Fernando M. Contreras-Moreno a, Mircea G. Hidalgo-Mihart b, *, Rafael Reyna-Hurtado c, Carlos A. López-González d, Alejandro Jesús-de la Cruz b
a Universidad Autónoma de Nayarit, Unidad Académica de Agricultura, Programa de Doctorado en Ciencias Biológico Agropecuarias, Carretera Tepic-Compostela Km. 9, 63780 Xalisco, Nayarit, México
b Universidad Autónoma de Nayarit, Unidad Académica de Agricultura, Programa de Maestría en Ciencias Biológico Agropecuarias, Carretera Tepic-Compostela Km. 9, 63780 Xalisco, Nayarit, México
c Universidad Autónoma de Nayarit, Secretaría de Investigación y Posgrado, Ciudad de la Cultura s/n, Centro, 63000 Tepic, Nayarit, México
d Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, Departamento de Plancton y Ecología Marina, Cátedras-Conacyt, Av. Instituto Politécnico Nacional s/n, Playa Palo de Santa Rita, 23090 La Paz, Baja California Sur, México
e Universidad Autónoma de Nayarit, Unidad Académica de Agricultura, Programa Académico de Biología, Carretera Tepic-Compostela Km. 9, 63780 Xalisco, Nayarit, México
*Corresponding author: ubisha@uan.edu.mx (O.U. Hernández-Almeida)
Received: 21 April 2020; accepted: 9 November 2020
Abstract
The main element to assess the potential risks associated with toxic cyanobacterial blooms is the precise identification of the species. As part of a water quality monitoring during 2015 in Santa María del Oro crater lake, Nayarit, a cyanobacterial bloom was detected. Thus, this work aimed to identify the bloom-forming cyanobacteria species using a polyphasic approach. The cyanobacteria that produced the bloom were Limnoraphis robusta, which represented the first record for Mexico, and Microcystis aeruginosa, which is a new record for Nayarit. During the bloom’s most intense phase in March and April, the filament and trichome width of L. robusta decreased by 50%, thus, resembling L. hieronymusii morphometry. Despite morphological variations, ecological and molecular data allowed us to assign, unambiguously, the morphotype to L. robusta. According to our data, it is important to trace the cell size of natural populations to assess their morphological variation limits.
Keywords: Santa María del Oro; Cyanobacteria bloom; Morphological variation; Polyphasic approach
Evaluación polifásica de las cianobacterias Limnoraphis robusta (Oscillatoriaceae) y Microcystis aeruginosa (Microcystaceae), formadoras de florecimientos en un lago cráter subtropical mexicano
Resumen
El principal elemento para evaluar los posibles riesgos asociados a los florecimientos de cianobacterias tóxicas es la precisa identificación de las especies. Como parte del monitoreo de la calidad del agua en el lago cráter de Santa María del Oro (Nayarit), en 2015 se detectó un florecimiento de cianobacterias. Así, el objetivo de este trabajo fue identificar las especies de cianobacterias formadoras de florecimientos mediante el enfoque polifásico. Las cianobacterias que produjeron el florecimiento fueron Limnoraphis robusta, que representa el primer registro para México y Microcystis aeruginosa, que es un nuevo registro para Nayarit. Durante la fase más intensa del florecimiento, marzo y abril, el ancho del filamento y del tricoma de L. robusta disminuyó un 50%, por lo que se asemeja a la morfometría de L. hieronymusii. A pesar de las variaciones morfológicas, los datos ecológicos y moleculares permitieron asignar, sin ambigüedades, el morfotipo a L. robusta. De acuerdo con nuestros datos, es importante trazar el tamaño celular de las poblaciones naturales para conocer sus límites de variación morfológica.
Palabras clave: Santa María del Oro; Florecimientos de cianobacterias; Variación morfológica; Evaluación polifásica
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The polyphasic approach has allowed a better assessment of cyanobacteria diversity and has been especially useful to discriminate between cryptotaxa, which are almost impossible to differentiate morphologically (Komárek, 2003, 2016). An example of the above is the genus Lyngbya which exhibits cytological (ultrastructural) and ecological variations, high morphological plasticity, and in some cases, lack of precision of the 16S rRNA gene at a specific level (Engene et al., 2010). Accordingly, Komárek et al. (2013) reviewed the Lyngbya species with planktic habitat and separated them into the genus Limnoraphis. This genus currently comprises 4 species: Limnoraphis hieronymusii (Lemmermann), L. birgei (G.M.Smith), L. cryptovaginata (Škorbatov), and L. robusta (Pakutty) (Komárek et al., 2013).
The genus Limnoraphis has 4 known species, of which only L. robusta produces blooms in freshwater bodies; these have been reported in Guatemala (Komárek et al., 2013), Peru (Komárková et al., 2016; Montoya et al., 2014), Cuba (Comas-González et al., 2017) and USA (Kurobe et al., 2013). It is worth noting that L. robusta blooms, both in Atitlán Lake, Guatemala, and Clear Lake, California, were accompanied by Microcystis aeruginosa (Kützing) Kützing 1846.
Cyanobacteria blooms are considered biological threats because their mass production often causes anoxia, but mainly because they can synthesize a wide variety of secondary metabolites known as cyanotoxins (Deblois & Juneau, 2010; Komárková et al., 2016). The presence of cyanotoxins is especially dangerous when the water body is used as a source of drinking water (Kuiper-Goodman et al., 1999).
Once a cyanobacteria bloom is detected, the main element for assessing the potential risk associated with the presence of cyanotoxins is the correct identification and quantification of cyanobacteria species (Lawton et al., 1999). However, cyanobacteria are organisms with very successful biological and ecological life strategies, with repeated rapid adaptation to various environmental conditions (Komárek, 2016), and so, many morphological characteristics used for identification have variations influenced by environmental conditions (Stoyanov et al., 2014; Wilmotte, 1994), leading to difficulties and confusion in the identification process. The way to address this problem is to combine morphological, ecological, and molecular traits (Komárek, 2003).
In Mexico, Cyanobacteria is the second most diverse group of freshwater phytoplankton, and blooms of these organisms, associated with eutrophic water bodies, are relatively common but poorly studied (Oliva-Martínez et al., 2014). According to Pérez-Morales et al. (2016), the main bloom-forming cyanobacteria are species of Microcystis, Cylindrospermopsis, Anabaena, Anabaenopsis, Nodularia, Phormidium, Planktothrix and Pseudoanabaena. In contrast, blooms of any Lyngbya or related species are uncommon, with the only report being in 2005 for Valle de Bravo, which is a reservoir used for recreational activities and water supply for Mexico City. This bloom was caused by an unidentified Lyngyba species along with Microcystis incerta and Anabaena (Dolichospermum) tenericaule (Nygaard) (Zapomelová et al., 2012), and was so intense that it forced Mexican authorities to close the reservoir to all water activities (Mercado-Borrayo, 2007).
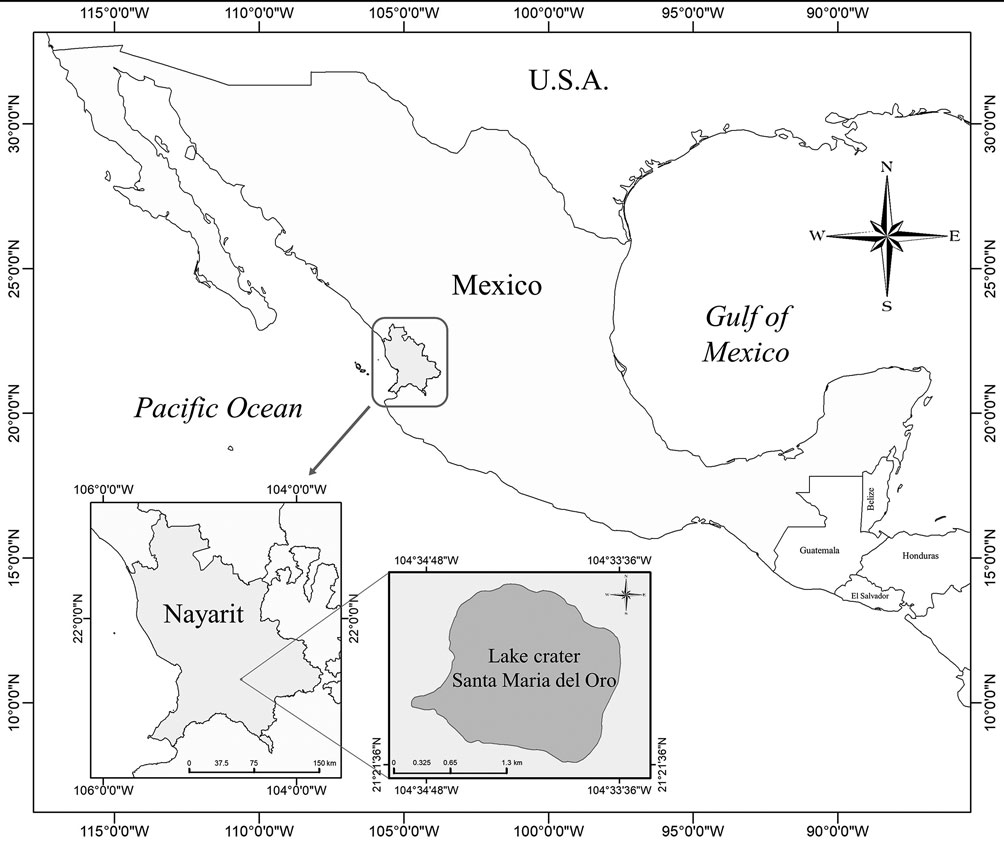
Santa María del Oro crater lake is located in the State of Nayarit. It is an important place for tourism that attracts thousands of visitors who perform ecotourism and recreational activities. During 2015, the presence of a possible microcystin-producing cyanobacteria bloom represented a risk for lake users; thus, arose the need to identify the species that generated the bloom. Hence, this work aims to identify the bloom-forming cyanobacteria species with the utmost precision using a polyphasic approach.
Materials and methods
Santa María del Oro crater lake, is a subtropical water body located southeast from the City of Tepic, in Nayarit, Mexico, (21°23’ N, 104°35’ W; 750 m asl) (Fig. 1). The lake is classified as warm monomictic, stratified for 7 months with anoxic hypolimnion (May to December) and complete mixing between January and March (Vázquez-Castro et al., 2008). It has a surface area of about 3.7 km2 and a maximum depth of 65 m. The annual average precipitation in the region is 1,214 mm year-1 and a pH mean of 8.8 (Vázquez & Caballero, 2013).
During 2015, we carried out monthly phytoplankton trawls with a 60 μm mesh net. One part of the sample was used for molecular analysis, and the other fixed with 4% formalin for morphological identification.
Observations were made with a phase-contrast microscope (Carl Zeiss Axioscope A1) and photographed with a digital camera (EOS Canon 6D); measurements were done with Adobe Photoshop CS6 (ver. 13.0.1.1). Observations were made immediately after collection, otherwise, during the next following days. Measurements and morphological traits were taken from the first 30 colonies. Diagnostic characteristics of the taxa were identified and enlisted using the taxonomic criteria of Komárek and Anagnostidis (1999, 2005), Komárek et al. (2013) and Comas-González et al. (2017).
Due to the extremely high density of the trawl samples, they were diluted with lake filtered water. After dilution, we used a stereomicroscope (Leica Zoom 2000) and natural bristles to isolate cyanobacteria filaments and coccoid colonies. Isolated colonies and filaments were washed in drops of filtered water to eliminate other organisms that may be present. The process was repeated until reaching ~50 mg of biomass. The biomass obtained in this way was stored in 1 ml tubes, and frozen at -80 °C until processed for molecular analyses.
DNA was extracted by the CTAB method (Allers & Lichten, 2000; Falcón & Valera, 2007). To improve cell lysis, due to a large amount of mucilage in samples, 1 mm diameter glass beads were used. For cell debris extraction a chloroform: octanol (24:1) solution was used.
The partial 16S rRNA gene region was amplified with cyanobacterial specific primers CYA106F and CYA781 (Nübel et al., 1997). The final volume of the PCR mixture was 50 μL and contained: 25 μL RedTaq Ready Mix PCR Reaction Mix [Sigma-Aldrich; 3 mM MgCl2, 20 mM Tris-HCl, pH 8.3, 100 mM KCl, 0.002% gelatin, 0.4 mM dNTP mix (dGTP, dCTP, dATP, dTTP), and 0.06 unit/μL of Taq DNA Polymerase], 1 μmol of each primer and 2 μL of template DNA. The amplification was carried out with the following setup: a cycle of 5 min at 94 °C; 35 cycles of 1 min at 94 °C, 1 min at 50 °C, 1 min at 72 °C and 5 min at 72 °C of final extension. Purification and sequencing were done by Macrogen (Seoul, Republic of Korea). The 16S rRNA partial sequences were manually edited to avoid reading errors, with the software BioEdit v.7.2.6 (Hall, 1999). Obtained sequences were compared with those in the GenBank NCBI (National Center for Biotechnology Information) using the Basic Local Alignment Search Tool (BLAST). Sequence alignment was performed with Clustal Omega 1.2.4 software (Sievers et al., 2011; Sievers & Higgins, 2018).
The analyses were performed based on maximum likelihood (ML) (Saitou & Nei, 1987) available in MEGA v7 software (Kumar et al., 2016). For ML, the evolutionary substitution model K2 + G was solved as the best one found using the MEGA v7 algorithms. The parameters (base frequencies, rate matrix of substitution types and shape of gamma distribution) were estimated from the data. One thousand bootstrap replicates were performed for ML analyses. Nucleotide sequences of the studied strains were deposited in GenBank under the accession numbers MH094657 (Limnoraphis robusta SAMAO-1), MH382818 (L. robusta SAMAO-2), and MH094658 (Microcystis aeruginosa SAMAO-3).
Results
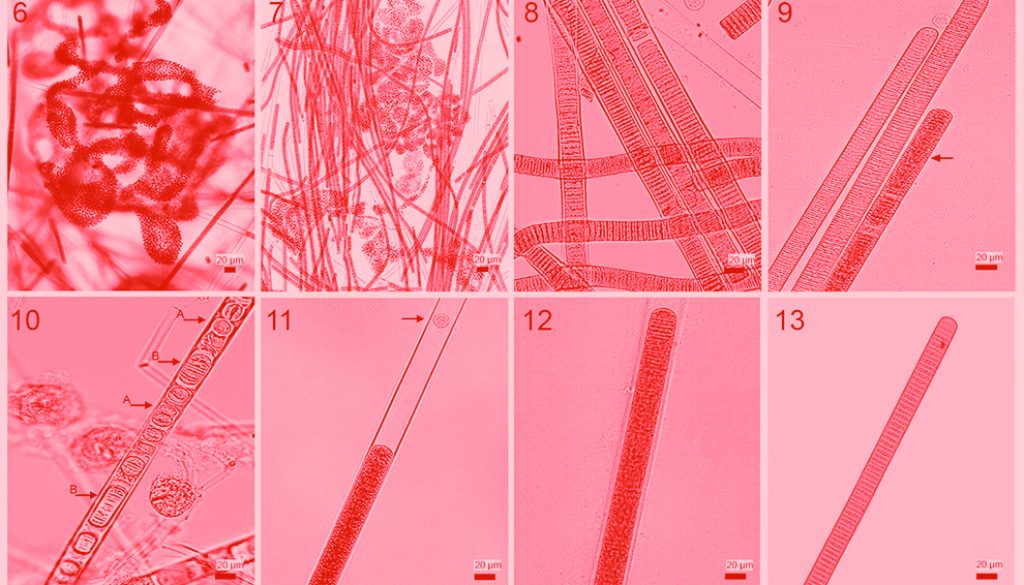
The bloom lasted from January to April 2015, with a peak during March and April (Figs. 2, 3). Visual inspection of surface water revealed a brownish colour and uneven distribution of cyanobacteria, with macroscopic scums mainly on the shore (Fig. 4) and clusters in the center (Fig. 5).
Microscopic observations of scums and clusters yielded 2 main cyanobacterial populations: one colonial coccoid form and one filamentous homocytous form. The colonial coccoid form was part of the planktonic ecosystem throughout the year. Colonies were macroscopic and mucilaginous with irregular or discoid form (Fig. 6). Cells were spherical, 3.4 to 7.9 µm (mean of 5.5 ± 0.8 µm) in diameter with many aereotopes, after division cells were hemispherical. Colour ranged between blue-green to pale green. These morphological traits correspond to those of Microcystis aeruginosa.
The filamentous individuals formed scums and irregular clusters intermingled with Microcystis aeruginosa (Fig. 7). Filaments were straight or slightly curved (Fig. 8), length ranged between 88.5 and 5,032.1 μm (mean of 912.7 ± 727.4 μm); width varied from 7.5 to 23.4 μm (mean of 16.8 ± 2.9 μm). Trichomes were straight, cylindrical and not constricted at cross-walls; width varied between 6.3 and 19.8 μm with a mean of 14.3 ± 3.2 μm; apical cells were widely rounded, without calyptra. Aerotopes were not regularly distributed along the whole trichome (Fig. 9). Hormogonia varied in length and cell numbers (2-12), also we observed monocytes (Fig. 10). Sheaths were firm, colorless, sometimes widened, surpassing the trichomes in form of a firm tube (Figs. 11, 12). The above morphological traits correspond to Limnoraphis robusta, however, morphometric characteristics show important variations over the year (Figs. 12, 13). Table 1 shows that during the bloom (February, March, and April), filament length and width and trichome width decreased considerably; then after the bloom, all morphometric characters stabilized. In April, we observed year minimum values for the filament mean length (377.1 ± 240.2 μm), width (9.9 ± 1.1 μm), and trichome width (8.2 ± 0.9 μm).

Sequences of ~ 640 bp were obtained from the morphological isolates of Limnoraphis robusta and Microcystis aeruginosa. For L. robusta SAMAO-1 and SAMAO-2, a 100% BLAST sequence identity was obtained with L. robusta (JX006093), while for M. aeruginosa SAMAO-3, BLAST identity was 99% with M. aeruginosa (KF287004.1). The phylogenetic analysis indicated that Limnoraphis from Santa María del Oro formed a monophyletic clade, with 99% of support, along with other species of the genus (Fig. 14). In contrast, Microcystis formed a clade with 98% bootstrap support; while M. aeruginosa formed a defined group with 71% bootstrap support (Fig. 15).
Table 1
Monthly range, mean and standard deviation values for Limnoraphis robusta filament length, width and trichome width measured in Santa María del Oro crater lake during 2015.
| Month | N | Filament length
min-max (μm) |
Filament
mean length (μm) |
Filament width
min-max (μm) |
Filament mean width
(μm) |
Trichome width
min-max (μm) |
Trichome
mean width (μm) |
| January | 30 | 167 – 995 | 626 ± 255 | 16 – 20 | 17.8 ± 1.3 | 14 – 18 | 16.2 ± 1.3 |
| February | 30 | 93 – 880 | 323 ± 243.6 | 9 – 23 | 16.6 ± 4.1 | 9 – 18 | 15.6 ± 1.3 |
| March | 30 | 89 – 1,608 | 472.2 ± 354 | 8 – 12 | 10.7 ± 4 | 7 – 9 | 8.3 ± 0.5 |
| April | 30 | 86 – 811 | 377.1 ± 240.2 | 7 – 20 | 9.9 ± 1.1 | 6 – 9 | 8.2 ± 0.9 |
| May | 30 | 145 – 3,069 | 690.2 ± 559.9 | 16 – 20 | 18.2 ± 1.4 | 12 – 20 | 16.4 ± 2.5 |
| June | 30 | 131 – 1,483 | 566.1 ± 326.3 | 16 – 21 | 17.9 ± 1.4 | 15 – 20 | 16.9 ± 1.6 |
| July | 30 | 236 – 3,013 | 830.8 ± 538.6 | 15 – 22 | 17.1 ± 1.5 | 13 – 18 | 14.7 ± 1.8 |
| August | 30 | 182 – 3,069 | 1,026.9 ± 854.9 | 15 – 19 | 16.2 ± 0.9 | 14 – 18 | 14.8 ± 1.5 |
| September | 30 | 150 – 2,692 | 931.9 ± 664.7 | 16 – 23 | 19.8 ± 1.5 | 15 – 18 | 16.5 ± 1.2 |
| October | 30 | 131 – 1,855 | 800.7 ± 398.1 | 15 – 19 | 16.6 ± 0.8 | 13 – 17 | 15.1 ± 1.1 |
| November | 30 | 475 – 3,679 | 1,409.8 ± 825.2 | 14 – 23 | 17.7 ± 2.3 | 13 – 18 | 15.5 ± 1.5 |
| December | 30 | 236 – 5,032 | 1,427.2 ± 1,044.9 | 15 – 22 | 17.1 ± 1.5 | 15 – 18 | 15.7 ± 0.9 |


Discussion
Besides being the first record of Limnoraphis robusta for Mexico, it is also the first record of a bloom of this species in a Mexican freshwater body. Hence, this study widens the biogeographic distribution of L. robusta and the bloom records for this species. It is also the first record for Nayarit of Microcystis aeruginosa, which is the main bloom-forming species in Mexican freshwater bodies (Pérez-Morales et al., 2016).
Despite the lack of specificity of phylogenetic analysis at the species level, morphological traits of the filamentous forms were those reported for L. robusta by Komárek et al. (2013). Regarding ecological data, in Santa María del Oro crater lake, Ochoa-Zamora (2018) observed that L. robusta was the dominant species whose density peak was on February, March, and April. Meanwhile, Salas-Betancourt (2018) estimated a mean temperature of 24.5 °C during the bloom, after it, rose to 26 °C in May and June, reaching its peak of 30 °C in August and September. On its side, Caballero et al. (2013) and Salas-Betancourt (2018) found that the trophic state of Santa María del Oro crater was mesotrophic, with elevated concentrations of phosphorus. Thus, based on our morphological measurements and ecological data of the study site, the identity of L. robusta is confirmed. Our result also supports Komárek et al. (2013) hypothesis that L. robusta blooms develop in oligo- to mesotrophic water bodies with increased phosphorus content.

Species of the genus Limnoraphis are not common in Mexico and most of the records are for Limnoraphis hieronymusii. Montejano et al. (2005) reported it for central Mexico, which comprises Balsas, Pánuco and Papaloapan rivers, although the authors did not specify a site. Novelo (2011) reported it for San Lorenzo spring (Tehuacán) and San Bernardino Lagunas and Laguna Mayor (Vicente Guerrero), Puebla. Finally, Nava-Ruiz and Valadez-Cruz (2012) and Valadez et al. (2013) reported it for Lagartos Lagoon, Quintana Roo. For Limnoraphis birgei, the only record was by Gaytán-Herrera et al. (2011) for the Valle de Bravo reservoir, Estado de Mexico; where L. birgei was one of the dominant species.
Cyanobacteria morphological variations due to environmental conditions are long known (Wilmotte, 1994); thus, trichome and filament width variations observed on Limnoraphis robusta fall within the expectations. According to Anagnostidis and Komárek (1988), cell dimensions are relatively stable within certain limits and, therefore, are useful for species-level distinctions; however, as our data clearly show, even cell size had wide variations in the same site under different natural conditions. In particular, cell width variations are influenced by environmental conditions. For example, Krüger et al. (1981) reported that Microcystis aeruginosa had larger cells under stress conditions, mainly caused by light intensity and changes in the state of the growth media. Tavera et al. (1994) found that temperature is the most important factor concerning morphological changes in some Phormidium strains (Oscillatoriales); they observed that above 30 °C cells in trichomes started to appear wider than average. Thus, considering the temperature variations (24-30 °C) observed in Santa María del Oro crater lake by Salas-Betancourt (2018), we can hypothesize that temperature could be the driving forces that produce cell width modifications in L. robusta. Therefore, the following questions remain: Is temperature the main driver of morphological changes? Which temperature range represents stress conditions for L. robusta, during the bloom or after it?
Recognition of some cyanobacteria species is not always possible with the 16s rRNA gene sequencing (Komárek, 2010); also, wrong strain identification is a common issue found in cyanobacteria phylogenetic analysis (Komárek, 2018). According to Engene et al. (2010), the above is related with at least 2 reasons: a) the short length of the gene sequences available in GenBank (minimally 600 bp), thus affecting the reliability of the analysis, and b) that cyanobacteria could contain multiple and variable copies of their 16S rRNA genes. Based on the above, our Limnoraphis robusta phylogeny results were not surprising; moreover, Komárek et al. (2013) and Kurobe et al. (2013) found similar results for their Limnoraphis strains. Meanwhile, Kurobe et al. (2013) found that their strains SWMP11-13 (JX006094.1) and -14 (JX006095.1) were distinct from any other sequence of L. robusta or L. cryptovaginata and tentatively named it as Limnoraphis sp. The above was unsupported by the present study since our results show that those strains form a single cluster along with all other species of the genus; nevertheless, a greater number of longer sequences are needed to perform more robust phylogenetic analysis.
For Microcystis, the outlook is almost the same. There are several efforts to disentangle the subgeneric relationships among the members of the genus, for example Kim et al. (2002), Komárek (2018), Neilan et al. (1997) and Otsuka et al. (2001); however, all attempts made with partial sequences of the 16S rRNA have not been capable of elucidating it. For example, Engene et al. (2012) found that the genus Moorea has relatively high levels of intra-genomic gene heterogeneity; that in combination with low subgeneric sequence divergence, makes the 16S rRNA gene inadequate for distinguishing species in some cyanobacteria genera.
Our findings showed that it is relevant to know cell size variation limits on natural cyanobacteria populations under different environmental conditions since, in most studies, the identification relies on morphology. So, even though cyanobacteria cultures are a common resource to make species description, it should be considered that descriptions based on enriched cultures alone may not indicate the natural limits of variation for in situ populations (Baker, 2007). Komárek et al. (2013) found that Limnoraphis robusta aerotopes are mostly reduced and mucilaginous sheaths are distinctly thinner and less developed in comparison with natural material. As stated by Krüger et al. (1981), the validity of cell size as a taxonomic character should be questioned unless environmental conditions are considered carefully; especially because it is known that modifications of the morphology in culture are directed by the selected culture conditions (Komárek & Anagnostidis, 1989; Tavera et al., 1994).
Based on morphological, ecological and molecular data, Limnoraphis robusta and Microcystis aeruginosa were identified as the species that formed the cyanobacterial bloom in the Santa María del Oro crater lake, Nayarit. Also, is the first bloom in Mexico with these 2 species associated.
Acknowledgments
To the Asociación de Colonos de la Cuenca de Santa María del Oro, and Asociación de hoteleros y restauranteros de Santa María del Oro, for supporting the sampling campaigns. Especially, we thank Enrique Arregui Alva, Eriberto Rodríguez Aguilar, Aurora Ramírez Castañeda, Ramona Castañeda Guerrero, and Blanca Estela Márquez Valdivia, and Unidad de Tecnología de Alimentos-UAN, for the use of laboratories and equipment. Also, we thank Thematic network on Harmful Algae Blooms-Conacyt (RedFAN) for the support and encouragement in this research field. To Eberto Novelo Maldonado and the Continental Algae Laboratory: Ecology and Taxonomy (LACET), for the aid in the morphological identification of cyanobacteria species. Finally, we thank David Alfaro Siqueiros Beltrones and Karina Q. Almeida Leñero for their comments and the English edition that helped to improve the manuscript. This study was part of the projects “Baseline for water quality monitoring of the Santa María del Oro lagoon, Nayarit-SIP14-185”, “Harmful algal bloom dynamics in Santa María del Oro, Nayarit-SIP17-294” funded by Patronato UAN con el Impuesto Especial del 12% destinado a la UAN. The sequencing of DNA samples was funded by the National Council for Science and Technology (Conacyt) project 248468.
References
Allers, T., & Lichten, M. (2000). A method for preparing genomic DNA that restrains branch migration of holliday junctions. Nucleic Acids Research, 28, 6e–6.
Anagnostidis, K., & Komárek, J. (1988). Modern approach to the classification system of cyanophytes. 3. Oscillatoriales. Archiv für Hydrobiologie, Supplement 80, 327–472.
Baker, K. K. (2007). Systematics and ecology of Lyngbya spp. and associated species (Cyanophyta) in a New England salt marsh. Journal of Phycology, 23, 201–208. https://doi.org/10.1111/j.1529-8817.1987.tb04445.x
Caballero, M., Rodríguez, A., Vilaclara, G., Ortega, B., Roy, P., & Lozano, S. (2013). Hydrochemistry, ostracods and diatoms in a deep, tropical, crater lake in Western Mexico. Journal of Limnology, 72, 512–523. https://doi.org/10.4081/jlimnol.2013.e42
Comas-González, A. A., Labaut-Betancourt, Y., & Peraza-Escarrá, R. (2017). Ocurrencia de Limnoraphis robusta (Parakutty) Komárek et al. (Oscillatoriales, Cyanobacteria) en el embalse Hanabanilla (Cuba Central). Anales de Biología, 39, 1–6. https://doi.org/10.6018/analesbio.39.01
Deblois, C. P., & Juneau, P. (2010). Relationship between photosynthetic processes and microcystin in Microcystis aeruginosa grown under different photon irradiances. Harmful Algae, 9, 18–24. https://doi.org/10.1016/j.hal.2009.07.001
Engene, N., Coates, R. C., & Gerwick, W. H. (2010). 16S rRNA gene heterogeneity in the filamentous marine cyanobacterial genus Lyngbya. Journal of Phycology, 46, 591–601. https://doi.org/10.1111/j.1529-8817.2010.00840.x
Engene, N., Rottacker, E. C., Kaštovský, J., Byrum, T., Choi, H., Ellisman, M. H. et al. (2012). Moorea producta gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. International Journal of Systematic and Evolutionary Microbiology, 62, 1171–1178. https://doi.org/10.1099/ijs.0.033761-0
Falcón, L. I., & Valera, A. (2007). Extracción de ácidos nucleicos. In L. E. Eguiarte, V. Souza, & X. Aguirre (Eds.), Ecología molecular (pp. 499–516). México D.F.: Semarnat/ INE/ UNAM/ CONABIO.
Gaytán-Herrera, M. L., Martínez-Almeida, V., Oliva-Martínez, M. G., Durán-Díaz, A., & Ramírez-García, P. (2011). Temporal variation of phytoplankton from the tropical reservoir Valle de Bravo, Mexico. Journal of Environmental Biology, 32, 117–126.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Kim, J. I., Lim, J. H., Lee, J. W., & Lee, H. B. (2002). Phylogenetic relationship of Microcystis (Cyanophyceae) based on partial 16S rRNA gene sequences in Korea. Algae, 17, 153–159. https://doi.org/10.4490/algae.2002.17.3.153
Komárek, J. (2003). Planktic Oscillatorialean cyanoprokaryotes (short review according to combined phenotype and molecular aspects). Hydrobiologia, 502, 367–382. https://doi.org/10.1023/B:HYDR.0000004294.17755.fe
Komárek, J. (2010). Recent changes (2008) in cyanobacteria taxonomy based on a combination of molecular background with phenotype and ecological consequences (genus and species concept). Hydrobiologia, 639, 245–259. https://doi.org/10.1007/s10750-009-0031-3
Komárek, J. (2016). A polyphasic approach for the taxonomy of cyanobacteria: principles and applications. European Journal of Phycology, 51, 346–353. https://doi.org/10.1080/09670262.2016.1163738
Komárek, J. (2018). Several problems of the polyphasic approach in the modern cyanobacterial system. Hydrobiologia, 811, 7–17. https://doi.org/10.1007/s10750-017-3379-9
Komárek, J., & Anagnostidis, K. (1989). Modern approach to the classification system of Cyanophytes 4 – Nostocales. Archiv für Hydrobiologie, 82, 247–345.
Komárek, J., & Anagnostidis, K. (1999). Cyanoprokaryota 1. Teil: Chroococcales. In G. Gärtner, H. Heynig & D. Mollenhauer (Eds.), Süsswasserflora von Mitteleuropa, 19/1 (pp. 1–548). Berlin: Elsevier Spektrum Akademischer Verlag.
Komárek, J., & Anagnostidis, K. (2005). Cyanoprokaryota 2. Teil: Oscillatoriales. In B. Büdel, G. Gärtner, L. Krienitz, & M. Schagerl (Eds.), Süßwasserflora von Mitteleuropa, 19/2 (pp. 1–759). Munich: Elsevier Spektrum Akademischer Verlag.
Komárek, J., Zapomělová, E., Šmarda, J., Kopecký, J., Rejmánková, E., Woodhouse, J., Neilan, B. A., & Komárková, J. (2013). Polyphasic evaluation of Limnoraphis robusta, a water – bloom forming cyanobacterium from Lake Atitlán, Guatemala, with a description of Limnoraphis gen. nov. Fottea, 13, 39–52. https://doi.org/10.5507/fot.2013.004
Komárková, J., Montoya, H., & Komárek, J. (2016). Cyanobacterial water bloom of Limnoraphis robusta in the Lago Mayor of Lake Titicaca. Can it develop? Hydrobiologia, 764, 249–258. https://doi.org/10.1007/s10750-015-2298-x
Krüger, G. H. J., Eloff, J. N., & Pretorius, J. A. (1981). Morphological changes in toxic and nontoxic Microcystis isolates at different irradiance level. Journal of Phycology, 17, 52–56. https://doi.org/10.1111/j.1529-8817.1981.tb00818.x
Kuiper-Goodman, T., Falconer, I., & Fitzgerald, J. (1999). Human health aspects. In I. Chorus & J. Bartram (Eds.) Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management (pp. 125–160). London: World Health Organization.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. https://doi.org/10.1093/molbev/msw054
Kurobe, T., Baxa, D. V., Mioni, C. E., Kudela, R. M., Smythe, T. R., Waller, S. et al. (2013). Identification of harmful cyanobacteria in the Sacramento-San Joaquin Delta and Clear Lake, California by DNA barcoding. SpringerPlus 2, 491. https://doi.org/10.1186/2193-1801-2-491
Lawton, L., Marsalek, B., Padisák, J., & Chorus, I. (1999). Determination of cyanobacteria in the laboratory. In I. Chorus & J. Bartram (Eds.), Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management (pp. 334–361). London: World Health Organization.
Mercado-Borrayo, B. M. (2007). Estudio sobre la remoción de cianobacterias y sus metabolitos en la planta potabilizadora ‘Los Berros’ Sistema Cutzamala (Tesis de maestría). Instituto de Ingeniería, Universidad Nacional Autónoma de México. México D. F.
Montejano, G., Gold-Morgan, M., & León-Tejera, H. (2005). Surveying the diversity of cyanoprokaryotes in poorly known regions: The case of the central region of Mexico. Algological Studies/Archiv Für Hydrobiologie, 117, 329–38. https://doi.org/10.1127/1864-1318/2005/0117-0329
Montoya, H., Komárková, J., & Komárek, J. (2014). Cyanobacterial species, potentially forming water-blooms in the Lake Titicaca (Peru). Arnaldoa, 21, 381–90.
Nava-Ruiz, V. M., & Valadez-Cruz, F. (2012). Flora planctónica de laguna Lagartos, Quintana Roo. Revista Mexicana de Biodiversidad, 83, 561–82. https://doi.org/10.7550/rmb.24868
Neilan, B. A., Jacobs, D., Dot, D. E. L., Blackall, L. L., Hawkins, P. R., Cox, P. T., & Goodman, A. E. (1997). rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. International Journal of Systematic Bacteriology, 47, 693–697. https://doi.org/10.1099/00207713-47-3-693
Novelo, E. (2011). Cyanoprokaryota. Fascículo 90. In R. Medina (Ed.), Flora del Valle de Tehuacán-Cuicatlán (pp. 1–101). México D.F.: Universidad Autónoma de México.
Nübel, U., García-Pichel, F., & Muyzer, G. (1997). PCR primers to amplify 16S rRNA genes from cyanobacteria. Applied and Environmental Microbiology, 63, 3327–3332.
Ochoa-Zamora, G. G. (2018). Variación espacio-temporal de las poblaciones de cianobacterias formadoras de florecimientos en el lago cráter de Santa María del Oro, Nayarit (Tesis). Unidad Académica de Agricultura, Universidad Autónoma de Nayarit. Xalisco, Nayarit, México.
Oliva-Martínez, M. G., Godínez-Ortega, J. L., & Zúñiga-Ramos, C. A. (2014). Biodiversidad del fitoplancton de aguas continentales en México. Revista Mexicana de Biodiversidad, 85, 54–61. https://doi.org/10.7550/rmb.32706
Otsuka, S., Suda, S., Oyaizu, H., Matsumoto, S., & Watanabe, M. M. (2001). A proposal for the unification of five species of the cyanobacterial genus Microcystis Kützing ex Lemmermann 1907 under the Rules of the Bacteriological Code. International Journal of Systematic and Evolutionary Microbiology, 51, 873–879. https://doi.org/10.1099/00207713-51-3-873
Pérez-Morales, A., Olivos-Ortiz, A., Quijano-Scheggia, S. I., Espinosa-Rodríguez, C. A., & Jiménez-Santos, M. A. (2016). Estado actual del estudio de cianobacterias dulceacuícolas formadoras de florecimientos en el centro de México. In E. García-Mendoza, S. I. Quijano-Scheggia, A. Olivos-Ortiz, & E. Núñez-Vázquez (Eds.), Florecimientos algales nocivos en México (pp. 408–421). Ensenada: CICESE.
Saitou, N., & Nei, M. (1987). The Neighbor-Joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–25. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Salas-Betancourt, A. (2018). Dinámica de nutrientes del lago Santa María del Oro, Nayarit (Tesis). Unidad Académica de Ciencias Químico Biológicas y Farmacéuticas, Universidad Autónoma de Nayarit. Tepic, Nayarit, México.
Sievers, F., & Higgins, D. G. (2018). Clustal Omega for making accurate alignments to many protein sciences. Protein Science, 27, 135–145. https://doi.org/10.1002/pro.3290
Sievers, F., Wilm, A., Dineen, D. G., Gibson, T. J., Karplus, K., Li, W. (2011). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology, 7, 539. https://doi.org/10.1038/msb.2011.75
Stoyanov, P., Moten, D., Mladenov, R., Dzhambazov, B., & Teneva, I. (2014). Phylogenetic relationships of some filamentous cyanoprokaryotic species. Evolutionary Bioinformatics, 10, EBO.S13748. https://doi.org/10.4137/EBO.S13748
Suda, S., Liu, Y., He, J., Hu, Z., Hiroki, M., & Watanabe, M. M. (1998). Morphological, biochemical and physiological characteristics of Lyngbya Hieronymusii var. hieronymusii (Oscillatoriales, Cyanobacteria). Phycological Research, 46, 51–55. https://doi.org/10.1046/j.1440-1835.1998.00125.x
Tavera, R., Kovácik, L., & Komárek, J. (1994). Ecophysiological and morphological characterization of some Oscillatorialean species from the Papaloapan Basin in Mexico. Algological Studies, 73, 23–41.
Valadez, F., Rosiles-González, G., Almazán-Becerril, A., & Merino-Ibarra, M. (2013). Planktonic cyanobacteria of the tropical karstic Lake Lagartos from the Yucatan Peninsula, Mexico. Revista de Biología Tropical, 61, 971–79.
Vázquez-Castro, G., Ortega-Guerrero, B., Rodríguez, A., Caballero, M., & Lozano-García, S. (2008). Mineralogía magnética como indicador de sequía en los sedimentos lacustres de los últimos ca. 2,600 años de Santa María Del Oro, occidente de México. Revista Mexicana de Ciencias Geológicas, 25, 21–38.
Vázquez, G., & Caballero, M. (2013). The structure and species composition of the diatom communities in tropical volcanic lakes of eastern Mexico. Diatom Research, 28, 77–91. https://doi.org/10.1080/0269249X.2012.739974
Wilmotte, A. (1994). Molecular evolution and taxonomy of the cyanobacteria. In: D. A. Bryant (Ed.), The molecular biology of cyanobacteria (pp. 1–25). Dordrecht: Springer. https://doi.org/10.1007/978-94-011-0227-8_1