Redescription of the velvet worm Oroperipatus eisenii (Onychophora: Peripatidae), through DNA sequencing, scanning electron microscopy and new collection records from Western Mexico
Gerardo A. Contreras-Félix a, *, Griselda Montiel-Parra b, Fabio G. Cupul-Magaña c, Tila M. Pérez b
a Colección Nacional de Arácnidos (CNAN), Departamento de Zoología, Instituto of Biología, Universidad Nacional Autónoma de México, 3er Circuito exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
b Colección Nacional de Ácaros (CNAC), Departamento de Zoología, Instituto of Biología, Universidad Nacional Autónoma de México, 3er Circuito exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
c Centro Universitario de la Costa, Universidad de Guadalajara, Av. Universidad 203, Delegación Ixtapa, 48280 Puerto Vallarta, Jalisco, Mexico
*Corresponding author: contrerasfelixga@gmail.com (G.A. Contreras-Félix)
Abstract
Specimens of Oroperipatus eisenii (Wheeler, 1898) were re-examined, including 13 new records for the species collected in the states of Nayarit, Jalisco, and Michoacán in Mexico. Scanning electron micrographs that illustrate most of the diagnostic characters were provided allowing a redescription of the species, as well as a map of the localities where the collections were carried out. DNA sequences were obtained from the collected specimens for the cytochrome C oxidase I (COI) gene; results of the similarity analysis showed that there was no genetic divergence for COI within the sampled onychophoran populations. Natural history information of the species is included.
Keywords:
Tropical forest; North America; Biodiversity; Species delimitation; Speciation
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Redescripción del gusano terciopelo Oroperipatus eisenii (Onychophora: Peripatidae), a través de secuenciación de ADN, microscopía electrónica de barrido y registros nuevos del occidente de México
Resumen
En este trabajo se reexaminaron ejemplares de Oroperipatus eisenii (Wheeler, 1898) y se presentan 13 registros nuevos de la especie en los estados de Nayarit, Jalisco y Michoacán, en México. Se proporcionan imágenes de microscopio electrónico de barrido que ilustran la mayoría de los caracteres diagnósticos y se incluye la redescripción de la especie, así como un mapa que muestra las localidades de recolecta. Se obtuvieron secuencias del gen citocromo C oxidasa I (COI) a partir de los ejemplares recolectados; los resultados del análisis de similitud muestran que no existe diferenciación genética entre las poblaciones de O. eisenii estudiadas. También se presenta información sobre la historia natural de la especie.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Keywords:
Bosque tropical; Norteamérica; Biodiversidad; Delimitación de especies; Especiación
Introduction
Velvet worms are terrestrial invertebrates traditionally known from studies on historical biogeography, conservation biology, and evolution (Allwood et al., 2010; Murienne et al., 2014). Their populations are usually low density in most habitats, so they are labeled as “rare” (Monge-Nájera & Morera-Brenes, 2015). Velvet worms are more common in tropical and temperate forests of the Southern Hemisphere and near the equator; these animals are more diverse and better known in southern locations than in the northern counterparts (Mayer et al., 2015; Oliveira, Read et al., 2012).
Several contributions on onychophoran in Mexico, Central America, and the Caribbean have recently appeared, but the species of Costa Rica are among the most studied taxonomically and in other fields such as morphology (Monge-Nájera & Morera-Brenes, 1994; Oliveira, Franke et al., 2012); physiology (Mayer & Harzsch, 2007; Monge-Nájera & Morera-Brenes, 1994); ecology (Monge-Nájera, 1994; Monge-Nájera & Alfaro, 1995); behavior (Monge-Nájera et al., 1993); biophysics (Concha et al., 2015); biochemistry (Baer et al., 2014); genetics and genomics (Jeffery et al., 2012; Morera-Brenes et al., 1992; Podsiadlowski et al., 2008); phylogeography (Morera-Brenes & Monge-Nájera, 2010; Murienne et al., 2014); taphonomy (Monge-Nájera & Xianguang, 2002); zooantropology (Monge-Nájera & Morera-Brenes, 2015); conservation (Monge-Nájera, 2018; Sosa-Bartuano et al., 2018); and digital photography (Barquero-González et al., 2016).
So far, 206 species of velvet worms have been described, of which 186 are valid species. The rest have been cataloged as nomina dubia due to taxonomic inconsistencies (Oliveira, Read et al., 2012; Oliveira et al., 2015, 2018). The diversity of velvet worms in Mexico is poorly known; only 3 species belonging to the neotropical Peripatidae have been reported. The first description of Oroperipatus goudoti (Bouvier, 1899) contained imprecise data, which only mentions “Mexico” as the collection locality and has been cataloged as nomina dubia. The second, Macroperipatus perrieri (Bouvier, 1899), has an original label that does not provide details specifying if it was found in the state of “Veracrus” (sic) or the city of “Veracrus”. The third species Oroperipatus eisenii (Wheeler, 1898), has the best locality description from Tepic, Nayarit. There are DNA sequences from unidentified onychophorans from the states of Chiapas (Murienne et al., 2014), and Campeche (BOLD Systems, 2014). Toledo-Matus et al. (2018) reported an undescribed species of Oroperipatus from the deciduous forest of Tuxtla Gutiérrez, Chiapas. Monge-Nájera (2018) reports specimens unidentified from the state of Nuevo León.
The species Oroperipatus eisenii was described in the original paper based on 87 specimens from Tepic, Nayarit (Wheeler, 1898). Later, Rucker (1900) described the external and internal morphology of males from the same locality. The information about the deposit of type specimens is scarce in both studies. A pair of specimens labeled as syntypes is conserved in the Muséum National d’Historie Naturelle in Paris, France (catalog number MY112, and MY113). No other information on the whereabouts of the rest of the material was published.
As part of this research, specimens of O. eisenii were reviewed at the American Museum of Natural History (AMNH), in New York. Several specimens had the label of Tepec (sic), Nayarit, Mexico. Knowing that William Wheeler was one of the first curators of this invertebrate collection (Parker, 1938), these specimens are presumably syntypes of the species, as they have a label by Monge-Nájera indicating so. Subsequent records of this species were reported from Rio Purus, Brazil (Fuhrmann, 1914), and El Cermeño, Panama City (Clark & Zetek, 1946), but these records require confirmation. No more information about O. eisenii was published until 2008. Five specimens were collected, although their sex was not determined, in a suburban area in the state of Jalisco in Western Mexico (Cupul-Magaña & Navarrete-Heredia, 2008), situated about 100 km from the type locality. Oliveira, Read et al. (2012) made an extensive checklist of the velvet worms of the world and suggested that the specimens and species required a detailed revision to discard a species misidentification. In the present work, a redescription of the species O. eisenii is done based on freshly collected specimens between 2014 and 2017 in several locations from the Mexican states of Jalisco, Nayarit, and Michoacán. The first scanning electron micrographs of this species are provided. Additionally, new DNA COI sequences from most localities were obtained and used in the analysis.
Materials and methods
The velvet worms were collected under scientific permits FAUT-0027 and FAUT-0209 from Secretaría del Medio Ambiente y Recursos Naturales (Semarnat) in the Mexican states of Nayarit, Jalisco, and Michoacán. All specimens were preserved in 96% ethanol and then examined morphologically. The specimens were deposited in the recently created “Onychophora Collection”, associated to the Colección Nacional de Ácaros (CNAC), Universidad Nacional Autónoma de México. Others were deposited at the Centro de Estudios en Zoología (CZUG), Universidad de Guadalajara, Jalisco, Mexico; the American Museum of Natural History (AMNH), New York, USA, and the Museum of Comparative Zoology (MCZ), Harvard University, Cambridge, MA.
The classification and terminology of morphological structures followed the works of Oliveira et al. (2010), Oliveira, Franke et al., (2012). The measurements of size were taken with an ocular micrometer calibrated at 10X and are given in millimeters. Photographs of a live specimen were taken with a Nikon D5500 digital camera with AF-S Micro Nikkor 105 and ring flash Metz 15 Ms-1 digital. The microphotographs were obtained with a Leica Z16 APOA microscope and a Leica DFC490 camera; they were processed with the Leica Application Suite E Program. Other microphotographs were obtained with an Axio Zoom V16 microscope including the Zeiss AxioCamMRc5 camera; and were processed with the Zeiss Efficient Navigation program. Scanning electron microscopy examination and photography were carried out with a Hitachi S-4700 Field Emission at the American Museum of Natural History, following the methodology by Ruhberg and Daniels (2013). Pictures were edited with the Adobe Photoshop CS6 software. The geographic map was generated with ESRI ArcGIS online suite.
The genomic DNA extractions were obtained following the procedure of the Qiagen DNeasy Blood and Tissue Kit by soaking a whole segment of each velvet worm from the juvenile specimens and a small part of the body from the adult specimens. In both cases, the middle portion of the body was used to obtain the tissue. The Cytochrome C Oxidase I sequence (COI) was amplified through polymerase chain reaction (PCR) using the following combination of primers HCO: TAAACTTCAGGGTGACCAAAAAATCA and LCO: GGTCAACAAATCATAAAGATATTGG. For PCR, the annealing temperature was 46ºC. Edition of the sequences was carried out with Bioedit (Hall, 2005) and genetic distances measurements were obtained with MEGA7 (Tamura et al., 2013). DNA sequences were deposited in GenBank under accession numbers (MH454602, MH454603, MH454604, MH454605, MH454606, MH454607, MH454608, MH454609).
Redescription
Family Peripatidae Evans, 1901.
Genus Oroperipatus (Cockerell, 1908).
Oroperipatus eisenii (Wheeler, 1898).
Taxonomic summary
Synonyms: Peripatus eisenii, original name (Wheeler, 1898), Oroperipatus eiseni (sic) (Clark, 1913).
Holotype: not designated.
Type locality: Tepic, Nayarit, Mexico.
Syntype: Tepec (sic), Mexico (AMNH 416). Part of the type series described by Wheeler in 1898, without further information; probably collected by Eisen.
Notes: the species name is commonly misspelled as “eiseni” (Clark, 1913; Fuhrmann, 1914; Peck, 1975; Sampaio-Costa et al., 2009; Vasconcellos et al., 2004).
Material examined: Mexico: Nayarit: Mpio. San Blas: Outside El Naranjo Cave, Jalcocotán (21°27’11.08” N, 105°0’0” W, elev. 361 m), 19-VI-2015, collectors: G. Montiel and G. Contreras, 1 male and 5 females (CNAC/Ony-00010). Same locality data except, 1 female (MCZ: IZ: 71297), and 1 female (CZUG). Same locality data except, 26-VI-2016, collectors: G. Montiel, L. Olguín, D. Guerrero, and G. Contreras, 2 males and 3 females (CNAC/Ony-00016). Same locality data, 1 male and 2 females (AMNH). Same locality data, 1 female (MCZ.IZ:74293). Río Tetepozco, Jalcocotán, (21°27’31.86” N, 105°0’0” W, elev. 362 m), 26-VI-2016, collectors: G. Montiel, L. Olguín, D. Guerrero, and G. Contreras, 3 females (CNAC/Ony-00015). Same locality data except 1 female (CNAC/Ony-00017). Mpio. Compostela: Mesillas (21°13’14.19” N, 105°0’0” W, elev. 441 m), 21-VIII-2017, collectors: R. Ramírez and J. Mendoza, 1 female (CNAC/Ony-00025). Mesillas (21°13’17.79” N, 105°0’0” W, elev. 442 m), 11-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, and R. Paredes, 1 male and 2 females (CNAC/Ony-00029). Mpio. Xalisco: nacimiento de agua Pantanal (21°25’36.44” N 104°0’0” W, elev. 917 m), 12-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, and R. Paredes, 6 male and 7 females (CNAC/Ony-00034). Same locality data except 1 female (CZUG). Tepec (sic), Mex., 8 females and 3 juveniles (AMNH 416). Jalisco: Mpio. Puerto Vallarta: Carretera Cihuatlán-Puerto Vallarta (20°35’23.85” N , 105°0’0” W, elev. 39 m), 23-II-2015, collectors: A. Flores and G. Contreras, 1 female (CNAC/Ony-00001). Same locality and collector data except 21-II-2015, 6 females (CNAC/Ony-00009). Same locality and collector data except 3-IV-2015, 1 female (CNAC/Ony-00011). Same locality data except 26-X-2016, collectors G. Montiel, D. Guerrero, and G. Contreras, 3 females (CNAC/Ony-00020). Same locality data except 11-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, and R. Paredes, 1 male and 4 females (CNAC/Ony-00031). Centro Universitario de la Costa (20°42’11.7” N, 105°0’0” W, elev. 11 m), 3-III-2009, collector: F. Cupul, 1 male and 1 juvenile (CNAC/Ony-00006). Same locality data except 26-X-2016, collectors: G. Montiel, D. Guerrero, F. Cupul, and G. Contreras, 14 females (CNAC/Ony-00019). Same locality data except, 11-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, and R. Paredes, 3 male and 5 females (CNAC/Ony-00030). Puerto Vallarta, 8-IX-2012, no collector data, 2 females (CNAC/Ony-00007). Mpio. Acatlán de Juárez: Acatlán de Juárez (20°25’4.51” N, 103°0’0” W, elev. 1,366 m), 16-VIII-2014, collector: G. Contreras, 3 females (CNAC/Ony-00004). Mpio. Unión de Tula: Unión de Tula (19°57’20.84” N, 104°0’0” W, elev. 1,351 m), 2-XI-2014, collector: A. Reyes, 1 female and 2 juveniles (CNAC/Ony-00005). Unión de Tula (19°57’19.54” N, 104°16’17.65” W, elev. 892 m), 28-X-2016, collectors: G. Montiel, D. Guerrero, and G. Contreras, 8 females (CNAC/Ony-00021). Same locality data except, 1 female (CZUG). Mpio. Tequila: Tequila, 16-VII-2016, collector: J. Carranza, 1 female (CNAN/Ony-00014). Aguacaliente, Santo Toribio Chapel (20°54’16.38” N, 103°0’0” W, elev. 1,294 m), 29-X-2016, collectors: G. Montiel, D. Guerrero, J. Carranza, and G. Contreras, 12 females (CNAC/Ony-00022). Same locality data except, 1 female (CZUG). Same locality data except 12-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, and R. Paredes, 1 male and 2 females (CNAC/Ony-00032). Ojo de Agua, Aguacaliente (20°54’39.13” N, 103°49’32.34” W, elev. 1,300 m), 12-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, and R. Paredes, 3 females (CNAC/Ony-00033). Mpio. Chapala: Chapala, Los Reyes street (20°17’37.39” N, 103°14’14.71” W, elev. 1,453 m), 10-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, R. Paredes, J. Barragán, R. Carrillo, and J. Ascensio, 1 male and 24 females (CNAC/Ony-00027). Same locality data except, 1 female (CZUG). Mpio. Ajijic: San Antonio Tlayacalpa, Ramón Velázquez street (20°17’37.39” N, 103°14’14.71” W, elev. 1,536 m), 10-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, R. Paredes, J. Barragán, R. Carrillo, and J. Ascensio, 2 males and 4 females (CNAC/Ony-00028). Michoacán: Mpio. Uruapan, Manuel Pérez Coronado street, Uruapan (19°23’41.06” N, 102°3’35.28” W, elev. 1,596 m), 9-IX-2017, collectors: G. Montiel, D. Guerrero, I. Salgado, R. Paredes, and R. Villalvazo, 1 male and 9 females (CNAC/Ony-00026).
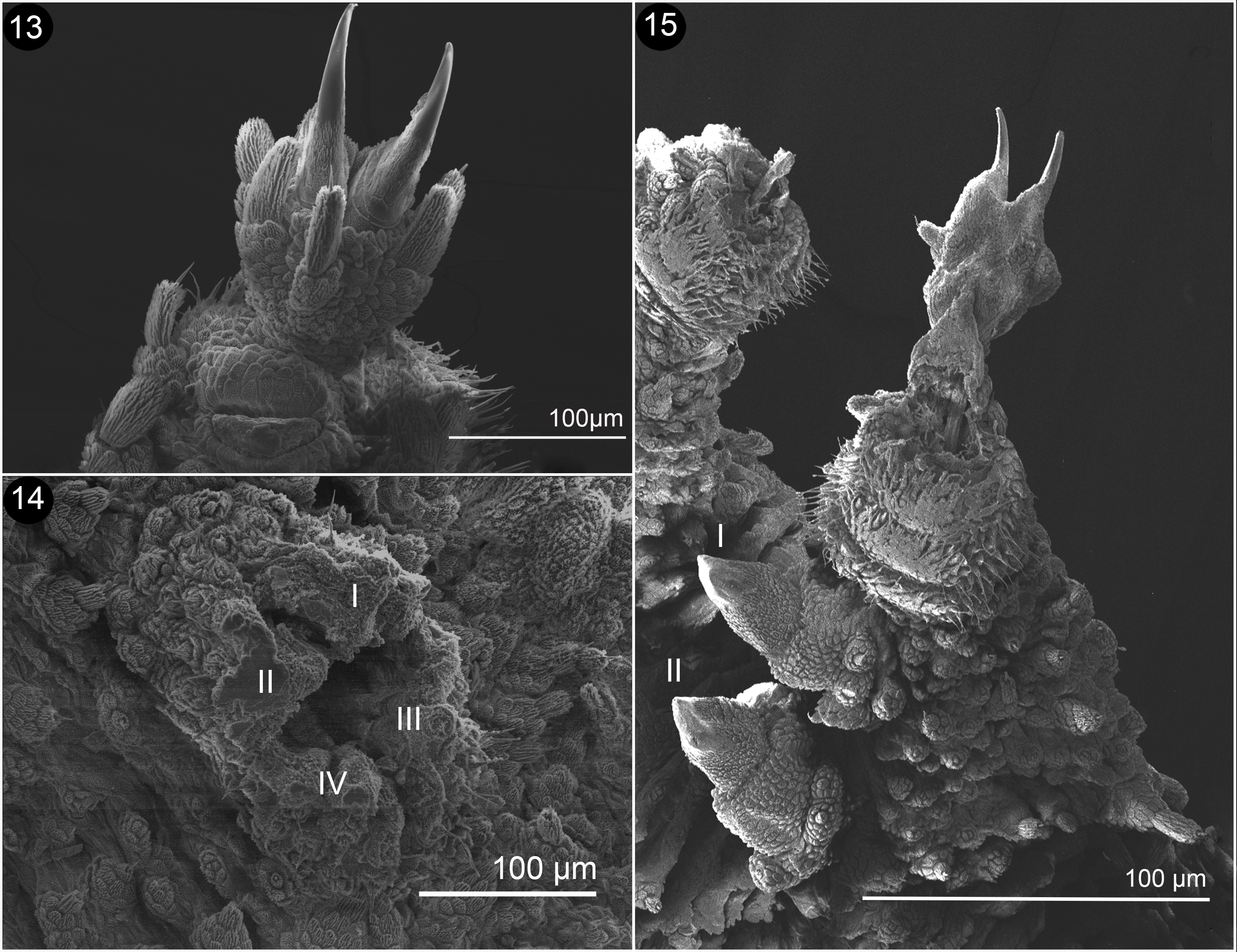
Diagnosis: both sexes of O. eisenii present a dark brown coloration on the dorsum, and a light whitish coloration on the ventral face. Each segment of the body presents 10 complete plicae; the primary papillae present 4 ranks of scales on the base and 4 ranks of longer scales on the apical portion. The antennae present 35 rings on the base and have a terminal button followed by 9 rings. The structure of the inner jaw blade, presents a diastema that separates the single principal tooth and the 2 accessory teeths, from 6 to 7 denticles, the second ones are bilobated. The nephridial tubercle is on the fourth and fifth pair of legs; each leg presents 4 spinous pads. Finally, males present a prominent crural tubercles on legs 21 and 22.
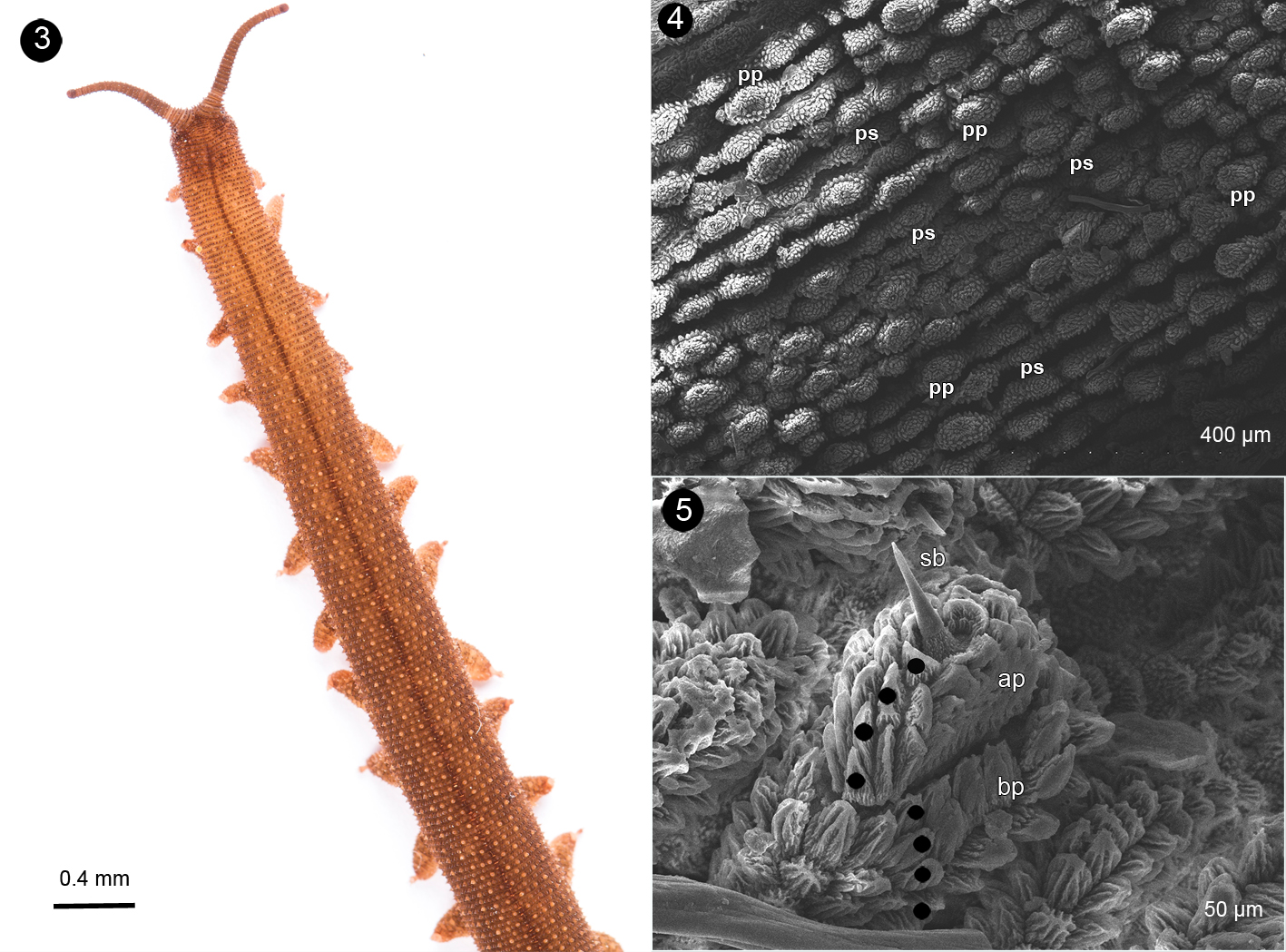
Description: maximum body size after fixation: length: 17 to 39 mm, and width: 2 to 5 mm in adults; length: 14 to 18 mm, and width: 2 mm in young. Color pattern: coloration of dorsal integument (in vivo) is dark brown without any discernible pattern. The dorsal midline is darker than the rest of the integument (Fig. 1). The ventral body surface is mainly pale reddish, brighter than the dorsal integument (Fig. 2).
Body dorsum: the dorsomedian furrow is very well defined; without the presence of any hyaline organs. Each body segment has 10 plicae. All plicae have the same width along the body (Fig. 3). Primary and secondary papillae are present on each plicae (Fig. 4). There are never 2 primary papillae together, they always have at least 1 secondary papillae separating them. The primary papillae are aligned longitudinally, with 1 plicae between each primary papillae (Fig. 4). Both papillae occur on the top of the plicae, there is never more than a single line of papillae for each plicae. Primary papillae present a wider, rounded shape at the base, and are shorter than the apical portion (Fig. 5); the apical portion has 4 scale ranks, and these are noticeable longer than the ones on the basal portion. The sensory bristle is on the primary papillae and is larger than the scales (Fig. 5). Accessory papillae are similar in shape to primary papillae. They almost maintain a proportion of 2 to 1 with respect to the primary papillae.

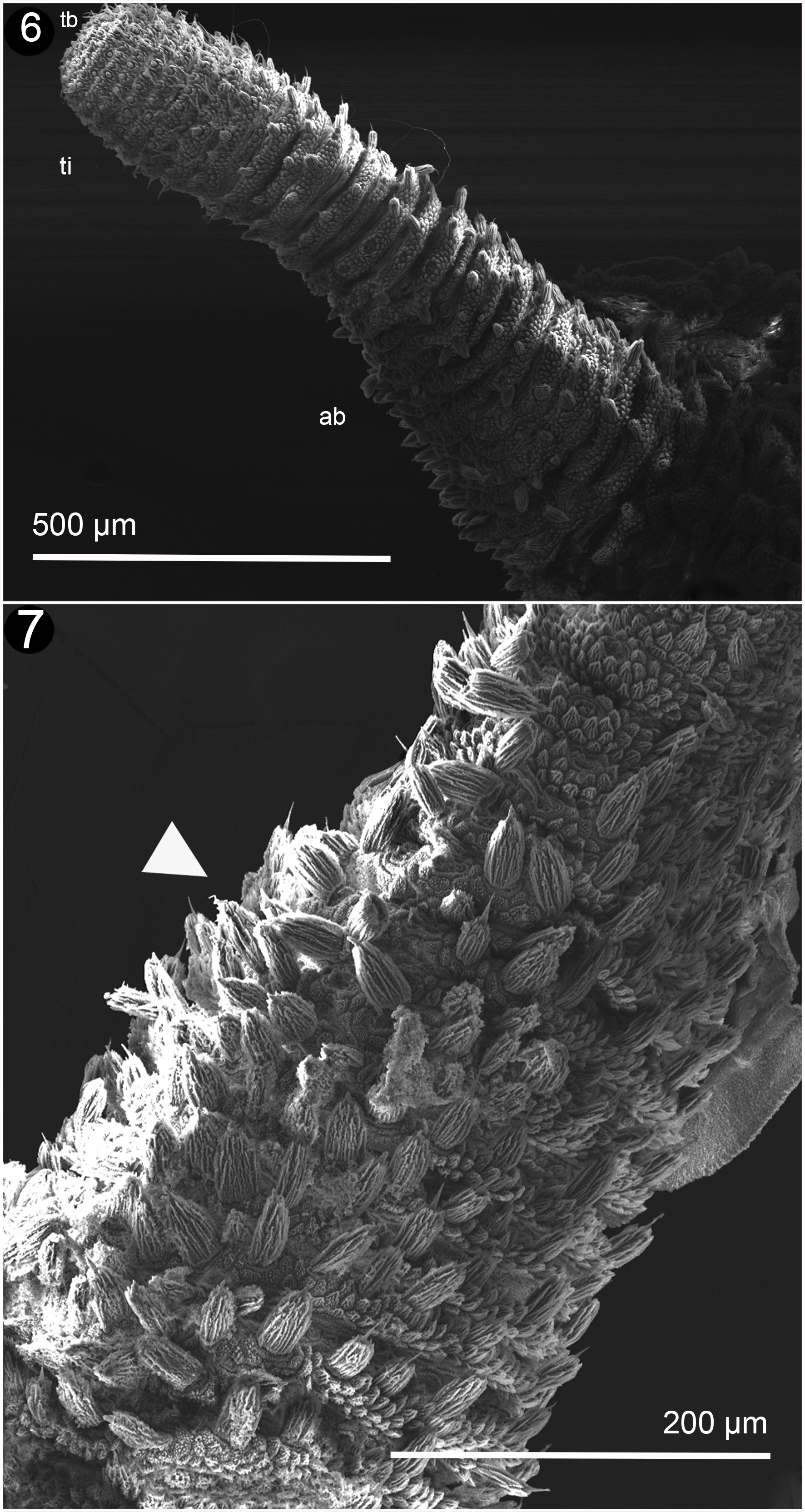
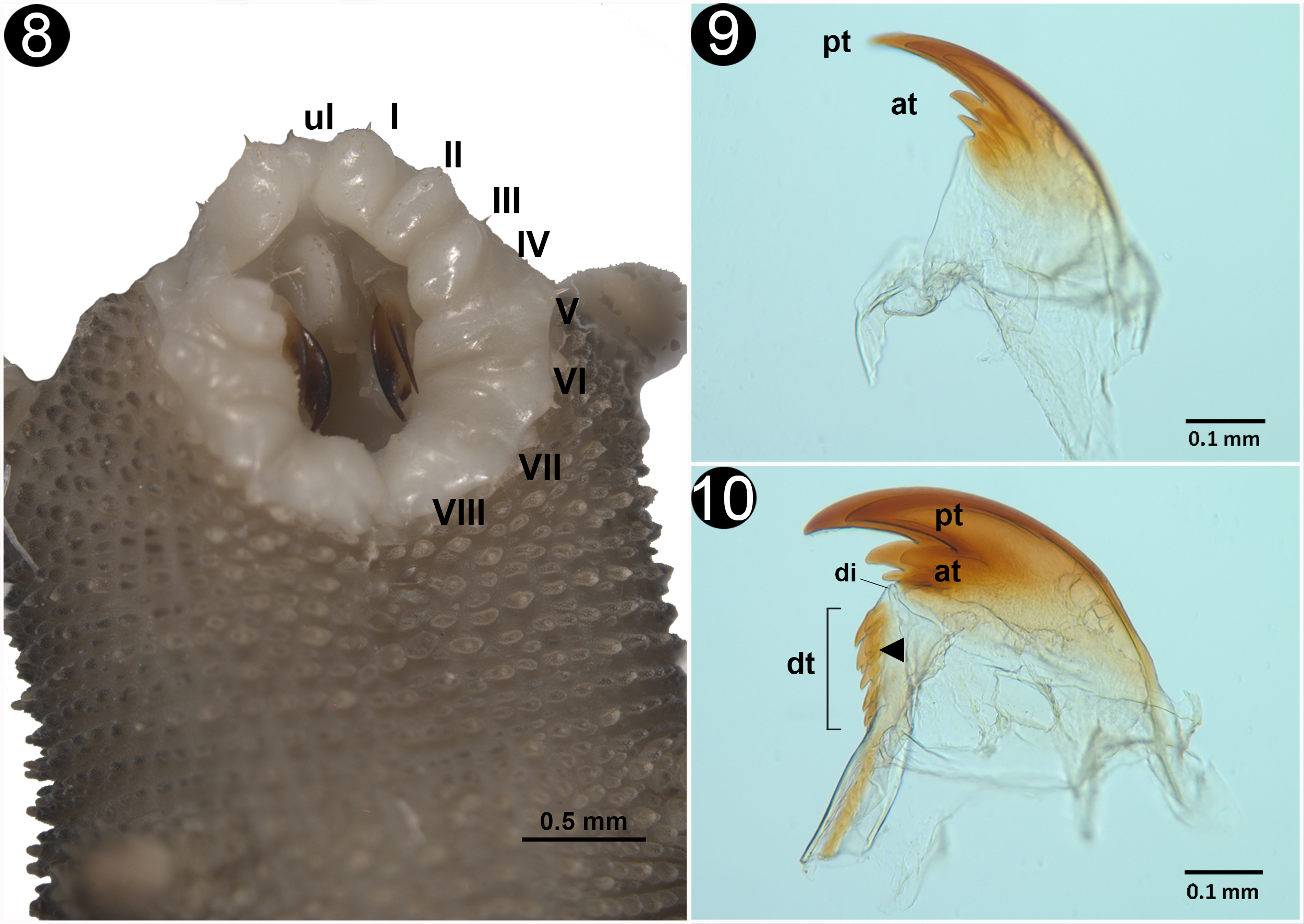
Body venter: females have 28 or 29 pairs of legs; males and juveniles have 24 pairs of legs. The legs have 3 complete spinous pads and a smaller one which is incomplete. They are situated distally on the ventral surface of each leg (Fig. 6). The nephridial tubercle is located on the posterior part of the third spinous pad, dividing it in 2. It is present on the fourth and fifth legs (Fig. 7). Each leg has several finger-like foot papillae around the claw, and 1 papillae situated on the dorsal face, between the claws (Fig. 8). Ventral and preventral organs are absent or not evident. The genital opening is located near the penultimate pair of legs; this is a characteristic of the Peripatidae family; it consists of 2 parallel lobes on each side, higher than the ventral integument (Fig. 9). Two pregenital legs have 2, big and prominent crural tubercles only found in males (Fig. 10).
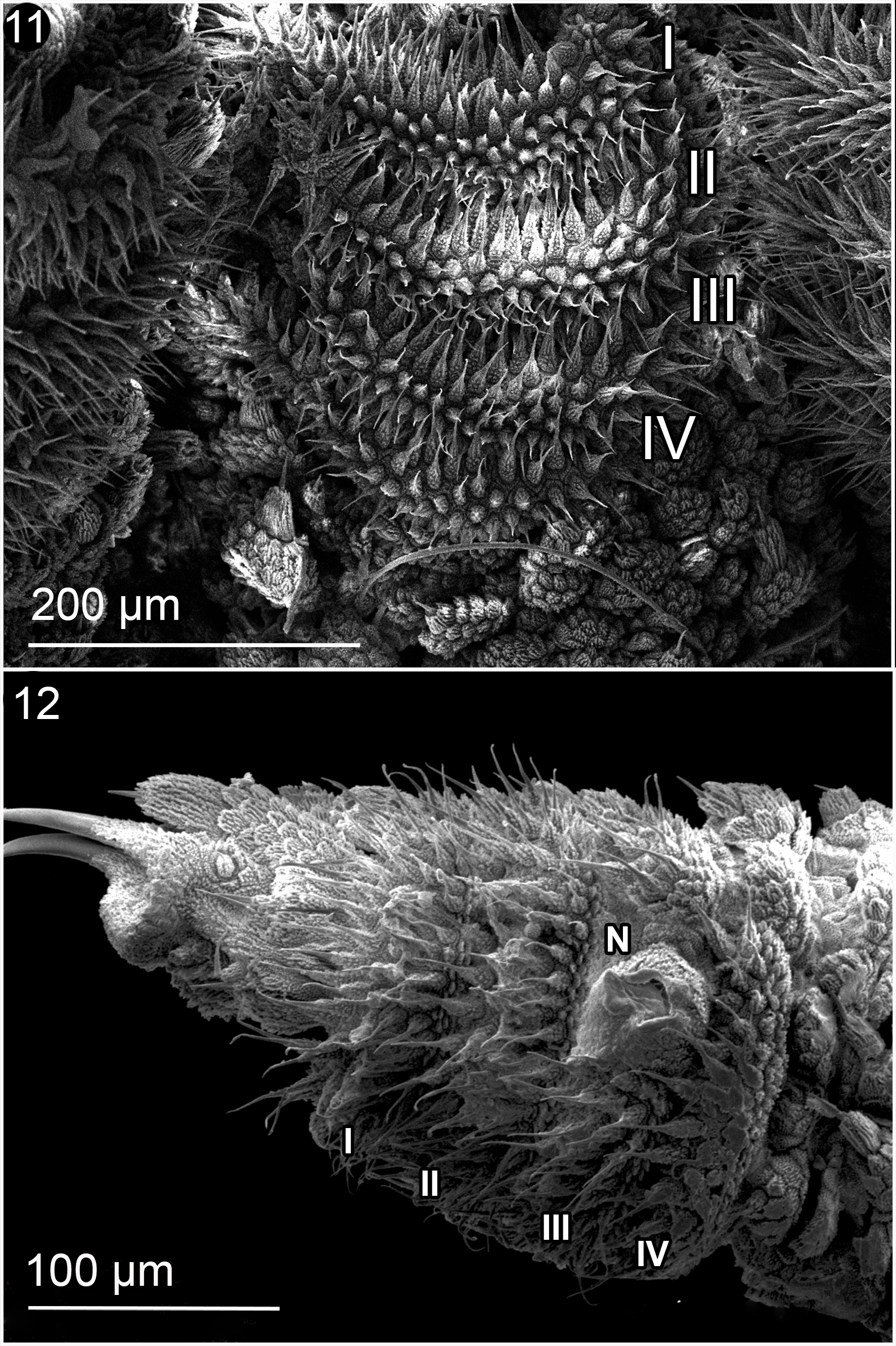
Head: the arrangement of the dermal papillae on the head is irregular. The eyes are present behind the antenna and the frontal organs on the ventrolateral base of the antenna.
Antennae: Antennal tip has a terminal button followed by 9 rings equal in size; the antennal body has a total of 35 rings in both sexes. The 19 rings at the base have the presence of finger-like sensillae; 10 rings are wide and 9 are thin (Fig. 11). Frontal organ appears to begin on the eleventh ring of the antennal body to the base (Fig. 12). Mouth: opening is surrounded by lip papilla, the anterior-most is unpaired and smaller, and is followed by 7 pairs of lobes posterior ones, equal in size; all of them have small spines (Fig. 13); the spines and the lip papilla are noted by Wheeler (1898) on the drawings made in the original description. Inner and outer jaw blades, both have an enlarged principal tooth, and 1 or 2 smaller accessory teeth (Figs. 14, 15). The structure of the inner jaw blade, presents a diastema that separates the single principal tooth and the 2 accessory teeths, from 6 to 7 denticles, the second ones are bilobated (Fig. 15).
Remarks
The species was only known from its type locality (Tepic, Nayarit) as well as from a suburban area in the State of Jalisco, situated about 100 km from the type locality (Cupul-Magaña & Navarrete-Heredia, 2008). Oliveira, Read et al. (2012) suggested that the specimens of Jalisco required a revision. In this work, we provide morphological and molecular information of the specimens from Nayarit and Jalisco. Our results show that the specimens belong to the same species O. eisenii. Therefore, the identification carried out by Cupul-Magaña and Navarrete-Heredia (2008) was corroborated.
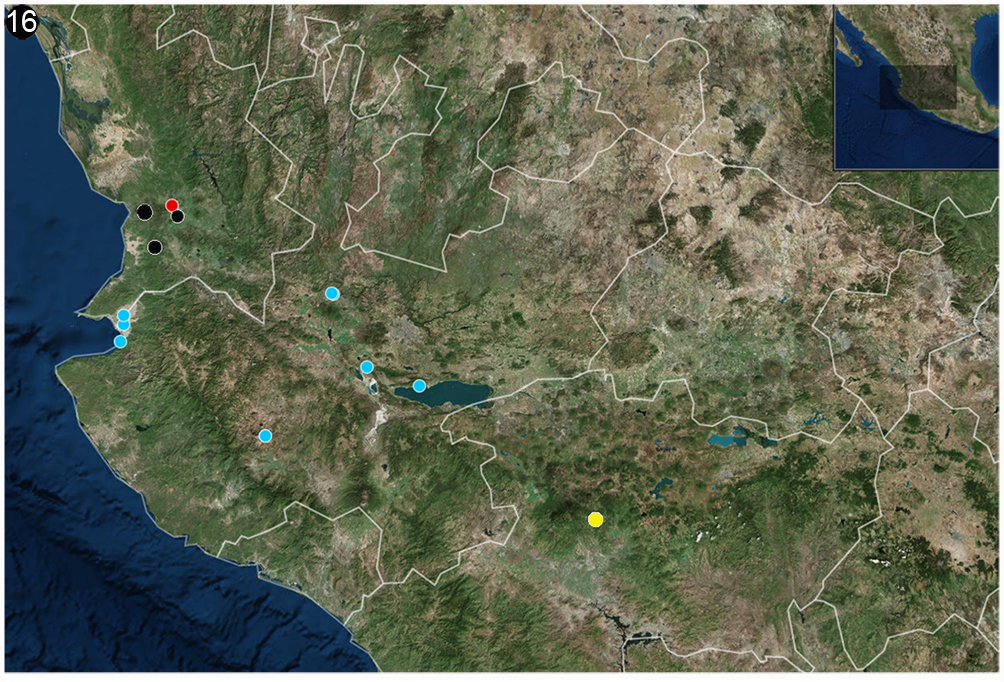
Geographical distribution: after our study, this species is now known to be present in 13 localities of 3 Mexican States: Jalisco, Nayarit, and Michoacán. Its distribution has a range of approximately 400 km (Fig. 16), including altitudes from the sea level to almost 1,600 m, with a preference for tropical habitats usually found close to water bodies.
Natural history: most of the specimens were found under stones, rotten logs, or leaf litter, close to mango (Manguifera indica), yaca (Artocarpus heterophyllus), and banana (Musa paradisiaca) plantations; usually in shady places in groups of 2 to 4 specimens (Fig. 17). Also, they were found in anthropogenic environments under bricks, stones, leaf litter, rotting wood, garbage, flowerpots, and crevices of the soil close to the roots of small plants (Fig. 18), These findings are added to the ones summarized by Monge-Nájera (2018) of peripatids occurring in urban areas of Latin America and Caribbean. The climate was between 20 ºC to 23 ºC and 46% to 50% relative humidity. During the fixation procedure and captivity of O. eisenii, it was common for females to have abortions. In some females under captivity, the parturition sequence was documented and will be reported in detail elsewhere; in short, during previous days the females showed movements of raising the anal region, the neonate came out with the antennas retracted and started moving after the fifth pair of legs was out; the newborn worms stayed with the mother for a few hours. The parturition sequence was relatively consistent requiring around 40 min. During the first weeks, the color of the juveniles was lighter than the females. There can be a difference of 3 to 14 days between each birth. The dissection of some gravid females showed up to 10 embryos in different stages of development.
Discussion
The genetic marker COI has been shown to be a reliable marker for species delimitation and differentiation of populations, in previous studies of velvet worms (Daniels et al., 2009; Lacorte et al., 2011; McDonald et al., 2012; Oliveira et al., 2011). Therefore, in this work the sequencing of COI on 8 genomic DNA samples was carried out, obtained from juvenile and adult worms. As a result of our analysis (Table 1), the genetic divergence values (0.2 divergence percentage) strongly suggested that all samples belonged to specimens within the same species. This value was far from the suggested limits for erection of new species: ranging between 1.1 and 13.5 (Hebert et al., 1991; Daniels et al., 2009; McDonald et al., 2012; Oliveira et al., 2011; Rockman et al., 2001). Therefore, variations obtained within the present populations of O. eisenii, did not fulfill the criteria for erection of a new species of Oroperipatus. It is well known, that velvet worms present low vagility (Allwood et al., 2010), and the morphological and molecular similarities found among the specimens from the different sampled areas for the present study, suggested that the populations have been recently separated; due to the fact that genetic divergence between the closest populations, separated by around 10 km (Puerto Vallarta and Centro Universitario de la Costa), is the same divergence found on specimens that are separated by a much longer distance, about 170 km and 93 km (Puerto Vallarta, Acatlán de Juárez, and outside El Naranjo Cave) (Table 1).
Table 1
The p-distances recorded between the different populations of velvet worms of the species Oroperipatus eisenii, from 8 localities in 2 states of Mexico, and based on sequences of the mitochondrial COI gene. Numbers in bold are the mayor genetic distances observed in all the samples. Locality of the samples: Jalisco: 1. Puerto Vallarta, 2. Acatlán de Juárez, 9. Centro Universitario de la Costa, 10. Carretera Cihuatlán-Puerto Vallarta, 11. Unión de Tula, and 12. Tequila. Nayarit: 5. Outside El Naranjo Cave, and 16. Mesillas.
|
Samples |
01 |
02 |
05 |
09 |
10 |
11 |
12 |
16 |
|
01 |
||||||||
|
02 |
0.000 |
|||||||
|
05 |
0.000 |
0.000 |
||||||
|
09 |
0.002 |
0.002 |
0.002 |
|||||
|
10 |
0.000 |
0.000 |
0.000 |
0.002 |
||||
|
11 |
0.000 |
0.000 |
0.000 |
0.002 |
0.000 |
|||
|
12 |
0.000 |
0.000 |
0.000 |
0.002 |
0.000 |
0.000 |
||
|
16 |
0.000 |
0.000 |
0.000 |
0.002 |
0.000 |
0.000 |
0.000 |
Since the description of O. eisenii, 120 years ago, this species has been collected in small numbers. Regarding the finding of the specimens in household locations, it is conceivable that these populations, are present in low densities (from 2 to 26 specimens per locality) in consequence of intensive human activities; almost half of our records were close to urban areas (courtyards and vacant lots). These findings support the hypothesis proposed by Monge-Nájera (2018) that places onychophorans living in urban and suburban areas in the category of “survivors of habitat modification.” We agree with the model he presented that explains the persistence of the onychophoran populations found in urban areas is due to nutrient abundance, artificial abiotic factors, and elimination of predators and pathogens, among others. However, the cause-effect relationship of such activities has not been quantified and requires further research. For now, onychophorans are still hard to find.
Acknowledgements
This work was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, Universidad Nacional Autónoma de México (PAPIIT-UNAM No. IN211626). The first author is recipient of a scholarship (269851) from CONACYT and other financial support from the Graduate Program in Biological Sciences of UNAM. We thank D. Guerrero, L. Olguín, R. Fierros, R. Paredes, R. Villalvazo, C. Guzmán, J.L. Barragán, R. Carrillo, J. J. Ascensio, A. Torres, I. Salgado, and M. Vargas for field assistance during the expeditions. E. Rodriguez and L. Berniker for providing access to the specimens at the AMNH invertebrates collection and SEM image facilities. We also thank M. Hill and A. K. Smith for their assistance on scanning electron microscopy. M. A. Sicilia and J. Arreguin for providing photographs of living specimens. A. Flores, for his kind help on the identification of plants. R. Paredes for his comments to improve this article. J. Mendoza who provided the specimen from Mesillas. M. Vásquez, A. Reyes, and P. Ramírez for providing some localities for collecting. S. Guzmán for assistance in the Laboratorio de Microscopía y Fotografía de la Biodiversidad (II) del Instituto de Biología. Finally, we thank two anonymous reviewers for useful comments.


References
Allwood, J., Gleeson, D., Mayer, G., Daniels, S., Beggs, J. R., & Buckley, T.R. (2010). Support for vicariant origins of the New Zeland Onychophora. Journal of Biogeography, 37, 669–681.
Baer, A., Oliveira, I. S., Steinhagen, M., Beck-Sickinger, A. G., & Mayer, G. (2014). Slime protein profiling: a non-invasive tool for species identification in Onychophora (velvet worms). Journal of Zoological Systematics and Evolutionary Research, 52, 265–272.
Barquero-González, J. P., Acosta-Chávez, V. J., Sotela, M. L., Villalobos, B. F., & Morera-Brenes, B. (2016). Evidencia fotográfica de especies desconocidas de onicóforos (Onychophora: Peripatidae) de Costa Rica. Cuadernos de Investigación UNED, 8, 139–147.
BOLD Systems. (2014). Barcode of life data system, v 3. International Barcode of Life. Accessed March 30, 2018 from: http://www.boldsystems.org
Bouvier, E. L. (1899). Sur les variations et les groupements spécifiques des Péripates américains. Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences, 128, 1344–1346.
Clark, A. H. (1913). A revision of the American species of Peripatus. Proceedings of the Biological Society of Washington, 26, 15–19.
Clark, A. H., & Zetek, J. (1946). The Onychophores of Panama and the Canal Zone. Proceedings of the United States National Museum, 96, 205–213.
Concha, A., Mellado, P., Morera-Brenes, B., Sampaio C. S., Mahadevan, L., & Monge-Nájera, J. (2015). Oscillation of the velvet worm slime jet by passive hydrodynamic instability. Nature Communications, 6, 1–6.
Cupul-Magaña, F. G., & Navarrete-Heredia, J. L. (2008). Rediscovery and new data for Oroperipatus eisenii (Wheeler, 1898) from Mexico (Onychophora: Peripatidae). Entomological News, 119, 545–549.
Daniels, S. R., Picker, M. D., Cowlin, R. M., & Hamer, M. L. (2009). Unravelling evolutionary lineages among South African velvet worms (Onychophora: Peripatopsis) provides evidence for widespread cryptic speciation. Biological Journal of the Linnean Society, 97, 200–216.
Fuhrmann, O. (1914). Quelques nouveaux Péripates américains. Mémoires de la Société des Sciences Naturelles de Neuchâtel, 5, 176–192.
Hall, T. (2005). BioEdit v7.0.5. Ibis therapeutics. Carlsbad, CA, USA.
Hebert, P. D. N., Billington, N., Finston, T. L., Boileau, M. G., Beaton, M. J., & Barrette, J. (1991). Genetic variation in the onychophora Plicatoperipatus jamaicensis. Heredity, 67, 221–229.
Jeffery, N. W., Oliveira, I. S., Gregory, T. R., Rowell, D. M., & Mayer, G. (2012). Genome size and chromosome number in velvet worms (Onychophora). Genetica, 140, 497–504.
Lacorte, G. A., Oliveira, I. S., & Fonseca, C. G. (2011). Phylogenetic relationships among the Epiperipatus lineages (Onychophora: Peripatidae) from the Minas Gerais State, Brazil. Zootaxa, 2755, 57–65.
Mayer, G., & Harzsch, S. (2007). Immunolocalization of serotonin in Onychophora argues against segmental ganglia being an ancestral feature of arthropods. BMC Evolutionary Biology, 7, 1–9.
Mayer, G., Oliveira, I. S., Baer, A., Hammel, J. U., Gallant, J., & Hochberg, R. (2015). Capture of prey, feeding, and functional anatomy of the jaws in velvet worms (Onychophora). Integrative and Comparative Biology, 55, 217–227.
McDonald, D. E., Ruhberg, H., & Daniels, S. R. (2012). Two new Peripatopsis species (Onychophora: Peripatopsidae) from the Western Cape province, South Africa. Zootaxa, 3380, 55–68.
Monge-Nájera, J. (1994). Reproductive trends, habitat type and body characteristics in velvet worms (Onychophora). Revista de Biología Tropical, 42, 611–622.
Monge-Nájera, J. (2018). City worms (Onychophora): Why do fragile invertebrates from an ancient lineage live in heavily urbanized areas? UNED Research Journal, 10, 91–94.
Monge-Nájera, J., & Alfaro, J. P. (1995). Geographic variation of habitats in Costa Rican velvet worms (Onychophora: Peripatidae). Biogeographica, 71, 97–108.
Monge-Nájera, J., Barrientos, Z., & Aguilar, F. (1993). Behavior of Epiperipatus biolleyi (Onychophora: Peripatidae) under laboratory conditions. Revista de Biología Tropical, 41, 689–696.
Monge-Nájera, J., & Morera-Brenes, B. (1994). Morphological and physiological characteristics of two species of Epiperipatus from Costa Rica (Onychophora: Peripatidae). Revista de Biología Tropical, 42, 181–188.
Monge-Nájera, J., & Morera-Brenes, B. (2015). Velvet worms (Onychophora) in folklore and art: geographic pattern, types of cultural reference and public perception. British Journal of Education, Society and Behavioural Science, 10, 1–9.
Monge-Nájera, J., & Xianguang, H. (2002). Experimental taphonomy of velvet worms (Onychophora) and implications for the Cambrian “explosion, disparity and decimation” model. Revista de Biología Tropical, 50, 1133–1138.
Morera-Brenes, B., Herrera, A., Mora, M., & León, P. (1992). Estudios genómicos de Epiperipatus biolleyi (Peripatidae, Onychophora). Revista Brasileira de Genética, 15 (Suppl.), 91.
Morera-Brenes, B., & Monge-Nájera, J. (2010). A new giant species of placented worm and the mechanism by which onychophorans weave their nets (Onychophora: Peripatidae). Revista de Biología Tropical, 58, 1127–1142.
Murienne, J., Daniels, S. R., Buckley, T. R., Mayer, G., & Giribet, G. (2014). A living fossil tale of Pangaean biogeography. Proceedings of the Royal Society B: Biological Sciences, 281, 1–9.
Oliveira, I. S., Franke, F. A., Hering, L., Schaffer, S., Rowell, D. M., Weck-Heimann, A. et al. (2012). Unexplored character diversity in Onychophora (velvet worms): a comparative study of three peripatid species. Plos One, 7, 1–20.
Oliveira, I. S., Hering, L., & Mayer, G. (2018). Onychophora. The University of Kassel. Accessed January 10, 2018 from http://www.onychophora.com/index.htm
Oliveira, I. S., Lacorte, G. A., Fonseca, C. G., Wieloch, A. H., & Mayer, G. (2011). Cryptic speciation in Brazilian Epiperipatus (Onychophora: Peripatidae) reveals an underestimated diversity among the peripatid velvet worms. Plos One, 6, 1–13.
Oliveira, I. S., Lacorte, G. A., Weck-Heimann, A., Cordeiro, L. M., Wieloch, A. H., & Mayer, G. (2015). A new and critically endangered species and genus of Onychophora (Peripatidae) from the Brazilian savannah –a vulnerable biodiversity hotspot. Systematics and Biodiversity, 13, 211–233.
Oliveira, I. S., Read, V. M. S. J., & Mayer, G. (2012). A world checklist of Onychophora (velvet worms), with notes on nomenclature and status of names. Zookeys, 211, 1–70.
Oliveira, I. S., Wieloch, A. H., & Mayer, G. (2010). Revised taxonomy and redescription of two species of the Peripatidae (Onychophora) from Brazil: a step towards consistent terminology of morphological characters. Zootaxa, 2493, 16–34.
Parker, G. H. (1938) Biographic memoir of William Morton Wheeler 1865-1937. National Academy of Sciences of the United States of America. Biographical Memoirs, 19, 201– 241.
Peck, S. B. (1975). A review of the New World Onychophora with the description of a new Cavernicolous genus and species from Jamaica. Psyche, 82, 241–358.
Podsiadlowski, L., Braband, A., & Mayer, G. (2008). The complete mitochondrial genome of the onychophoran Epiperipatus biolleyi reveals a unique transfer RNA set and provides further support for the ecdysozoa hypothesis. Molecular Biology and Evolution, 25, 42–51.
Rockman, M. W., Rowell, D. M., & Tait, N. N. (2001). Phylogenetics of Planipapillus, lawn-headed onychophorans of the Australian Alps, based on nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution, 21, 103–116.
Rucker, A. (1900). A description of the male of Peripatus eisenii Wheeler. Biological Bulletin, 1, 251–259.
Ruhberg, H., & Daniels, S. R. (2013). Morphological assessment supports the recognition of four novel species in the widely distributed velvet worm Peripatopsis moseleyi sensu lato (Onychophora: Peropatopsidae). Invertebrate Systematics, 27, 131–145.
Sampaio-Costa, C., Chagas-Junior, A., & Baptista, R. L. C. (2009). Brazilian species of Onychophora with notes on their taxonomy and distribution. Zoologia, 26, 553–561.
Sosa-Bartuano, A., Monge-Nájera, J., & Morera-Brenes, B. (2018). A proposed solution to the species problem in velvet worm conservation (Onychophora). UNED Research Journal, 10, 204–208.
Tamura, T., Stecher, G., Paterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Toledo-Matus, X., Rivera-Velázquez, G., Monge-Nájera, J., & Morera-Brenes, B. (2018). An undescribed species of velvet worm from Chiapas, Mexico (Onychophora: Peripatidae). UNED Research Journal, 10, 178–179.
Vasconcellos, A., Oliveira, A. W., & Costa, E. E. C. (2004). Onychophora de florestas úmidas do complexo da Mata Atlantica do Nordeste Brasileriro e sua importancia para conservaçâo e estudos sistemáticos, Série Biodiversidade, 9. En K. C. Pôrto, J.J.P. Cabral, & M. Tabarelli (Eds.), Brejos de altitude: historia natural, ecologia e conservaçãcao (pp. 139–144). Brasilia: Ministerio do Meio Ambiente.
Wheeler, W. M. (1898). A new Peripatus from Mexico. Journal of Morphology, 15, 2–9.



