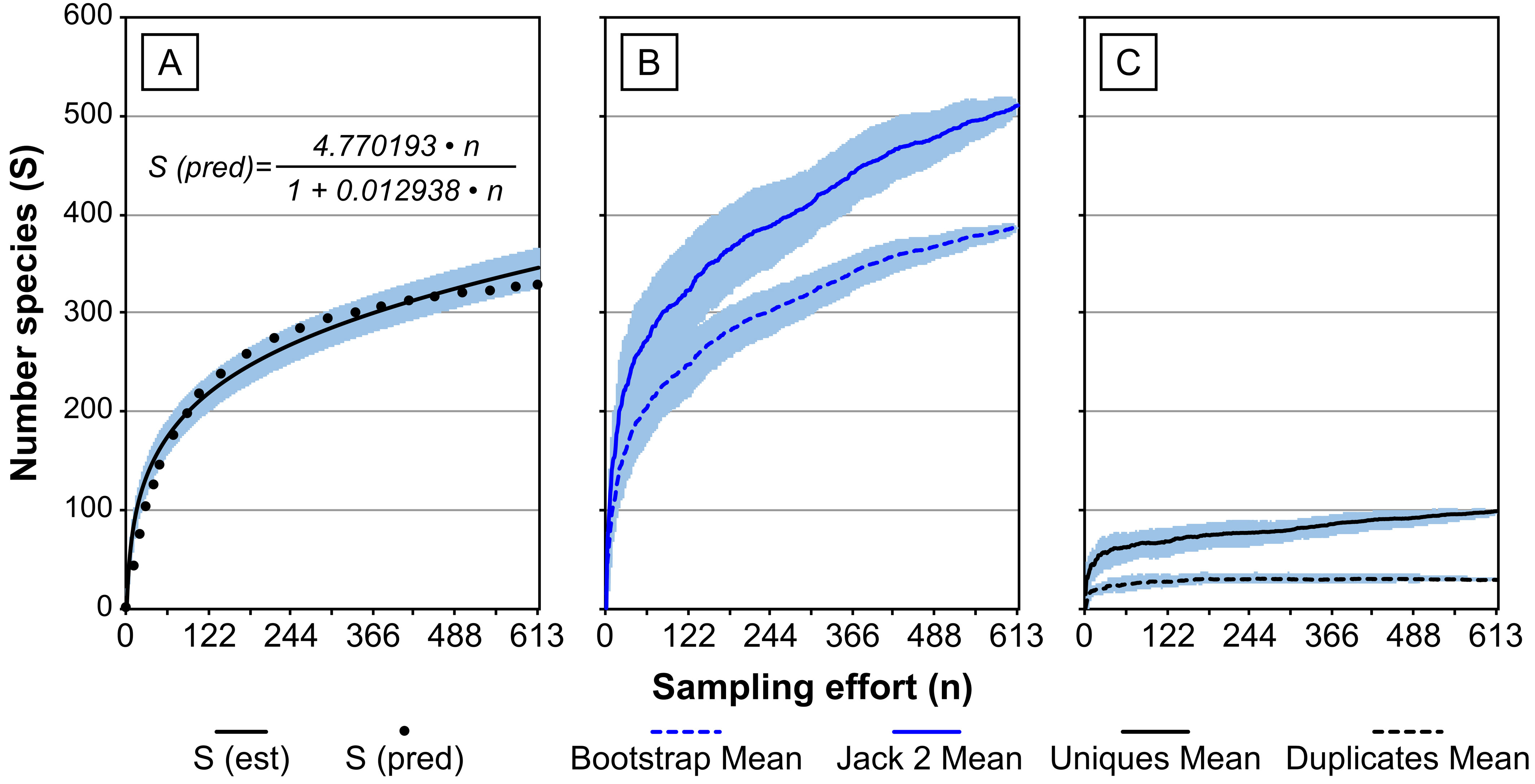
From the information compiled, it was possible to model the species rarefaction curve (Fig. 4A). With the selected data (n = 613), the greatest estimated species richness was S = 346.0 ± 10.5 (mean ± SD) which, as expected, coincides with observed species richness (346). With Clench’s model we obtained the fitted curve (R2 = 0.9593, n = 613) whose estimated asymptote (a/b) reaches 368.7 species. Thus, we determined that the theoretical observed richness (q%) represents 93.9% of the inventory of species reported in the SCA. The value of the calculated slope S’ is 0.06 species by sampling unit.
Table 2
New records of pelagic copepods for the Southern Caribbean (SCA). Most copepods were captured with the giant Clark-Bumpus sampler, the asterisk (*) indicates vertical tows with a plankton net. Date: year-month-day. CV = Copepodite stage V. The other specimens are adults.
| Specie | Specimen | Station | Date | Depth (m) |
| Arietellus setosus Giesbrecht, 1893 | 1 ♂ | 63 | 1968-03-28 | 200 |
| 1 CV | 63 | 1967-04-10 | 200 | |
| 1 CV | 63 | 1968-01-31 | 1,000 | |
| 1 ♂ | 76 | 1967-11-25 | 200 | |
| Candacia elongata (Boeck, 1872) | 1 ♀ | 63 | 1967-12-08 | 500 |
| Candacia tenuimana (Giesbrecht, 1889) | 1 ♀ | 63 | 1967-08-12 | 500 |
| Euchaeta pubera Sars G.O., 1907 | 1 ♀ | 63 | 1967-08-12 | 50 |
| Euchirella maxima Wolfenden, 1905 | 1 ♀ | 63 | 1967-08-12 | *1,000-0 |
| Mimocalanus cultrifer Farran, 1908 | 7 ♀ | 63 | 1967-08-12 | 100 |
| Paraeuchaeta barbata (Brady, 1883) | 1 ♀ | 63 | 1967-08-12 | *1,000-0 |
| 1 ♀ | 63 | 1968-01-31 | 1,000 | |
| Paraeuchaeta bisinuata (Sars, 1907) | 1 ♀ | 63 | 1967-08-12 | *1,000-0 |
| Pleuromamma borealis Dahl, 1893 | 1 ♀ | 63 | 1968-01-31 | 1,000 |
| Pontoeciella abyssicola (Scott, 1893) | 1 ♀ | 63 | 1967-08-12 | 500 |
| Pseudoamallothrix ovata (Farran, 1905) | 1 ♀ | 76 | 1968-01-30 | 200 |
| 1 ♀ | 76 | 1968-01-30 | 1,000 | |
| 1 ♀ | 76 | 1968-03-27 | 500 |
The most conservative estimator of richness was the Bootstrap, with 387.5 species expected (n = 613), whereas the most extreme value was obtained for Jack 2 (second-order Jackknife) with 511.7 expected species. These values predict that the inventory could increase by 12 to 48% over the currently reported figure (Fig. 4B). The curves of these estimators show a clear increasing tendency, mostly related to rare species incorporation (i.e., “uniques” and “duplicates”) (Fig. 4C). In the case of species that appear only once in the sample (“uniques”), we noticed that their number increases proportionately to the sampling effort (98 spp.), thus representing 28.3% of the biodiversity to be evaluated, whereas the species appearing twice (“duplicates”) stabilize at 30 species and progressively decrease with increasing sampling effort.
Discussion
Diversity of pelagic copepods in the Southern Caribbean (SCA)
The diversity found in this work (346 species and 2 subspecies) represents a substantial increase compared to previous reports made in the SCA ecoregion (Márquez-Rojas et al., 2020; Miloslavich et al., 2005; Rodríguez & Suárez, 2003). This value corresponds to 48% of the 724 copepods species documented by Razouls et al. (2021) in the TNWA province (including Venezuela, western Caribbean Sea, Gulf of Mexico, Florida and Sargasso Sea). The copepod species richness found differs numerically from similar inventories conducted in other ecoregions of the TNWA province. For example, 216 species have been recorded in Florida and adjacent waters (Owre & Foyo, 1967), 201 in the Western Caribbean (Mexican Caribbean) (Suárez-Morales & Gasca, 1998), and 247 in the Southwestern Caribbean (Colombian Caribbean), as reported by Medellín-Mora and Navas (2010), and subsequently updated by Gaviria et al. (2019) and Dorado-Roncancio et al. (2021). For the Gulf of Mexico, the 335 species reported represent a value close to that found here and as expected, the species composition between these ecoregions have similarities (Campos-Hernández & Suárez-Morales, 1994; Suárez-Morales et al., 2009). Following Reid’s (1990) review of the Gulf of Mexico and the Caribbean Sea pelagic copepods, we recognize that these ecoregions share a total of 155 species (44.7%) with the present work. While species reported here in 12 families for the Southern Caribbean coincide with all those documented in the Tropical Northwestern Atlantic (Razouls et al., 2021).
It is recognized that 40 species are often reported, so that, as a whole, they can be considered characteristics of the ecoregion community. Corycaeidae species have been continuously reported in the literature, which includes tropical and subtropical species (Campos-Hernández & Suárez-Morales, 1994; Owre & Foyo, 1967), which are very frequent and sometimes abundant in the eastern region of Venezuela (Cervigón, 1964; Márquez-Rojas et al., 2014a, 2014b). This has been attributed to the great adaptive capacity of these copepods under the changing conditions of coastal areas (Álvarez-Cadena et al., 1998; Björnberg, 1981; Suárez, 1989) and their affinity to tropical water masses (Márquez-Rojas et al., 2014a).
The greatest diversity of marine copepods species in the Atlantic is found in the tropical and subtropical latitudes (Piontkovski & Landry, 2003; Rombouts et al., 2009; Wilson, 1942), a pattern also valid for other pelagic groups like: fish, ostracods, decapods and euphausiids (Angel, 1993). The SCA is considered the area with the highest nutrient input in the TNWA because of the widespread upwelling processes along its coastline (Castellanos et al., 2002; Correa-Ramírez et al., 2020; Okolodkov, 2003; Rueda-Roa et al., 2018; Villamizar & Cervigón, 2017), which adds to the huge nutrient input from the Orinoco and Amazon rivers (Muller-Karger & Varela, 1990; Rueda-Roa et al., 2018). This condition may promote species diversity because of the increased energy flow of these ecosystems (Saiz et al., 2007). While for oceanic environments, Angel (1993) indicates that the high values in the pelagic species numbers are usually associated with oligotrophic conditions. Consistent with this pattern, most of the rare species and new reports in the SCA resulted from studies conducted in oceanic environments and mesopelagic samples (MHI Archive, 1970; Owre & Foyo, 1964; Park, 1970; Wilson, 1942). In these areas, the greatest diversity can be reached at depths close to 1000 m, well below the depth where the ecological processes supporting it take place (Angel, 1993), it may therefore be appropriate to evaluate how these conditions interact and influence the diversity of the pelagic components of the SCA. Unfortunately, studies of the deep-water pelagic fauna of tropical latitudes are still scarce, it is expected that future work in these strata will allow us to expand our knowledge of the marine diversity of the ecoregion.
Quantitative tools application for biodiversity estimation allow to test the accuracy species inventories (Colwell et al., 2004; Soberón & Llorente, 1993; van der Spoel, 1994). They improve the confidence in the methodologies employed and allow proving adequate sample sizes for analysis (Branco et al., 2018). Our analysis is deemed as an adequate approximation of the SCA pelagic copepods diversity, as indicated by the 93.9% of the expected inventory and the low slope value obtained (i.e., species incorporation rate by sampling unit S’ = 0.06). Jiménez-Valverde and Hortal (2003) stated that, in order to consider that the inventory is representative of the species diversity, it is necessary to obtain a slope of less than 0.10 species by sampling unit. It has been hitherto reported that community attributes, such as spatial aggregation or uneven distribution of abundance (both characteristics typical of planktonic communities) tend to weaken the predictive trait of non-parametric estimators (Branco et al., 2018; Jiménez-Valverde & Hortal, 2003; Reese et al., 2014). The values obtained in the present study from non-parametric estimators show that the present copepod inventory for the SCA is underestimated between 12 and 48%. This is shown by the increase in the number of rare species (“uniques” and “duplicates”) that are incorporated to the inventory at increasing sampling efforts. Although another characteristic of pelagic ecosystems is their high proportion of rare species (Angel, 1993), this fact must be weighted when predicting species richness using non-parametric estimators. These rare species can also be considered transient members of the community resulting from local dispersal (McManus & Woodson, 2012; van der Spoel, 1994).
Distribution of copepods in the Southern Caribbean (SCA)
Large Marine Ecosystems (LME) arrangement is based on coastal areas classification and continental shelf (neritic zone) (Spalding et al., 2007, 2012). On the other hand, the biogeographic approaches to the mesopelagic fauna proposed by Sutton et al. (2017) we consider may be more suitable to understand the ecological processes that determine the structure and distribution of the zooplankton community in the SCA. According to this biogeographic classification, the Tropical Northwestern Atlantic (TNWA) province (Greater Caribbean) is part of a larger ecoregion called the Central North Atlantic, bounded between the North Atlantic Drift and the Equatorial Atlantic ecoregions. In contrast to the LME classification, where the SCA ecoregion is geographically well delimited, based on Sutton et al. (2017), it is possible to consider that distribution in some planktonic species present greater geographic amplitude, extending throughout the Central North Atlantic ecoregion, as they are influenced by factors such as the physiology of organisms, and environmental factors such as the water mass transport, temperature and salinity (Angel, 1993; McManus & Woodson, 2012; Rombouts et al., 2009). For example, the South Atlantic (Bradford-Grieve et al., 1999) shares 47.8% of the species reported in this work, despite presenting differentiable environmental characteristics such as oligotrophic waters and complex current circulation patterns (Sutton et al., 2017).
The similarity in species composition with contiguous areas, such as the Equatorial Current, has been previously hypothesized (Björnberg, 1981). This is possible due to the relationship between hydrographic regime patterns between major ocean basins (Angel, 1993). In addition, biogeographic boundaries may be asymmetric/semi-permeable by concerning to specific components of the planktonic community (Sutton et al., 2017). In this sense, the Amallothrix tenuiserrata, Eucalanus elongatus and Pareucalanus attenuatus distribution is documented in different works conducted in the SCA and areas adjacent to the Tropical Northwestern Atlantic (TNWA) province (Cervigón & Marcano, 1965; Legaré, 1964; MHI Archive, 1970; Owre & Foyo, 1964, 1972). For this reason, we consider the inclusion of these species justified as part of the biota of the TNWA. Rendón et al. (2003), suggest that E. elongatus may be an introduced species, indicating that it is present in ship ballast waters in Cartagena Bay, Colombia (Southwestern Caribbean). This statement is in contrast to the records of the species in the province (Casanova et al., 2007; Cervigón & Marcano, 1965; MHI Archive, 1970; Owre & Foyo, 1964, 1967; Wilson, 1942; Zoppi de Roa et al., 2007), which we consider enough to rule out that it is an introduced species and instead consider the species part of pelagic copepods community of the TNWA province.
On the other hand, reports of the following species have been eventual: Calanus propinquus, Calocalanus elongatus, C. gresei, C. kristalli, C. longisetosus, C. neptunus, C. pseudocontractus, Candacia tenuimana, Canthocalanus pauper, Cymbasoma rigidum, Ditrichocorycaeus cf. affinis, D. americanus, D. subtilis, Euchirella formosa, Farranula curta, F. gibbula, Labidocera wollastoni, Lucicutia grandis, Oithona fallax, Oncaea gracilis, Onychocorycaeus pumilus, Paracalanus pygmaeus, Pontella lobiancoi, P. mediterranea, Sapphirina maculosa, Scolecitrichopsis tenuipes (Márquez-Rojas et al., 2014a; MHI Archive, 1970; Owre & Foyo, 1964; Wilson, 1942). Despite these few specific reports, the documented distribution areas for the species are contiguous to the TNWA (Razouls et al., 2021), which may explain their presence in the SCA. The opposite is the case for Ditrichocorycaeus andrewsi (Farran), which is not included in the present list because its known natural distribution is centered in the Western Pacific, with some reports from the Indian and Eastern Pacific (GBIF.org, 2021; Razouls et al., 2021). We do not rule out the species presence in the Gulf of Cariaco (Márquez-Rojas et al., 2020; Morales, 2008), but we await its confirmation in the TNWA or adjacent provinces.
Information gaps and perspectives
The underestimation in the number of species mentioned above is notorious in the documented reports of genera in the SCA that so far have not been associated with species present in the TNWA, as is the case with the benthopelagic and bipolar Bradyidius (Legaré, 1964) and Monstrilla (Cervigón & Marcano, 1965). Legaré (1961) indicates that 8 to 10 undetermined species of the order Harpacticoida have been found in samples, although most of them probably correspond to demersal species.
The families Oithonidae and Paracalanidae stand out due to their species richness and unique reports (Ferrari & Bowman, 1980; MHI Archive, 1970). In particular, identification of these species has represented a difficulty and they correspond to groups in which greater efforts must be made to evaluate their real diversity. Oithona hebes reported by Ferrari and Bowman (1980) has also been recorded in brackish waters of the coastal lagoon of Tacarigua (Zacarías & Zoppi de Roa, 1981), an environment in which 2 or more species of the genus are presumed to be present. Other examples of the taxonomic difficulty associated with Oithona species is evidenced in the work of Cervigón (1963) and more recently by Segovia-Kancev et al. (2022), who reports an aggregate of unidentified species belonging to the genus. Regarding Paracalanus, Reid (1990) indicates that there is a probability that P. indicus has been misidentified in the literature as P. parvus and P. quasimodo. Therefore, we do not rule out the possibility that this may be the case in some of the historical reports and we consider of interest the more detailed evaluation of this genus in the Southern Caribbean. In order to support species presence with eventual reports, we urge the various research groups working in the ecoregion to confirm and incorporate the specimens in biological collections during future activities.
Finally, we consider that knowledge of meso and bathypelagic copepods are in the early stages of development; a study of these communities in the Gulf of Mexico and the Northwestern Caribbean provided 58 new records of planktonic copepods for these ocean basins (Park, 1970), while more recently Dorado-Roncancio et al. (2021) reported 33 new records for the Southwestern Caribbean (Colombian Caribbean). Most research on plankton in the Southern Caribbean (SCA) has focused on the neritic zone east of Venezuela. Therefore, to increase the knowledge of the community and associated ecological processes, it is necessary to extend the research area to the western and oceanic zone of the SCA. This work was intended to cover the deficit in the pelagic copepod information in the SCA, which we consider of major importance to carry out with intention of facilitating additional studies of the group. We hope that this work represents a starting point for documentation of species with few records and that it allows a better understanding of the associated oceanographic processes, due to the great relevance of ecological studies in meso and bathypelagic waters for global changes evaluation.
From the information analyzed, we can conclude that the value we give for pelagic copepods diversity is an adequate reflection of the current documented knowledge. However, as suggested by the richness estimators, it is very likely that the actual diversity of pelagic copepods is underestimated. One element that supports this fact is the high number of rare species, typical of pelagic ecosystems. The most conservative value of expected richness was obtained from the non-parametric Bootstrap estimator, while Jack 2 (second-order Jackknife) expresses the most extreme increase in expected richness. In the pelagic copepod community of the Southern Caribbean, the members of Corycaeidae stand out with a significant number of species that are often reported. We consider that presence of Amallothrix tenuiserrata, Eucalanus elongatus and Pareucalanus attenuatus in the Tropical Northwestern Atlantic is supported by documented reports from the ecoregion.
Acknowledgments
This publication is a posthumous tribute to the professors and researchers of pelagic ecosystems, Evelyn Zoppi de Roa (1931-2019) and Fernando Cervigón (1930-2017). It was Fernando Cervigón’s wish to thank the John Simon Guggenheim Memorial Foundation, who granted him the scholarship during 1967-1968 by which it was possible to obtain the samples for this work. We would also like to thank Pablo J. Rodríguez, who collaborated in the dissections of the copepods. To the Fundación La Salle de Ciencias Naturales, for the logistic support during the expeditions carried out in the Motor Ship “La Salle”. To Jeannette Pérez for her excellent and careful organization of the data. We would like to thank the anonymous reviewers for their valuable contributions that allowed us to improve the quality of this work.
Appendix. Systematic list of copepods species registered in the Southern Caribbean (SCA). The number corresponds to the reference where the species was reported (Table 1). The dot [●] indicates reports of the species in the present work (stations on Cariaco Basin, La Tortuga and La Blanquilla Islands, and Los Roques Archipelago 1967-1968).
| Subclass Copepoda Milne-Edwards, 1840 | References |
| Infraclass Neocopepoda Huys & Boxshall, 1991 | |
| Superorder Gymnoplea Giesbrecht, 1882 | |
| Order Calanoida Sars, 1903 | |
| Family Acartiidae Sars, 1900 | |
| Acartia (Acanthacartia) spinata Esterly, 1911 | 9, 20, 21, 22, 25 |
| Acartia (Acanthacartia) tonsa Dana, 1849 | 5, 9, 21, 24, 28 |
| Acartia (Acartia) danae Giesbrecht, 1889 | 3, 5, 7, 8, 9, 11, 19, 24, ● |
| Acartia (Acartia) negligens Dana, 1849 | 8, 11, 12, 25, 28 |
| Appendix. Continues | |
| Subclass Copepoda Milne-Edwards, 1840 | References |
| Acartia (Acartiura) bermudensis Esterly, 1911 | 9 |
| Acartia (Acartiura) clausi Giesbrecht, 1889 | 3, 7, 14 |
| Acartia (Acartiura) longiremis (Lilljeborg, 1853) | 1 |
| Acartia (Odontacartia) lilljeborgi Giesbrecht, 1889 | 3, 4, 7, 9, 19, 20, 24, 25, 28, ● |
| Family Aetideidae Giesbrecht, 1893 | |
| Aetideopsis multiserrata (Wolfenden, 1904) | 8, 9, ● |
| Aetideus acutus Farran, 1929 | 7, 9, ● |
| Aetideus armatus (Boeck, 1872) | 1, 5, 7, 8, 9, 11, 12, ● |
| Aetideus bradyi Scott A., 1909 | 9 |
| Aetideus giesbrechti Cleve, 1904 | 3, 4, 7, 8, 9, 11, ● |
| Chiridius poppei Giesbrecht, 1893 | 9, ● |
| Chirundina streetsii Giesbrecht, 1895 | 9, 12, ● |
| Euchirella amoena Giesbrecht, 1888 | 7, 8, 9, 11, 12, 14, 22, ● |
| Euchirella bitumida With, 1915 | 8 |
| Euchirella curticauda Giesbrecht, 1888 | 8, 12, ● |
| Euchirella formosa Vervoort, 1949 | 9 |
| Euchirella maxima Wolfenden, 1905 | ● |
| Euchirella messinensis (Claus, 1863) | 8, ● |
| Euchirella pulchra (Lubbock, 1856) | 8, 9, 12, ● |
| Euchirella rostrata (Claus, 1866) | 9, 21, 24 |
| Euchirella venusta Giesbrecht, 1888 | 8, ● |
| Gaetanus miles Giesbrecht, 1888 | 8, 9, 12, ● |
| Gaetanus minor Farran, 1905 | 8, 9, 11, ● |
| Paivella inaciae Vervoort, 1965 | 9, ● |
| Pseudeuchaeta brevicauda Sars G.O., 1905 | 9 |
| Pseudochirella obesa Sars G.O., 1920 | 8 |
| Undeuchaeta major Giesbrecht, 1888 | 8, 11, ● |
| Undeuchaeta plumosa (Lubbock, 1856) | 8, 11, 12, ● |
| Valdiviella brevicornis Sars G.O., 1905 | 8 |
| Valdiviella insignis Farran, 1908 | 8, 12 |
| Family Arietellidae Sars, 1902 | |
| Arietellus armatus Wolfenden, 1911 | 8 |
| Arietellus giesbrechti Sars G.O., 1905 | 8 |
| Arietellus setosus Giesbrecht, 1893 | ● |
| Arietellus simplex Sars G.O., 1905 | 8, 12 |
| Family Augaptilidae Sars, 1905 | |
| Augaptilus longicaudatus (Claus, 1863) | 9, 12, ● |
| Centraugaptilus horridus (Farran, 1908) | 8 |
| Euaugaptilus bullifer (Giesbrecht, 1889) | 10 |
| Euaugaptilus fosaii Pineda-Polo, 1979 | 15 |
| Euaugaptilus hecticus (Giesbrecht, 1893) | 7, 8, 9, 11, 12, ● |
| Euaugaptilus laticeps (Sars G.O., 1905) | 10 |
| Euaugaptilus latifrons (Sars G.O., 1907) | 8 |
| Euaugaptilus magnus (Wolfenden, 1904) | 8 |
| Euaugaptilus nodifrons (Sars G.O., 1905) | 9, 12 |
| Euaugaptilus oblongus (Sars G.O., 1905) | 10 |
| Euaugaptilus palumboi (Giesbrecht, 1889) | 9 |
| Euaugaptilus rigidus (Sars G.O., 1907) | 8 |
| Euaugaptilus tenuispinus Sars G.O., 1920 | 12 |
| Haloptilus acutifrons (Giesbrecht, 1893) | 3, 4, 7, 8, 9, 12, ● |
| Haloptilus austini Grice, 1959 | 9 |
| Haloptilus fertilis (Giesbrecht, 1893) | 9 |
| Haloptilus longicirrus Brodsky, 1950 | 9 |
| Haloptilus longicornis (Claus, 1863) | 3, 7, 8, 9, 11, 12, 14, 19, 20, 22, ● |
| Haloptilus mucronatus (Claus, 1863) | 11, 18 |
| Haloptilus ornatus (Giesbrecht, 1893) | 1, 9, 11, 18, ● |
| Haloptilus paralongicirrus Park, 1970 | 11 |
| Haloptilus spiniceps (Giesbrecht, 1893) | 8, 9, 11, 19, 22, 26, ● |
| Heteroptilus acutilobus (Sars G.O., 1920) | 10 |
| Pseudhaloptilus eurygnathus (Sars G.O., 1920) | 12 |
| Family Bathypontiidae Brodsky, 1950 | |
| Temorites brevis Sars G.O., 1900 | 27 |
| Temorites elongata (Sars G.O., 1905) | 12 |
| Temorites minor (Wolfenden, 1906) | 8 |
| Family Calanidae Dana, 1846 | |
| Calanus helgolandicus (Claus, 1863) | 1 |
| Calanus propinquus Brady, 1883 | 1 |
| Canthocalanus pauper (Giesbrecht, 1888) | 11 |
| Cosmocalanus darwinii (Lubbock, 1860) | 26 |
| Mesocalanus tenuicornis (Dana, 1849) | 7, 9, 11, ● |
| Nannocalanus minor (Claus, 1863) | 1, 3, 4, 5, 7, 8, 9, 11, 12, 14, 18, 19, 20, 21, 22, 26, 28, ● |
| Neocalanus gracilis (Dana, 1852) | 3, 5, 7, 8, 9, 11, 12, 18, 20, 22, 25, 26, 28, ● |
| Neocalanus robustior (Giesbrecht, 1888) | 8, 9, 11, 19, 21, ● |
| Undinula vulgaris (Dana, 1849) | 1, 5, 7, 8, 9, 11, 12, 14, 18, 19, 20, 22, 25, 26, 28, ● |
| Family Candaciidae Giesbrecht, 1893 | |
| Candacia bipinnata (Giesbrecht, 1889) | 5, 7, 9, 18, 25, ● |
| Candacia bispinosa (Claus, 1863) | 1, 8, 9, 11, ● |
| Candacia curta (Dana, 1849) | 3, 4, 5, 7, 8, 9, 11, 14, 20, 22, 25, 26, ● |
| Candacia elongata (Boeck, 1872) | ● |
| Candacia longimana (Claus, 1863) | 9, 11, 12, 19, ● |
| Candacia norvegica (Boeck, 1865) | 1 |
| Candacia pachydactyla (Dana, 1849) | 1, 3, 5, 7, 8, 9, 11, 12, 18, 19, 20, 21, 22, 25, 26, ● |
| Candacia paenelongimana Fleminger & Bowman, 1956 | 28, ● |
| Candacia simplex (Giesbrecht, 1889) | 1, 7, 9, 11, 18, ● |
| Candacia tenuimana (Giesbrecht, 1889) | ● |
| Candacia varicans (Giesbrecht, 1893) | 3, 7, 9, 18, 19, ● |
| Family Centropagidae Giesbrecht, 1893 | |
| Centropages bradyi Wheeler, 1900 | 18, 19 |
| Centropages caribbeanensis Park, 1970 | 11 |
| Centropages furcatus (Dana, 1849) | 1, 3, 4, 5, 7, 8, 9, 11, 14, 21, 24, ● |
| Centropages hamatus (Lilljeborg, 1853) | 1 |
| Centropages velificatus (Oliveira, 1947) | 18, 19, 20, 21, 24, 25, 26, 28 |
| Centropages violaceus (Claus, 1863) | 5, 7, 8, 9, 11, 12, 19, 25, ● |
| Family Clausocalanidae Giesbrecht, 1893 | |
| Clausocalanus arcuicornis (Dana, 1849) | 1, 3, 4, 5, 7, 8, 9, 11, 12, 14, 18, 19, 20, 21, 22, 24, 25, 26, 28, ● |
| Clausocalanus furcatus (Brady, 1883) | 3, 4, 5, 7, 8, 9, 11, 12, 14, 20, 21, 25, 28, ● |
| Clausocalanus jobei Frost & Fleminger, 1968 | 11 |
| Clausocalanus lividus Frost & Fleminger, 1968 | 11 |
| Clausocalanus mastigophorus (Claus, 1863) | 11 |
| Clausocalanus paululus Farran, 1926 | 11 |
| Clausocalanus pergens Farran, 1926 | 11 |
| Ctenocalanus vanus Giesbrecht, 1888 | 9, 11, ● |
| Microcalanus pusillus Sars G.O., 1903 | 1 |
| Pseudocalanus minutus (Krøyer, 1845) | 1 |
| Family Eucalanidae Giesbrecht, 1893 | |
| Eucalanus elongatus (Dana, 1848) | 1, 8, 9, 11, 12, 19, 20, 22, ● |
| Pareucalanus attenuatus (Dana, 1849) | 3, 4, 5, 7, 8, 9, 11, 14, ● |
| Pareucalanus sewelli (Fleminger, 1973) | 18, 19, 20, 22, 25, 26 |
| Family Euchaetidae Giesbrecht, 1893 | |
| Euchaeta acuta Giesbrecht, 1893 | 20 |
| Euchaeta marina (Prestandrea, 1833) | 1, 3, 4, 5, 7, 8, 9, 11, 12, 14, 18, 19, 20, 22, 25, 26, 28, ● |
| Euchaeta media Giesbrecht, 1888 | 9, 11, ● |
| Euchaeta paraconcinna Fleminger, 1957 | 7, 9, ● |
| Euchaeta pubera Sars G.O., 1907 | ● |
| Euchaeta spinosa Giesbrecht, 1893 | 18, 25 |
| Paraeuchaeta barbata (Brady, 1883) | ● |
| Paraeuchaeta bisinuata (Sars G.O., 1907) | ● |
| Family Fosshageniidae Suárez-Morales & Iliffe, 1996 | |
| Temoropia mayumbaensis Scott T., 1894 | 7, 8, 9, 11, 19, 20, 21, 28, ● |
| Family Heterorhabdidae Sars, 1902 | |
| Disseta palumbii Giesbrecht, 1889 | 8 |
| Heterorhabdus abyssalis (Giesbrecht, 1889) | 9 |
| Heterorhabdus papilliger (Claus, 1863) | 7, 8, 9, 11, ● |
| Heterorhabdus spinifer Park, 1970 | 11 |
| Heterorhabdus spinifrons (Claus, 1863) | 7, 8, 9, 11, 12, ● |
| Heterostylites longicornis (Giesbrecht, 1889) | 8 |
| Paraheterorhabdus vipera (Giesbrecht, 1889) | 9 |
| Family Lucicutiidae Sars, 1902 | |
| Lucicutia bicornuta Wolfenden, 1905 | 8 |
| Lucicutia clausi (Giesbrecht, 1889) | 3, 4, 5, 7, 8, 9, 11, 20, ● |
| Lucicutia flavicornis (Claus, 1863) | 1, 3, 5, 7, 8, 9, 11, 12, 14, 18, 20, 21, 22, ● |
| Lucicutia gaussae Grice, 1963 | 9 |
| Lucicutia gemina Farran, 1926 | 9, 11, ● |
| Lucicutia grandis (Giesbrecht, 1895) | 1 |
| Lucicutia magna Wolfenden, 1903 | 8, 9, 12 |
| Lucicutia maxima Steuer, 1904 | 12 |
| Lucicutia ovalis (Giesbrecht, 1889) | 9, 11 |
| Family Megacalanidae Sewell, 1947 | |
| Megacalanus princeps Wolfenden, 1904 | 8, 12 |
| Family Metridinidae Sars, 1902 | |
| Gaussia princeps (Scott T., 1894) | 8, 12 |
| Metridia brevicauda Giesbrecht, 1889 | 8, 9, 12, ● |
| Metridia princeps Giesbrecht, 1889 | 8, 9, ● |
| Metridia venusta Giesbrecht, 1889 | 8, 12, ● |
| Pleuromamma abdominalis (Lubbock, 1856) | 7, 8, 9, 11, 12, 18, 19, 22, 26, ● |
| Pleuromamma borealis Dahl F., 1893 | ● |
| Pleuromamma gracilis Claus, 1863 | 3, 8, 9, 11, 12, 18, 19, 22, ● |
| Pleuromamma piseki Farran, 1929 | 7, 8, 9, 12, ● |
| Pleuromamma quadrungulata (Dahl F., 1893) | 8, 9, ● |
| Pleuromamma xiphias (Giesbrecht, 1889) | 8, 9, 11, 12, ● |
| Family Mormonillidae Giesbrecht, 1893 | |
| Neomormonilla minor (Giesbrecht, 1891) | 8, 11 |
| Mormonilla phasma Giesbrecht, 1891 | 8, 11, 12 |
| Family Nullosetigeridae Soh, Ohtsuka, Imbayashi & Suh, 1999 | |
| Nullosetigera bidentata (Brady, 1883) | 8, 9, ● |
| Nullosetigera helgae (Farran, 1908) | 8 |
| Nullosetigera impar (Farran, 1908) | 8 |
| Nullosetigera mutica (Sars G.O., 1907) | 8 |
| Family Paracalanidae Giesbrecht, 1893 | |
| Acrocalanus andersoni Bowman, 1958 | 9, ● |
| Acrocalanus gibber Giesbrecht, 1888 | 11 |
| Acrocalanus gracilis Giesbrecht, 1888 | 11 |
| Acrocalanus longicornis Giesbrecht, 1888 | 3, 7, 8, 9, 12, 18, 21, 22, 25, ● |
| Calocalanus contractus Farran, 1926 | 9, 11, 18, 20, 25 |
| Calocalanus elegans Shmeleva, 1965 | 11 |
| Calocalanus elongatus Shmeleva, 1968 | 11 |
| Calocalanus gresei Shmeleva, 1973 | 11 |
| Calocalanus kristalli Shmeleva, 1968 | 11 |
| Calocalanus longisetosus Shmeleva, 1965 | 11 |
| Calocalanus neptunus Shmeleva, 1965 | 11 |
| Calocalanus pavo (Dana, 1852) | 1, 5, 7, 8, 9, 11, 18, 19, 20, 22, 25, 26, 28, ● |
| Calocalanus pavoninus Farran, 1936 | 7, 8, 9, 11 |
| Calocalanus plumulosus (Claus, 1863) | 7, 9, 11, 18, 20, 22, 25, 28, ● |
| Calocalanus pseudocontractus Bernard, 1958 | 11 |
| Calocalanus styliremis Giesbrecht, 1888 | 3, 9, 11, 20, ● |
| Delibus nudus (Sewell, 1929) | 11 |
| Mecynocera clausi Thompson I.C., 1888 | 1, 7, 8, 9, 11, 18, 20, 21, 22, 25, 28, ● |
| Mecynocera gracilis (Tanaka, 1956) | 11 |
| Paracalanus aculeatus Giesbrecht, 1888 | 3, 4, 5, 7, 8, 9, 11, 12, 14, 18, 19, 20, 21, 22, 24, 25, 26, 28, ● |
| Paracalanus nanus Sars G.O., 1925 | 7, 11 |
| Paracalanus parvus (Claus, 1863) | 1, 3, 4, 9, 11, 14, ● |
| Paracalanus pygmaeus (Claus, 1863) | 11 |
| Paracalanus quasimodo Bowman, 1971 | 20, 21, 24, 28 |
| Parvocalanus crassirostris (Dahl F., 1894) | 14, 20, 25, 28 |
| Parvocalanus scotti (Früchtl, 1923) | 9 |
| Family Phaennidae Sars G.O., 1902 | |
| Cephalophanes frigidus Wolfenden, 1911 | 8 |
| Cephalophanes refulgens Sars G.O., 1907 | 27 |
| Onchocalanus affinis With, 1915 | 8 |
| Onchocalanus cristatus (Wolfenden, 1904) | 8 |
| Phaenna spinifera Claus, 1863 | 1, 9, 11, ● |
| Xanthocalanus agilis Giesbrecht, 1893 | 18 |
| Family Pontellidae Dana, 1852 | |
| Calanopia americana Dahl F., 1894 | 1, 7, 8, 9, 11, 14, 18, 19, 20, 25, 26, 28, ● |
| Calanopia biloba Bowman, 1957 | 2 |
| Labidocera acutifrons (Dana, 1849) | 3, 4, 5, 7, 8, 9, 11, 14, 19, 26, ● |
| Labidocera aestiva Wheeler, 1900 | 18, 19, 25 |
| Labidocera detruncata (Dana, 1849) | 24 |
| Labidocera fluviatilis Dahl F., 1894 | 9 |
| Labidocera nerii (Krøyer, 1849) | 1, 7, 8, 9, 12, ● |
| Labidocera scotti Giesbrecht, 1897 | 5, 7, 9, 14, 18, 19, 24, 25, ● |
| Labidocera wilsoni Fleminger & Tan, 1966 | 25 |
| Labidocera wollastoni (Lubbock, 1857) | 1 |
| Pontella atlantica (Milne Edwards, 1840) | 1, 18 |
| Pontella lobiancoi (Canu, 1888) | 1 |
| Pontella mediterranea (Claus, 1863) | 9 |
| Pontella mimocerami Fleminger, 1957 | 9, 14, 18, ● |
| Pontellina platychela Fleminger & Hulsemann, 1974 | 13 |
| Pontellina plumata (Dana, 1849) | 1, 5, 7, 8, 9, 11, 18, 22, ● |
| Pontellopsis brevis (Giesbrecht, 1889) | 3, 7, 9, 14, ● |
| Pontellopsis perspicax (Dana, 1849) | 9, ● |
| Pontellopsis villosa Brady, 1883 | 5, 9 |
| Family Pseudodiaptomidae Sars, 1902 | |
| Pseudodiaptomus acutus (Dahl F., 1894) | 9, 14, 17, 28, ● |
| Pseudodiaptomus cokeri González & Bowman, 1965 | 17 |
| Pseudodiaptomus marshi Wright S., 1936 | 17, 24 |
| Pseudodiaptomus pelagicus Herrick, 1884 | 24 |
| Family Rhincalanidae Geletin, 1976 | |
| Rhincalanus cornutus (Dana, 1849) | 1, 3, 4, 5, 7, 8, 9, 11, 14, 18, 19, 20, 22, 25, 26, 28, ● |
| Rhincalanus cornutus atlanticus Schmaus, 1917 | 12, ● |
| Rhincalanus nasutus Giesbrecht, 1888 | 1, 8, 18, 22 |
| Family Scolecitrichidae Giesbrecht, 1893 | |
| Amallothrix tenuiserrata (Giesbrecht, 1893) | 8, 9, 11, ● |
| Archescolecithrix auropecten (Giesbrecht, 1893) | 9 |
| Lophothrix frontalis Giesbrecht, 1895 | 8, ● |
| Lophothrix humilifrons Sars G.O., 1905 | 8, 12 |
| Lophothrix latipes (Scott T., 1894) | 7, 8 |
| Lophothrix quadrispinosa Wolfenden, 1911 | 8, ● |
| Pseudoamallothrix emarginata (Farran, 1905) | 8 |
| Pseudoamallothrix ovata (Farran, 1905) | ● |
| Scaphocalanus brevirostris Park, 1970 | 11 |
| Scaphocalanus echinatus (Farran, 1905) | 9, ● |
| Scaphocalanus magnus (Scott T., 1894) | 8 |
| Scaphocalanus major (Scott T., 1894) | 9 |
| Scolecithricella abyssalis (Giesbrecht, 1888) | 9 |
| Scolecithricella dentata (Giesbrecht, 1893) | 8, 9, 11, ● |
| Scolecithricella longifurca (Giesbrecht, 1888) | 21 |
| Scolecithricella vittata (Giesbrecht, 1893) | 8, 9, 11, ● |
| Scolecitrichopsis ctenopus (Giesbrecht, 1888) | 7, 8, 9, 11, 14, ● |
| Scolecitrichopsis tenuipes (Scott T., 1894) | 9 |
| Scolecithrix bradyi Giesbrecht, 1888 | 8, 9, 11, 19, ● |
| Scolecithrix danae (Lubbock, 1856) | 1, 3, 5, 7, 8, 9, 11, 14, 18, 19, 20, 22, 25, 26, 28, ● |
| Scottocalanus corystes Owre & Foyo, 1967 | 10, ● |
| Scottocalanus helenae (Lubbock, 1856) | 8, 9, 25 |
| Scottocalanus persecans (Giesbrecht, 1895) | 8, ● |
| Scottocalanus securifrons (Scott T., 1894) | 8, 9, 11, 22, ● |
| Scottocalanus thomasi Scott A., 1909 | 8 |
| Family Spinocalanidae Vervoort, 1951 | |
| Mimocalanus cultrifer Farran, 1908 | ● |
| Monacilla tenera Sars G.O., 1907 | 8, 12 |
| Monacilla typica Sars G.O., 1905 | 8, 12, ● |
| Spinocalanus abyssalis Giesbrecht, 1888 | 9, ● |
| Spinocalanus angusticeps Sars G.O., 1920 | 7 |
| Spinocalanus spinosus Farran, 1908 | 9, ● |
| Family Subeucalanidae Giesbrecht, 1893 | |
| Subeucalanus crassus (Giesbrecht, 1888) | 7, 9, 11, 14, 20, 24, ● |
| Subeucalanus monachus (Giesbrecht, 1888) | 3, 4, 5, 7, 8, 11, 14, 18, 20, 22, 25, ● |
| Subeucalanus mucronatus (Giesbrecht, 1888) | 19 |
| Subeucalanus pileatus (Giesbrecht, 1888) | 7, 9, 12, 14, ● |
| Subeucalanus subcrassus (Giesbrecht, 1888) | 11, 18, 19, 20, 21, 22, 24, 25, 26, 28 |
| Subeucalanus subtenuis (Giesbrecht, 1888) | 5, 7, 9, 14, 20, 22, 24, 28, ● |
| Family Temoridae Giesbrecht, 1893 | |
| Temora longicornis (Müller O.F., 1785) | 1 |
| Temora stylifera (Dana, 1849) | 1, 3, 4, 5, 7, 8, 9, 11, 12, 14, 18, 19, 20, 21, 22, 24, 25, 26, 28, ● |
| Temora turbinata (Dana, 1849) | 3, 4, 5, 7, 8, 9, 11, 12, 14, 18, 20, 21, 22, 24, 25, 28, ● |
| Family Tharybidae Sars G.O., 1902 | |
| Parundinella manicula Fleminger, 1957 | 9 |
| Parundinella spinodenticula Fleminger, 1957 | 7 |
| Superorder Podoplea Giesbrecht, 1882 | |
| Order Cyclopoida Burmeister, 1834 | |
| Family Clausidiidae Embleton, 1901 | |
| Saphirella tropica Wolfenden, 1906 | 10, 22, 25 |
| Saphirella sp. Owre & Foyo, 1967 | 10, 28, ● |
| Family Corycaeidae Dana, 1852 | |
| Agetus flaccus (Giesbrecht, 1891) | 1, 3, 6, 7, 8, 9, 11, 12, 24, ● |
| Agetus limbatus (Brady, 1883) | 6, 8, 9, 11, 12, 24, 28, ● |
| Agetus typicus Krøyer, 1849 | 1, 3, 6, 7, 8, 9, 11, 14, 18, 19, 20, 21, 22, 23, 24, 25, 26, 28, ● |
| Corycaeus clausi Dahl F., 1894 | 1, 6, 7, 8, 9, 11, 19, 23, 24, 25, 26, 28, ● |
| Corycaeus crassiusculus Dana, 1849 | 1, 7 |
| Corycaeus speciosus Dana, 1849 | 1, 3, 4, 5, 6, 7, 8, 9, 11, 12, 14, 18, 19, 20, 21, 22, 23, 24, 25, 26, 28, ● |
| Corycaeus subulatus Herrick, 1887 | 9, ● |
| Ditrichocorycaeus cf. affinis (McMurrich, 1916) | 23 |
| Ditrichocorycaeus africanus (Dahl F., 1894) | 7 |
| Ditrichocorycaeus amazonicus (Dahl F., 1894) | 6, 9, 21, 23, 24, ● |
| Ditrichocorycaeus americanus (Wilson M.S., 1949) | 6, ● |
| Ditrichocorycaeus anglicus (Lubbock, 1857) | 1, 6, 9, ● |
| Ditrichocorycaeus dubius (Farran, 1911) | 1, 11 |
| Ditrichocorycaeus minimus (Dahl F., 1894) | 1, 11 |
| Ditrichocorycaeus subtilis (Dahl M., 1912) | 11 |
| Farranula carinata (Giesbrecht, 1891) | 1, 5, 8, 12, 18, 23, 24 |
| Farranula curta (Farran, 1911) | 1, 11 |
| Farranula gibbula (Giesbrecht, 1891) | 1 |
| Farranula grkacilis (Dana, 1849) | 3, 4, 6, 7, 8, 9, 11, 18, 19, 20, 22, 23, 25, 26, ● |
| Farranula rostrata (Claus, 1863) | 1, 7, 8, 21, 23, 28 |
| Monocorycaeus robustus (Giesbrecht, 1891) | 23 |
| Onychocorycaeus agilis (Dana, 1849) | 6, 7, 9, 11, ● |
| Onychocorycaeus catus (Dahl F., 1894) | 18, 19, 20, 21, 22, 23, 24, 25, 26, 28 |
| Onychocorycaeus giesbrechti (Dahl F., 1894) | 3, 4, 6, 7, 9, 11, 14, 21, 23, ● |
| Onychocorycaeus latus (Dana, 1849) | 11, 12, 18, 19, 20, 22, 23, 24, 25, 26, 28 |
| Onychocorycaeus pacificus (Dahl F., 1894) | 6, 7, 9, ● |
| Onychocorycaeus pumilus (Dahl M., 1912) | 11 |
| Urocorycaeus furcifer (Claus, 1863) | 1, 6, 7, 9, 11, 20, 22, 24, 25, 28, ● |
| Urocorycaeus lautus (Dana, 1849) | 6, 7, 8, 9, 11, 12, 20, 22, 23, 24, 25, 26, 28, ● |
| Urocorycaeus longistylis (Dana, 1849) | 3, 7, 23, 24 |
| Family Cyclopoida Incertae Sedis | |
| Pachos punctatum (Claus, 1863) | 9, 18, 22 |
| Family Lubbockiidae Huys & Böttger-Schnack, 1997 | |
| Lubbockia aculeata Giesbrecht, 1891 | 8, 9, ● |
| Lubbockia squillimana Claus, 1863 | 1, 3, 4, 7, 8, 9, 11, 19, 20, 22, 26, ● |
| Family Oithonidae Dana, 1852 | |
| Dioithona oculata (Farran, 1913) | 16, 25 |
| Oithona atlantica Farran, 1908 | 3, 4, 7, 11, 19, 20, 24, 26 |
| Oithona brevicornis Giesbrecht, 1891 | 11 |
| Oithona decipiens Farran, 1913 | 11, 16 |
| Oithona fallax Farran, 1913 | 11 |
| Oithona flemingeri (Ferrari & Bowman, 1980) | 16 |
| Oithona hamata Rosendorn, 1917 | 11 |
| Oithona hebes Giesbrecht, 1891 | 16 |
| Oithona nana Giesbrecht, 1893 | 1, 11, 14, 16, 18, 19, 20, 21, 22, 24, 25 |
| Oithona oswaldocruzi Oliveira, 1945 | 16 |
| Oithona parvula (Farran, 1908) | 11 |
| Oithona plumifera Baird, 1843 | 1, 3, 4, 7, 8, 11, 12, 14, 16, 18, 19, 20, 21, 22, 24, 25, 26 |
| Oithona robusta Giesbrecht, 1891 | 8, 9, 11, 20 |
| Oithona setigera (Dana, 1849) | 1, 7, 8, 9, 11, 14, 16, 18, 20, 22, 24, 25, 26 |
| Oithona similis Claus, 1866 | 1, 11, 24, 26 |
| Oithona simplex Farran, 1913 | 11 |
| Oithona tenuis Rosendorn, 1917 | 11 |
| Oithona vivida Farran, 1913 | 11 |
| Oithona sp. 1 Ferrari & Bowman, 1980 | 16 |
| Oithona sp. 2 Ferrari & Bowman, 1980 | 16 |
| Family Oncaeidae Giesbrecht, 1893 | |
| Conaea rapax Giesbrecht, 1891 | 9, ● |
| Monothula subtilis (Giesbrecht, 1893) | 1 |
| Oncaea curta Sars G.O., 1916 | 11 |
| Oncaea gracilis (Dana, 1852) | 8, 12 |
| Oncaea latimana Gordeeva K.T., 1975 | 19 |
| Oncaea media Giesbrecht, 1891 | 1, 7, 9, 11, 18, 22, 24, 25, 26, 28, ● |
| Oncaea mediterranea (Claus, 1863) | 3, 7, 8, 9, 11, 12, 14, 18, 19, 20, 21, 22, 24, 25, 26, 28, ● |
| Oncaea notopus Giesbrecht, 1891 | 8, 11, 12, 20 |
| Oncaea ornata Giesbrecht, 1891 | 8, 12 |
| Oncaea tenella Sars G.O., 1916 | 1 |
| Oncaea venusta Philippi, 1843 | 1, 7, 8, 9, 11, 12, 18, 19, 20, 21, 22, 24, 25, 26, 28, ● |
| Oncaea venusta venella Farran, 1929 | 11 |
| Triconia conifera (Giesbrecht, 1891) | 1, 3, 4, 7, 8, 9, 11, 12, 20, 28, ● |
| Triconia dentipes (Giesbrecht, 1891) | 11 |
| Triconia minuta (Giesbrecht, 1893 [“1892”]) | 1, 7, 11 |
| Triconia similis (Sars G.O., 1918) | 11 |
| Family Sapphirinidae Thorell, 1860 | |
| Copilia mirabilis Dana, 1852 | 3, 4, 7, 8, 9, 11, 12, 14, 18, 19, 20, 22, 25, 26, ● |
| Copilia quadrata Dana, 1849 | 1, 8, 9, 11, 18, 19, 22 |
| Copilia vitrea (Haeckel, 1864) | 7 |
| Sapphirina angusta Dana, 1849 | 3, 4, 7, 8, 9, 18 |
| Sapphirina auronitens Claus, 1863 | 1, 7, 19 |
| Sapphirina darwinii Haeckel, 1864 | 7 |
| Sapphirina gemma Dana, 1852 | 7 |
| Sapphirina intestinata Giesbrecht, 1891 | 3, 7, 19 |
| Sapphirina maculosa Giesbrecht, 1893 | 9 |
| Sapphirina metallina Dana, 1849 | 7, 8, 9, 11 |
| Sapphirina nigromaculata Claus, 1863 | 3, 7, 9, 11, 19, 28 |
| Sapphirina opalina Dana, 1849 | 3, 7, 11, 19 |
| Sapphirina ovatolanceolata Dana, 1849 | 11, 26 |
| Sapphirina scarlata Giesbrecht, 1891 | 7, 9, 18, 21, 22, 25 |
| Sapphirina sinuicauda Brady, 1883 | 7 |
| Sapphirina stellata Giesbrecht, 1891 | 26 |
| Vettoria granulosa (Giesbrecht, 1891) | 8, 9, 11, ● |
| Vettoria parva (Farran, 1936) | 9, 10, 11 |
| Order Harpacticoida Sars, 1903 | |
| Family Aegisthidae Giesbrecht, 1893 | |
| Aegisthus aculeatus Giesbrecht, 1891 | 8, 12, 25, ● |
| Aegisthus mucronatus Giesbrecht, 1891 | 8, 9, 12, ● |
| Family Ectinosomatidae Sars, 1903 | |
| Microsetella norvegica (Boeck, 1865) | 1, 7, 9, 11, ● |
| Microsetella rosea (Dana, 1847) | 1, 3, 4, 7, 9, 11, 18, 19, 20, 21, 22, 25, 26, ● |
| Family Miraciidae Dana, 1846 | |
| Distioculus minor (Scott T., 1894) | 8, 9, 11, 12, 18, 28, ● |
| Macrosetella gracilis (Dana, 1847) | 1, 3, 4, 5, 7, 8, 9, 11, 12, 14, 18, 19, 20, 22, 25, 26, 28, ● |
| Miracia efferata Dana, 1849 | 3, 7, 8, 9, 11, 12, 19, 22 |
| Oculosetella gracilis (Dana, 1849) | 9, 11, ● |
| Family Peltidiidae Sars, 1904 | |
| Clytemnestra scutellata Dana, 1847 | 1, 7, 8, 9, 11, 12, 18, 25, 28, ● |
| Goniopsyllus rostratus Brady, 1883 | 3, 4, 7, 9, 11, 26 |
| Family Tachidiidae Sars G.O., 1909 | |
| Euterpina acutifrons (Dana, 1847) | 4, 7, 9, 11, 14, 18, 19, 20, 22, 24, 25, 26, 28, ● |
| Order Monstriloida Sars G.O., 1901 | |
| Family Monstrillidae Dana, 1849 | |
| Cymbasoma rigidum Thompson I.C., 1888 | 25 |
| Order Siphonostomatoida Thorell, 1859 | |
| Family Pontoeciellidae Giesbrecht, 1895 | |
| Pontoeciella abyssicola (Scott T., 1893) | ● |
| Family Rataniidae Giesbrecht, 1897 | |
| Ratania flava Giesbrecht, 1893 | 11 |
References
Alvarez-Cadena, J. N., Suárez-Morales, E., & Gasca, R. (1998). Copepod assemblages from a reef-related environment in the Mexican Caribbean Sea. Crustaceana, 71, 411–433. https://doi.org/10.2307/20106009
Angel, M. V. (1993). Biodiversity of the pelagic ocean. Conservation Biology, 7, 760–772.
Björnberg, T. K. S. (1981). Copepoda. In D. Boltovskoy (Ed.), Atlas del zooplancton del Atlántico sudoccidental y métodos de trabajo con el zooplancton marino (pp. 587–679). Mar del Plata: Argentina: Publ. Esp. I.N.I.D.E.P.
Bowman, T. E. (1957). A new species of Calanopia (Copepoda: Calanoida) from the Caribbean Sea. Proceedings of The United States National Museum, 107, 39–45. https://doi.org/10.5479/si.00963801.107-3382.39
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to copepod diversity. Dorchester, UK: The Ray Society.
Bradford-Grieve, J. M., Markhaseva, E. L., Rocha, C. E. F., & Abiahy, B. (1999). Copepoda. In D. Boltovskoy (Ed.), Zooplankton of the South Atlantic Ocean. Volume 2 (pp. 869–1098). Leiden, Netherlands: Backhuys Publishers.
Branco, M., Figueiras, F. G., & Cermeño, P. (2018). Assessing the efficiency of non-parametric estimators of species richness for marine microplankton. Journal of Plankton Research, 40, 230–243. https://doi.org/10.1093/plankt/fby005
Camisotti, H., & Pérez, J. (2022). Algunos copépodos de la Fachada Atlántica de Venezuela. v1.8. Caribbean OBIS Node. Dataset/Samplingevent https://doi.org/10.15468/qwwn64 accessed via GBIF.org on 2022-08-21
Campos-Hernández, A., & Suárez-Morales, E. (1994). Copépodos pelágicos del Golfo de México y Mar Caribe. I. Sistemática y biología. Chetumal, Quintana Roo: CIQRO/ Conacyt/ Regina de los Angeles S.A.
Casanova, E. (2017). Dinámica de la comunidad planctónica en relación a las perturbaciones naturales en el Refugio de Fauna Silvestre Isla de Aves, Venezuela (Ph. D. Thesis), Universidad Central de Venezuela (UCV). Caracas, Venezuela.
Casanova, E., Zoppi de Roa, E., & Montiel, E. (2007). Caracterización espacial y temporal del zooplancton en el Archipiélago Los Roques, Venezuela. Boletín del Instituto Oceanográfico de Venezuela, 46, 51–65.
Casanova, E., Zoppi de Roa, E. & Montiel, E. (2021). Spatial and temporal characterization of zooplankton in Los Roques Archipelago (Venezuela). v1.5. Caribbean OBIS Node. Dataset/Samplingevent. https://doi.org/10.15468/22yzcn accessed via GBIF.org on 2022-08-21.
Castellanos, P., Varela, R. J., & Muller-Karger, F. (2002). Descripción de las áreas de surgencia al sur del Mar Caribe examinadas con el sensor infrarrojo AVHRR. Memoria de La Fundación La Salle de Ciencias Naturales, 154, 55–76.
Cervigón, F. (1963). Contribución al conocimiento de los copépodos pelágicos de las costas de Venezuela. Memoria de La Sociedad de Ciencias Naturales La Salle, 22, 181–197.
Cervigón, F. (1964). Los Corycaeidae del Caribe sur-oriental (Copepoda, Cyclopoida). Memoria de La Sociedad de Ciencias Naturales La Salle, 24, 163–201.
Cervigón, F., & Marcano, P. J. (1965). Chapter VI – Zooplancton. In: Estudios sobre el ecosistema pelágico del Noreste de Venezuela. Memoria de La Sociedad de Ciencias Naturales La Salle, 25, 263–358.
Chen, M., Kim, D., Liu, H., & Kang, C. K. (2018). Variability in copepod trophic levels and feeding selectivity based on stable isotope analysis in Gwangyang Bay of the southern coast of the Korean Peninsula. Biogeosciences, 15, 2055–2073. https://doi.org/10.5194/bg-15-2055-2018
Chiarucci, A., Bacaro, G., & Scheiner, S. M. (2011). Old and new challenges in using species diversity for assessing biodiversity. Philosophical Transactions of the Royal Society B, 366, 2426–2437. https://doi.org/10.1098/rstb.2011.0065
Colwell, R. K. (2013). EstimateS: Statistical estimation of species richness and shares species from samples (v. 9.1). Accessed via http://viceroy.eeb.uconn.edu/estimates/ on 2021-05-20.
Colwell, R. K., Mao, C. X., & Chang, J. (2004). Interpolando, extrapolando y comparando las curvas de acumulación de especies basadas en su incidencia. In G. Halffter, J. Soberón, P. Koleff, & A. Melic (Eds.), Sobre diversidad biológica: el significado de las diversidades alfa, beta y gamma. (m3m: Monog, pp. 73–84). Ciudad de México: Conabio/ SEA España/ Grupo Diversitas-México/ Conacyt. www.sea-entomologia.org/m3m
Comeau, S., Gorsky, G., Jeffree, R., Teyssié, J.-L., & Gattuso, J.-P. (2009). Impact of ocean acidification on a key arctic pelagic mollusc (Limacina helicina). Biogeosciences, 6, 1877–1882. https://doi.org/10.5194/bg-6-1877-2009
Correa-Ramirez, M., Rodriguez-Santana, Á., Ricaurte-Villota,
C., & Paramo, J. (2020). The Southern Caribbean upwelling
system off Colombia: Water masses and mixing processes. Deep-Sea Research Part I, 155, 1–16. https://doi.org/10.
1016/j.dsr.2019.103145
Díaz, O. (2000). Copépodos ectoparásitos del pez luna Mola mola (Giglioli 1883) (Pisces: Molidae) en el golfo de Cariaco, Venezuela. Boletín del Instituto Oceanográfico de Venezuela, 39, 11–17.
Dorado-Roncancio, E. F., Medellín-Mora, J., & Mancera-Pineda, J. E. (2021). Taxonomic diversity and ecological attributes of copepods of the Colombian Caribbean Sea. Neotropical Biodiversity, 7, 491-502. https://doi.org/10.1080/23766808.2021.2000295
Farran, G. P. (1936). Copepoda. Great Barrier Reef Expedition 1928-29, Scientific Reports, British Museum (Natural History), London, 5, 73–142.
Ferrari, F. D., & Bowman, T. E. (1980). Pelagic copepods of the family Oithonidae (Cyclopoida) from the east coasts of Central and South America. Smithsonian Contributions to Zoology, 312, 1–27. https://doi.org/10.5479/si.00810282.312
Flanders Marine Institute. (2021). Maritime boundaries geodatabase: maritime boundaries and Exclusive Economic Zones (200NM), Version 11. https://doi.org/10.14284/386 on 2021-05-20
Fleminger, A., & Hulsemann, K. (1974). Systematics and distribution of the four sibling species comprising the genus Pontellina Dana (Copepoda, Calanoida). Fishery Bulletin, 72, 63–120.
Gaviria, S., Dorado-Roncancio, J., & Ahrens, M. J. (2019). Revision and update of the checklist of copepods (Crustacea: Hexanauplia) of the Colombian Caribbean. Boletín de Investigaciones Marinas y Costeras, 48, 119–151. https://doi.org/10.25268/bimc.invemar.2019.48.1.761
GBIF.org. (2021). Global Biodiversity Information Facility (GBIF) – Home page. Accessed on 2021-08-11: https://www.gbif.org
Glud, R. N., Grossart, H. P., Larsen, M., Tang, K. W., Arendt, K. E., Rysgaard, S. et al. (2015). Copepod carcasses as microbial hot spots for pelagic denitrification. Limnology and Oceanography, 60, 2026–2036. https://doi.org/10.1002/lno.10149
González-Cebrero, L., Varela, R. J., & Rojas-Márquez, J. (2017). Eufáusidos epipelágicos de la Fosa de Cariaco. Memoria de La Fundación La Salle de Ciencias Naturales, 74, 125–141.
González, L. W., Granado, A. A., & Sánchez, L. A. (1986). Incidencia del género Lernanthropus Blainville, 1822 (Copepoda) en branquias de juveniles de Pargo cebal, Lutjanus analis (Cuvier, 1828). Boletín del Instituto Oceanográfico de la Universidad de Oriente, 25, 95–98.
Ho, J.-S., & Bashirullah, A. K. M. (1977). Two species of caligid copepods (Crustacea) parasitic on marine fishes of Venezuela, with discussion of Metacaligus Thomsen, 1949. Journal of Natural History, 11, 703–714. https://doi.org/10.1080/00222937700770601
Humes, A. G. (1973). Tychidion guyanense n. gen., n. sp. (Copepoda, Cyclopoida) associated with an annelid off Guyana. Zoologische Mededelingen, 46, 27–34.
Huys, R., & Boxshall, G. A. (1991). Copepod evolution. London: Ray Society (Publications).
Jiménez-Valverde, A., & Hortal, J. (2003). Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Revista Ibérica de Aracnología, 8, 151–161.
Khodami, S., McArthur, J. V., Blanco-Bercial, L., & Martinez-Arbizu, P. (2017). Molecular phylogeny and revision of copepod orders (Crustacea: Copepoda). Scientific Reports, 7, 9164. https://doi.org/10.1038/s41598-017-06656-4
Kim, I.-H., Suárez-Morales, E., & Márquez-Rojas, B. (2019). Caligid copepods (Copepoda: Siphonostomatoida: Caligidae) as zooplankters off the Venezuelan Coast, Western Caribbean Sea. Thalassas, 35, 607–618. https://doi.org/10.1007/s41208-019-00130-w
Kiørboe, T. (2011). What makes pelagic copepods so successful? Journal of Plankton Research, 33, 677–685. https://doi.org/10.1093/plankt/fbq159
Lagarde, G. (1989). Crustáceos parásitos en peces marinos de la zona central de Venezuela. Boletín del Instituto Oceanográfico de la Universidad de Oriente, 28, 135–144.
Legaré, J. E. H. (1961). Preliminary survey of the zooplankton of the Cariaco region. Boletín del Instituto Oceanográfico de la Universidad de Oriente, 1, 191–218.
Legaré, J. E. H. (1964). The pelagic copepod of eastern Venezuela. 1. The Cariaco Trench. Boletín del Instituto Oceanográfico de la Universidad de Oriente, 3, 15–81.
Marquez, B., Allen Peña, T., Troccoli, L., & Marín, B. (2021). IOV copépodos del golfo de Cariaco, Venezuela. v1.5. Dataset/Samplingevent. https://doi.org/10.15468/akt3bc
Márquez-Rojas, B. (2016). Dinámica del mesozooplancton en el sector oriental (saco) del golfo de Cariaco, estado Sucre, Venezuela (Ph. D. Thesis). Universidad Central de Venezuela (UCV). Caracas, Venezuela.
Márquez-Rojas, B., Díaz-Díaz, O., Troccoli, L., Morales, J., & Marcano, L. M. (2014a). Corycaeidae Dana, 1852 (Copepoda: Poecilostomatoida) del Golfo de Cariaco, Venezuela. Métodos en Ecología y Sistemática, 9, 1–21.
Márquez-Rojas, B., Díaz-Díaz, O., Troccoli, L., Morales, J., & Marcano, L. M. (2014b). Distribución espacial y abundancia de la familia Corycaeidae Dana, 1852 (Copepoda: Poecilostomatoida) en el Golfo de Cariaco, Venezuela. Boletín del Instituto Oceanográfico de Venezuela, 53, 221–233.
Márquez-Rojas, B., Zoppi de Roa, E., & Zegarra-Narro, J. (2020). An updated checklist of copepod species (Arthropoda: Crustacea) from the Gulf of Cariaco, Venezuela. Pan-American Journal of Aquatic Sciences, 15, 143–150.
Márquez-Rojas, B., Díaz-Díaz, O., Troccoli, L., Morales, J. & Marcano, L. M. (2021). Distribución espacial y abundancia de la familia Corycaeidae Dana, 1852 (Copepoda: Cyclopoida) en el golfo de Cariaco, Venezuela. Caribbean OBIS Node. https://doi.org/10.15468/afw98k
McManus, M. A., & Woodson, C. B. (2012). Plankton distribution and ocean dispersal. The Journal of Experimental Biology, 215, 1008–1016. https://doi.org/10.1242/jeb.059014
Medellín-Mora, J., & Navas, G. R. (2010). Listado taxonómico de copépodos (Arthropoda: Crustacea) del mar Caribe colombiano. Boletín de Investigaciones Marinas y Costeras, 39, 265–306.
MHI Archive. (1970). Marine Hydrophysical Institute, NASU, Sebastopol (Ukraine). IBSS Biomass Collection. 90VE-701108-ru-0503 (Cruise AV03), R/V Akademik Vernadskiy. COPEPOD: The Global Plankton Database. Online, 2004-2021. Accessed on 2021-05-20 via: https://www.st.nfms.noaa.gov/copepod
Miloslavich, P., Klein, E., Martín, A., Bastidas, C., Marin, B., & Spiniello, P. (2005). Venezuela. In P. Miloslavich, & E. Klein (Eds.), Caribbean marine biodiversity – the known and unknown (pp. 109–136). Lancaster, Pennsylvania: DEStech Publications, Inc.
Morales, J. (2008). Adundancia, composición y biomasa de los espectros de tallas del zooplancton en la plataforma Pariche-Manicuare, Golfo de Cariaco, estado Sucre, Venezuela (Thesis). Universidad of Oriente (UDO). Cumaná, Venezuela.
Morales, J. (2014). Distribución vertical de los copépodos en la depresión de Guaracayal, golfo de Cariaco, Venezuela (M. Sc. Thesis). Universidad of Oriente (UDO). Cumaná, Venezuela.
Mori, T. (1937). The pelagic Copepoda from the neighbouring waters of Japan. Published by the autor. Tokyo: The Soyo Co. Inc.
Muller-Karger, F., & Varela, R. J. (1990). Influjo del río Orinoco en el mar Caribe: Observaciones con el CZCS desde el espacio. Memoria de La Fundación La Salle de Ciencias Naturales, 49–50, 361–390.
Ohtsuka, S., Madinabeitia, I., Yamashita, H., Venmathi-Maran, B. A., Suárez-Morales, E., & Ho, J. (2018). Planktonic phases in symbiotic copepods: A review. Bulletin Southern California Academy of Sciences, 117, 104–119. https://doi.org/10.3160/3616.1
Okolodkov, Y. B. (2003). A review of Russian plankton research in the Gulf of Mexico and the Caribbean Sea in the 1960-1980s. Hidrobiológica, 13, 207–221.
Orrell, T. & Informatics Office (2022). NMNH Extant Specimen Records (USNM, US). Version 1.58. National Museum of Natural History, Smithsonian Institution. https://doi.org/10.15468/hnhrg3
Owre, H. B., & Foyo, M. (1964). Report on a collection of Copepoda from the Caribbean Sea. Bulletin of Marine Science of the Gulf and Caribbean, 14, 359–372.
Owre, H. B., & Foyo, M. (1967). Copepods of the Florida Current. Fauna Caribaea No. 1: Crustacea, I: Copepoda. Miami: Inst. of Mar. Sci. Univ. Miami.
Owre, H. B., & Foyo, M. (1972). Studies on Caribbean zooplankton. Description of the program and results of the first cruise. Bulletin of Marine Science, 22, 483–521.
Park, T. S. (1970). Calanoid copepods from the Caribbean Sea and Gulf of Mexico. 2. New species and new records from plankton samples. Bulletin of Marine Science, 20, 472–546.
Pineda-Polo, F. H. (1979). A new species of Euaugaptilidae (Copepoda-Calanidae) from the Cariaco Trench. Boletín del Instituto Oceanográfico de la Universidad de Oriente, 18, 13–15.
Piontkovski, S. A., & Landry, M. R. (2003). Copepod species diversity and climate variability in the tropical Atlantic Ocean. Fisheries Oceanography, 12, 352–359. https://doi.org/10.1046/j.1365-2419.2003.00250.x
Razouls, C., de Bovée, F., Kouwenberg, J., & Desreumaux, N. (2021). Diversity and geographic distribution of marine planktonic copepods (2005-2021). Sorbonne University, CNRS. http://copepodes.obs-banyuls.fr/en
Reese, G. C., Wilson, K. R., & Flather, C. H. (2014). Performance of species richness estimators across assemblage types and survey parameters. Global Ecology and Biogeography, 23, 585–594. https://doi.org/10.1111/geb.12144
Reid, J. W. (1990). Continental and coastal free-living Copepoda (Crustacea) of Mexico, Central America and the Caribbean region. In D. Navarro, & J. G. Robison (Eds.), Diversidad biológica en la Reserva de Biosfera de Sian Ka’an, Quintana Roo, México (pp. 175–213). Chetumal, Quintana Roo: CIQRO/ University of Florida.
Rendón, S., Vanegas, T., & Tigreros, P. C. (2003). Contaminación en la Bahía de Cartagena por agua de lastre de los buques. Boletín Científico CIOH, 21, 91–100. https://doi.org/10.26640/22159045.118
Rodríguez, G., & Suárez, H. (2003). Crustáceos. In M. Aguilera, A. Azocar, & E. González-Jiménez (Eds.), Biodiversidad en Venezuela, Volume 1 (pp. 288–311). Caracas: Fundación Polar and Ministerio de Ciencias y Tecnología/ Fondo Nacional de Ciencia, Tecnología e Innovación (Fonacit).
Rojas-Márquez, J. (2008). Variación temporal en la oferta-demanda de carbono de una fracción de la comunidad planctónica durante eventos de surgencia en la Fosa de Cariaco (Thesis). Universidad Central de Venezuela (UCV). Caracas, Venezuela, pp. 99.
Rombouts, I., Beaugrand, G., Ibañez, F., Gasparini, S., Chiba, S., & Legendre, L. (2009). Global latitudinal variations in marine copepod diversity and environmental factors. Proceedings of the Royal Society B, 276, 3053–3062. https://doi.org/10.1098/rspb.2009.0742
Rose, M. (1933). Copepodes pelagiques. Faune de France, 26, 1–374.
Rueda-Roa, D. T., Ezer, T., & Muller-Karger, F. E. (2018). Description and mechanisms of the mid-year upwelling in the southern Caribbean Sea from remote sensing and local data. Journal of Marine Science and Engineering, 6, 10–12. https://doi.org/10.3390/jmse6020036
Saiz, E., Calbet, A., Atienza, D., & Alcaraz, M. (2007). Feeding and production of zooplankton in the Catalan Sea (NW Mediterranean). Progress in Oceanography, 74, 313–328. https://doi.org/10.1016/j.pocean.2007.04.004
Segovia-Kansev, D., Scott-Frías, J., & Torres-Parra R. (2022). Density and distribution of phytoplankton, copepods and cladocerans in Mochima Bay (Venezuela) on March 2016. Version 1.2. Caribbean OBIS Node. Sampling event dataset https://doi.org/10.15468/cxykg6
Sewell, R. B. S. (1947). The free-swimming planktonic Copepoda: Systematic account. Scientific Reports of the John Murray Expedition, 8, 1–303.
Smith, E. P., & van Belle, G. (1984). Nonparametric estimation of species richness. Biometrics, 40, 119–129. https://doi.org/10.2307/2530750
Soberón, J., & Llorente, J. E. (1993). The use of species accumulation functions for the prediction of species richness. Conservation Biology, 7, 480–488.
Spalding, M. D., Agostini, V. N., Rice, J., & Grant, S. M. (2012). Pelagic provinces of the world: A biogeographic classification of the world’s surface pelagic waters. Ocean & Coastal Management, 60, 19–30. https://doi.org/10.1016/j.ocecoaman.2011.12.016
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdaña, Z. A., Finlayson, M. et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience, 57, 573–583. https://doi.org/10.1641/b570707
StatSoft. (2011). STATISTICA (data analysis software system and computer program manual) (v. 10). Accessed on 2021-05-20 via: www.statsoft.com
Stock, J. H. (1995). Two new copepods parasitic on Caribbean polychaetes. Studies on the Natural History of the Caribbean Region, 72, 1–11.
Suárez-Morales, E., Camisotti, H., & Martín, A. (2012). A new species of Caligus (Copepoda, Siphonostomatoida) from the plankton of the Caribbean coast of Venezuela with a key to species. Zookeys, 201, 59–71. https://doi.org/10.3897/zookeys.201.3099
Suárez-Morales, E., De Troch, M., & Fiers, F. (2006). A checklist of the marine Harpacticoida (Copepoda) of the Caribbean Sea. Zootaxa, 1285, 1–19. https://www.biotaxa.org/Zootaxa/article/view/zootaxa.1285.1.1/0
Suárez-Morales, E., Fleeger, J. W., & Montagna, P. A. (2009). Free-living Copepoda (Crustacea) of the Gulf of Mexico. In D. L. Felder & D. K. Camp (Eds.), Gulf of Mexico origins, waters, and biota. Volume 1, Biodiversity (pp. 841–870). College Station: Texas A&M University Press.
Suárez-Morales, E., & Gasca, R. (1998). Updated checklist of the free-living marine Copepoda (Crustacea) of Mexico. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología, 69, 105–119.
Suárez, E. (1989). Distribución, abundancia y nuevos registros de Corycaeidae (Copepoda: Cyclopoida) en el Banco de Campeche y Mar Caribe mexicano. Boletín del Instituto Oceanográfico de Venezuela, 28, 3–7.
Sutton, T. T., Clark, M. R., Dunn, D. C., Halpin, P. N., Rogers, A. D., Guinotte, J. et al. (2017). A global biogeographic classification of the mesopelagic zone. Deep-Sea Research Part I, 126, 85–102. https://doi.org/10.1016/j.dsr.2017.05.006
Tanaka, O. (1960). Pelagic Copepoda. Special Publications from the Seto Marine Biological Laboratory, 10, 1–177.
Tilley, J. D., Butler, C. M., Suárez-Morales, E., Franks, J. S., Hoffmayer, E. R., Gibson, D. P. et al. (2016). Feeding ecology of larval Atlantic bluefin tuna, Thunnus thynnus, from the central Gulf of Mexico. Bulletin of Marine Science, 92, 321–334. https://doi.org/10.5343/bms.2015.1067
Turner, J. T. (2004). The importance of small planktonic copepods and their roles in pelagic marine food webs. Zoological Studies, 43, 255–266.
van der Spoel, S. (1994). A biosystematic basis for pelagic biodiversity. Bijdragen Tot de Dierkunde, 64, 3–31. https://doi.org/10.1163/26660644-06401001
Vervoort, W. (1963). Pelagic Copepoda Part I. Atlantide Report, 7, 77–194.
Vervoort, W. (1965). Pelagic Copepoda Part II. Atlantide Report, 8, 9–216.
Villamizar, E. Y., & Cervigón, F. (2017). Variability and sustainability of the Southern Subarea of the Caribbean Sea large marine ecosystem. Environmental Development, 22, 30–41. https://doi.org/10.1016/j.envdev.2017.02.005
Walter, T. C. (1989). Review of the new world species of Pseudodiaptomus (Copepoda: Calanoida), with a key to the species. Bulletin of Marine Science, 45, 590–628.
Walter, T. C., & Boxshall, G. A. (2021). World of copepods database. https://doi.org/10.14284/356 on 2021-08-11
Wilson, C. B. (1942). The copepods of the plankton gathered during the last cruise of the Carnegie. Scientific results of cruise VII of the Carnegie during 1928-1929 under the command of Captain J.P. Ault. Publications of the Carnegie Institution of Washington, 536, 1–237.
Zacarías, D., & Zoppi de Roa, E. (1981). Desarrollo larval y postlarval de Oithona hebes Giesbrecht (Copepoda: Cyclopoida). Acta Biologica Venezuelica, 11, 109–123.
Zoppi de Roa, E. (1977). El zooplancton marino de la región oriental de Venezuela. Part. I & II (Ph. D. Thesis). Universidad Central de Venezuela. Caracas, Venezuela.
Zoppi de Roa, E., Díaz, Y. J., Marín, B., & Márquez-Rojas, B. (2007). Componente zooplanctónico. In A. Martín & D. Bone (Eds.), Línea base ambiental plataforma deltana (pp. 83–95). Caracas: PDVSA and Universidad Simón Bolívar.
Zoppi de Roa, E., & Palacios-Cáceres, M. (2005). Evaluación preliminar de la comunidad zooplanctónica del Frente Atlántico de Venezuela. In M. G. Gómez, M. Capaldo, C. Yanes, & A. Martín (Eds.), Frente Atlántico venezolano. Investigaciones Geoambientales. Tome I: Ciencias Ambientales. (pp. 127–140). Caracas: PDVSA/ Fondo Editorial Fundambiente.
Zoppi, E. (1961). Distribución vertical del zooplancton en el golfo y extremo este de la fosa de Cariaco. Boletín del Instituto Oceanográfico de la Universidad de Oriente, 1, 219–247.