Sandra Milena Ospina-Garcés *, Livia León-Paniagua
Universidad Nacional Autónoma de México, Facultad de Ciencias, Museo de Zoología “Alfonso L. Herrera”, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Mexico City, Mexico
*Corresponding author: ospinagarcess@gmail.com (S.M. Ospina-Garcés)
Received: 17 May 2020; accepted: 18 August 2020
Abstract
The fishing bat Noctilio leporinus Linnaeus, 1758, represents a complex of subspecies with a discontinuous lowland distribution in Central, South America and the Caribbean. Although Mexican populations are currently included in the subspecies N. l. mastivus, the morphological variation in these groups has been poorly studied and only the body size differences with other subspecies have been documented. In addition, sex differences in cranial morphology for this complex of subspecies have been identified previously. To determine whether there are geographic differences between 2 isolated Mexican populations of N. l. mastivus and quantify the cranial sexual dimorphism in this subspecies, we performed geometric morphometric analyses of 2 dimensional landmark configurations describing cranial shapes. Our results support significant shape differences between the Pacific coast (west) and Gulf of Mexico-Yucatán Peninsula (east) populations, but no differences in cranial size were found. There were differences between sexes in the size and shape of the sagittal crest, in both populations, and these results suggest a continuous trend of development of this character in males, which imply functional differences in masticatory function between sexes. Morphological differences between populations could be related to genetic isolation and may be accentuated by differences in habitat structure between the dry (west) and humid (east) slopes of the Mexican mountains.
Keywords: Geometric morphometrics; Noctilionoidea; Sexual characters
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Dimorfismo sexual y variación geográfica del cráneo del murciélago pescador Noctilio leporinus (Chiroptera: Noctilionidae) en México
Resumen
El murciélago pescador Noctilio leporinus Linnaeus, 1758, representa un complejo de subespecies con una distribución discontinua en tierras bajas de América Central, América del Sur y el Caribe. Aunque las poblaciones mexicanas están actualmente incluidas en la subespecie N. l. mastivus, la variación morfológica en estos grupos ha sido poco estudiada y solo se han documentado las diferencias del tamaño corporal con otras subespecies. Además, previamente han sido identificadas diferencias entre sexos en la morfología craneal para este complejo de subespecies. Para determinar si existen diferencias geográficas entre 2 poblaciones mexicanas aisladas de N. l. mastivus y cuantificar el dimorfismo sexual craneal en esta subespecie, realizamos análisis de morfometría geométrica a partir de configuraciones de marcas en 2 dimensiones, que describen las formas craneales. Nuestros resultados apoyan diferencias significativas en la forma entre las poblaciones de la costa del Pacífico (oeste) y la del golfo de México y península de Yucatán (este), pero no en el tamaño craneal. Se encontraron diferencias entre sexos en el tamaño y la forma de la cresta sagital en ambas poblaciones, y este resultado sugiere una tendencia continua de desarrollo de este carácter en los machos, lo que implica diferencias funcionales en la función masticatoria entre sexos. Las diferencias morfológicas entre las poblaciones podrían estar relacionadas con el aislamiento genético y verse acentuadas por las diferencias en la estructura del hábitat entre las laderas secas (oeste) y húmedas (este) de los sistemas montañosos mexicanos.
Palabras clave: Morfometría geométrica; Noctilionoidea; Caracteres sexuales
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The fishing bat Noctilio leporinus Linnaeus, 1758 is one of the 2 species included in the family Noctilionidae. It is widely distributed in continental America and the Caribbean, and found from western (Sinaloa) and eastern (Veracruz) Mexico through northern Argentina (Hood & Jones, 1984). N. leporinus shows a pattern of discontinuous distribution restricted to humid and lowland habitats, including coastal areas and river basins, owing to its dietary preferences and foraging habits, which require water bodies (Altenbach, 1989; Hood & Jones, 1984; Kalko et al., 1998). Given its wide distribution, the taxonomy of the species has been revised, and it has been divided into 3 subspecies: N. l. mastivus, N. l. leporinus and N. l. rufescens; whichare morphologically differentiated in their body size and are restricted to different geographic regions within its range of distribution (Davis, 1973).
Within the species complex, the subspecies N. l. mastivus is found in the North, Central American and Caribbean group, including Mexico. This subspecies presents both the widest distribution and the highest variation in size and coloration (Davis, 1973). There is some disagreement as to whether N. l. mastivus is a single group, or whether it actually combines several subspecies with more restricted distributions (Goldman, 1915; Hall & Kelson, 1959). For example, specimens collected in western Mexico (Guerrero) were described by Goldman (1915) as a distinct subspecies (N. l. mexicanus) because of its smaller size compared to the populations from the West Indies. However, based on an extensive review of specimens over the complete distribution range, Davis (1973) suggested that these differences are small and insignificant, and classified N. l. mexicanus as a minor synonym of N. l. mastivus.
Although Mexican populations are currently included in the mastivus group, the only evidence analyzed so far is variation in body size (Davis, 1973). There are no phylogeographic studies for these populations and the few sequences analyzed come from a narrow geographic range in the Mexican Pacific Coast, so there is little molecular evidence to determine whether there has been divergence between Mexican populations (Lewis-Oritt et al., 2001; Pavan et al., 2013). Studies on the genetic structure and geographic diversification processes in other lowland mammal species (Arteaga et al., 2011; Hernández-Canchola & León-Paniagua, 2017; López-Wilchis et al., 2012; Rojas et al., 2016) and other vertebrates distributed in lowlands (Mulcahy et al., 2006), have suggested geographic isolation of populations of lowland species in Mexico through vicariant events such as the emergence of the Sierra Madre Occidental and the Sierra Madre Sur, and later the Trans-Volcanic Belt (during the Pliocene). This isolation and the resulting genetic differentiation between populations of N. l. mastivus between the western and eastern slopes could be reflected in cranial morphology, and differences between the western (Pacific) and eastern (Gulf of Mexico and Yucatán Peninsula) slopes of the Mexican mountains have not been explored.
Differences in cranial size between populations of N. l. mastivus have been previously mentioned, as well as sexual size dimorphism within populations, with males being larger than females (Davis, 1973). In this sense, there are also marked differences between sexes in several cranial morphological features associated with the piscivorous diet of N. leporinus. For example, males have greater sagittal crests and more developed processes of insertion of the masticatory muscles (Davis, 1973), such characters indicates the change in the temporal muscle to a more vertical position and the increase of masticatory muscles volume in fishing bat species (Freeman, 1988; Ospina-Garcés et al., 2016). The presence of sexually dimorphic characters in the cranial morphology of N. leporinus could be related to the differences in foraging patterns and the size of prey consumed, between sexes, that have been reported for this species (Bordignon, 2006), which is socially structured by cooperative stable female groups and males foraging alone (Brooke, 1997). However, the variation in cranial morphology (shape of the complete structure) remains little explored and has only been addressed by traditional morphometrics, and in the context of animalivorous diets (Davis, 1973; Freeman, 1988).
Geometric morphometrics comprises a set of tools to quantitatively analyze shape independent of size, and it has been successfully used to analyze shape variability in relation to a wide range of taxonomic, evolutionary, environmental, and geographical aspects of different bat groups (Dumont et al., 2011; Marchán-Rivadeneira et al., 2010; Nogueira et al., 2009; Ospina-Garcés & De Luna, 2017). Geometric morphometrics could be useful to quantify geographic variation in Mexican populations of N. leporinus, as well as the differences between the sexes in cranial morphology. Given the lack of morphological evidence and possible geographic isolation of the Pacific and Gulf of Mexico-Yucatán Peninsula populations, we implemented a geometric morphometric protocol to analyze the variation in cranial morphology of N. l. mastivus across different geographic regions in Mexico to: 1) evaluate the variability of cranial characters based on the geographic variation suggested for this subspecies in Mexico, 2) quantify sexual shape dimorphism (SShD) and sexual size dimorphism (SSD) of the cranial characters within each of the geographic groups considered, and 3) to describe the trends of morphological change between sexes and geographic groups of Mexico.
Materials and methods
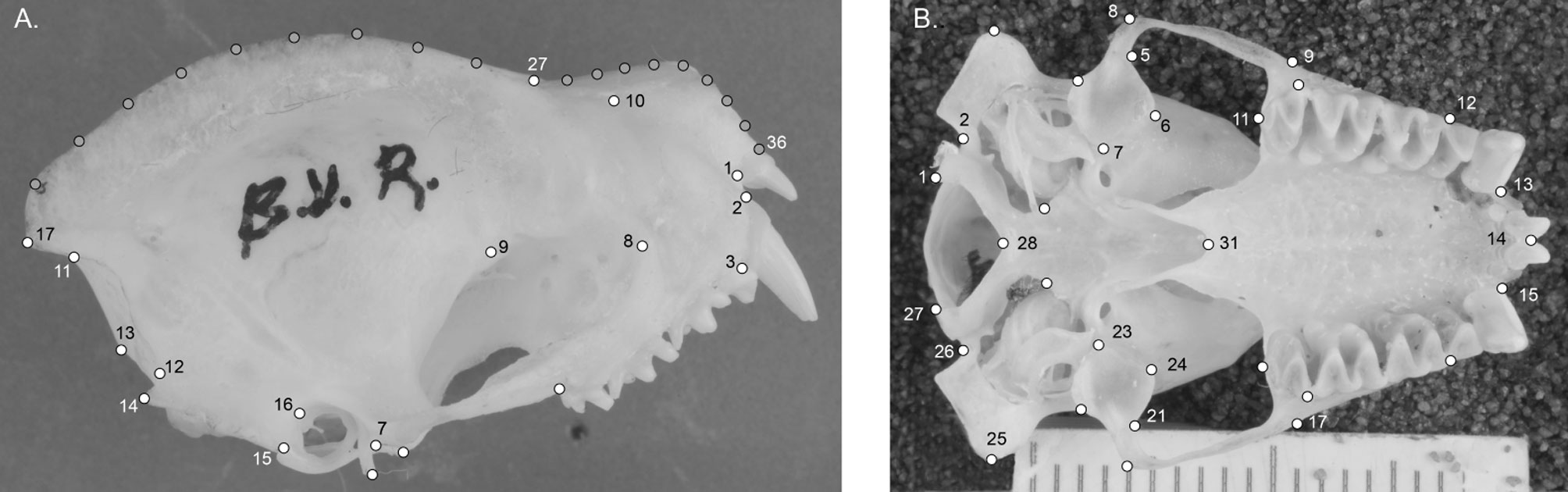
We obtained photographs of the lateral and ventral views of N. leporinus skulls from Mexican specimens (Appendix 1) deposited in the mammal collections of the Museo de Zoología Alfonso L. Herrera, Facultad de Ciencias, Universidad Nacional Autónoma de México (MZFC-UNAM), the Instituto Nacional de Antropología e Historia (INAH), the National Collection of Mammals (CNM-UNAM), Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional sede Durango of the Instituto Politécnico Nacional (CIIDIR Durango-IPN), Colecciones Biológicas del Campus de Ciencias Biológicas y Agropecuarias de la Universidad Autónoma de Yucatán (CZOO-CCBA-UADY), and the University of Kansas (UK). We included only adult specimens, defined by the criteria of fused sutures in the basisphenoid cranial region and the complete ossification of the phalanges in the wing (Lim, 1997). We took the images with a Nikon D3100 camera, with an adapted macro lens (Nikkor 60mm 2.8 F) and saved in TIF format with 300 dpi quality. We obtained a total of 80 photographs for the ventral view and 76 for the lateral view of the skull (Table 1).
Table 1
Sample sizes for each sex and geographic group compared.
|
|
Lateral view of skull |
Ventral view of skull |
||
|
Region |
Males |
Females |
Males |
Females |
|
Western |
29 |
20 |
33 |
21 |
|
Eastern |
15 |
11 |
15 |
11 |
From the photographs obtained, we designed a 2D configuration of landmarks and semilandmarks to describe the lateral view of the skull and one configuration of landmarks was designed to describe the ventral view of the skull. We defined landmarks based on anatomical characters visible in all of the specimens, which represent homology hypotheses, and the semilandmarks were located using the equidistant division of the curves drawn on the contours of the structures, configurations of landmarks and semilandmarks were digitized in the program TpsDig 2.3 (Rohlf, 2017). The lateral view of the skull was represented with 10 landmarks; 11 semilandmarks were used to describe the contour of the sagittal crest and 10 to describe the contour of the rostrum (Fig. 1A). The ventral view of the skull was represented with a configuration of 31 semilandmarks covering both sides of the skull (Fig. 1B), and later the 2 sides of the skull were averaged to analyze the symmetrical variation of the structure. Only the average of the 2 sides was included in statistical models. We described the anatomical definition of landmarks in Table 2.
We performed a superimposition of configurations to eliminate the effect of scale, position and rotation of all configurations. Before this, we obtained the size descriptor “Centroid Size” (CS) for each configuration (Zelditch et al., 2012). We performed a Procrustes superimposition of landmarks, and we aligned semilandmarks using the Bending energy matrix minimization criterion, which optimized the deformation energy from each curve configuration with respect to a consensus curve (Gunz & Mitteroecker, 2013). From these procedures, we obtained aligned configurations that were used in exploratory analyses and statistical comparisons in the package geomorph 3.1.1 (Adams et al., 2019).
We explored the position within the morphospace of each configuration of specimens from different sexes and geographic groups compared, the first group, the western population, comprises localities from the Pacific coast and the second group, the eastern population, includes specimens from the Gulf of Mexico and Yucatán Peninsula (Supplementary data 1), using a Principal Component Analysis (PCA) (Zelditch et al., 2012). The trends of cranial shape variation were described from deformation grids derived from the first 2 principal components of each configuration. In the ventral view of the skull, one side was reflected to construct deformation grids. We explored the patterns of size variation using boxplots of each character, comparing between sexes and between geographic groups. In view of the skull size dimorphism, previously mentioned for N. leporinus, we explored the relationship between the greater length of the skull (GLS, from the posterior-most margin to the anterior-most margin of the base of the skull), as a body size descriptor, and the braincase height (BCH, from the base of the glenoid fossa to the highest sagittal crest elevation), between sexes (Yom-Tov & Geffen, 2006).
Table 2
Anatomical definition of landmark and semilandmarks in both cranial configurations.
|
Character |
Landmarks |
Anatomical definition |
|
Lateral view of the skull |
1 |
Posterior alveolar border of the I2 |
|
|
2 |
Anterior alveolar border of the canine |
|
|
3 |
Posterior alveolar border of the canine |
|
|
4 |
Posterior alveolar border of the M3 |
|
|
5, 7 |
Vertices of the glenoid fossa |
|
|
6 |
Deepest point of the glenoid fossa in lateral view |
|
|
8 |
Anteriormost point of the ocular fossa |
|
|
9 |
Medial process of the orbitosphenoid |
|
|
10 |
Inner juntion between the premaxilla and maxilla |
|
|
11 |
Posteriormost point of the sagital crest |
|
|
12, 14 |
Posteriormost edge of the mastoid process |
|
|
13 |
Posteriormost vertice of the foramen magnum view |
|
|
15, 16 |
Anterior edge of the mastoid process |
|
|
17:27 |
Landmarks and semilandmarks describing the external edge of the sagittal crest |
|
|
22:38 |
Landmarks and semilandmarks describing the external edge of the nasal bone |
|
Ventral view of the skull |
1, 27 |
Lateral vertices of the foramen magnum |
|
|
2, 26 |
Internalmost point of the mastoid exposure of the petrosal |
|
|
3, 25 |
Externalmost point of the mastoid exposure of the petrosal |
|
|
4:7, 21:24 |
Vertices of the glenoid fossa |
|
|
8, 20 |
Maximum zigomatic width |
|
|
9, 17 |
Externalmost point of the zygomatic process of the maxilla |
|
|
10, 18 |
Width across the third upper molars |
|
|
11, 19 |
Posteriormost point of the third upper molars |
|
|
12, 16 |
Union between the first premolar upper molar and premolar |
|
|
13, 15 |
Internalmost point of the base of the canine |
|
|
14 |
Union between the upper internal insicives |
Considering the traditional morphometric variation previously reported among N. leporinus populations (Davis, 1973), in the maxillary tooth row (MTR, alveolar length of tooth row on the right side of maxilla) and the width across the third upper molars (M3-M3, distance between the external border of molars), we measured both distances in a subsample of 10 specimens of each sex and populations. Both distances were obtained from scaled images, using the program TpsDig 2.3 (Rohlf, 2017), and were compared between sexes and populations using ordinary linear models. All analyses were done in R 3.3.4 (R Core Team, 2017).
To evaluate sexual dimorphism in cranial shape characters we used a Procrustes ANOVA model to test the effects of CS, sex and the interaction between CS and sex. We used a permutation procedure with 1,000 replicates on the residuals of this model to assign the significance of the variable and factor included, using the package RRPP 1.02 (Collyer & Adams, 2018). We compared the allometric vectors from sexes using the regression score of the first principal component for shape on the observed CS and described patterns of shape change with increasing size. We evaluated differences in CS between sexes and geographic groups using an ordinary linear model and performed the permutations of the residuals to assign the significance of the estimated coefficients to each group, using the package RRPP (Collyer & Adams, 2018).
To evaluate differences in shape between sexes and geographic groups we performed a Canonical Variable Analysis (CVA) for each configuration. We used the first 5 principal components from a general shape PCA to minimize the problem of smaller sample size in some groups. We performed a cross-validation to obtain posterior probabilities for each group of comparison (sexes and geographic group) in the package Mass (Ripley et al., 2019). Finally, we tested for significant differences in shape among groups using Mahalanobis distances and performing a permutation resample test with 100 replicates to obtain the significance of distances in the package Morpho (Schlager, 2019).
Additionally, to explore similarities between individuals belonging to the localities of Chiapas, Oaxaca and Veracruz, which are very close regions between the 2 study populations, we performed a cluster analysis with individual shape characters, labeling localities and populations. We used the paired Procrustes distances between configurations and the Unweighted Pair Group Method using Arithmetic averages (UPGMA), as the aggrupation criterion.
Results
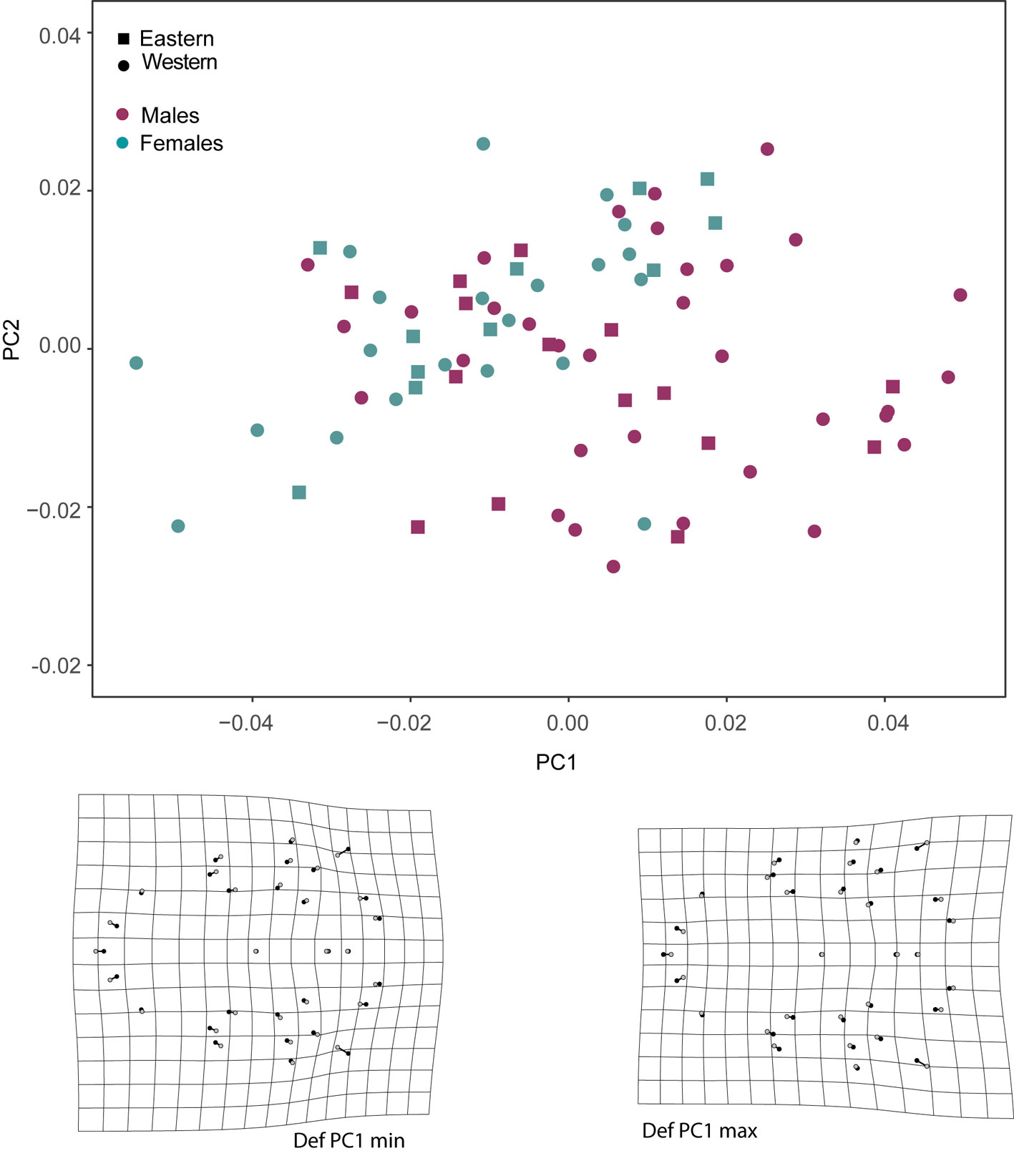
There was more SShD and geographic variance in the lateral view of the skulls than in the ventral view. The morphospace of the lateral view of the skull indicated that the first principal component (PC1) explained the most shape variance between the sexes, showing mixed individuals from different populations. Deformation grids obtained from PC1 indicated higher sagittal crests (landmarks 17-27) and shorter rostrum (landmarks 28-36) in males (positive direction on the PC1 axis), and lower sagittal crest in females, with a posteriorly displaced occipital region (landmarks 12-16). This region of the morphospace is also occupied by some males (negative direction on the PC1 axis; Fig. 2). The second PC described the geographic variance in the shape of the lateral view of skull; deformation grids derived from the second PC indicated a downward lengthening of the base of the skull (landmarks 5-6, 14-16), inducing a higher braincase in the eastern group (more positive PC2 values) and lower braincase in the western group (more negative PC2 values).
In the case of the ventral view of the skull, shape variance was observed between sexes in PC1. Deformation grids derived from the PC1 indicated major changes at the mastoid process and shorter and broader palatines in males (positive PC1 values), compared to females and some smaller males (negative PC1 values; Fig. 3). We observed little deformation on the grids derived from the PC2 axis (grids not presented).
SShD was found in the lateral view of the skull. The Procrustes ANOVA model showed significant effects on shape variation of CS, sex and their interaction. CS explained the highest percent of variance (21%) followed by sex (5%), and their interaction also explained a significant but lower percent of shape variance. In this sense, the allometric vectors for each sex indicated differences in the magnitude of the shape change explained by the CS increase (heterogeneity of slopes). There was a stronger change in shape with increasing CS in females than in males, demonstrated by the significance of the interaction of CS and sex (Fig.4A, Table 3).
In the case of the ventral view of the skull, there was a significant effect of CS on shape (Table 3). Sex differences were not significant on this view of the skull and the homogeneity of slopes was supported by a non-significant interaction (Fig. 4B).
SSD was found in both shape characters. A significant effect of sex was observed from the ordinary least squares model for the lateral (F1,74 = 31.40, p = 0.001, r2 = 0.31), and ventral views of the skull (F1,79 = 53.12, p = 0.001 r2 = 0.41). In general, females had smaller skull CS than males, and a bimodal distribution was apparent in the violin plot for the eastern populations (Fig. 5). Moreover, a lower correlation was observed between the body size estimator (GLS) and the BCH for males (R2 = 0.27, F1,42 =15.29, p < 0.001), than for females (R2 = 0.70, F1,29 = 68.48, p < 0.001).
The traditional morphometric variation between N. leporinus populations indicated shorter distances in the eastern group (MTR: males 10.657 mm +/- 0.186, females 10.29 +/- 0.232 and M3-M3: males 13.631 mm +/- 0.284, females 13.121 mm +/- 0.405), and larger distances in the western group (MTR: males 10.667 mm +/- 0.27, females 10.433 mm +/- 0.295 and M3-M3: males 13.663 +/- 0.426, females 13.379 mm +/- 0.268). However, no significant differences in both distances were found between populations (F1,39 < 1.66, p > 0.20) or the interaction between populations and sexes (F1,39 < 1.07, p > 0.31).
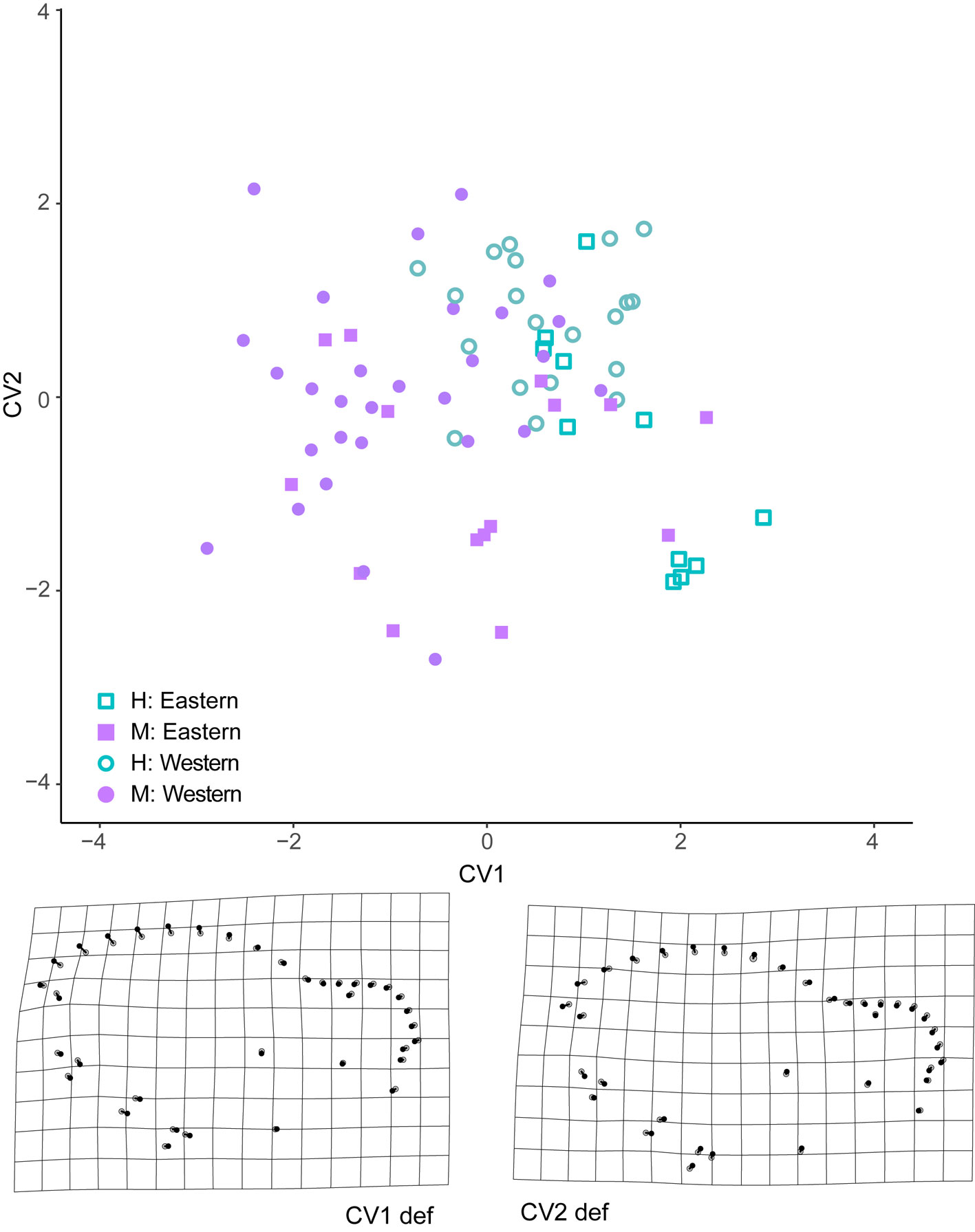
The results of the CVA analysis showed significant differences among the compared groups in both the ventral and lateral views of the skull. In the lateral view, we found the lowest percent of correct assignments (56%), but sexes and geographic groups were significantly discriminated (Fig.6). The greatest Mahalanobis distance between sexes was found in the lateral view of the skull in specimens from the eastern group (D2 = 1.842, p = 0.001). A lower but still significant distance was found between sexes in specimens from the western group (D2 = 1.735, p = 0.001). Distances between geographic groups (within each sex) were also significant (D2 >1.782 and p < 0.05). The deformation grids derived from CV1 recovers a trend of higher sagittal crests in males. The deformation grid derived from CV2 indicated lower braincase (landmarks 17-22) and slightly higher rostrum (landmarks 27-36) in specimens from the Pacific coast.
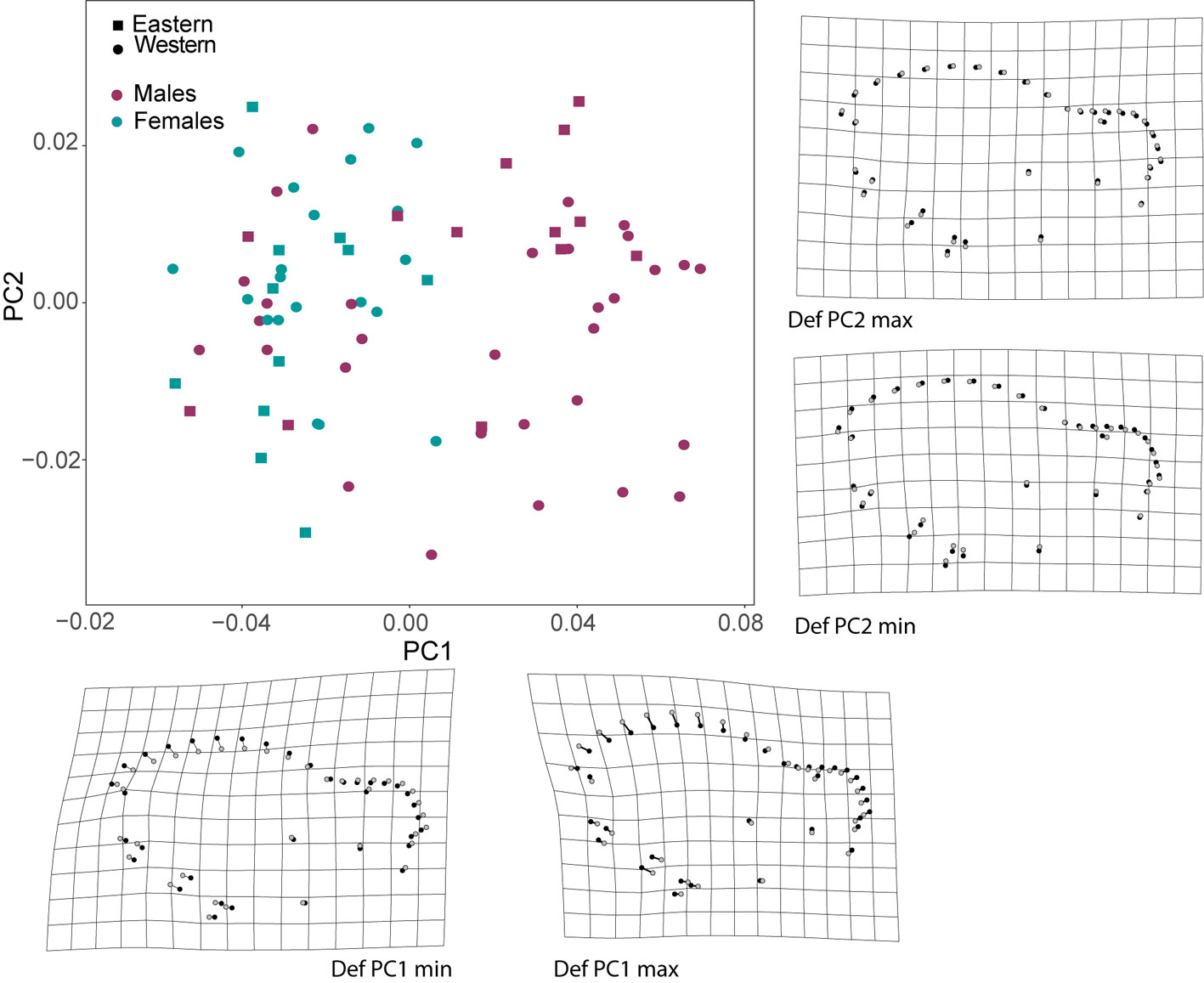
In the ventral view of the skull, only geographic differences were tested, and significant differences between regions were recovered (D2 = 1.690, p = 0.001). This shape configuration showed a higher percent of correct reassignment (63%) than in the lateral view. The deformation grid derived from the only CV obtained showed landmark displacements in the palatine (landmark 31, 14), the maxillary tooth row (landmarks 9-11 on the left side and 17-19 on the right side) and the position of glenoid fossa (landmarks 4-7 on the left side and 21-24 on the right side), indicating larger palatine and anteriorly displaced tooth row and glenoid fossa (positive direction of this axis) in the Pacific coast population (Supplementary data 2). In contrast, no significant differences in CS were found between geographic groups in the lateral view (F1,74 = 0.079, p = 0.776) or ventral view of the skull (F1,79 = 0.888, p = 0.335).
The dendrogram representation of the cluster results for the lateral view of the skull indicates that there is no pattern of similarities between individuals from Chiapas, Oaxaca and Veracruz (Supplementary data 3), we found similar results in ventral view of the skull. In general, the similarities are greater between individuals of the same population, but there is not a biased aggrupation of individuals from particular localities.
Discussion
Considering that morphological characters are the basis for taxonomic descriptions and represent valuable characters for reconstructing the history of mammals (Springer et al., 2007), we evaluated morphological differences, sexual dimorphism in cranial shape and size between populations of the fishing bat N. leporinus mastivus. We quantify sexual dimorphism based on cranial shape and size and present evidence of the differences between western and eastern Mexican populations. The cranial differences and the non-sympatric distribution of N. leporinus mastivus populations in Mexico (Supplementary data 2) suggest that there may be different lineages within this subspecies. However, there is need for further molecular evidence to confirm this assumption.
Additionally, we found that the main cranial shape differences between sexes are the ridges of the skull, which represent functional dietary characters. Remarkably, there were adult males with lower crests, a mixed characteristic with females, in the skull lateral view morphospace. This could indicate that these characters continue to develop even after reaching adulthood, and that they could be secondary sexual characters involved in sexual selection in N. leporinus.
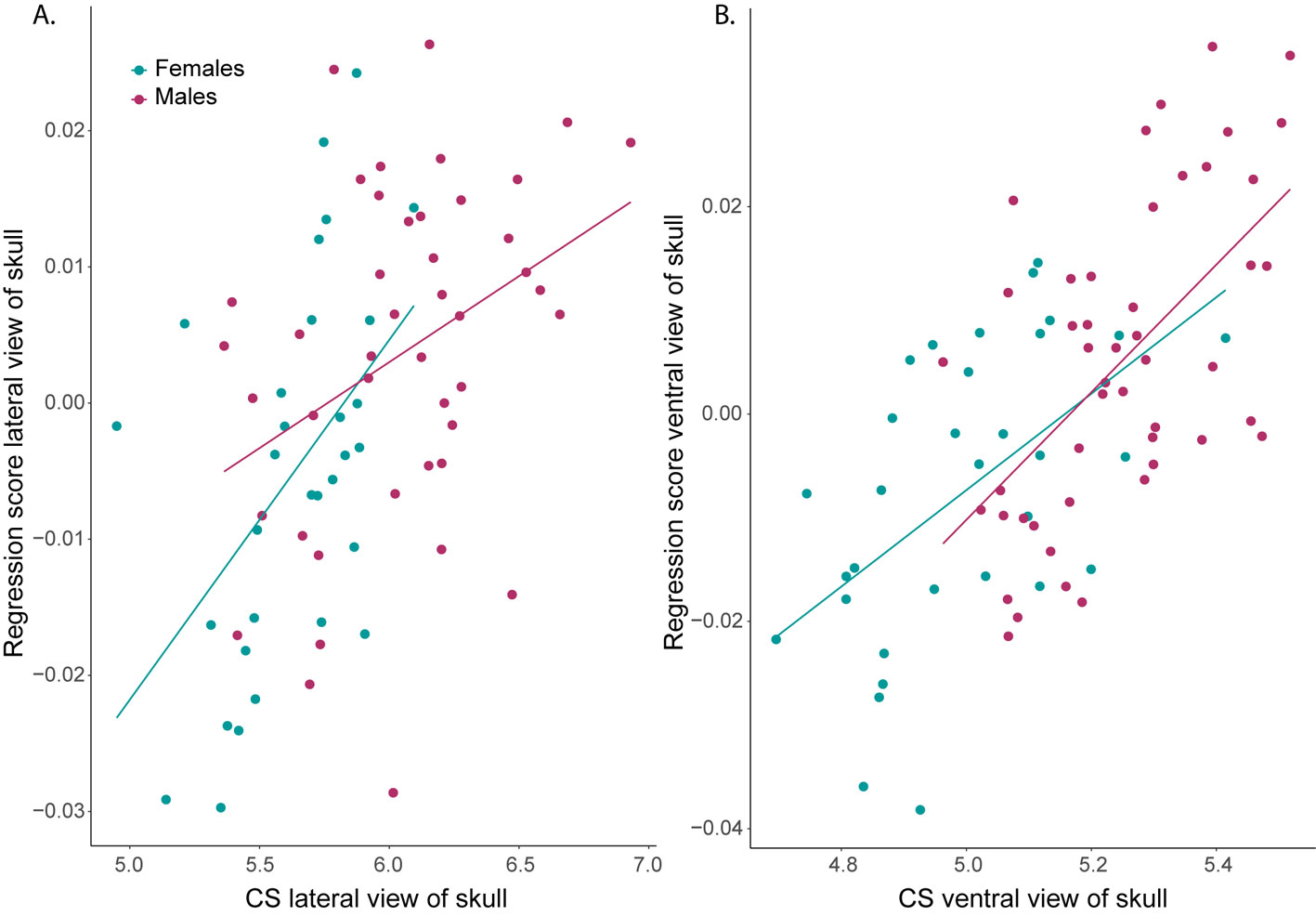
Table 3. Results of the Procrustes ANOVA model testing the shape variance explained by CS+Sex+CS*Sex for each cranial configuration. *Significant term of the model.
|
Lateral View |
Df |
SS |
MS |
Rsq |
F |
Pr (> SS) |
|
CS |
1 |
0.035 |
0.035 |
0.213 |
21.348 |
0.001* |
|
Sex |
1 |
0.008 |
0.008 |
0.050 |
5.043 |
0.002* |
|
CS:Sex |
1 |
0.005 |
0.005 |
0.029 |
2.914 |
0.022* |
|
Residuals |
71 |
0.115 |
0.002 |
0.708 |
||
|
Total |
74 |
0.163 |
||||
|
Ventral view |
Df |
SS |
MS |
Rsq |
F |
Pr (> SS) |
|
CS |
1 |
0.010 |
0.010 |
0.224 |
22.542 |
0.001* |
|
Sex |
1 |
0.000 |
0.000 |
0.010 |
0.958 |
0.395 |
|
CS:Sex |
1 |
0.000 |
0.000 |
0.011 |
1.089 |
0.346 |
|
Residuals |
76 |
0.032 |
0.000 |
0.756 |
||
|
Total |
79 |
0.043 |

The marked SShD found for the lateral view of the skulls and the SSD in the general shape of the skull support the idea of larger and more developed crests in N. leporinus males (Davis, 1973; Hood & Jones, 1984). However, a mixture of females and males was observed in the morphospace of shape characters sharing lower sagittal crests and smaller mastoid processes. These “minor” males, term used in this context to refer to males with lower sagittal crest, could be expressing smaller secondary sexual characters since sexual selection for other characters like body size and testicle size has been demonstrated in some bat populations (McCracken & Wilkinson, 2000; Voigt, 2014). The characters that show the most variation between sexes are the sagittal crest and the petrosal exposition of the mastoid process in the occipital region, which have functional relevance because they are insertion zones for the masticatory muscles (Dumont et al., 2009; Freeman, 1988; Herring & Herring, 1974; Santana & Cheung, 2016). The fact that males have a larger crest than females suggest sexual or functional selective pressures operating within some populations of N. leporinus.
The sexual dimorphism in bats has been discussed in relation to sperm production in males. A broad revision of mating systems in bats concludes that males are not more physically ornamented than females, but males are equipped with relatively large testes, which has been considered an adaptive response to postcopulatory sexual selection (McCracken & Wilkinson, 2000). Sexual selection in bats might favor greater sperm production when females are promiscuous (Wilkinson & McCracken, 2003), and those species with promiscuous females have relatively smaller brains than species with female mate fidelity (Willig & Hollander, 1995). Although N. leporinus presents a polygynous social organization (Brooke, 1997), our results could suggest smaller heads of females in this mating system, or functional differences in cranial size between sexes. However, more evidence about differences in the sexual behavior of N. leporinus should be collected.
Even though, the evidence about sex differences in foraging patterns in this species is scarce, previous works suggest sex differences in feeding and foraging habits (Bordignon, 2006; Brooke, 1997). In Noctilio species, female groups remain together for a long time and males reside with females groups for a shorter period of time, usually or 2 years (Brooke, 1997). The long-term stability of female groups, which forage together in the same area while males forage solitarily in a larger and different area (Brooke, 1997), could be the key to understanding differences in cranial morphology, with functional implications for diet, and the natural selective pressures on sexes. Since a positive relationship has been found between cranial size and shape (related to masticatory muscles) and the hardness of prey in bats (Dumont & Herrel, 2003; Herrel et al., 2008; Santana et al., 2010), greater cranial crests and size in males could suggest differences in dietary preferences between sexes, and also explain that males forage in larger areas, where they could find larger and more variable prey (Brooke, 1997).
The observed stronger shape changes of the skull lateral view with increasing CS in females than in males could be explained by a smaller range of CS variation in females. This broad range of cranial CS in males, with some gaps in CS distribution, reveals differences in male size. In accordance, results from Brooke (1997) indicate that bachelor males (young males), which roost singly or in small groups, differ from adult males roosting with female groups in the amount of tooth wear, body and testes sizes; and these larger males may have more developed crests. These findings are supported by the positive relationship between linear distances describing the size of the skull (GLS) and the size of the sagittal crest (BCH). In adult males, the size of the skull explains a lower proportion of the height of the sagittal crest, suggesting that the development of this character in males could be related to other factors besides the body size.
Variation in male size and cranial development could also be explained by the presence of developmental stages from subadult to adult specimens, which both show fused cranial sutures and are separated by size variables (Monrroy et al., 2020; Reyes-Amaya et al., 2017). In the case of N. leporinus, it has been suggested that drastic changes in cranial traits like the development of the sagittal crest with an increase in the temporalis muscle size, accompany size differences, and are related to the acquisition of morphological specializations when the fishing bats begin to feed independently (Monrroy et al., 2020). Although all the males satisfied the criterion of fused sutures in the basisphenoid, considering the measurements of braincase height (BCH) and greatest length of the skull (GLS) proposed by Monrroy et al. (2020), most of the specimens are in the final adult developmental stages (BCH > 13.4 mm and GLS > 24.8mm), and few specimens (2 males) are in the last subadult developmental stage (BCH: 13.09-13.72 mm and GLS: 23.5-24.06 mm).These results indicate that the development of cranial crests could continue during the adult phase, as noted for other bats (Simmons com. pers., 2020), or this character could represent a secondary character involved in sexual selection by females of N. leporinus, with consequences for the feeding performance between sexes, and between bachelor and “major” males.
Both cranial characters present significant differences between the Pacific coast (west) and the Gulf Yucatán (east) populations. Specimens from the western group had a lower braincase, larger palatine and anteriorly displaced maxillar-glenoid fossa region compared to the eastern group specimens. These changes could have functional consequences in dietary performance, since they involve the masticatory muscle attachment regions, the position of the mandible in relation to the maxillary tooth row and the bite points (Dumont & Herrel, 2003; Freeman, 1988). These morphological differences between populations could be the consequence of genetic isolations and accentuated changes in environmental conditions (Arteaga et al., 2011; Hernández-Canchola & León-Paniagua, 2017), because of vicariant events in Central America (Gutiérrez-García & Vázquez-Domínguez, 2013). Given that habitats on the Pacific coast are dominated by deciduous dry forest (Maass et al., 2005), in contrast to tropical rainforest dominating the Gulf of Mexico and Yucatán regions (Engle, 2011), morphological differences could be maintained by differences in this environmental conditions, or even by differences in the dietary resources available within these habitats. However, a phylogeographic analysis of genetic divergence is necessary to confirm these assumptions.
In relation to the traditional morphometric variation previously analyzed among N. leporinus populations (Davis, 1973), the maxillary tooth row (MTR) and the width across the third upper molars (M3-M3) could be affected by the morphological changes in the rostrum (larger palatine), recovered by the geometric morphometric analyses. We observed shorter distances in specimens from the eastern group than specimens of the western group, and this trend of cranial size variation is congruent with the results found by Davis (1973) in N. leporinus. However, no significant differences in distances were observed by Davis (1973) or here, and this pattern was also recovered for the cranial size descriptor (CS).Similar results were observed comparing cranial size between the Mexican gulf and the Pacific coast subspecies of the bat species Artibeus jamaicensis, but differences in size between subspecies from humid and dry habitats were found at a larger geographic scale, which suggest that cranial size differences could not be detected at this geographic scale, but shape differences were found (Davis 1970).
The effect of wet versus dry habitat in relation to the intraspecific variation of body size has been discussed in widely distributed species, body size is also modulated by seasonality and has been negatively related to temperature and positively related to humidity (Marchán-Rivadeneira et al., 2010; Storz et al., 2008). Although the Mexican populations of the subspecies N. leporinus mastivus have similar cranial size, the cranial shape differences revealed an opposite pattern, with specimens having a larger rostrum in the western populations (drier habitat). These results are supported by a similar trend in cranial size variation in the humid Amazon basin, where the smallest subspecies (N. leporinus leporinus) is distributed (Davis, 1973). Our results revealed interesting patterns of morphological variation between populations in a species reported in Mexico at a maximum elevation of 500 m (Sabaté & Paniagua, 2004), but more studies are necessary to understand size and shape variation at a broader geographic scale, which has been poorly documented for lowland bat species with discontinuous distributions.
We present differences in the cranial shape between western and eastern populations of N. leporinus mastivus in Mexico. These morphological differences, not documented until now, have been found thanks to the sensitivity of geometric morphometrics to assess morphological differences between populations. Additionally, we present morphological evidence of sexual dimorphism for this subspecies and a gradient of development of the sagittal crest in males. This may be due to sexual selection, making the crest a secondary sexual character, natural selection due to the dietary relevance trait, or both. Our results show interesting patterns of cranial size and shape variation in the fishing bat N. leporinus that need to be analyzed through new molecular evidence and at a broader geographic scale.
Acknowledgments
We thank the curatorial staff of the mammal collections where samples were obtained: Julieta Vargas (CNM, UNAM), Maria Eifler (the University of Kansas), Yire Gómez (Facultad de Ciencias, UNAM), Colección Nacional de Mamíferos (UNAM), Celia López-Gonzáles (CIIDIR Durango), Eréndira Estrella Martínez (UADY), Joaquin Arroyo-Cabrales (INAH), and Cristina Mac Swiney, for facilitating the loan of specimens. S. M. Ospina-Garcés is supported by the postdoctoral fellowship program from the Dirección General de Asuntos del Personal Académico (DGAPA, Universidad Nacional Autónoma de México).
Appendix 1. List of collection codes and catalog number of the 80 specimens examined.
Facultad de Ciencias, UNAM: MZFC–M 101, 103, 6291, 933, 934, 10433, 10435, 10676, 13823, 15586.
National Collection of Mammals, UNAM: CNM 5921, 6209, 6210, 6211, 6212, 6213, 6214, 6215, 6216, 6217, 6218, 6219, 6221, 6222, 6490, 6931, 6998, 7399, 7817, 7818, 7819, 8186, 17133, 17135, 17410, 17412, 17413, 17414, 17415, 18118, 19116, 19117, 19119, 23701, 23766, 27886, 28413, 28704, 31758, 34400, 34401, 34402, 34403, 44184, 48760, 48761, 48762.
UADY: YUCC–CC–250–11/HER12, 13, 107, 108, 1174, 1175, 1177, 1178.
INAH: DP– INAH 1583, 5188, 8115.
University of Kansas: KUM 41490, 41491, 41492, 90585, 90586, 90589, 91485, 91488.
CIIDIR–Durango: CRD 6174, 7133, 7134, 7135.
References
Adams, D. C., Collyer, M. L., & Kaliontzopoulou, A. (2019). Geomorph: Software for geometric morphometric analyses. R package version 3.1.0. https://cran.r–project.org/package=geomorph
Altenbach, J. S. (1989). Prey capture by the fishing bats Noctilio leporinus and Myotis vivesi. Journal of Mammalogy, 70, 421–424. https://doi.org/10.2307/1381532
Arteaga, M. C., McCormack, J. E., Eguiarte, L. E., & Medellín, R. A. (2011). Genetic admixture in multidimensional environmental space: asymmetrical niche similarity promotes gene flow in armadillos (Dasypus novemcinctus): niche similarity promotes genetic admixture. Evolution, 65, 2470–2480. https://doi.org/10.1111/j.1558–5646.2011.01329.x
Bordignon, M. O. (2006). Diet of the fishing bat Noctilio leporinus (Linnaeus)(Mammalia, Chiroptera) in a mangrove area of southern Brazil. Revista Brasileira de Zoologia, 23, 256-260. http://dx.doi.org/10.1590/S0101-81752006000100019
Brooke, A. P. (1997). Social organization and foraging behaviour of the fishing bat, Noctilio leporinus (Chiroptera:Noctilionidae). Ethology, 103, 421–436. https://doi.org/10.1111/j.1439–0310.1997.tb00157.x
Collyer, M. L., & Adams, D. C. (2018). RRPP: An r package for fitting linear models to high–dimensional data using residual randomization. Methods in Ecology and Evolution, 9, 1772–1779. https://doi.org/10.1111/2041–210X.13029
Davis, W. B. (1970). The large fruit bats (genus Artibeus) of Middle America, with a review of the Artibeus jamaicensis complex. Journal of Mammalogy, 51, 105–122.
Davis, W. B. (1973). Geographic variation in the fishing bat, Noctilio leporinus. Journal of Mammalogy, 54, 862–874. https://doi.org/10.2307/1379081
Dumont, E. R., Dávalos, L. M., Goldberg, A., Santana, S. E., Rex, K., & Voigt, C. C. (2011). Morphological innovation, diversification and invasion of a new adaptive zone. Proceedings of the Royal Society of London B: Biological Sciences, 279, 1797–1805. https://doi.org/10.1098/rspb.2011.2005
Dumont, E. R., & Herrel, A. (2003). The effects of gape angle and bite point on bite force in bats. Journal of Experimental Biology, 206, 2117–2123. https://doi.org/10.1242/jeb.00375
Dumont, E. R., Herrel, A., Medellín, R. A., Vargas-Contreras, J. A., & Santana, S. E. (2009). Built to bite: cranial design and function in the wrinkle–faced bat. Journal of Zoology, 279, 329–337. https://doi.org/10.1111/j.1469–7998.2009.00618.x
Engle, V. D. (2011). Estimating the provision of ecosystem services by Gulf of Mexico Coastal Wetlands. Wetlands, 31, 179–193. https://doi.org/10.1007/s13157–010–0132–9
Freeman, P. W. (1988). Frugivorous and animalivorous bats (Microchiroptera): dental and cranial adaptations. Biological Journal of the Linnean Society, 33, 249–272. https://doi.org/10.1111/j.1095–8312.1988.tb00811.x
Goldman, E. A. (1915). Five new mammals from Mexico and Arizona. Proceedings of the Biological Society of Washington, 28, 133–137.
Gunz, P., & Mitteroecker, P. (2013). Semilandmarks: a method for quantifying curves and surfaces. Hystrix, the Italian Journal of Mammalogy, 24, 103–109. http://doi:10.4404/hystrix-24.1-6292
Gutiérrez-García, T. A., & Vázquez-Domínguez, E. (2013). Consensus between genes and stones in the biogeographic and evolutionary history of Central America. Quaternary Research (United States), 79, 311–324. http://dx.doi.org/10.1016/j.yqres.2012.12.007
Hall, E., & Kelson, K. R. (1959). The mammals of North America. New York: Ronald Press.
Hernández-Canchola, G., & León-Paniagua, L. (2017). Genetic and ecological processes promoting early diversification in the lowland Mesoamerican bat Sturnira parvidens (Chiroptera: Phyllostomidae). Molecular Phylogenetics and Evolution, 114, 334–345. https://doi.org/10.1016/j.ympev.2017.06.015
Herrel, A., De Smet, A., Aguirre, L. F., & Aerts, P. (2008). Morphological and mechanical determinants of bite force in bats: Do muscles matter? Journal of Experimental Biology, 211, 86–91. https://doi:10.1242/jeb.012211
Herring, S. W., & Herring, S. E. (1974). The superficial masseter and gape in mammals. The American Naturalist, 108, 561–576. https://doi.org/10.1086/282934
Hood, C. S., & Jones, J. K. (1984). Noctilio leporinus. Mammalian Species, 216, 1–7. https://doi.org/10.2307/3503809
Kalko, E. K. V., Schnitzler, H. U., Kaipf, I., & Grinnell, A. D. (1998). Echolocation and foraging behavior of the lesser bulldog bat Noctilio albiventris: preadaptations for piscivory? Behavioral Ecology and Sociobiology, 42, 305–319. https://doi.org/10.1007/s002650050443
Lewis-Oritt, N., Van Den Bussche, R. A., & Baker, R. J. (2001). Molecular evidence for evolution of piscivory in Noctilio (Chiroptera: Noctilionidae). Journal of Mammalogy, 82, 748–759. https://doi.org/10.1644/15451542(2001)082<0748:MEFEOP>2.0.CO;2
Lim, B. K. (1997). Morphometric differentiation and species status of the Allopatric fruit-eating bats Artibeus jamaicensis and A. planirostris in Venezuela. Studies on Neotropical Fauna and Environment, 32, 65–71. https://doi.org/10.1080/01650521.1997.9709606
López-Wilchis, R., Guevara-Chumacero, L. M., Pérez, N. Á., Juste, J., Ibáñez, C., & Sosa, I. B. D. (2012). Taxonomic status assessment of the Mexican populations of funnel–eared bats, genus Natalus (Chiroptera: Natalidae). Acta Chiropterologica, 14, 305–316. https://doi.org/info:doi/10.3161/150811012X661639
Maass, J. M., Balvanera, P., Castillo, A., Daily, G. C., Mooney, H. A., Ehrlich, P. et al. (2005). Ecosystem services of tropical dry forests. Ecology and Society, 10, 17. https://www.jstor.org/stable/10.2307/26267750
Marchán-Rivadeneira, M. R., Phillips, C. J., Strauss, R. E., Antonio-Guerrero, J., Mancina, C. A., & Baker, R. J. (2010). Cranial differentiation of fruit-eating bats (genus Artibeus) based on size-standardized data. Acta Chiropterologica, 12, 143–154. https://doi.org/10.3161/150811010X504644
McCracken, G. F., & Wilkinson, G. S. (2000). Eight-bat mating systems. In E. G. Crichton, & P. H. Krutzsch (Eds.), Reproductive biology of bats (pp. 321–362). San Diego: Academic Press. https://doi.org/10.1016/B978–012195670–7/50009–6
Monrroy, G. A., Reyes-Amaya, N., & Jerez, A. (2020). Postnatal cranial ontogeny of the greater bulldog bat Noctilio leporinus (Chiroptera: Noctilionidae). Acta Zoológica, 101, 412–430. https://doi.org/10.1111/azo.12309
Mulcahy, D. G., Morrill, B. H., & Mendelson III, J. R. (2006). Historical biogeography of lowland species of toads (Bufo) across the Trans-Mexican Neovolcanic Belt and the Isthmus of Tehuantepec. Journal of Biogeography, 33, 1889–1904. https://doi.org/10.1111/j.1365–2699.2006.01546.x
Nogueira, M. R., Peracchi, A. L., & Monteiro, L. R. (2009). Morphological correlates of bite force and diet in the skull and mandible of phyllostomid bats. Functional Ecology, 23, 715–723. https://doi.org/10.1111/j.1365–2435.2009.01549.x
Ospina-Garcés, S. M., & De Luna, E. (2017). Phylogenetic analysis of landmark data and the morphological evolution of cranial shape and diets in species of Myotis (Chiroptera: Vespertilionidae). Zoomorphology, 136, 251–265. https://doi.org/10.1007/s00435–017–0345–z
Ospina-Garcés, S. M., De Luna, E., Herrera, M., L. G., & Flores-Martínez, J. J. (2016). Cranial shape and diet variation in Myotis species (Chiroptera: Vespertilionidae): testing the relationship between form and function. Acta Chiropterologica, 18, 163–180. https://doi.org/10.3161/15081109ACC2016.18.1.007
Pavan, A. C., Martins, F. M., & Morgante, J. S. (2013). Evolutionary history of bulldog bats (genus Noctilio): recent diversification and the role of the Caribbean in Neotropical biogeography. Biological Journal of the Linnean Society, 108, 210–224. https://doi.org/10.1111/j.1095–8312.2012.01979.x
R Core Team. (2017). R: a language and environment for statistical computing (Versión 3.3.3) [Computer software]. https://stat.ethz.ch/pipermail/r–announce/2017/000611.html
Reyes-Amaya, N., Jerez, A., & Flores, D. (2017). Morphology and postnatal development of lower hindlimbs in Desmodus rotundus (Chiroptera: Phyllostomidae): a comparative study. The Anatomical Record, 300, 2150–2165. https://doi.org/10.1002/ar.23646
Ripley, B., Venables, B., Hornik, K., Gebhardt, A., & Firth, D. (2019). MASS package | R Documentation (Versión 7.3) [Computer software]. https://www.rdocumentation.org/packages/MASS/versions/7.3–47
Rohlf, F. J. (2017). Tpsdig, version 2.3, tpsUtil version 1.74. Department pf Ecology and Evolution, Stony Brook.
Rojas, D., Warsi, O. M., & Dávalos, L. M. (2016). Bats (Chiroptera: Noctilionoidea) challenge a recent origin of extant neotropical diversity. Systematic Biology, 65, 432–448. https://doi.org/10.1093/sysbio/syw011
Sabaté, D. M., & Paniagua, L. L. (2004). Estudio comparativo de los patrones de riqueza altitudinal de especies en mastofaunas de áreas montañosas mexicanas. Revista Mexicana de Mastozoología (Nueva Época), 6, 60–82.
Santana, S. E., Dumont, E. R., & Davis, J. L. (2010). Mechanics of bite force production and its relationship to diet in bats. Functional Ecology, 24, 776–784.
Santana, S. E., & Cheung, E. (2016). Go big or go fish: morphological specializations in carnivorous bats. Proceedings of the Royal Society B: Biological Sciences, 283, 20160615. https://doi.org/10.1098/rspb.2016.0615
Schlager, S. (2019). Package morpho. Academic Press. https://doi.org/10.1016/B978–0–12–810493–4.00011–0
Springer, M. S., Burk-Herrick, A., Meredith, R., Eizirik, E., Teeling, E., O’Brien, S. J. et al. (2007). The adequacy of morphology for reconstructing the early history of placental mammals. Systematic Biology, 56, 673–684. https://doi.org/10.1080/10635150701491149
Storz, J. F., Balasingh, J., Bhat, H. R., Nathan, P. T., Doss, D. P. S., Prakash, A. A. et al. (2008). Clinal variation in body size and sexual dimorphism in an Indian fruit bat, Cynopterus sphinx (Chiroptera: Pteropodidae). Biological Journal of the Linnean Society, 72, 17–31. https://doi.org/10.1111/j.1095–8312.2001.tb01298.x
Voigt, C. C. (2014). Chapter 16-sexual selection in neotropical bats. In R. H. Macedo, & G. Machado (Eds.), Sexual selection: perspectives and models from the Neotropics (pp. 409–432). Oxford: Academic Press. https://doi.org/10.1016/B978–0–12–416028–6.00016–5
Wilkinson, G. S., & McCracken, G. F. (2003). Bats and balls: sexual selection and sperm competition in the Chiroptera. In T. H. Kunz, & B. Fenton (Eds.), Bat ecology (pp. 128–155). Chicago: University of Chicago Press.
Willig, M. R., & Hollander, R. R. (1995). Secondary sexual dimorphism and phylogenetic constraints in bats: a multivariate approach. Journal of Mammalogy, 76, 981–992. https://doi.org/10.2307/1382592
Yom-Tov, Y., & Geffen, E. (2006). Geographic variation in body size: the effects of ambient temperature and precipitation. Oecologia, 148, 213–218. https://10.1007/s00442-006-0364-9
Zelditch, M. L., Swiderski, D. L., & Sheets, H. D. (2012). Geometric morphometrics for biologists: a primer. Amsterdam: Academic Press.