Ilse E. Segura-Zarzosa a, Alejandro M. Maeda-Martínez b, Gopal Murugan b, Hortencia Obregón-Barboza b, *
a Universidad Autónoma de Nuevo León, Facultad de Ciencias Biológicas, Avenida Universidad s/n, Ciudad Universitaria, 66455 San Nicolás de los Garza, Nuevo León, Mexico
b Centro de Investigaciones Biológicas del Noroeste, S.C., Calle IPN 195, 23096 La Paz, Baja California Sur, Mexico
*Corresponding autor: hobregon04@cibnor.mx (H. Obregón-Barboza)
Received: 29 April 2021; accepted: 23 August 2021
Abstract
For the Mexican oniscideans of the family Armadillidae, 6 species of the genus Cubaris Brandt, 1833 and 18 species of the genus Venezillo Verhoeff, 1928 have been recorded. Of them, only Venezillo stuckchensis (Mulaik, 1960) has been registered for the Baja California Peninsula (BCP). This species was described upon a single male and no biological studies exist on this taxonomic entity since its original description in 1960. In this work, we redescribe V. stuckchensis including the designation of a neotype and paratopotypes using specimens from the type locality. We report this species from 5 additional wetlands of southern BCP in the state of Baja California Sur. The type locality is corrected in its formal name and its geographical location. The morphological identity of V. stuckchensis is documented using SEM micrographs, and data on its molecular identity with 16S ribosomal RNA mitochondrial gene fragments are given.
Keywords: Malacostraca; Isopoda; Neotype; Taxonomy; North America
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Redescripción de Venezillo stuckchensis (Crustacea: Oniscidea: Armadillidae), un isópodo terrestre de la península de Baja California, México
Resumen
Para los isópodos terrestres mexicanos de la familia Armadillidae se han registrado 6 especies del género Cubaris Brandt, 1833 y 18 especies del género Venezillo Verhoeff, 1928. De ellas, solo Venezillo stuckchensis (Mulaik, 1960) ha sido reportada para la península de Baja California (PBC). Esta especie fue descrita con base en un solo ejemplar macho y desde su descripción en 1960, no existen estudios biológicos sobre esta entidad taxonómica. En este trabajo se redescribe V. stuckchensis con la designación de un neotipo y paratopotipos con especímenes de la localidad tipo. Se reporta la especie de 5 humedales adicionales del sur de la PBC en Baja California Sur. Se corrige el nombre formal y la localización geográfica de la localidad tipo. La identidad morfológica de V. stuckchensis se documenta con micrografías de MEB y se proporcionan datos sobre su identidad molecular con base en fragmentos del gene mitocondrial 16S ribosomal ARN.
Palabras clave: Malacostraca; Isopoda; Neotipo; Taxonomía; Norteamérica
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
For the Mexican species inventory of the family Armadillidae, Schmalfuss (2003) and Jass and Klausmeier (2004) listed Cubaris Brandt, 1833 with 6 species, and Venezillo Verhoeff, 1928 with 18 species. For the Baja California Peninsula (BCP) and islands of northwestern Mexico, Schmalfuss (2003) catalogued the 3 Armadillo species of Mulaik (1960) as Cubaris benitensis (Mulaik, 1960), Venezillo macrosoma (Mulaik, 1960) and V. stuckchensis (Mulaik, 1960). Recently we revised scientific oniscidean collections at 4 academic institutions in northern Mexico and found that most of the 1,820 specimens examined belong to 6 exotic species (Segura-Zarzosa et al., 2020). In that revision we detected specimens collected from several wetlands (oases) of the southern part of the BCP, whose morphology fit well with that of Venezillo stuckchensis. Mulaik (1960) described this species using a single male specimen (holotype) collected in the BCP in “Santiago, Baja California”, mentioning that this specimen would be deposited in the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional (IPN) in Mexico City. One of us (AMMM) revised the Isopoda IPN collection and none of the lot labeled as the original Armadillo stuckchensis Mulaik, 1960 was found, therefore we conclude that the holotype is lost. A detailed taxonomic revision of the material deposited at the IPN collection is in progress and the results will be published elsewhere.
In this work, we redescribe Venezillo stuckchensis and designate a selected male as neotype using specimens from the type locality, and also report this species from 5 additional wetlands in the southern portion of the BCP in the state of Baja California Sur. The type locality is corrected in its formal name and its geographical location (see below the type locality section). This work fulfills the requirements of the Article 75 of the Fourth Edition International Code of Zoological Nomenclature to designate a neotype: 1) no name-bearing type specimen (i.e., holotype, lectotype, syntype, nor prior neotype) is believed to be extant, 2) a name-bearing type is necessary to define the nominal taxon objectively, 3) to designate the neotype with the express purpose of clarifying its type locality, 4) to present a statement and its bibliographic reference of the characters that differentiate it from other taxa, 5) to present evidence that the neotype is consistent with what is known of the former name-bearing type from the original description, 6) the neotype comes from the original type locality, and 7) the neotype is deposited in a recognized scientific institution. We describe and document the morphological identity of Venezillo stuckchensis using SEM micrographs. In addition, we present data on its molecular identity upon the analysis of 16S ribosomal RNA (16S) mitochondrial gene fragments of 4 paratopotype specimens.
Materials and methods
We revised oniscidean lots deposited at the following Mexican institutions: Centro de Investigaciones Biológicas del Noroeste, S.C. (CIB); Departamento de Biología, Universidad Autónoma de Aguascalientes; Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León; and Facultad de Ciencias Biológicas, Universidad Juárez del Estado de Durango. From these lots, we found specimens of 3 species of the Armadillidae: Cubaris murina Brandt, 1833 recorded from the states of Baja California Sur, Nuevo León, and Tamaulipas (Segura-Zarzosa et al., 2020), Venezillo stuckchensis, subject of the present work, and Venezillo sp. from the northern-central state of Durango whose taxonomic study will be published elsewhere. Besides Cubaris murina, specimens of 3 additional exotic species were found with the neotype and paratopotypes in the type locality.
For the morphological study of oniscideans we carried out the following steps: 1) separation of morphotypes according to the habitus type, general shape of cephalothorax, second antennae, pereion, pleon, uropods and pleuronite 6 (pleotelson); 2) specimens of each morphotype were sexed according to the sexual dimorphism (Schmidt, 2002); only adult specimens were considered; 3) each adult specimen was examined in a stereomicroscope, and measured for total length (TL) using a digital caliper (Mitutoyo 700-113, Kawasaki, Japan). For the SEM analysis male and female specimens were dehydrated individually in 100% ethanol for 24 h and critical-point dried (Samdri-PVT-3B, Tousimis, Rockville, MD, USA), sputter coated with gold (desk II, Denton Vacuum, Moorestown, NJ, USA), and analyzed with a scanning electron microscope S-300N Hitachi at CIB. The anatomical terminology is mainly according to Schmidt (2002, 2003). All the material of V. stuckchensis comes from the state of Baja California Sur. The material examined section for V. stuckchensis is organized with the name of the site, collection date, collector’s name, catalog code, number of males and females examined, and range values of TL and maximum body width (MW). The material examined of exotic and native species was determined following morphological descriptions of Budde-Lund (1885), Richardson (1902, 1905), Barnard (1932), Van Name (1936), Mulaik (1960), Green (1961), Schultz (1984), Shultz (2018), Karasawa (2012), Treviño-Flores and Rodríguez-Almaraz (2012), and Segura-Zarzosa et al. (2020). The classification follows Ahyong et al. (2011). The place of the species to its respective genus and family is after Taiti et al. (1998), Schmalfuss (2003), and Schmidt and Leistikow (2004).
We carried out molecular and phylogenetic analyses limited to the study of 16S fragments. Genomic DNA was extracted from the pereiopods with Gentra Puregene tissue kit (Qiagen) following manufacturer’s protocol. Using specimens from the type locality we applied standard protocols to amplify fragments of the mitochondrial gene 16S. We obtained amplified products from 4 specimens, 2 males and 2 females. The PCR reaction mixture contained 75 ng DNA, 30 pmol of each primers 16Sar and 16Sbr (CGC CTG TTT ATC AAA AAC AT and CCG GTC TGA ACT CAG ATC ACG T; Palumbi et al., 1991), 1x PCR buffer (200 mM Tris HCl pH 8.4, 500 mM KCl), 2 mM MgCl2, 0.15 mM dNTP’s, and 1 U Platinum Taq DNA polymerase (Invitrogen, Sao Paulo, Brazil). Amplification conditions were 94 °C for 4 min, followed by 35 cycles of 94 °C for 30s, 50 °C for 30s and 72 °C for 1 min and a final extension for 5 min at 72 °C. Amplified products were sequenced at Macrogen, South Korea. We edited 16S sequences in DNA Baser 4.5 (www.dnabaser.com) and aligned them under default settings in Clustal X (Thompson et al., 1997). Haplotypic determination was carried out in DnaSP 5.10 (Librado & Rozas, 2009). We further explored the phylogenetic relationships of V. stuckchensis using GenBank sequences of other species of Armadillidae, and of Armadillidiidae, Porcellionidae and Tylidae (Table 1). The analysis was carried out using MrBayes v3.2 (Ronquist et al., 2012). Poorly aligned and divergent positions were trimmed using automated 1 method in TrimAl v1.2 (Capella-Gutiérrez et al., 2009) implemented in Phylomon2 (Sánchez et al., 2011). A best nucleotide substitution model for the phylogenetic analysis was selected under Bayesian information criterion from the jModeltest 2.1.4 (Darriba et al., 2012; Guindon & Gascuel, 2003) to implement in Bayesian Inference (BI) method. The BI was run in MrBayes for 10 million generations and a consensus tree was generated after burning 0.25 of the trees generated during the analysis. The consensus tree was viewed using the program FigTree v1.4.3.
Results
Order Isopoda Latreille, 1817
Suborder Oniscidea Latreille, 1802
Armadillidae Brandt & Ratzeburg, 1833
Venezillo Verhoeff, 1928
Venezillo stuckchensis (Mulaik, 1960)
(Figs. 1-15)
Armadillo stuckchensis Mulaik, 1960: 194 (original description).
Venezillo stuckchensis (Mulaik, 1960): Souza-Kury, 2000: 244, 246; Schmalfuss, 2003: 293.
Venezillo stuckchensis Mulaik 1960: Jass & Klausmeier, 2004: 5, 17, 18, 69.
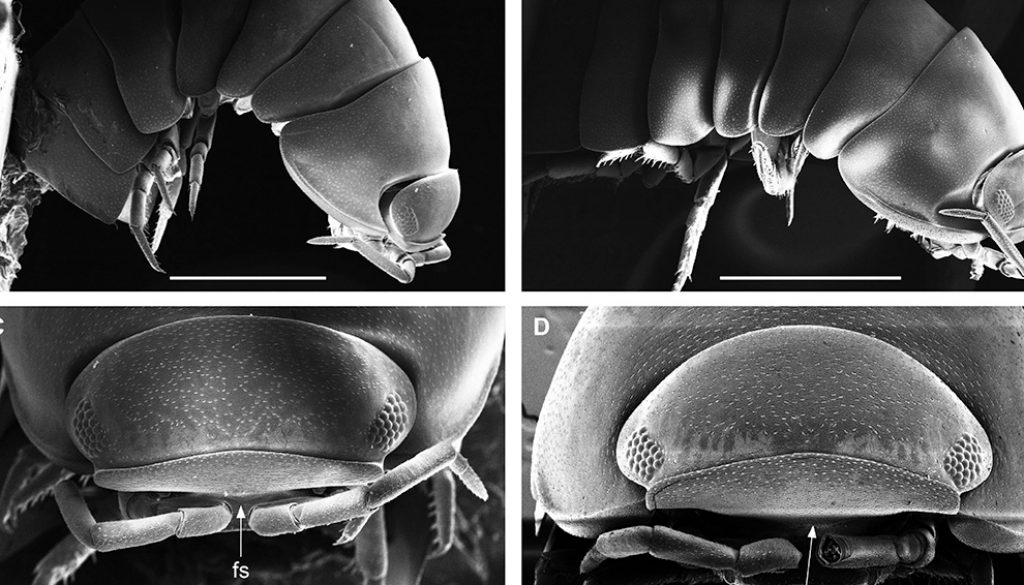
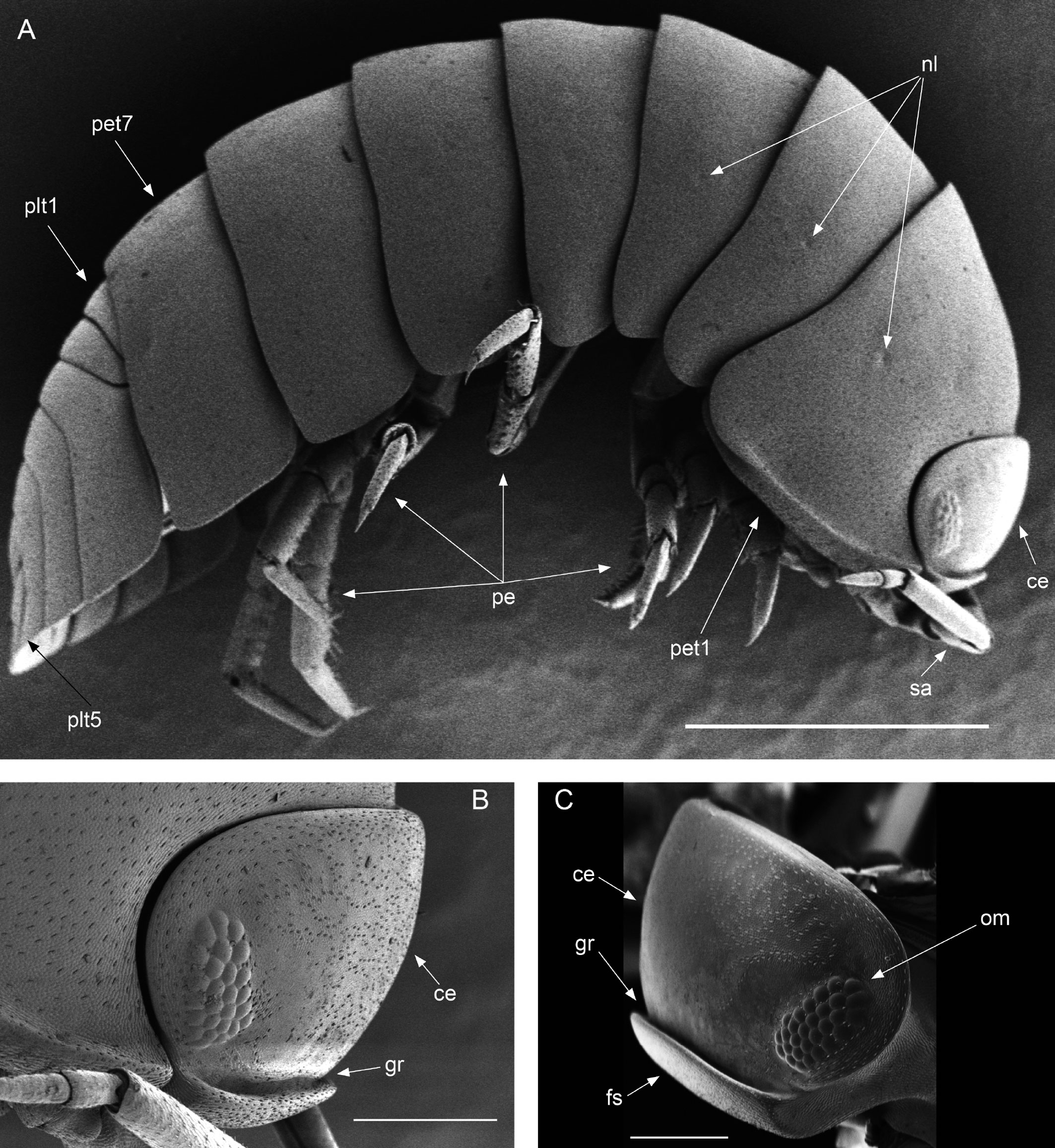
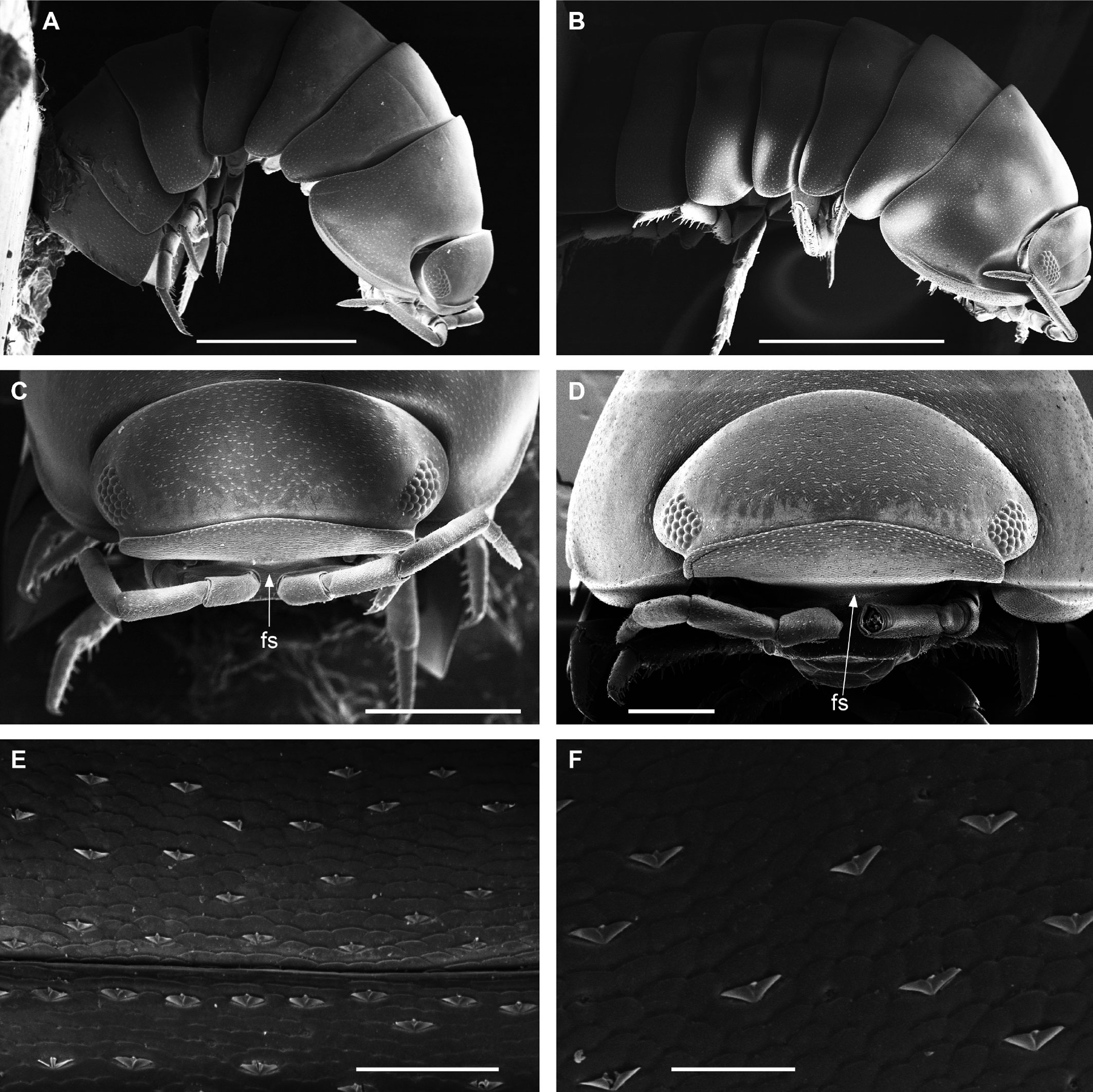
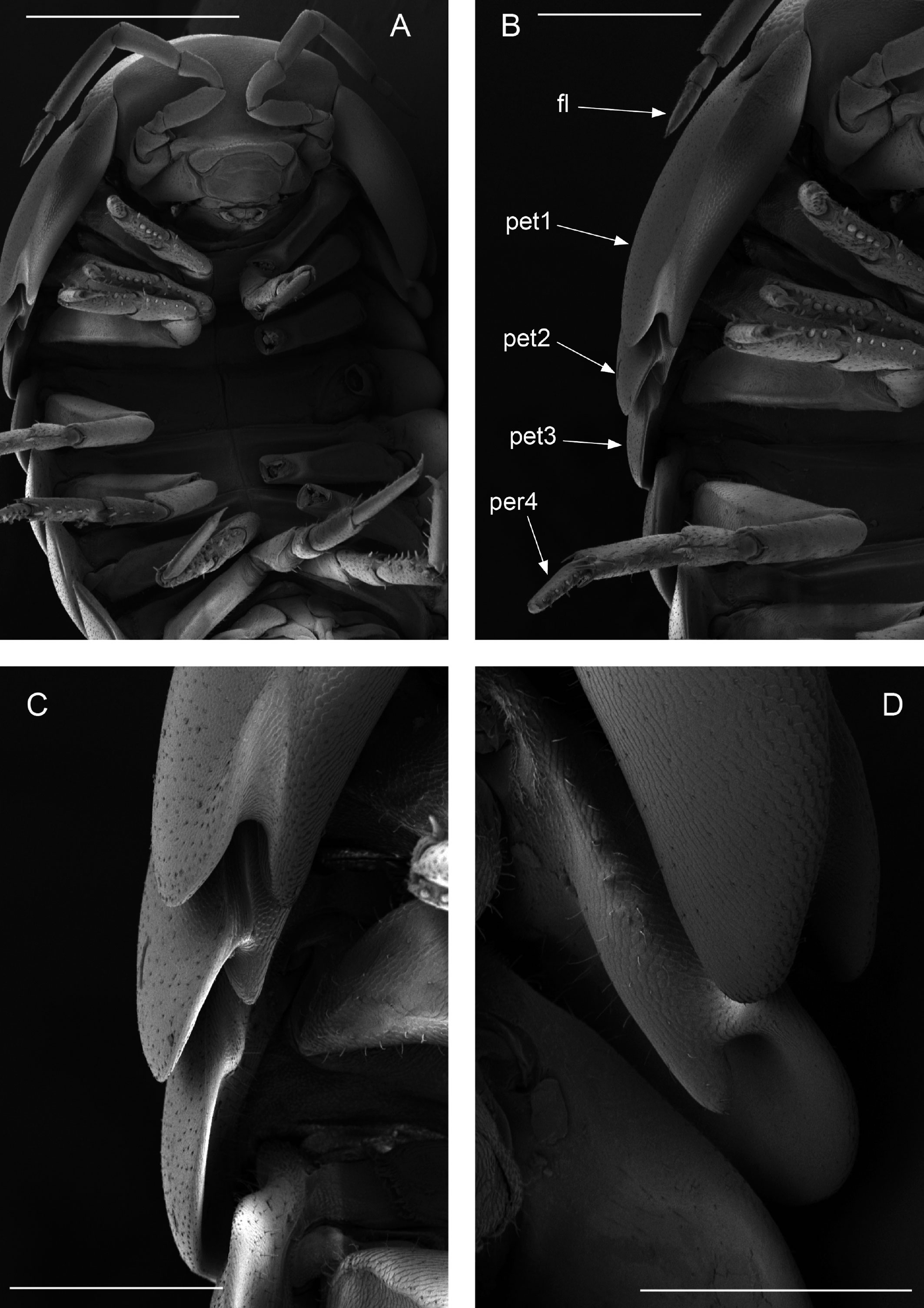
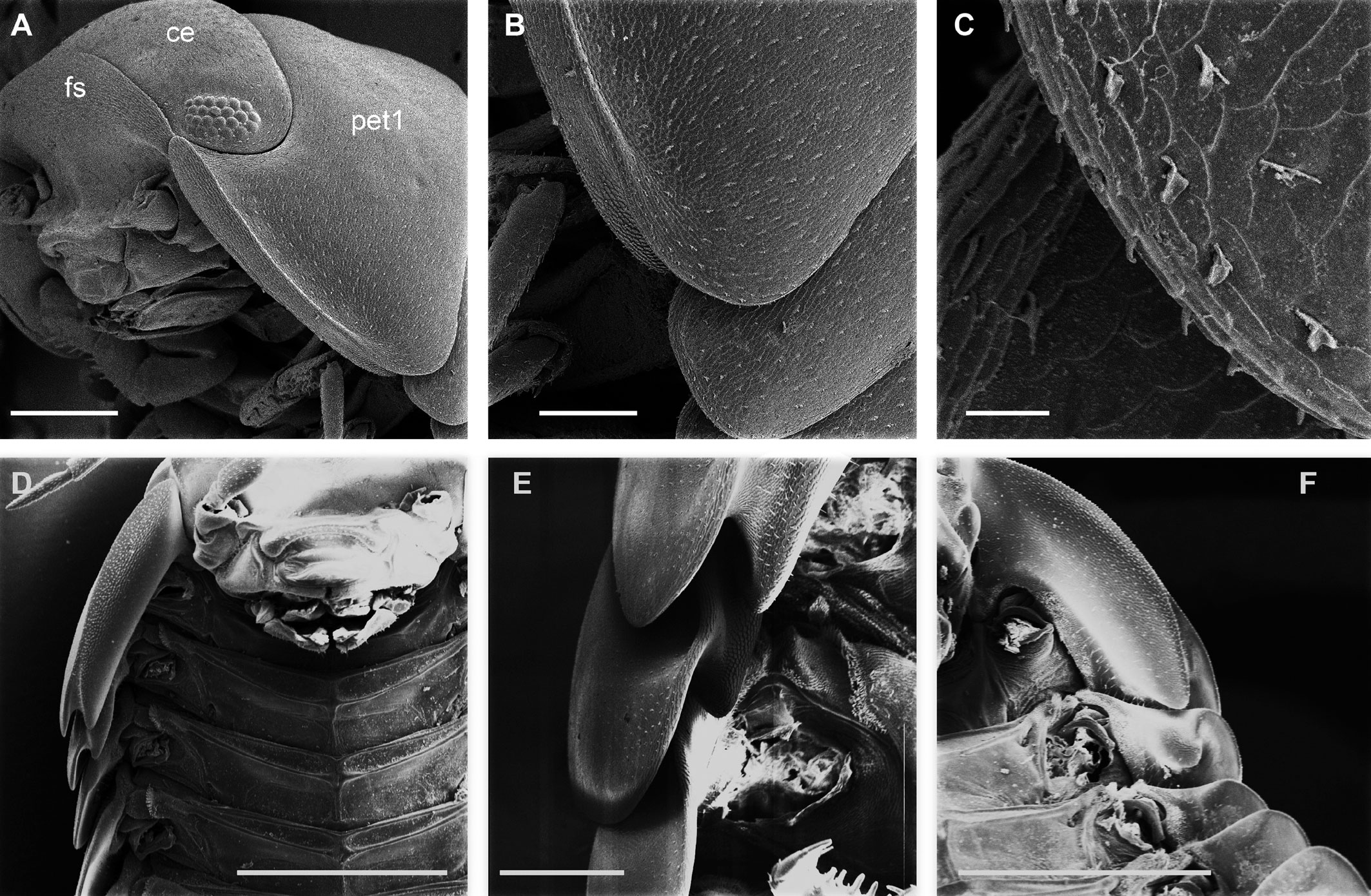
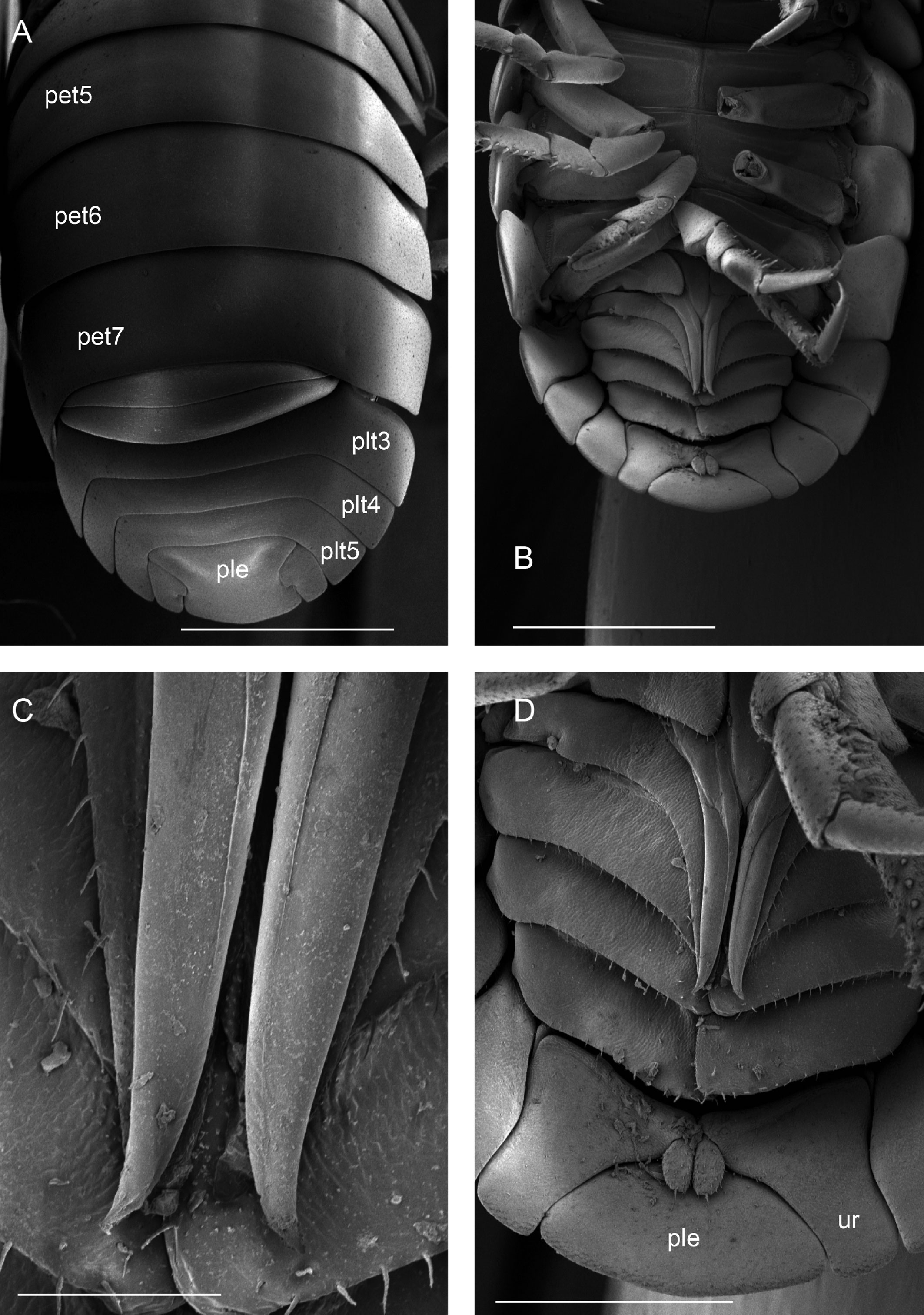
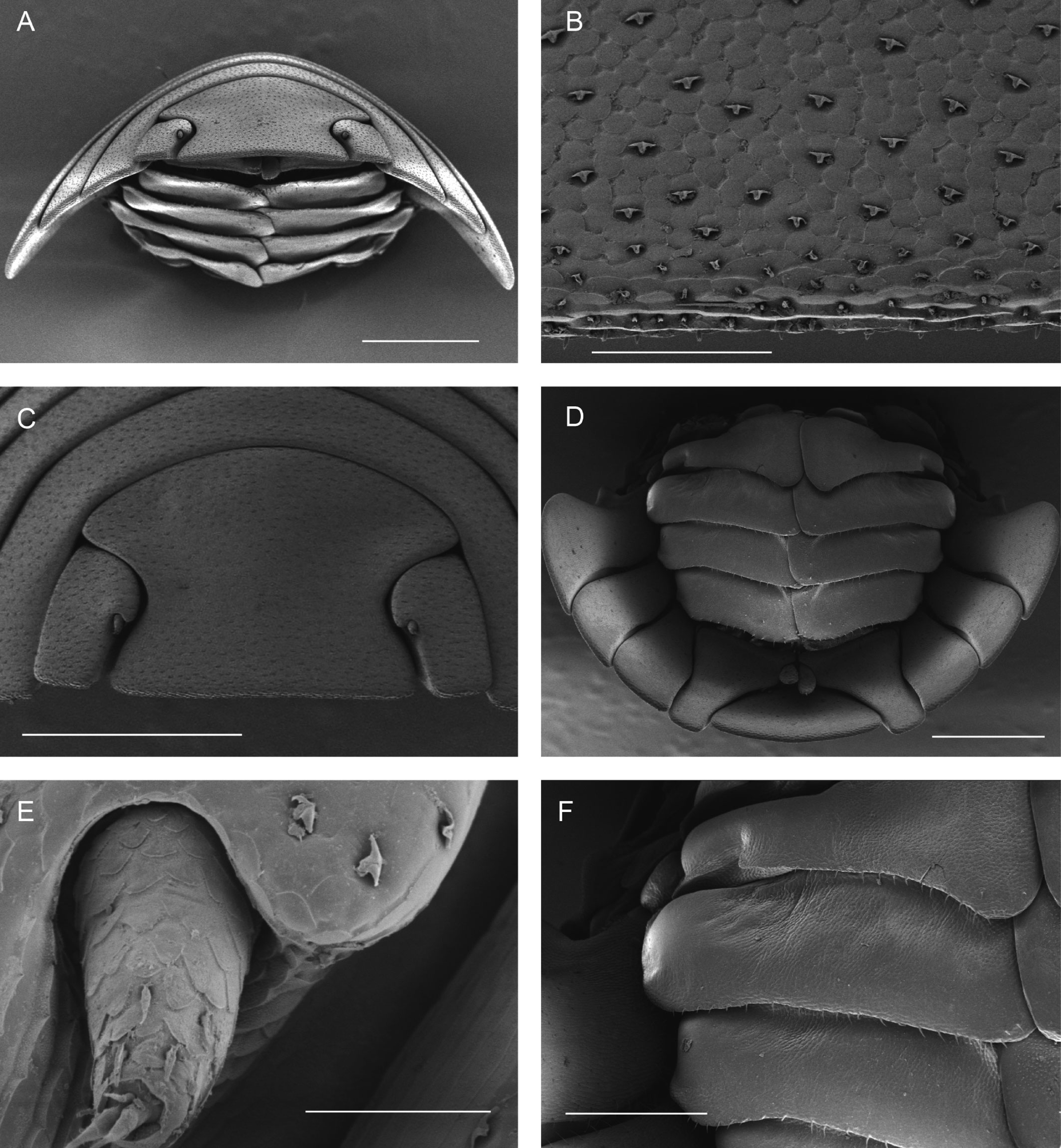
Diagnosis. Habitus type endoantennal conglobator. Body ovate, strongly convex, more than twice as long as wide. Total body length 5-20 mm, maximum width is in pereion-tergite 5. Color normally dark bluish gray, some light gray. Pair of small noduli laterales on each pereion-tergite 1-7, all at same distance to lateral margin near posterior border of tergites. Cephalothorax compressed antero-posteriorly, 2 times as wide as long, vertex convex in the middle. Complex eyes situated at the sides of cephalothorax, halfway between frontal and caudal margins, each one with 23-27 ommatidia normally distributed in 4 rows. Frontal shield (lamina frontalis) trapezoidal in frontal view, with proximo-lateral antennal lobes for holding proximal portion of second antenna during conglobation, posterior margin expanded, remaining a groove behind frontal shield. First antenna 3-jointed, basal article longest and broadest. Second antennae with first article short, second about 3 times as long as first, third and fourth subequal shorter than second, fifth about 1.5 times longer than fourth. Flagellum 2-jointed, second article lanceolate with apical cone, about 2 times longer than first; complete flagellum about 1/2 length than fifth article. Pereion-tergite 1 with notch (schisma) along 1/4 of the epimeron at the posterior corner into which the anterior corner of the pereion-tergite 2 epimeron fits when the animal conglobates. Pereion-tergite 2 with large digitiform lobe on anterior ventral side. Pleotelson wide at base, becoming constricted about the middle, expanding posteriorly to a truncate caudal margin. Uropod sympodites enlarged, flattened, about twice as wide as long, filling space between caudal side of pleon-tergite 5 and lateral side of pleotelson; exopodites inserted on medial margin of sympodites, about halfway between anterior and caudal margins; endopodites inserted on anteromedial margin of sympodites not visible dorsally, elongated, flattened, distally rounded, extending one third the length of pleotelson, their bases covered by an extension of anteromedial margin of sympodite.
Table 1
GenBank sequences of species of Armadillidae, Armadillidiidae, Porcellionidae and Tylidae included in the phylogenetic analysis.
|
Family |
Species |
Accession No. |
Reference |
|
Armadillidae |
Cubaris murina |
AB646775 |
|
|
C. murina |
AB646776 |
||
|
Merulana helmsiana |
KC706379 |
Lee et al., 2014 |
|
|
Spherillo grossus |
KC706381 |
Lee et al., 2014 |
|
|
S. grossus |
KC706384 |
Lee et al., 2014 |
|
|
S. grossus |
KC706387 |
Lee et al., 2014 |
|
|
Venezillo stuckchensis |
MW692078- |
This study |
|
|
MW692081 |
|||
|
Armadillidiidae |
Armadillidium pelagicum |
AJ639742 |
|
|
A. pelagicum |
AJ639750 |
||
|
A. vulgare |
AB646793 |
||
|
A. vulgare |
EF643519 |
Marcadé et al., 2007 |
|
|
Porcellionidae |
Porcellio laevis |
MG887957 |
Dimitriou et al., 2018 |
|
P. laevis |
MG887958 |
Dimitriou et al., 2018 |
|
|
Porcellio nasutus |
MG887954 |
Dimitriou et al., 2018 |
|
|
Porcellionides pruinosus |
MG887949 |
Dimitriou et al., 2018 |
|
|
P. pruinosus |
MG887950 |
Dimitriou et al., 2018 |
|
|
Proporcellio vulcanius |
MG887948 |
Dimitriou et al., 2018 |
|
|
Tylidae |
Tylos granulatus |
MK603226 |
Mbongwa et al., 2019 |
|
|
Tylos punctatus |
KF007428 |
Hurtado et al., 2013 |
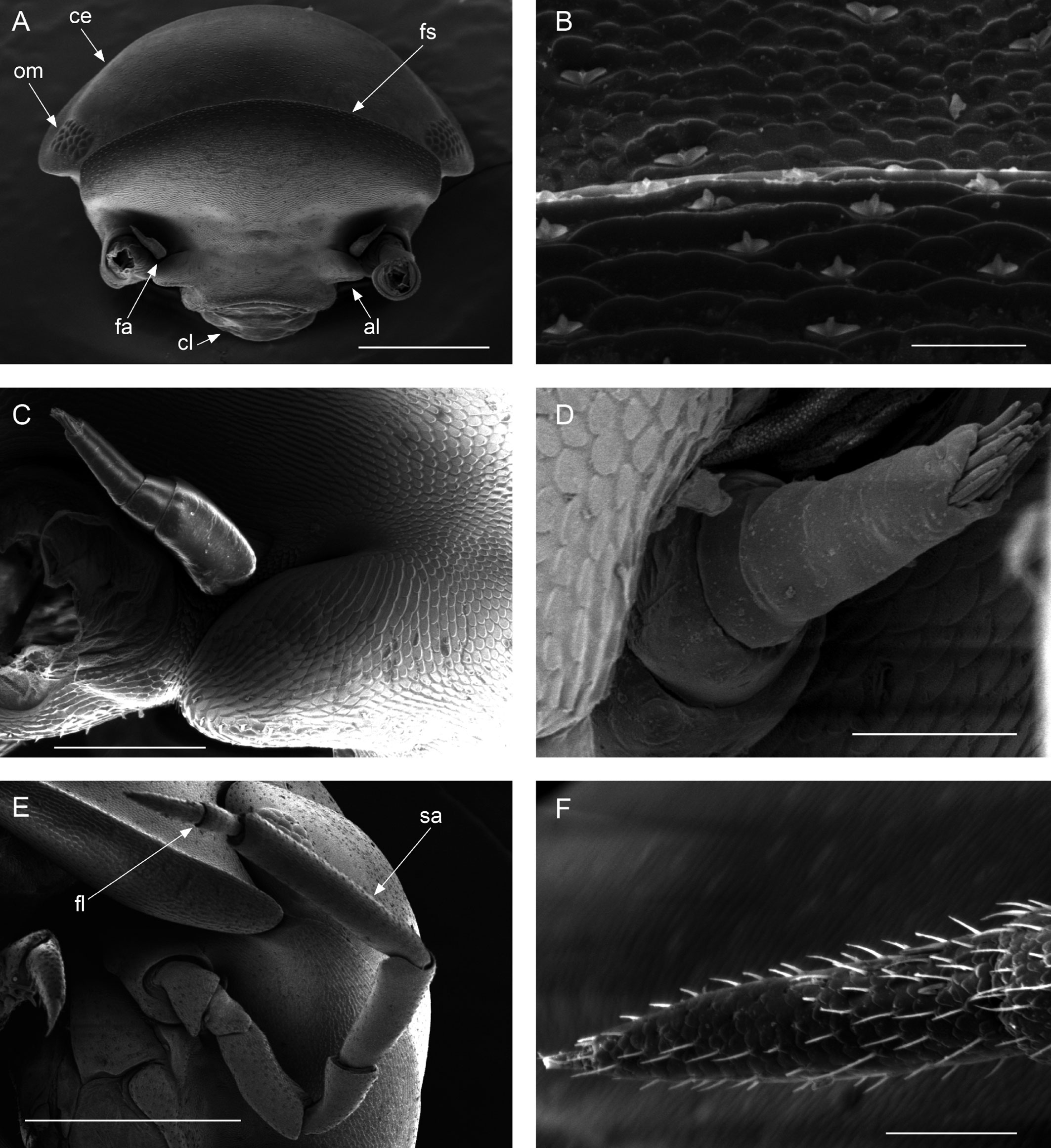
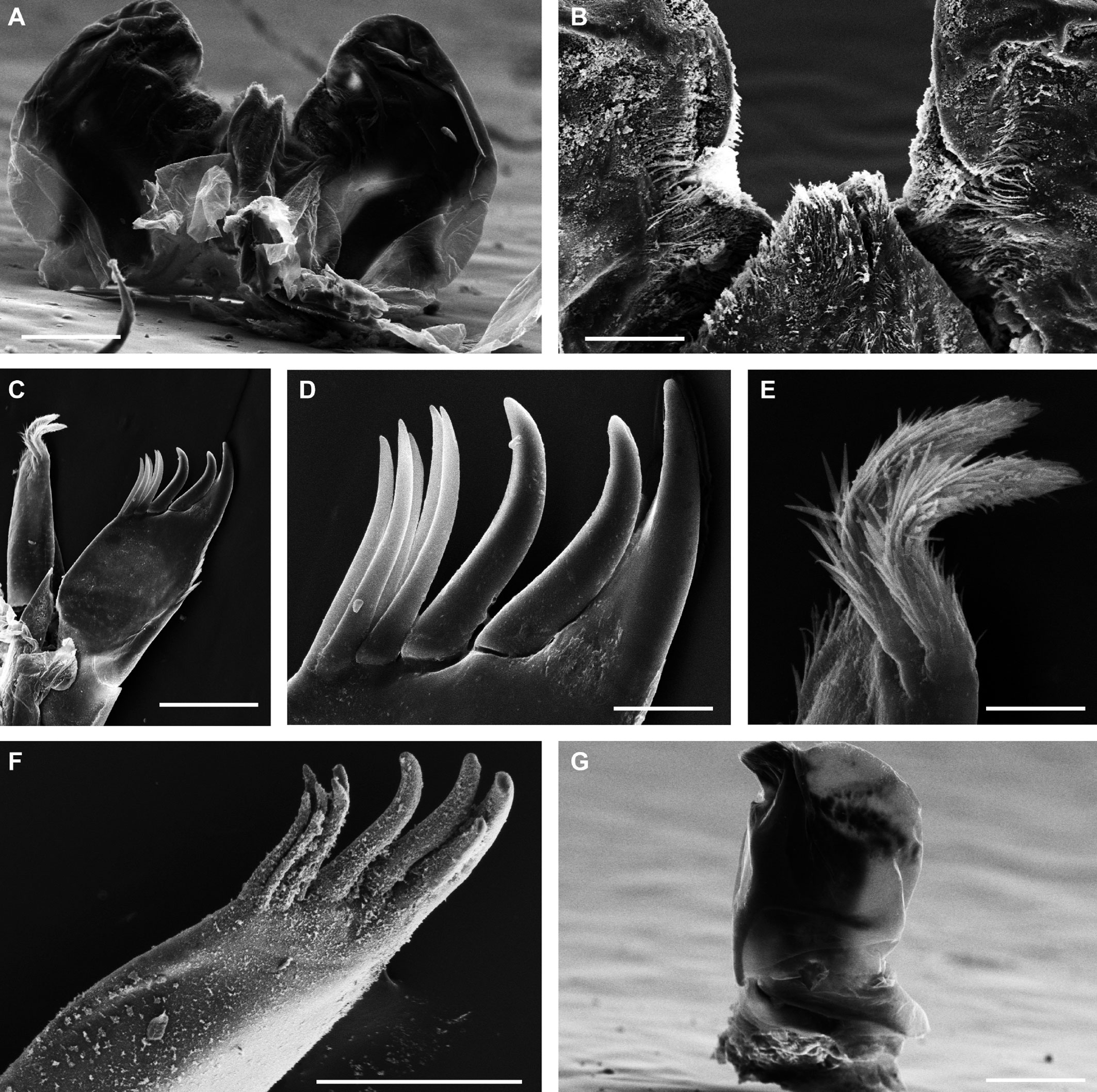
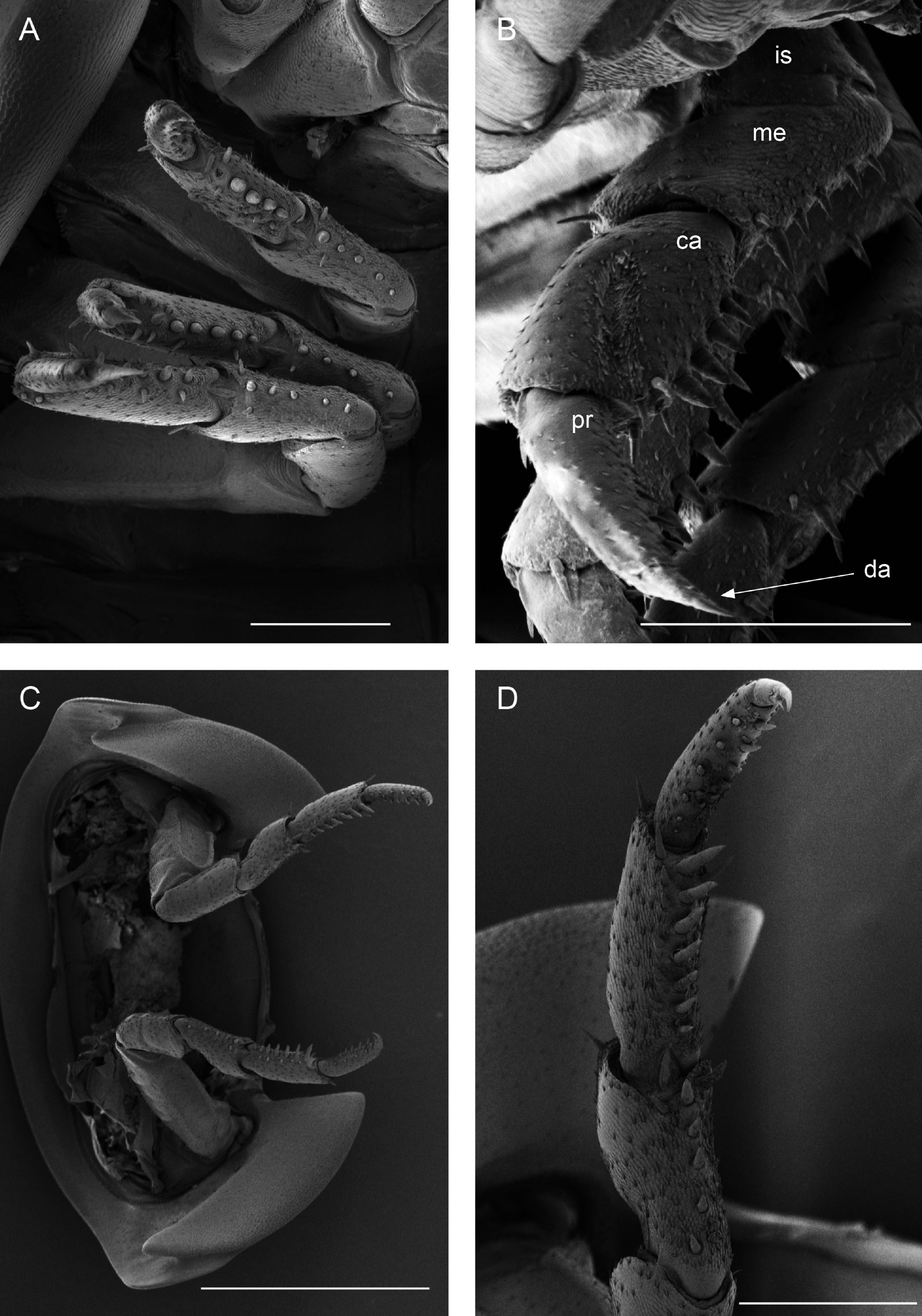
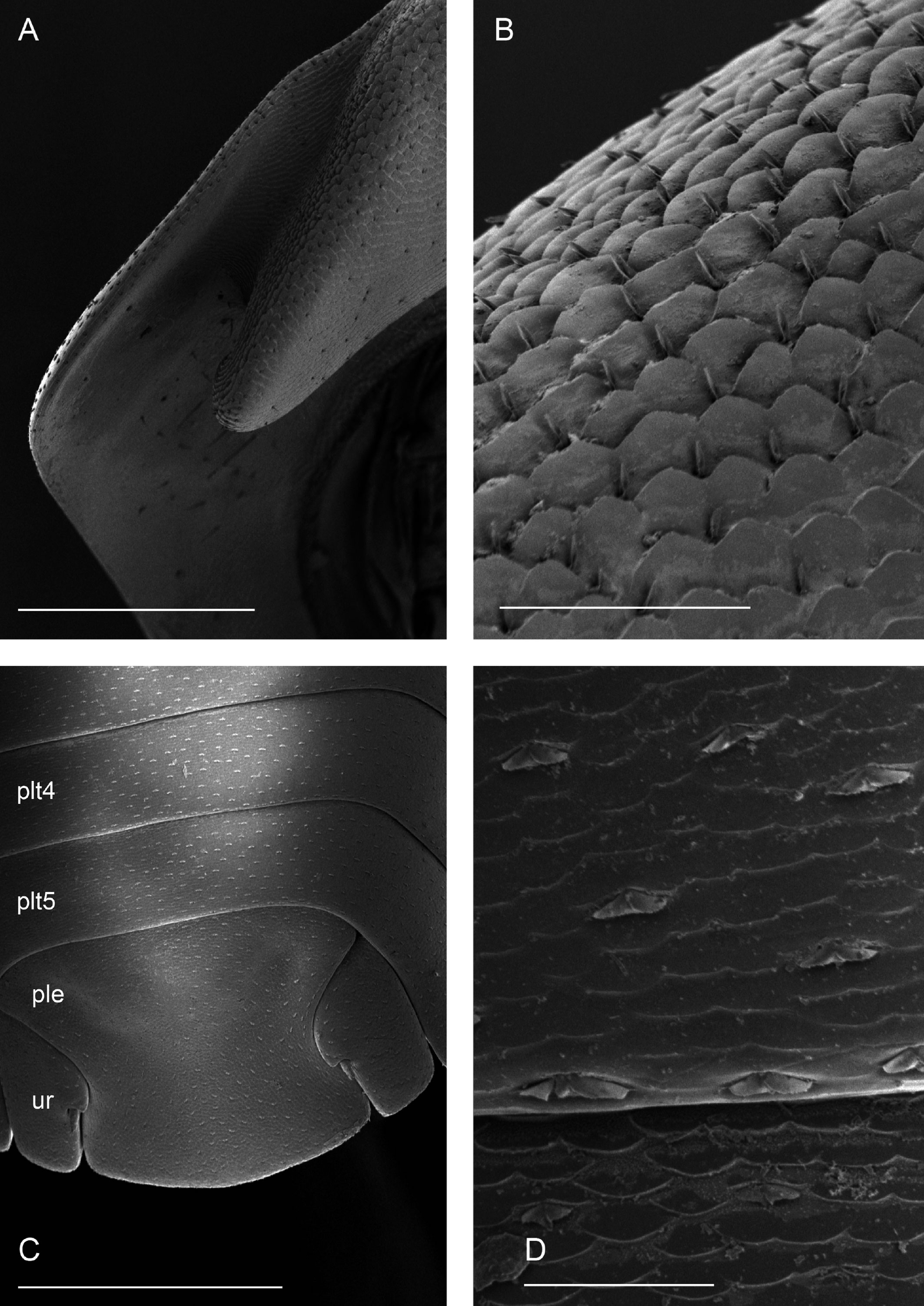
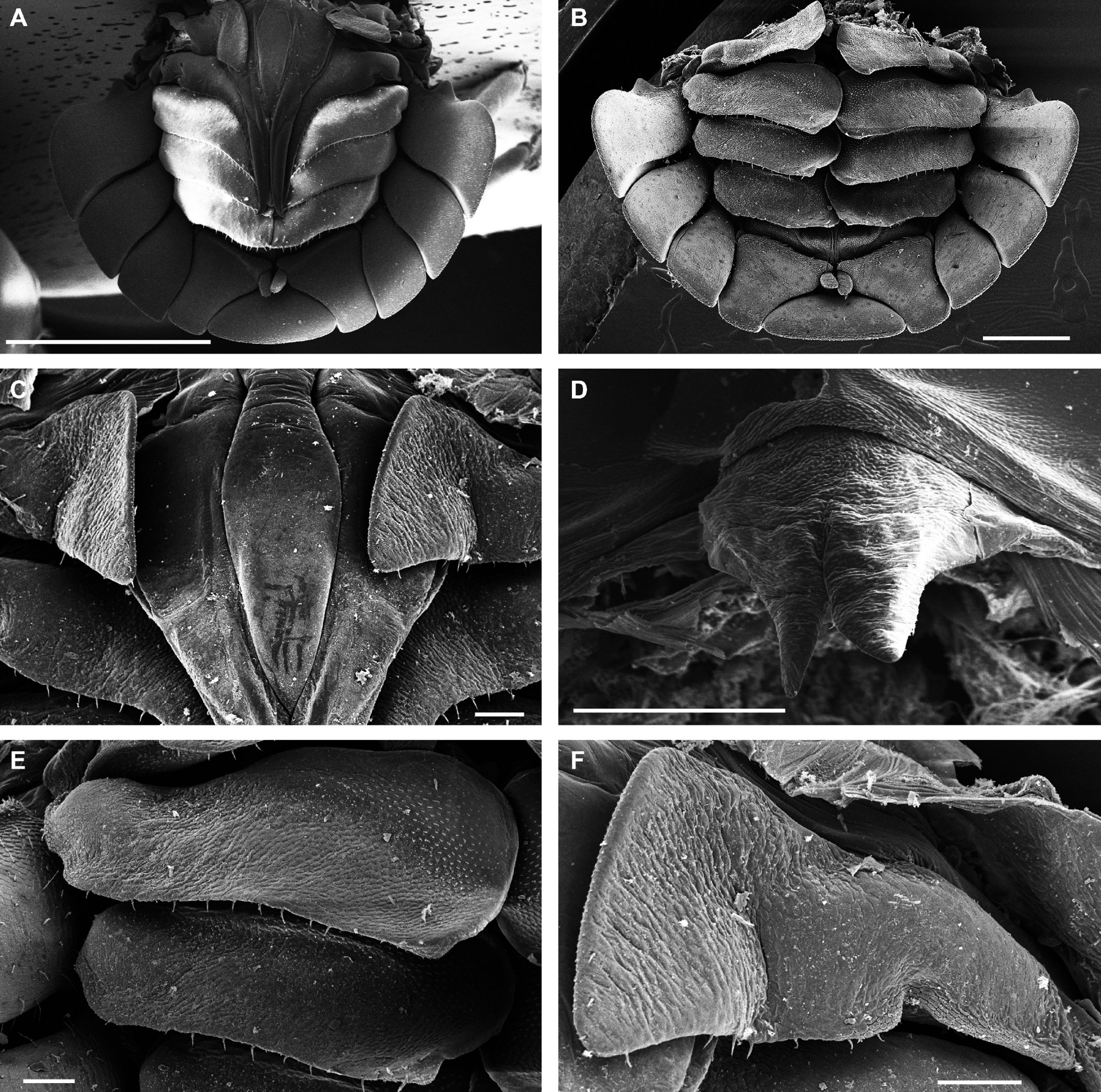
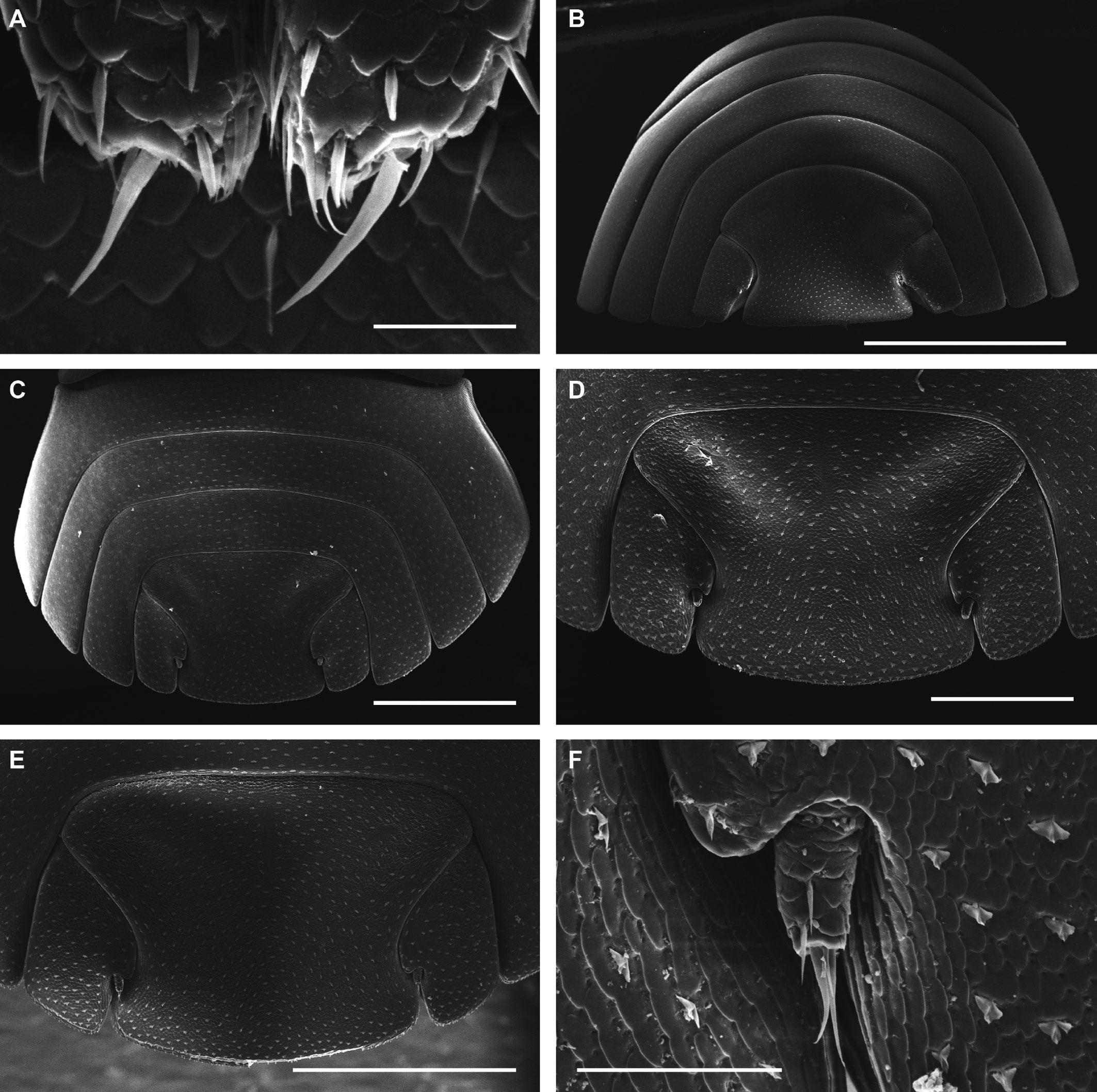
Description. Habitus, measurements and color as described in Diagnosis (Fig. 1A). Dorsal surface of cephalothorax and tergites glossy, without tubercles, covered with semi-curved scales and scattered tricorn scales (Figs. 2E-F, 3B). Pair of small noduli laterales on each pereion-tergite 1-7 all at same distance to lateral margin near posterior border of tergites (Fig. 1A). Cephalothorax compressed antero-posteriorly, 2 times as wide as long, vertex convex in the middle (Figs. 1B-C, 2C-D). Complex eyes situated laterally, halfway between frontal and caudal margins, each with 23-27 ommatidia normally distributed in 4 rows (Figs. 1B-C, 2C-D). Frontal shield (lamina frontalis) covered by semi-curved scales with scattered tricorn scales (Figs. 2E-F, 3B), trapezoid in frontal view with proximal lateral antennal lobes for holding the proximal portion of the second antenna during conglobation; posterior margin expanded, remaining a groove behind the frontal shield (Figs. 1A-C, 2A-D). First antenna 3-jointed with aesthetascs on distal article, basal article longest and broadest (Fig. 3C-D). Second antennae with first article short, second about 3 times as long as first, third and fourth subequal and each a little shorter than second, fifth about 1.5 times longer than fourth (Fig. 3E). Flagellum 2-jointed, second article lanceolate with apical cone, about 2 times longer than first; complete flagellum about 1/2 length of fifth article (Fig. 3F). Clypeus with anterior margin straight, posterior margin convex (Fig. 4A). Right mandible with pars incisive with 3 round projections, central one largest, lacinia mobilis composed by 2 cusps with short lightly rounded portion, pars molaris with tuft of plumose setae. Left mandible with pars incisive with 3 crown shaped projections, central one largest, lacinia mobilis short with irregular cusp with basal hairy lobe, pars molaris composed by tuft of plumose hairy setae, external surface of mandibles with rounded and lanceolate scales (Fig. 4B). Labium with 4 lobes, medial lobes small, lateral lobes larger (Fig. 5A-B). First maxilla outer endite with outer group of 4 stout teeth, and inner group of 6 slender teeth (Figs. 4C, 5C-D, F); inner endite with 2 penicils (Fig. 5C, E), external margin with row of short setae (Fig. 5C). Second maxilla membranous and laminate (Fig. 5G), inner lobe smaller, covered by abundant short setae, outer lobe wide, rounded, covered by pubescence. Maxilliped palp with 3 articles (Fig. 4D-E); basal article bearing 2 large setae; medial article with 1 large proximal seta and 1 small apical seta, distal article with an apical seta (Fig. 4E); ventral surface covered by semi-curved scales with lanceolate scales (Fig. 4F). Pereion-tergite 1 with notch (schisma) along 1/4 of the epimeron at the posterior corner (Figs. 6A-D, 8C, 11A) into which the anterior corner of the pereion-tergite 2 epimeron fits when the animal conglobates (Fig. 7A-F). Pereion-tergite 2 with large digitiform lobe on anterior ventral side (Fig. 6C-D). Pereion-tergite 3 with low protuberance on anterior ventral side (Fig. 6C, D). Pereiopod 1 with antennal brush composed by a groove covered by transversal scales on 2/3 of anterior side of carpus and basal part of propodus (Figs. 8B, 9A-D), inner side of merus, carpus and propodus with spines (Fig. 8A-D). Sternite 7 with medial process apically bilobate covering proximal portion of genital papilla (Fig. 12D). Pleon-tergites (pleon-segments) 1 and 2 with lateral parts undeveloped, covered laterally by pereion-tergite 7 (Figs. 1A, 10A). Pleon-tergites 3-5 broadly expanded laterally, lateral margins forming continuous line with lateral margins of pereion-tergites (Figs. 1A, 10A-B). Male genital papilla with ventral shield lanceolate (Figs. 13B, 12A, C). Pleopod 1 endopodite (copulatory appendage) elongated with acute apex (Figs. 10B-D, 12A, 13C), exopodite trapezoid with perispiracular area (Figs. 12C, F, 13A). Pleotelson wide at base, becoming constricted about the middle, expanding posteriorly to a truncate caudal margin (Figs. 11C, 14A, C, 15B, C-E), external surface covered by rounded scales and scattered tricorn scales (Figs. 11D, 14B). Uropod sympodites enlarged, flattened about twice as wide as long, filling space between caudal side of pleon-tergite 5 and lateral side of pleotelson (Figs. 10D, 11C, 12A-B, 13D-E, 14A, C-D, 15B-D); exopodites inserted on medial margin of sympodites, about halfway between anterior and caudal margins (Figs. 14C, E, 15F); endopodites inserted on anteromedial margin of sympodites not visible in dorsal view, elongated, flattened, distally rounded, covered by semirounded scales and lanceolate scales, apex with curved spine, extending one third length of pleotelson, their bases are covered by an extension of the anteromedial margin of the sympodite (Figs. 10D, 13D-F, 15A).
Taxonomic summary
Type locality. Santiago, municipality of Los Cabos, Baja California Sur, Mexico (23°29’02” N, 109°43’01” W, 186 m asl). In the original description, Mulaik (1960) wrote “Santiago, Baja California; holotipo macho, debajo de rocas, 23-III-1945, M. Correa”. In 1945, the BCP was politically divided into 2 entities: the state of Baja California, covering the northern part from the parallel 28° in the south, up to the international border with the USA in the north, and the territory (not a formal state) of Baja California Sur covering the southern part from the parallel 28° in the north, up to its southern tip in Cabo San Lucas. This territory was declared as the formal state of Baja California Sur in 1974 (Del Río & Altable-Fernández, 2000). In the northern state of Baja California there was not a place with the name of Santiago, whilst in the southern state of Baja California Sur there was, as up today, a small town named Santiago, founded as a Jesuit mission in 1721 (Del Río & Altable-Fernández, 2000). The name “M. Correa”, indicated as the collector by Mulaik (1960) corresponds to Mr. Manuel Correa, a field-work assistant at IPN, who along with recognized biologists carried out several expeditions to the BCP during those years (Pardo-Teijeiro & Álvarez-Lires, 2010). According to Mulaik (1960), M. Correa was also the collector of Ligia exotica specimens from Isla Pichilingue, La Paz, in the current state of Baja California Sur in the date of “24-IV-1945”. These data document that M. Correa visited the state of Baja California Sur in 1945, and thus we conclude, that the type locality of Venezillo stuckchensis corresponds to Santiago, Baja California Sur, Mexico.
Type material. Neotype male from the type locality, collection date 19.07.2019, TL 15.8 mm, MW 4.9 mm, cephalothorax length 1.3 mm, pereion length 6.8 mm, pleon length 3.3 mm, number of ommatidia 27 (CNCR 35786); paratopotypes, 3 males: TL 10-13.3 mm, MW 4.3-5.4 mm (CNCR 35787), 2 males: TL 9.6-12.2 mm, MW 3.8-4.9 mm (CIB-161B), 3 females: TL 11.4-17.5 mm, MW 5-7.7 (CNCR 35788), 3 females: TL 10.6-16.3 mm, MW 4.8-6.9 (CIB-162B). Type material collected by H. Obregón-Barboza.
Additional Venezillo stuckchensis specimens examined. Baja California Sur, El Chorro, Agua Caliente, municipality of Los Cabos, 23°26′20′′ N, 109°48′15′′ W, 206 m asl, 18.03.2005, C. Palacios, 3 females: TL 10.5-16.4 mm, MW 6.2-6.9 mm (CIB-42A); Presa Buena Mujer, municipality of La Paz, 24°05′20′′ N, 110°11′36.99′′ W, 180 m asl, 21.04.2004, C. Palacios, 1 male: TL 10.3 mm, MW 5.3 mm, 1 female: TL 14.8 mm, MW 6.8 mm (CIB-20A); Las Pocitas, El Pilar, municipality of La Paz, 24°28′19′′ N, 111°00′09′′ W, 148 m asl, 17.09.2008, C. Palacios, 8 females: TL 13.3-19.2 mm, MW 5.4-8.2 mm (CIB-16A); San Pedro de la Presa, municipality of La Paz, 24°50′17′′ N, 110°59′41′′ W, 158 m asl, 25.11.2008, C. Palacios, 2 males: TL 6-10.3 mm, MW 3.5-5.3 mm, 3 females: TL 13-14.8 mm, MW 6.8-7 mm (CIB-54A); Carambuche, La Purísima, municipality of Comondú, 26°14′13.2′′ N, 112°00′08.5′′ W, 119 m asl, 31.12.2006, A. Maeda, 17 males: TL 5.6-11 mm, MW 2.5-4.9 mm, 4 females: TL 6.2-8.1 mm, MW 2.5-3 mm (CIB-160B).

Remarks
Venezillo stuckchensis (Mulaik, 1960) fulfills the characteristics of the Armadillidae defined by Schmalfuss and Ferrara (1983), Schmidt (2003), and Taiti et al. (1998), with endoantennal conglobation ability, cephalothorax constricted in longitudinal direction, wide frontal shield, pleotelson with quadrangular distal part, second antenna flagellum 2-jointed, inner branch of the maxillule with 2 penicils, bilobate lamella in sternite 5 (seventh male pereion segment), lungs in all pairs of exopodites, uropod sympodite (protopodite) flattened and with distinctly concave medial margin and exopodite reduced in size, inserted dorsally on the medial margin of the protopod, and noduli laterales on the dorsal surface of pereion-tergites. The morphological features mentioned by Mulaik (1960) for the holotype are concordant with those of the designated neotype and the specimens here examined and determined as V. stuckchensis. Mulaik (1960) mentioned the holotype with a large size of more than 18 mm in length and 7 mm wide, and 27 ommatidia in each eye distributed in 4 irregular rows. The size range of the material examined, including the male neotype, is TL 5.5-15.8 mm, and MW 2.5-5.4 mm in males, and TL 6.2-19.5 mm, MW 2.5-8.2 mm in females, with 23-27 ommatidia normally distributed in 4 rows (Figs. 1B-C, 2C-D). Similar to the specimens examined in this work, Mulaik (1960) noted the holotype with a trapezoid frontal shield with proximo-lateral antennal lobes and posterior margin expanded, remaining a groove behind the frontal shield, a second antenna 2-jointed flagellum shorter than the antennal fifth article with the distal article longer than the proximal one, the pereion-tergite 1 with a notch along 1/4 of the epimeron at the posterior corner and the pereion-tergite 2 with a large digitiform lobe on the anterior ventral side (Figs. 6C-D, 7A-F). Mulaik (1960) also reported and figured the presence of 2 types of spines, pointed and stout on the inner side of carpus, and the antennal brush in the carpus of pereiopod 1. The specimens examined have pointed spines on the inner side of merus, carpus and propodus of pereiopod 1 (Figs. 8A-D, 9A-F) with antennal brush on the anterior side of carpus and basal part of propodus (Fig. 9A-D). In this work we describe features not mentioned by Mulaik (1960) in the original description such as the color (dark bluish-light gray) and the morphology of the dorsal surface of cephalothorax and tergites with semi-curved scales and scattered tricorn scales (Figs. 2E-F, 3B), the noduli laterales (Fig. 1A), first antenna 3-jointed (Fig. 3C-D), the morphology of clypeus (Fig. 4A), mandibles (Fig. 4B), labium (Fig. 5A-B), first maxilla (Figs. 4C, 5C-D, F), inner endite with penicils (Fig. 5C, E), second maxilla (Fig. 5G), maxilliped (Fig. 4D-F), pereion-tergite 3 with a low protuberance on the anterior ventral side (Fig. 6C, D), sternite 7 with bilobate medial process (Fig. 12D), male genital papilla with lanceolate ventral shield (Figs. 12A, C, 13B), pleopod 1 endopodite (copulatory appendage) (Figs. 10B-D, 12A, 13C), exopodite trapezoid with perispiracular area (Figs. 12C, F, 13A), uropod sympodites enlarged and flattened (Figs. 10D, 11C, 12A-B, 13D-E, 14A, C-D, 15B-D), exopodites on the medial margin of sympodites, about halfway between anterior and caudal margins (Figs. 14C, E, 15F), and endopodites (Figs. 10D, 13D-F, 15A).
Mulaik (1960) recognized V. stuckchensis as a different entity after the revision of specimens of 22 Mexican species which belong to the Armadillidae. He mentioned that, although based on a single specimen (the holotype), this represented a distinct species by the combination of the large size, high number of ommatidia, and the length of the flagellum compared to the length of the other second antenna articles. Based on the revision of the original descriptions of the Venezillo species reported for Mexico and the USA by Leistikow and Wägele (1999), and Schmalfuss (2003), we conclude that V. stuckchensis is morphologically similar to 2 geographically distant species, V. oaxacanus (Van Name, 1936) from Oaxaca state in southern Mexico, and V. tanneri (Mulaik, 1942 in Mulaik & Mulaik, 1942) from Texas, USA. As in V. stuckchensis, these 2 species have the dorsal surface of cephalothorax and tergites smooth without tubercles, the pereion-tergite 1 with a notch on the epimeron at the posterior corner and the pereion-tergite 2 with a lobe or cleft on the anterior ventral side. V. oaxacanus is different with a smaller number of ommatidia (as much as 18) and its color is light gray with narrow yellowish borders and small yellowish markings on the dorso-lateral parts of the thoracic segments (Mulaik, 1960; Van Name, 1936), and V. tanneri has smaller number of ommatidia (8) and its color is reddish-brown (Mulaik & Mulaik, 1942). The geographically closer Venezillo species to V. stuckchensis is V. macrosoma (Mulaik, 1960), a species recorded from Isla San Pedro Mártir, Sonora, an island situated between the BCP and mainland Mexico (Fig. 16). According to Mulaik (1960) V. macrosoma has the dorsal surface of cephalothorax and tergites with rows of tubercles on their posterior margins, the eyes composed by 14 ommatidia, the pereion-tergite 2 has no lobe or cleft on the anterior ventral side, and the pleotelson bears a dorsal triangular elevation on the medial side.
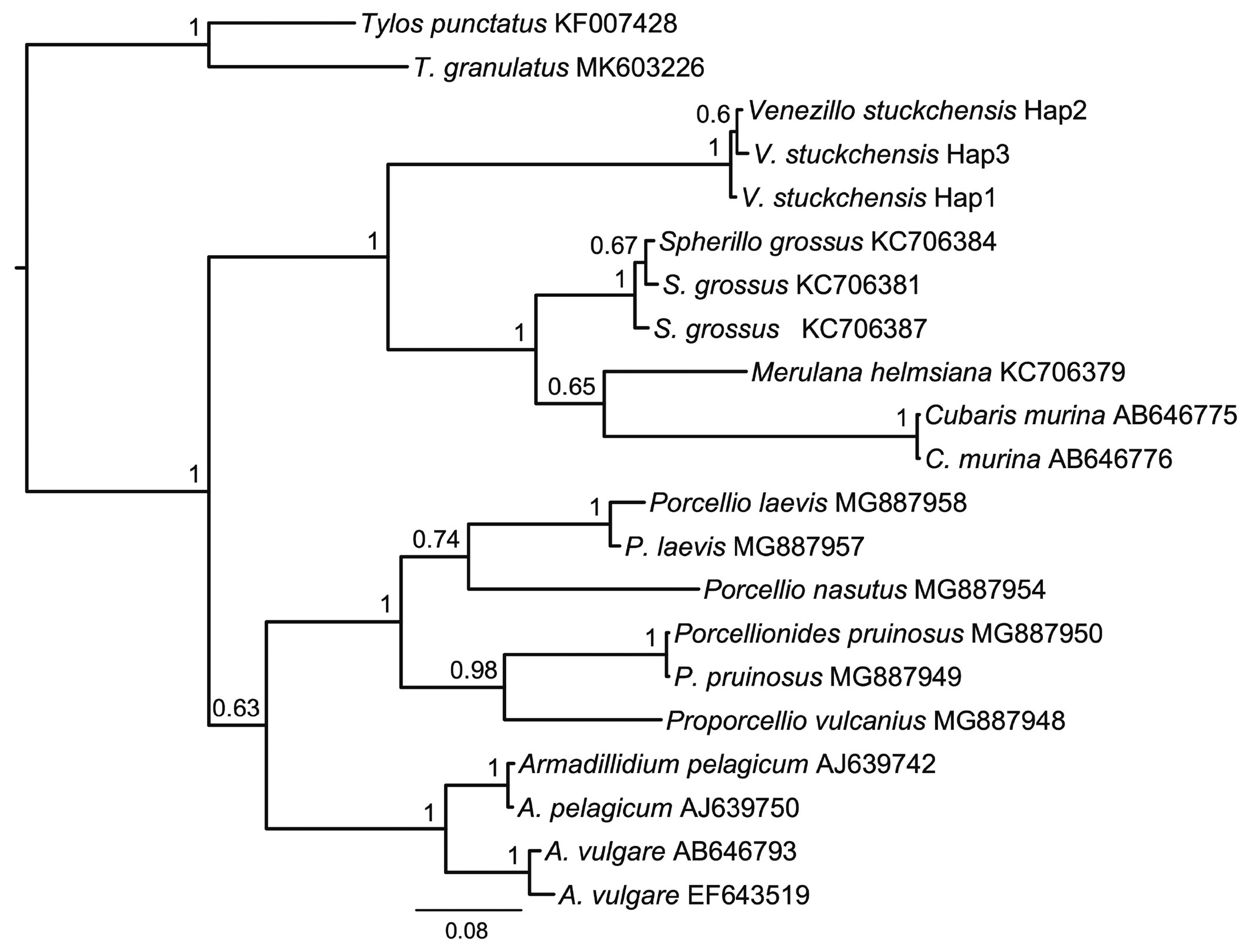
Haplotypic identity. From the 439 bp 16S sequences of V. stuckchensis, 3 haplotypes were identified. Their uncorrected pairwise genetic distances ranged from 0.68 to 1.14%. These sequences are the first deposited in the GenBank for the genus Venezillo (Table 1); therefore it was not possible to compare them with other congeneric species. The Bayesian phylogenetic analysis with the best fit nucleotide substitution model TIM2+I+G for the dataset containing 404 characters showed that V. stuckchensis formed a monophyletic clade with the 3 species of Armadillidae which was well-supported by posterior probability, confirming its place within this family. The node separating the species of Armadillidae from species of the other 2 families, Armadillidiidae and Porcellionidae, was also well supported (Fig. 17). The closer phylogenetic relationship between the Armadillidiidae and Porcellionidae, separated from the Armadillidae has been already reported in molecular phylogenetic studies using the mitochondrial large subunit rRNA gene (Michel-Salzata & Bouchona, 2000), and the nuclear 18S and 28S, and mitochondrial COI genes (Lins et al., 2017).
Distribution and habitat remarks. The distribution of the species covers a maximum lineal distance of about 450 km in the state of Baja California Sur, from the southernmost site in El Chorro, Agua Caliente to the northernmost site in Carambuche, La Purísima (Fig. 16). Oniscidean samples collected from wetlands located just to the south (oasis Estero San José del Cabo) and to the north (oasis San Ignacio) of this area contained specimens of the exotic Porcellio laevis Latreille, 1804, and Porcellionides pruinosus (Brandt, 1833) (Segura-Zarzosa et al., 2020). We report populations of Venezillo stuckchensis from 6 wetlands which are distributed in the southern part of the BCP. The characteristics of these wetlands are as follows, from south to north: 1) El Chorro, Agua Caliente, Los Cabos. This oasis belongs to the Santiago basin whose drainage ends in the Gulf of California (Mar de Cortés). It is located in the Agua Caliente creek with a permanent hot spring. Phytogeographically, it is in the Cape Lowland Tropical Region surrounded by typical riparian vegetation of the tropical dry forest with Palo Blanco (Lysiloma candidum), Encino Negro (Quercus brandegeei), Shine-Leaf Lomboy (Jatropha vernicosa) and the native palm species Brahea brandegeei and Washingtonia robusta, as the most relevant species (Garcillán et al., 2012). No additional oniscidean species were recorded in this site. 2) Santiago, Los Cabos (type locality). The oasis belongs to the Santiago drainage basin. Phytogeographically, it is located in the Cape Lowland Tropical Region (Garcillán et al., 2012). The town of Santiago is growing around a wetland with a forest of native palms (Brahea brandegeei and Washingtonia robusta) and exotic date palms (Phoenix dactylifera) as the most relevant species (Fig. 18). During the recent visit to the type locality (year 2019) only 13 Venezillo stuckchensis specimens were found under a dead palm trunk along with many specimens of 4 exotic species as follows: Armadillidae: Cubaris murina: 44 males, TL 4-9.8 mm, 46 females, TL 4.3-10.3 mm (CIB-173B); Porcellionidae: Agabiformius lentus (Budde-Lund, 1885): 1 female, TL 3.5 mm (CIB-174B); Porcellio laevis: 40 males, TL 4.9-15.1 mm, 30 females, TL 4-14 mm (CIB-175B); Porcellionides pruinosus: 11 males: TL 2.1-10.3 mm, 20 females: TL 3.2-11.1 mm (CIB-176B). 3) Presa Buena Mujer, La Paz. The site belongs to La Paz basin, whose drainage ends in the Gulf of California in the southeastern coast of the BCP. There is an artificial wetland as a result of a dam constructed with a concrete curtain.

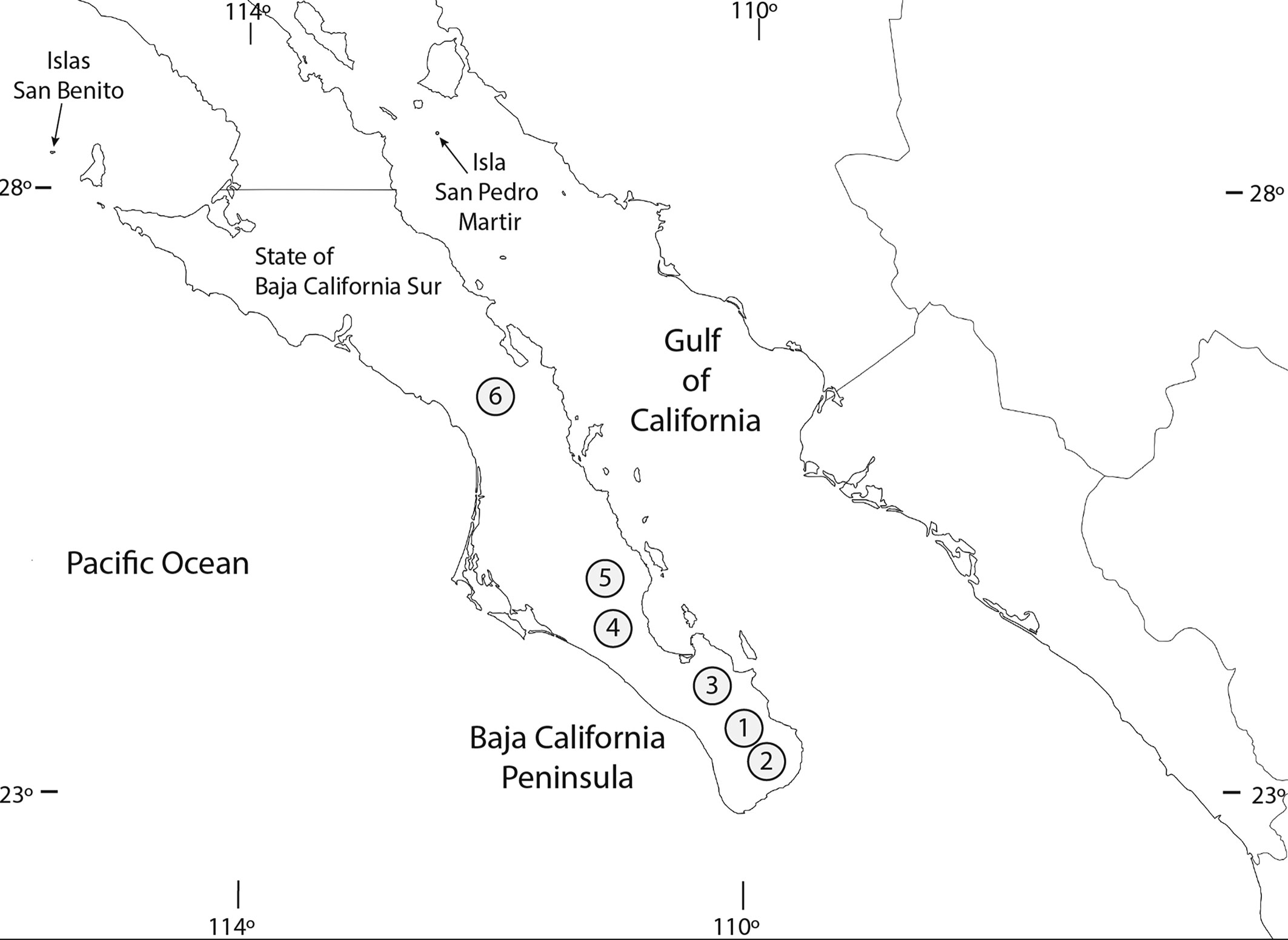
Phytogeographically, it is located in the Central Gulf Coast of the Sonoran Desert Region with sarcocaulescent plants with Ashy Limberbush (Jatropha cinerea), Palo Blanco (Lysiloma candidum), the Elephant Cactus or Cardón (Pachycereus pringlei), and the native palms Brahea brandegeei and Washingtonia robusta, as the most relevant species (Garcillán et al., 2012). No additional oniscidean species were recorded in this site. 4) Las Pocitas, El Pilar, La Paz. The site is in the El Pilar creek. It belongs to Las Pocitas-San Hilario basin whose drainage ends in the Pacific slope in the western coast of the BCP. The oasis has permanent springs surrounded by typical riparian vegetation of the southern BCP. Phytogeographically it is located in the Magdalena Plains of the Sonoran Desert Region with desert trees like the Western Honey Mesquite (Prosopis glandulosa), Adam’s tree (Fouquieria diguetii), Peninsular Palo Verde (Parkinsonia florida), the Elephant Cactus or Cardón (Pachycereus pringlei), Galloping Cactus (Stenocereus gummosus), the native palms Brahea brandegeei and Washingtonia robusta, and exotic Date palms (Phoenix dactylifera) as the most relevant species (Garcillán et al., 2012). Specimens of Porcellionides pruinosus were collected from this site (Segura-Zarzosa et al., 2020). 5) San Pedro de la Presa, La Paz. The site is in the Las Ánimas creek. It belongs to the Santa Rita basin whose drainage ends in the Pacific slope in the western coast of the BCP. This oasis has large permanent springs surrounded by a forest of native (Brahea brandegeei and Washingtonia robusta) and exotic Date palms (Phoenix dactylifera) as the most relevant species. Phytogeographically it is located in the Magdalena Plains of the Sonoran Desert Region (Garcillán et al., 2012). Specimens of Porcellionides pruinosus were collected from this site (Segura-Zarzosa et al., 2020). 6) Carambuche, La Purísima, Comondú. The site is in the La Purísima creek. It belongs to La Purísima basin whose drainage ends in the Pacific slope in the western coast of the BCP. This oasis has permanent springs surrounded by typical riparian vegetation of southern BCP with native (Brahea brandegeei and Washingtonia robusta) and exotic Date palms (Phoenix dactylifera) as the most relevant species. Phytogeographically it is located in the Sonoran Desert in the border between the Magdalena Plains and the Vizcaíno Desert ecological regions (Garcillán et al., 2012). Specimens of Porcellionides pruinosus were collected from this site (Segura-Zarzosa et al., 2020).

Acknowledgments
To María Luisa Jiménez Jiménez and Carlos Palacios Cardiel, Laboratorio de Aracnología y Entomología, CIB, for the donation of isopod material, and to Aurora Cristina González Pedraza, and Juan Antonio García Oviedo, Laboratorio de Ecología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Ciudad de México, for their permission to study the oniscidean collection. Special thanks to Ariel A. Cruz Villacorta, Laboratorio de Microscopía Electrónica, CIB, and Araceli Adabache Ortiz, Laboratorio Institucional de Microscopía Electrónica, Universidad Autónoma de Aguascalientes for their expert assistance in the SEM analyses. Also, to Marcelo Silva Briano for his kind support to carry out the SEM study at UAA. Many thanks to Gerardo Rafael Hernández García, Departamento de Extensión y Divulgación Científica, CIB, for editing SEM plates, cladogram, and map. We thank José Ángel Camacho Durán for his help in field work. Field collection permit was given by the Secretaría de Medio Ambiente y Recursos Naturales (SGPA/DGVS/01059/16). Ilse E. Segura-Zarzosa received a doctoral fellowship from Consejo Nacional de Ciencia y Tecnología (No. 551538).
References
Ahyong, S. T., Lowry, J. K., Alonso, M., Bamber, R. N., Boxshall, G. A., Castro, P. et al. (2011). Subphylum Crustacea Brünnich, 1772. In Z. Q. Zhang (Ed.), Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148, 165–191. http://dx.doi.org/10.11646/zootaxa.3148.1.33
Barnard, K. H. (1932). Contributions to the Crustacean fauna of South Africa, No. 11, Terrestrial Isopoda. Annals of the South African Museum, 30, 179–388. https://doi.org/10.5962/ bhl.part.9314
Brandt, J. F. (1833). Conspectus monographiae crustaceorum oniscidorum latreillii. Bulletin de la Société Impériale des Naturalistes de Moscou, 6, 171–193.
Brandt, J. F., & Ratzeburg, J. T. C. (1833). Isopoda. Gleichfüßler. In Medizinische Zoologie oder getreue Darstellung und Beschreibung der Thiere die in der Arzneimittellehre in Betracht kommen, in systematischer Folge herausgegeben, 2, 70–84.
Budde-Lund, G. (1885). Crustacea Isopoda terrestria per familias et genera et species descripta. Copenhagen: Nielsen & Lydiche. https://doi.org/10.5962/bhl.title.109769
Capella-Gutiérrez, S., Silla-Martínez, J. M., & Gabaldón, T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics, 25, 1972–1973. https://doi.org/10.1093/bioinformatics/btp348
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest2: more models, new heuristics and parallel computing. Nature Methods, 9, 772. https://doi.org/10.1038/nmeth.2109
Del Río, I., & Altable-Fernández, M. E. (2000). Breve historia de Baja California Sur. México D.F.: El Colegio de México, Fideicomiso Historia de las Américas, Fondo de Cultura Económica.
Dimitriou, A. C., Taiti, S., Schmalfuss, H., & Sfenthourakis, S. (2018). A molecular phylogeny of Porcellionidae (Isopoda, Oniscidea) reveals inconsistencies with present taxonomy. In E. Hornung, S. Taiti, & K. Szlavecz (Eds.), Isopods in a Changing World. Zookeys, 801, 163–176. https://doi.org/10.3897/zookeys.801.23566
Garcillán, P. P., González-Abraham, C., & Ezcurra, E. (2012). Phytogeography, vegetation, and ecological regions. In J. P. Rebman, & N. C. Roberts (Eds.), Baja California plant field guide (pp. 23–34). San Diego: San Diego Natural History Museum Publication.
Green, A. J. A. (1961). A study of Tasmanian Oniscoidea (Crustacea: Isopoda). Australian Journal of Zoology, 9, 258–365. https://doi.org/10.1071/zo9610258
Guindon, S., & Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52, 696–704. https://doi.org/10.1080/10635150390235520
Hurtado, L. A., Lee, E. J., & Mateos, M. (2013). Contrasting phylogeography of sandy vs. rocky supralittoral isopods in the megadiverse and geologically dynamic Gulf of California and adjacent areas. Plos One, 8, e67827. https://doi.org/10.1371/journal.pone.0067827
Jass, J., & Klausmeier, B. (2004). Terrestrial isopod (Crustacea: Isopoda) atlas of Mexico. Milwaukee Public Museum Contributions in Biology and Geology, 100, 1–77.
Karasawa, S. (2012). Cubaris iriomotensis, a junior synonym of the pantropical species Cubaris murina (Crustacea: Isopoda: Oniscidea). Edaphologia, 91, 21–30. https://doi.org/10.20695/edaphologia.91.0_21
Latreille, P. A. (1802). Histoire naturelle générale et particulière, des crustacés et des insectes. Tome troisième. Paris. https://www.biodiversitylibrary.org/page/24884921
Latreille, P. A. (1804). Histoire naturelle generale et particulière, des crustacés et des insectes. Tome septiéme. Paris. https://www.biodiversitylibrary.org/page/24882991
Latreille, M. (1817). Les crustacés, les arachnides et les insectés. In M. le Cher. Cuvier, Le Règne Animal, distribué d’après son organisation, pour servir de base a l’histoire naturelle des animaux et d’introduction a l’anatomie comparée. Tome III. Paris.
Lee, T. R. C., Ho, S. Y. W., Wilson, G. D. F., & Lo, N. (2014). Phylogeography and diversity of the terrestrial isopod Spherillo grossus (Oniscidea: Armadillidae) on the Australian east coast. Zoological Journal of the Linnean Society, 170, 297-309. https://doi.org/10.1111/zoj.12105
Leistikow, A., & Wägele, J. W. (1999). Checklist of the terrestrial isopods of the new world (Crustacea, Isopoda, Oniscidea). Revista Brasileira de Zoologia, 16, 1–72. https://doi.org/10.1590/s0101-81751999000100001
Librado, P., & Rozas, J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25, 1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Lins, L. S. F., Ho, S. Y. W., & Lo, N. (2017). An evolutionary timescale for terrestrial isopods and a lack of molecular support for the monophyly of Oniscidea (Crustacea: Isopoda). Organisms, Diversity & Evolution, 17, 813–820. https://doi.org/10.1007/s13127-017-0346-2
Marcadé, I., Cordaux, R., Doublet, V., Debenest, C., Bouchon, D., & Raimond, R. (2007). Structure and evolution of the atypical mitochondrial genome of Armadillidium vulgare (Isopoda, Crustacea). Journal of Molecular Evolution, 65, 651–659. https://doi.org/10.1007/s00239-007-9037-5
Mbongwa, N. A., Hui, C., Pulfrich, A., & von der Heyden, S. (2019). Every beach an island-deep population divergence and possible loss of genetic diversity in Tylos granulatus, a sandy shore isopod. Marine Ecology Progress Series, 614, 111–123. https://doi.org/10.3354/meps12882
Michel-Salzata, A., & Bouchon, D. (2000). Phylogenetic analysis of mitochondrial LSU rRNA in oniscids. Comptes Rendus de l’Academie des Sciences, Serie III, 323, 827–837. https://doi.org/10.1016/s0764-4469(00)01221-x
Mulaik, S. (1960). Contribución al conocimiento de los isópodos terrestres de México (Isopoda, Oniscoidea). Revista de la Sociedad Mexicana de Historia Natural, 21, 79–292. https://doi.org/10.18268/bsgm2009v61n2a15
Mulaik, S., & Mulaik, D. (1942). New species and records of American terrestrial isopods. Bulletin of the University of Utah, 32, 1–11. https://collections.lib.utah.edu/ark:/87278/s67w6x03
Palumbi, S. R., Martin, A. P., Romano, S. L., McMillan, W. O., Stice, L., & Grabowski, G. (1991). The simple fool’s guide to PCR. Special publication of Department of Zoology. Honolulu, Hawaii: University of Hawaii, USA.
Pardo-Tejeiro, X. F., & Álvarez-Lires, M. M. (2010). La presencia de Bibiano F. Osorio-Tafall en la Revista Ciencia. Llull, 33, 315–332.
Richardson, H. (1902). The marine and terrestrial isopods of the Bermudas, with descriptions of new genera and species. Transactions of the Connecticut Academy of Arts and Sciences, 11, 277–310.
Richardson, H. (1905). A monograph on the isopods of North America. Bulletin of the United States National Museum, 54, 1–727. https://doi.org/10.5479/si.03629236.54.i
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S. et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
Sánchez, R., Serra, F., Tárraga, J., Medina, I., Carbonell, J., Pulido, L. et al. (2011). Phylemon 2.0: a suite of web-tools for molecular evolution, phylogenetics, phylogenomics and hypotheses testing. Nucleic Acids Research, 39 (Web Server issue), W470–W474. https://doi.org/10.1093/nar/gkr408
Schmalfuss, H. (2003). World catalog of terrestrial isopods (Isopoda: Oniscidea). Stuttgarter Beiträge zur Naturkunde, Serie A, 654, 1–341. https://doi.org/10.1002/mmnz.20030790102
Schmalfuss, H., & Ferrara, F. (1983). Terrestrial isopods from west Africa. Monitore Zoologico Italiano, Supplemento, 18, 111–157. https://doi.org/10.1080/00269786.1983.11758568
Schmidt, C. (2002). Contribution to the phylogenetic system of the Crinocheta (Crustacea, Isopoda). Part 1. (Olibrinidae to Scyphacidae s. str.). Mitteilungenausdem Museum für Naturkunde in Berlin, Zoologische Reihe, 78, 275–352. https://doi.org/10.1002/mmnz.4850780207
Schmidt, C. (2003). Contribution to the phylogenetic system of the Crinocheta (Crustacea, Isopoda). Part 2. (Oniscoidea to Armadillidiidae). Mitteilungenausdem Museum für Naturkunde in Berlin, ZoologischeReihe, 79, 3–179. https://doi.org/10.1002/mmnz.20030790102
Schultz, G. A. (1984). Brackenridgia sphinxensis n. sp. from a cave with notes on other species from Arizona and California (Isopoda, Oniscoidea). The Southwestern Naturalist, 29, 309–319. https://doi.org/10.2307/3671362
Segura-Zarzosa, I. E., Rodríguez-Almaraz, G. A., Obregón-Barboza, H., Murugan, G., Treviño-Florez, J. A., & Maeda-Martínez, A. M. (2020). New records of exotic species of Oniscidea (Crustacea: Isopoda) from northern Mexico. Revista Mexicana de Biodiversidad, 91, e913098. https://doi.org/10.22201/ib.20078706e.2020.91.3098
Shultz, J. W. (2018). A guide to the identification of the terrestrial Isopoda of Maryland, U.S.A. (Crustacea). Zookeys, 801, 207–228. https://doi.org/10.3897/zookeys.801.24146
Schmidt, C., & Leistikow, A. (2004). Catalogue of genera of the terrestrial Isopoda (Crustacea: Isopoda: Oniscidea). Steenstrupia, 28, 1–118.
Souza-Kury, L. (2000). Oniscidea. In J. E. L. Bousequets, E. G. Soriano, & N. Papavero (Eds.), Biodiversidad, taxonomía y biogeografía de artrópodos de México: hacia una síntesis de su conocimiento, Vol. II (pp. 239–246). Ciudad de México: Universidad Nacional Autónoma de México. https://doi.org/10.1111/j.1539-6924.2005.00655.x
Taiti, S., Pasquino P., & Ferrara, F. (1998). Morphology, biogeography, and ecology of the family Armadillidae (Crustacea, Oniscidea). Israel Journal of Zoology, 44, 291–301. https://doi.org/10.1080/00212210.1998.10688952
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882. https://doi.org/10.1093/nar/25.24.4876
Treviño-Flores, J. A., & Rodríguez-Almaraz, G. (2012). Primeros registros de Porcellio laevis y Porcellio scaber (Crustacea: Oniscidea) del Noreste de México. In M. López-Mejía, & L. M. Mejía-Ortiz (Eds.), La carcinología en México: el legado del Dr. Alejandro Villalobos 30 años después (pp. 13–21). Cozumel, Quintana Roo: Universidad de Quintana Roo. https://doi.org/10.18226/21789061.v11i2p372
Van Name, W. (1936). The American land and freshwater isopod Crustacea. Bulletin of the American Museum of Natural History, 71, 1–535. http://hdl.handle.net/2246/1185
Verhoeff, K. W. (1928). Über einige Isopoden der Zoologischen Staatssammlung in München. Zoologischer Anzeiger, 76, 25–36, 113–123.