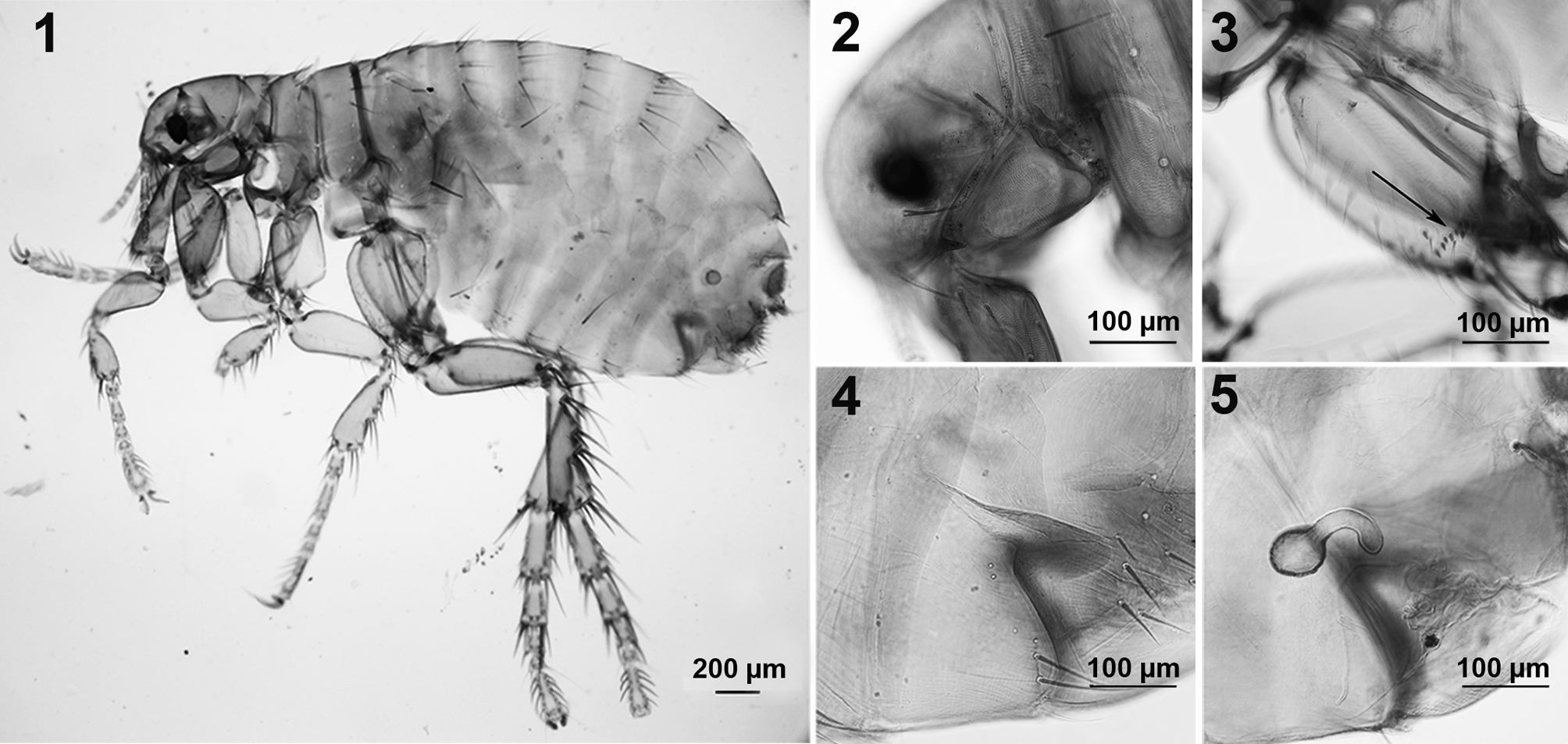
The human flea Pulex irritans (Siphonaptera: Pulicidae) in northwestern Argentina, with an investigation of Bartonella and Rickettsia spp.
Marcela Lareschi a, b, *, José Manuel Venzal c, Santiago Nava b, d, Atilio José Mangold b, d, Aránzazu Portillo e, Ana María Palomar-Urbina e, José Antonio Oteo-Revuelta e
a Centro de Estudios Parasitológicos y de Vectores, CONICET, Universidad Nacional de La Plata. Blv. 120 e/ 60 y 64 S/N, 1900 La Plata, Argentina
b Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Godoy Cruz 2290 (C1425FQB) CABA, Argentina
c Laboratorio de Vectores y Enfermedades Transmitidas, Facultad de Veterinaria, Universidad de la República, Gral. Fructuoso Rivera 1350, 50000 Salto, Departamento de Salto, Uruguay
d Laboratorio de Parasitología e Inmunología, Estación Experimental Agropecuaria Rafaela, Instituto Nacional de Tecnología Agropecuaria, RN34 227, Rafaela, Santa Fe, Argentina
e Centro de Rickettsiosis y Enfermedades Transmitidas por Artrópodos Vectores, Hospital San Pedro- Centro de Investigación Biomédica de La Rioja, Calle Piqueras, 98, 26006 Logroño, La Rioja, Spain
*Corresponding author: mlareschi@yahoo.com.ar (M. Lareschi)
Abstract
Pulex irritans is the only cosmopolitan flea species and the most studied one within the genus Pulex. It has importance in public health since it commonly parasitizes humans causing dermatitis, and it has been also implicated in the transmission of bacterial pathogens. Pulex irritans has been confused with the closely related Pulex simulans species for years. Herein, Pulex specimens collected from a Pampas fox and a Chacoan peccary from northwestern Argentina were identified by comparison with type specimens. In addition, the presence of Bartonella spp. and Rickettsia spp. was investigated using PCR assays. Our results provided characters of diagnostic importance to identify P. irritans, which include the shape of sternite VII in the females, and of the aedeagal sclerite, clasper and crochet in the males. Besides, we report for the first time P. irritans parasitizing a peccary. This finding reinforces the hypothesis of the origin of this flea associated with this mammal, and then colonizing humans and domestic mammals. There was no evidence of Bartonella or Rickettsia DNA in the analyzed fleas. This information even if negative may be considered relevant for P. irritans from Argentina.
Keywords:
Flea; Siphonaptera; Pulex; Bacteria; Pathogens; Argentina
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
La pulga humana Pulex irritans (Siphonaptera: Pulicidae) en el noroeste argentino, una investigación de Bartonella y Rickettsia spp.
Resumen
Pulex irritans es la única especie cosmopolita y la más estudiada dentro del género Pulex. Tiene importancia en la salud pública ya que comúnmente parasita a los seres humanos causando dermatitis y también ha sido implicada en la transmisión de patógenos bacterianos. Pulex irritans se ha confundido con la especie cercana Pulex simulans durante años. En este sentido, se identificaron los especímenes de Pulex recolectados de un zorro pampeano y un pecarí del Chaco del noroeste de la Argentina por comparación con los ejemplares tipo. Además, se investigó la presencia de Bartonella spp. y Rickettsia spp. utilizando ensayos de PCR. Nuestros resultados aportaron caracteres de importancia diagnóstica para identificar a P. irritans, que incluyen la forma del esternito VII en las hembras y del esclerito aedeagal, clasper y crochet en los machos. Además, se reporta por primera vez a P. irritans parasitando un pecarí. Este hallazgo refuerza la hipótesis del origen de esta pulga asociada con este mamífero y luego coloniza humanos y mamíferos domésticos. No hubo evidencia de ADN de Bartonella ni de Rickettsia en las pulgas analizadas. Esta información, si bien negativa, puede ser considerada relevante para P. irritans de Argentina.
Palabras clave:
Pulga; Siphonaptera; Pulex; Bacterias; Patógenos; Argentina
Introduction
Fleas (Siphonaptera) are holometabolous, laterally flattened, wingless insects, highly specialized to ectoparasitic life. Male and female adults are obligate hematophagous ectoparasites of mammals and birds. Fleas are important in public health as parasites, and as vectors of pathogens (Bitam et al., 2010; Marshall, 1981). About 2,574 species of fleas belonging to 16 families and 238 genera have been described, but only a minority lives in close association with humans (Lewis, 1999). The genus Pulex Linnaeus, 1758 (Pulicidae) contains 7 recognized species. Within this genus, Pulex irritans Linnaeus, 1758 is the most studied species and the only one which occurs in Europe (Hopla, 1980). This flea was originally described on the basis of specimens collected from humans from Uppsala, Sweden, and consequently called “the human flea”. Subsequently, Pulex simulans Baker, 1895 was described on the basis of specimens collected from America, and the author remarked that, although distinct, this species was morphologically very close and easily confused with P. irritans. However, P. simulans was again considered a synonym of P. irritans (Jordan & Rothschild, 1908), and it was in 1958 when P. simulans was considered a valid species since the morphological differences in the aedeagal sclerite and crochet in males of the 2 species were reported (Smit, 1958). However, in America P. irritans and P. simulans have been confused for years. Possibly these fleas continue to be confused up to the present. The original description of P. irritans included specimens from Europe and America, and probably specimens of P. simulans erroneously confused with P. irritans (Smit, 1958). Since the type specimen of P. irritans was lost, a male neotype from Budapest, Hungary, deposited in the Rothschild Collection of fleas, was designated (Smit, 1958). Confusion between close species of Pulex continues and a revision in order to enable a correct identification of P. irritans and P. simulans is necessary.
The relationship between Pulex irritans and humans could suggest a long history of association (Marshall, 1981). Currently, P. irritans has a cosmopolitan distribution, probably due to human transport. However, the genus Pulex is of American origin (Hopla, 1980). Pulex irritans has been recovered from archaeological sediments in Viking York (England), Dublin (Ireland) and abandoned farm sites in Norse Greenland (Iceland), but the origins of the flea appear to be Central to South American, where several congeners are known. The probable routes by which the species reached Western Europe are discussed and resolved in favor of a Beringian and Asiatic one, at any time during the postglacial era. Although this flea is presently relatively promiscuous, initial evolution is likely to have involved a single host, probably a peccary, closely associated with humans (Buckland & Sadler, 1989). Currently, a variety of mammals are known as hosts of P. irritans, and because of its close association with domestic mammals such as pigs and dogs, P. irritans can bite humans. Pulex irritans is able to transmit several zoonotic pathogens, namely the agents of plague, and the flea-borne spotted rickettsiosis (Brouqui & Raoult, 2006), and it has been important in transmitting Yersinia pestis from man to man and possibly from domestic animals to man (Hopla, 1980).
Because of its association with humans and domestic animals, and with disease transmission, P. irritans has been the most studied species within the genus Pulex. However, in Argentina most of the records of this flea were accidental and no morphological studies have been carried out in order to establish a correct identification of the species. In Argentina, P. irritans have been mentioned parasitizing wild mammals such as marsupials, carnivores, lagomorphs, rodents and even-toed ungulates, and the role of this flea as vector of pathogens had not been investigated (Lareschi et al., 2016).
The aim of this work is to show the results of the study of Pulex specimens from northwestern Argentina which involves a correct identification. In addition, we investigate the presence of bacteria from Bartonella and Rickettsia genera using molecular biology techniques.
Materials and methods
Forty-nine fleas were recovered from carcasses of a Chacoan peccary Catagonus wagneri (Rusconi, 1930) (Artiodactyla, Tayassuidae) and a Pampas fox Lycalopex gymnocercus (Fischer, 1814) (Carnivora, Canidae). Both animals were found dead at Rivadavia Banda Sur (24°11’ S, 62°53’ W), Salta Province, Argentina. Fleas were preserved in vials with 96% ethanol. All fleas (12 specimens) collected from the Chacoan peccary were mounted for their identification at the microscope. Since fleas from the Pampas fox were more abundant (37 specimens), 12 were prepared for morphological studies whereas the remaining 25 were preserved for molecular studies.
All fleas were identified using a binocular stereoscopic microscope. Twenty-four selected fleas were cleared in KOH, dehydrated in a graduated series of alcohol, diaphanized in eugenol and mounted on slides in Canada balsam for their detailed examination using the compound microscope. Photographs were taken by using a Microscope Olympus BX51 equipped with Photographic Camera Olympus DP71, BX51TF, Tokyo, Japan. Morphology was studied comparing our specimens with the male neotype of P. irritans and the female lectotype of P. simulans, as well as other specimens of both sexes of these species deposited at the Natural History Museum (NHM), London, U.K. The neotype of P. irritans (C. Rothschild Coll. 1923-615) and a male of P. simulans (Navarro Coll., Texas. Brit. Mus. 1949-268) (the last although not in very good condition) were photographed by using a dino lite Digital Microscope. Female specimens of both species were not in good enough condition to be photographed. For comparative purposes, specimens of other species of Pulex deposited at the NHM were also examined. In addition, figures, keys and descriptions of species of Pulex given in Hopkins & Rothschild (1953), Barrera (1955), and Hopla (1980) were also considered. Morphological terminology follows the 2 first authors. Representative specimens of fleas from northwestern Argentina were deposited in the Department of Entomology, Museo de La Plata (MLP), Argentina.
Twenty-five fleas obtained from L. gymnocercus were examined for the presence of Rickettsia and Bartonella species. Fleas were rinsed in distilled water and dried on sterile filter paper under a laminar-flow hood (Pérez-Martínez et al., 2009). Each flea was crushed with a sterile pestle, and DNA was extracted by lysis with 0.7 M ammonium hydroxide (Rijpkema et al., 1996). Detection of Bartonella spp. was tested using PCR primers targeting the RNA polymerase β-subunit-encoding gene (rpoB) and the citrate synthase (gltA) partial genes as well as the 16S-23S rRNA intergenic region (ITS) (Jensen et al., 2000, Norman et al., 1995; Renesto et al., 2001). Rickettsia DNA was tested with 2 PCR assays targeting the rickettsial gltA gene (Labruna et al., 2004; Regnery et al., 1991). A positive control consisting of Bartonella henselae (DNA extracted from a cat flea —Ctenocephalides felis— from La Rioja, Spain), or Rickettsia slovaca strain S14ab DNA (obtained from Vero cells inoculated with a Dermacentor marginatus tick at the Center of Rickettsiosis and Arthropod Borne Diseases from La Rioja, Spain), and a negative control (sterile water instead of template DNA) were used for each PCR assay.
Results
All 49 fleas were identified as P. irritans (Figs. 1-7). Five male specimens (2 from the Chacoan peccary, and 3 from the Pampas fox) and 19 females (10 from the Chacoan peccary, and 9 from the Pampas fox) were studied in more detailed: fleas from both genders presented large, dark and conspicuous eyes, without ventral sinus, falax extending down to the base of antennal fossa, shorter than the wider diameter of the eye, occipital groove not evident, labial palp and lacinia half to three-quarters length of anterior margin of fore coxa, lacinia with weak teeth, thinner at every level than the base of the first segment of the maxillary palps, second and fourth segments of maxillary palp subequal in length (Fig. 1); apex of clypeal apodeme dorsal to the implementation of the preocular seta, genal ctenidium constituted by a single dark tooth; occiput with only 1 strong bristle, with a single ocular bristle and a single bristle near base of maxilla (Fig. 2); with a row of small spines on inside of hind coxa near apex, consisting of 13-16 spines forming a patch (Fig. 3), break of mesocoxa incomplete.
Female (Figs. 1, 4, 5): length: 2.0-3.5 mm (Fig. 1); sternite VII with a sinus and with 4/6 setae on each side the sinus (Fig. 4); spermatheca as in Figure 5. No differences were observed between female fleas from the Chacoan peccary and those from the Pampas fox. In contrast, the lectotype of P. simulans presented a greater number of setae on each side the sinus of sternite VII.
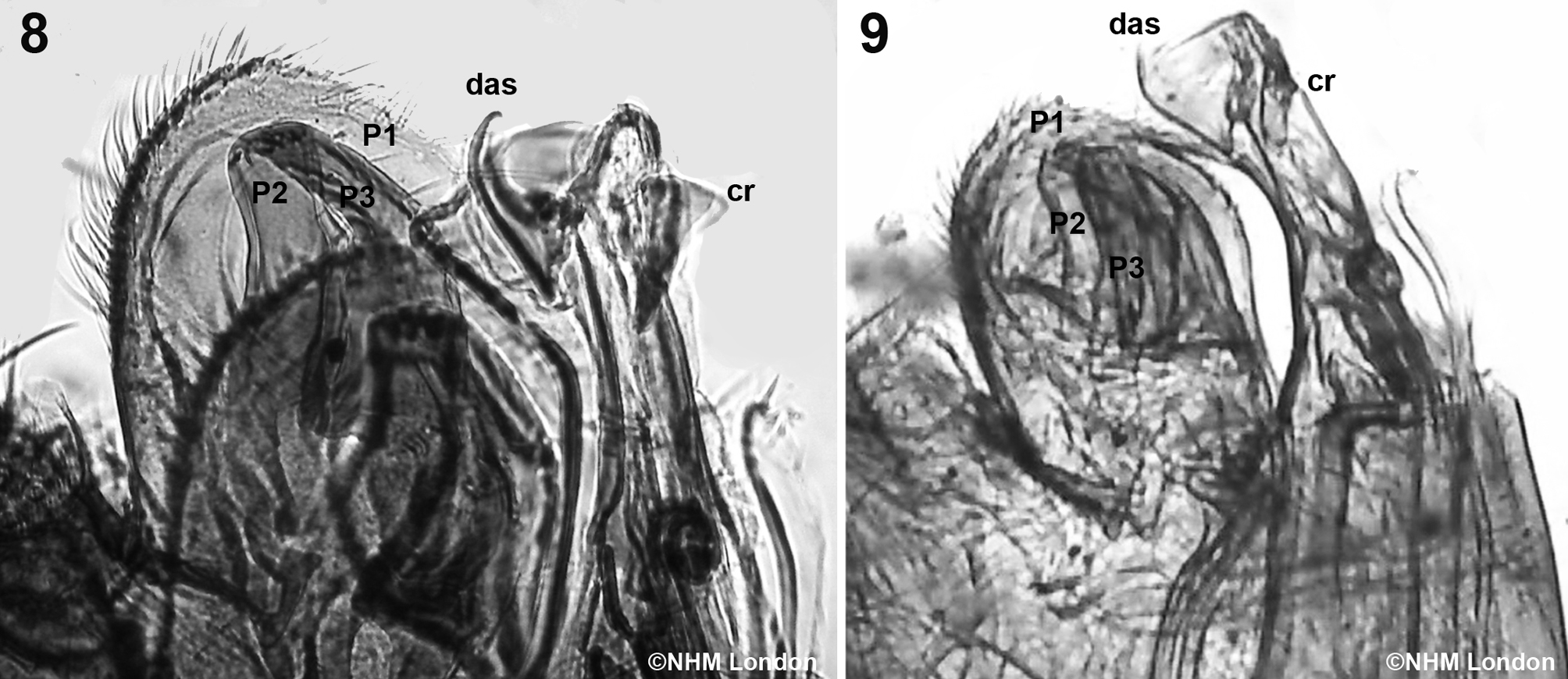
Male (Figs. 6-7): length 1.8-2.0 mm; with dorsal aedeagal sclerite (das) relatively long and slender; clasper with processes 1 (P1) very large and completely covering processes 2 (P2) and 3 (P3), ovoid but with postero-distal angle nearly straight; processes 2 and 3 shorter than 1; the crochet (cr) expanded apically, slightly different in the length of the expansion between fleas (shorter —on those from the Chacoan peccary; figure 6— than in those from the Pampas fox; figure 7); proximal arm of sternite IX thin, slightly curved, with parallel margins, but with the apex dilated; distal arm of sternite IX almost as long as the proximal arm, with parallel edges, straight, except in the apical portion is blunt (Fig. 7). Males of P. irritans from Argentina were similar to the neotype (Fig. 8).
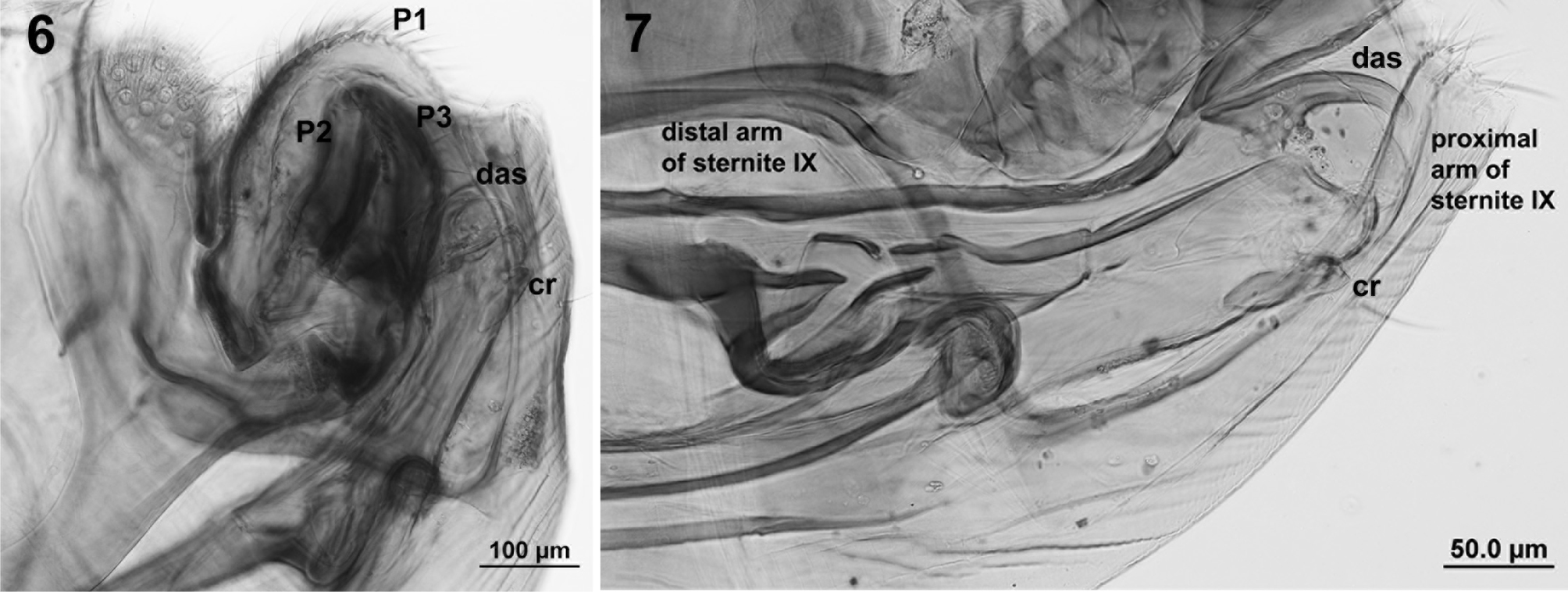
In contrast, they differ with P. simulans (Fig. 9) because of the shape of the aedeagal sclerite (das) (broad, although not well visible at the photograph); processes 1 (P1) of the clasper shorter, not covering completely processes 2 (P2) and 3 (P3); and the crochet (cr) almost straight and rodlike, with a very little expansion.
Rickettsia and Bartonella DNA was not detected in any flea tested by PCR.
Discussion
Specimens of P. irritans and P. simulans have been commonly confused in numerous studies because of the difficulty to identify them with certainty (Barrera, 1955). Smit (1958) recognized that there are usually more setae on each side of sternite VII of the females of P. simulans than in P. irritans. However, some authors (Durden et al., 2012; Hopla, 1980) do not recognize discriminatory female features for these 2 species. We support that the combination of the following characters obtained from the literature and confirmed by the examination of specimens deposited at the NHM, allows differentiating specimens of P. irritans from those of P. simulans: number of setae on each side the sinus of sternite VII in females (4-6 in irritans vs. 7-9 in simulans); shape of aedeagal sclerite (das) (slender in irritans vs strong in simulans), length of processes 1 (P1) of the clasper (long and covering completely processes 2 and 3 in irritans vs short and not covering the other processes in simulans), and the presence (in irritans) or absence (in simulans) of an expansion in the crochet (cr). According with these diagnostic characteristics, we confirmed that fleas from northwestern Argentina herein studied were P. irritans. Slight differences in the expansion of the apical part of the aedeagal crochet observed between fleas from the Chacoan peccary and those from the Pampas fox are in accordance with the variation of this structure mentioned in the literature (Hopla, 1980; Smit, 1958).
Pulex irritans is the only species of the genus reported from Argentina and was previously mentioned parasitizing L. gymnocercus in the country without more information about the locality (Hopkins & Rothschild, 1953). Besides, P. irritans was reported from Salta Province associated with Puma concolor (Linnaeus, 1771) (Carnivora, Felidae) in captivity (Hopkins & Rothschild, 1953). The Chacoan peccary, also known as tagua, was first described in 1930 based on fossils and it was originally thought to be an extinct species (Rusconi, 1930). In 1971, it was discovered to still be alive in the Argentinean Chaco region, in the Province of Salta. The species was well known to the native people, but it took a while for scientists to acknowledge its existence (Mayer & Wetzel, 1986). The Chacoan peccary is one of the 3 living species of the family Tayassuidae, all of them found in Argentina (Gasparini et al., 2006). Peccaries entered North America from Eurasia and then extended their range into South America during the Great American Biotic Interchange. The peccaries had great taxonomic diversity and wide geographic distribution in the past. However, in the modern fauna, peccaries have a lower generic and specific diversity, and they are distributed in the American continent from the southwestern USA to North-Central Argentina (Gasparini, 2013). Even though peccaries are suggested to be the original hosts of P. irritans (Buckland & Sadler, 1989), there is no evidence of this parasite-host association. In Argentina, and among Artiodactyla, P. irritans was collected only from brocket deer (Mazama spp.) (Lareschi et al., 2016). In contrast, collared peccaries (Pecari tajacu [Linnaeus, 1758]) from Texas were found parasitized with Pulex porcinus Jordan & Rothschild, 1923 (Samuel & Low, 1969).
Pulex irritans is the only cosmopolitan flea in the genus Pulex, and this species parasitizes a variety of mammals including guinea pigs, domestic dogs, cats, rats, domestic pigs and goats (Bitam et al., 2010; Hopla, 1980; Lareschi et al., 2016). Pulex irritans is a very aggressive flea and its infestations can reach tremendous levels, particularly when farmers are in contact with corrals or buildings adjacent to their homes (Bitman et al., 2010). In addition, P. irritans is involved in the natural cycle of different Bartonella species. Currently Bartonella species have been recognized associated with mammalian hosts and 11 species have been implicated in human diseases (Bitam et al., 2010). A Bartonella species, closely related to Bartonella rochalimae, was found in pools of P. irritans from foxes in the Iberian Peninsula. This bacterium is implicated as an agent of human diseases, and carnivores were confirmed as major reservoirs for Bartonella spp. in Spain (Márquez et al., 2009). Moreover, the identification of B. rochalimae in P. irritans from dogs in Chile supported the possibility that this flea could be a vector of this human pathogen (Pérez-Martínez et al., 2009). Bartonella quintana was also detected in P. irritans (Rolain et al., 2005). Bartonella quintana is a re-emerging pathogen mainly among homeless in the USA and Europe, which causes infections ranging from asymptomatic to severe illness, but classical symptoms correspond to an acute febrile illness, often accompanied by severe headache and pain in the long bones of the legs (Bitam et al., 2010). In addition, P. irritans is an intermediate host of some nematodes and platyhelminths (Hopla, 1980). Pulex irritans is also involved in the transmission of Rickettsia felis, which causes the flea-borne spotted rickettsiosis in humans (Brouqui & Raoult, 2006). Although few confirmed human cases have been reported, probably because they were misdiagnosed, this infection occurs worldwide (Bitam et al., 2010). In our study, Rickettsia and Bartonella DNA was not detected in any flea from the fox tested by PCR. In this sense, the results obtained are not representative of what could occur in other regions or other host species. However, since it is the first survey of these pathogens in specimens of P. irritans collected in Argentina, we believe that the information, although restricted and negative, may be considered relevant.
Buckland and Sadler (1989) propose that initial evolution of P. irritans may have involved a single host, probably a peccary. Our results report for the first time P. irritans parasitizing a peccary. In this sense, our results are new and may reinforce the hypothesis about the origin of P. irritans (Buckland & Sadler, 1989). In addition, we mentioned for the first time P. irritans in Salta Province associated with free ranging wild animals. Fleas from the Pampas fox were negative for Bartonella and Rickettsia DNA by PCR. More studies considering a bigger sample of fleas may be necessary for detection of the pathogens. The role of P. irritans as a human parasite as well as its association with the transmission of diseases implicated in public health support the necessity of new studies of this flea in Argentina, which may involve new locations and host species, and a correct identification of specimens.
Acknowledgments
We thank Luis Giambelluca and María Laura Morote (both CEPAVE) for their help with the photographs, an anonymous English person, and Theresa Howard and Erica McAlister (NHM) for their assistance to M.L. during her visit to study specimens deposited at the Rothschild Collection at the Natural History Museum (NHM), London. The visit of M.L. to the NHM was funded by the Universidad Nacional de La Plata and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
References
Barrera, A. (1955). Las especies mexicanas del género Pulex Linnaeus (Siph., Pulic.). Anales de la Escuela Nacional de Ciencias Biológicas, 6, 219–236.
Bitam, I., Dittmar, K., Parola, P., Whiting, M. F., & Raoult, D. (2010). Fleas and flea-borne diseases. International Journal of Infectious Diseases, 14, 667–676.
Brouqui, P., & Raoult, D. (2006). Arthropod-borne diseases in homeless. Annals of the New York Academy of Sciences, 1078, 223–235.
Buckland, P. C., & Sadler, J. P. (1989). A biogeography of the human flea, Pulex irritans L. (Siphonaptera: Pulicidae). Journal of Biogeography, 16, 115–120.
Durden, L. A., Wilson, N., Eckerlin, R. P., & Baker, W. W. (2012). The flea (Siphonaptera) fauna of Georgia, U.S.A.: hosts, distribution and medical-veterinary importance. Annals of Carnegie Museum, 80, 83–113.
Gasparini, G. M. (2013). Records and stratigraphical ranges of South American Tayassuidae (Mammalia, Artiodactyla). Journal of Mammalian Evolution, 20, 57–68.
Gasparini, G. M., Ortiz Jaureguizar, E., & Carlini, A. A. (2006). Orden Artiodactyla. In R. Bárquez, M. M. Díaz, & R. Ojeda (Eds.), Mamíferos de Argentina. Sistemática y distribución (pp. 114–122). Tucumán, Argentina: SAREM.
Hopkins, G. H. E., & Rothschild, M. (1953). An illustrated catalogue of the Rothschild Collection of fleas (Siphonaptera) in the British Museum (Natural History). Volume I. Tungidae and Pulicidae. London, U.K: The Trustees of the British Museum.
Hopla, C. E. (1980). A study of the host associations and zoogeography of Pulex. In R. Traub, & H. Starcke (Eds.), Fleas (pp. 185–207). Rotterdam, The Netherlands: Proceedings of the International Conference on Fleas, AA Balkema Publishers.
Jensen, W. A., Fall, M., Rooney, J., Kordick, D. L., & Breitschwerdt, E. B. (2000). Identification and differentiation of Bartonella species using a single step PCR assay. Journal of Clinical Microbiology, 38, 1717–1722.
Jordan, K., & Rothschild, N. C. (1908). Revision of the non-combed eyed Siphonaptera. Parasitology, 1, 1–100.
Labruna, M. B., Whitworth T., Horta, M. C., Bouyer , D. H., McBride, J. W., Pinter, A., et al. (2004). Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. Journal of Clinical Microbiology, 42, 90–98.
Lareschi, M., Sánchez, J., & Autino, A. (2016). A review of the fleas (Insecta- Siphonaptera) from Argentina. Zootaxa, 4103, 239–258.
Lewis, R. E. (1999). Resume of the Siphonaptera (Insecta) of the World. Journal of Medical Entomology, 35, 377–389.
Márquez, F. J., Millán, J., Rodríguez-Liébana, J. J., García-Egea, I., & Muniain, M. A. (2009). Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Medical and Veterinary Entomology, 23, 393–398.
Marshall, A. G. 1981. The ecology of ectoparasitic insects. New York: Academic Press.
Mayer, J. J., & Wetzel, R. M. (1986). Catagonus wagneri. Mammalian Species, 259, 1–5.
Norman, A. F., Regnery, R., Jameson, P., Greene, C., & Krause, D. C. (1995). Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. Journal of Clinical Microbiology, 33, 1797–1803.
Pérez-Martínez, L., Venzal, J. M., González-Acuña, D., Portillo, A., Blanco, J. R., & Oteo, J. A. (2009). Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerging Infectious Diseases, 15, 1150–1152.
Regnery, R., Spruill, C., & Plikaytis, B. (1991). Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. Journal of Bacteriology, 173, 1576–1589.
Renesto, P., Gouvernet J., Drancourt M., Roux V., & Raoult, D. (2001). Use of rpoB gene analysis for detection and identification of Bartonella species. Journal of Clinical Microbiology, 39, 430–437.
Rijpkema, S., Golubic D., Molkenboer, M., Verbeek-De Kruif, N., & Schelleken, J. (1996). Identification of four genomic groups of Borrelia burgdorferi sensu lato Ixodes ricinus ticks collected in a Lyme borreliosis Endemic region of northern Croatia. Experimental and Applied Acarology, 20, 23–30.
Rolain, J. M., Bourry, O., Davoust, B., & Raoult, D. (2005). Bartonella quintana and Rickettsia felis in Gabon. Emerging Infectious Diseases, 11, 1742–1744.
Rusconi, C. (1930). Las especies fósiles argentinas de pecaríes y sus relaciones con las del Brasil y Norteamérica. Anales del Museo Nacional de Historia Natural Bernadino Rivadavia, 36, 121–241.
Samuel, W. M., & Low, W. A. (1969). Parasites of the collared peccary from Texas. Journal of Wildlife Diseases, 6, 16–23.
Smit, F. G. A. M. (1958). A preliminary note on the occurrence of Pulex irritans L. and Pulex simulans Baker in North America. Journal of Parasitology, 44, 523–526.