Valentin Mar-Silva a, b, Yvonne Herrerías-Diego b, c, *, Martina Medina-Nava b, Juan Pablo Ramírez-Herrejón d, Luis Mendoza-Cuenca b, c, Rubén Hernández-Morales b, Omar Domínguez-Domínguez b, c
a Universidad Michoacana de San Nicolás de Hidalgo, Programa Institucional de Doctorado en Ciencias Biológicas, Francisco J. Múgica s/n, 58030 Morelia, Michoacán, Mexico
b Universidad Michoacana de San Nicolás de Hidalgo, Facultad de Biología, Francisco J. Múgica s/n, 58030 Morelia, Michoacán, Mexico
c Universidad Nacional Autónoma de México, Escuela Nacional de Estudios Superiores Unidad Morelia, Laboratorio Nacional de Análisis y Síntesis Ecológica, Antigua Carretera a Pátzcuaro Núm. 8701, Ex Hacienda de San José de la Huerta, 58190 Morelia, Michoacán, Mexico
d Universidad Autónoma de Querétaro, Conacyt-Facultad de Ciencias Naturales, Campus UAQ-Aeropuerto, Carretera a Chichimequillas s/n, Ejido Bolaños, 76140 Santiago de Querétaro, Querétaro, Mexico
*Corresponding author: yonnediego@gmail.com (Y. Herrerías-Diego)
Received: 18 March 2020; accepted: 24 December 2020
Abstract
The introduction of non-native fish into the Teuchitlán River, in west-central Mexico, was suggested as a key factor behind the change in the fish assemblage, and even in the extinction of endemic species. The characterization of the fish assemblage and the relationship between fish abundance and the anthropogenic conditions were evaluated over 2-year period. Fish assemblage structure, species replacement, and dominance were examined along a longitudinal environmental gradient. Physical and chemical parameters of the water were recorded. The relationship between fish abundance and abiotic factors was inferred from multivariate analysis. The results showed that the non-native poecilid Pseudoxiphophorus bimaculatus was dominant, and the abundance and biomass of non-native species exceeded 50% in all sites and seasons. Species diversity and native fish abundance were greater in upstream sites, which presented higher dissolved oxygen and lower nitrates. The native ichthyofauna of the Teuchitlán River has been replaced by non-native species. This decline of the native fish species seems to be due not only to the interaction with non-native species but also to the multiple human impacts on the lotic system.
Keywords: Biodiversity conservation; Biotic homogenization; Human impact; Native ichthyofauna
Variación espacial y temporal de la estructura del ensamblaje de peces en un río neotropical mexicano
Resumen
En el río Teuchitlán, centro oeste de México, se sugirió la introducción de peces no nativos como factor clave en el cambio del ensamblaje de peces e incluso en la extinción de especies endémicas. Durante 2 años se caracterizó el ensamblaje de peces y la relación de la abundancia íctica con las condiciones antropogénicas. Se evaluó la estructura del ensamblaje de peces, el recambio y la dominancia a lo largo del gradiente ambiental longitudinal. Se registraron los parámetros físicos y químicos del agua. La relación de la abundancia de los peces con las características abióticas se infirió con análisis multivariado. El poecílido no-nativo Pseudoxiphophorus bimaculatus fue dominante, la abundancia y biomasa de las especies no-nativas fue mayor a 50% en todos los sitios y temporadas. La diversidad y la abundancia de las especies nativas fue mayor en los sitios de manantial con mayor contenido de oxígeno y menor concentración de nitratos. La ictiofauna nativa del río Teuchitlán ha sido reemplazada por especies no nativas. Sin embargo, la disminución de las especies de peces nativas parece deberse no solo a la interacción con las especies no nativas, sino también al resultado de los múltiples impactos humanos sobre el sistema de lótico.
Palabras clave: Conservación de biodiversidad; Homogenización biótica; Impacto humano; Ictiofauna nativa
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The highest global biodiversity of fish occurs in the Neotropics, which comprises the region from central Mexico to the southern limits of South America, where more than 5,000 species are found (Reis et al., 2003). The unsustainable application of environmental policies in Latin American countries and the consequent negative human impacts on the native biota and habitat imperils the conservation of freshwater fish (Pelicice et al., 2017). Anthropic effects such as species invasions, habitat degradation, deforestation, pollution, climate change, fragmentation, and overfishing are now reaching a planetary scale (Collen et al., 2014; Dudgeon et al., 2006). Human pressure on freshwater ecosystems thus represents the main negative impact on inland aquatic biota (Arthington et al., 2016).
The Teuchitlán River, in the headwaters of the Ameca River basin in central Mexico, is of considerable biological importance due to its 4 endemic freshwater fish species (Domínguez-Domínguez et al., 2006; Miller & Smith, 1986). The establishment of non-native species since, at least, 1977 is considered a key factor in the loss of the fish biodiversity at the Teuchitlán River (De La Vega-Salazar et al., 2003b; Domínguez-Domínguez et al., 2008; Dzul-Caamal et al., 2013; López-López & Paulo-Maya, 2001; Webb & Miller, 1998). These introductions include the aquatic weeds Eichhornia crassipes and Pistia stratiotes (Semadet Jalisco, 2014), aquatic snails of the genera Pomacea and Melanoides and the fish species Cyprinus carpio (Linnaeus, 1758), Lepomis macrochirus (Rafinesque, 1819), Oreochromis aureus (Steindachner, 1864), Poecilia sphenops (Valenciennes, 1846), Xiphophorus hellerii (Heckel, 1848), Xiphophorus maculatus (Günther, 1866), Pseudoxiphophorus bimaculatus (Heckel, 1848) and Chapalichthys encaustus (Jordan & Snyder, 1899) (López-López & Paulo-Maya, 2001; Mar-Silva et al., 2019; Ramírez-García et al., 2017). However, the contribution of non-native species to the fish assemblage is unknown.
The upper portion of the Ameca River basin has a long history of human perturbation due to land-use change for agricultural purposes (De La Mora-Orozco et al., 2013). Moreover, 6 decades of modification of the Teuchitlán River by human activities has had a negative effect on the fauna in the area. This includes the interruption of the natural watercourse due to the construction of the “La Vega” Dam in the 1950s, one of the strongest human impacts on the river (De La Mora-Orozco et al., 2014); alterations of the river banks to prevent flooding and for recreational purposes, the construction of bridges and netting to control aquatic weeds (Herrerías-Diego et al., 2019). Consequently, the riverbed has changed and undergone an increased accumulation of sediment with reduced riverbank interaction with the riparian system, and the river presents disruption of its habitat structure (Herrerías-Diego et al., 2019). Although there is no industrial activity near the river, pollution from the unregulated application of fertilizers and agrochemicals in the surrounding areas affects the aquatic environment via the indirect and direct discharge of contaminants into water bodies (Favari-Perozzi et al., 2003). In addition, unplanned human population growth leads to higher pollution along the length of the river, which is exacerbated by the lack of successful application of environmental policies (Semadet Jalisco, 2014).
As a result of the above, and due to the interaction with non-native fish species and processes of human disturbance, the fish species assemblage at Teuchitlán River has changed over time (Dzul-Caamal et al., 2013; López-López & Paulo-Maya, 2001). In the early 1960s, 12 native fish species and no exotic species were reported, and the river was described as highly polluted and strongly used for human consumption and irrigation (Miller & Fitzsimons, 1971). In 1976, 12 native fish species and 2 non-native species (Cyprinus carpio and Xiphophorus maculatus) were located in the river (Kingston, 1978). A subsequent survey, in 1977, showed that the native fishes were reduced drastically in number, possibly through competition with introduced species, and that the riverbank was strongly modified with a continued presence of heavy pollution (Kingston, 1978). By the early 1990s, the same 12 native species were still found, but the number of exotic species had increased to 6. By 1996, the numbers had declined to 4 native and 3 non-native species (Dzul-Caamal et al., 2013; López-López & Paulo-Maya, 2001).
The Teuchitlán endemic species Notropis amecae (Chernoff, Miller, 1986), Skiffia francesae (Kingston, 1978) and Zoogoneticus tequila (Webb, Miller, 1998) are not currently found in the Teuchitlán River (De La Vega-Salazar et al., 2003a; Domínguez-Domínguez et al., 2008; IUCN, 2017). The native species Chirostoma jordani (Woolman, 1894), Poeciliopsis infans (Woolman, 1894) and Xenotoca melanosoma (Fitzsimons, 1972) have not been found in the headwaters of the basin for the past 20 years, and could, therefore, be locally extinct (López-López & Paulo-Maya, 2001).
This study aimed to characterize the spatial and temporal fish assemblages along a longitudinal environmental gradient and to explore the relationship between the local physicochemical water parameters and changes in the fish assemblages along the Teuchitlán River. Considering the critical status of fish conservation in the river, this research is fundamental for future management plans.
Materials and methods
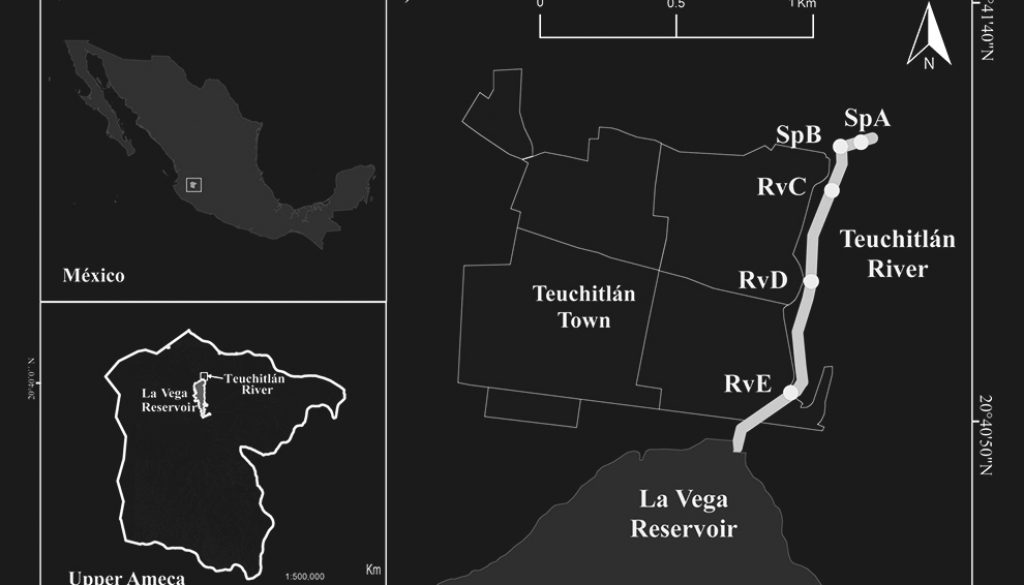
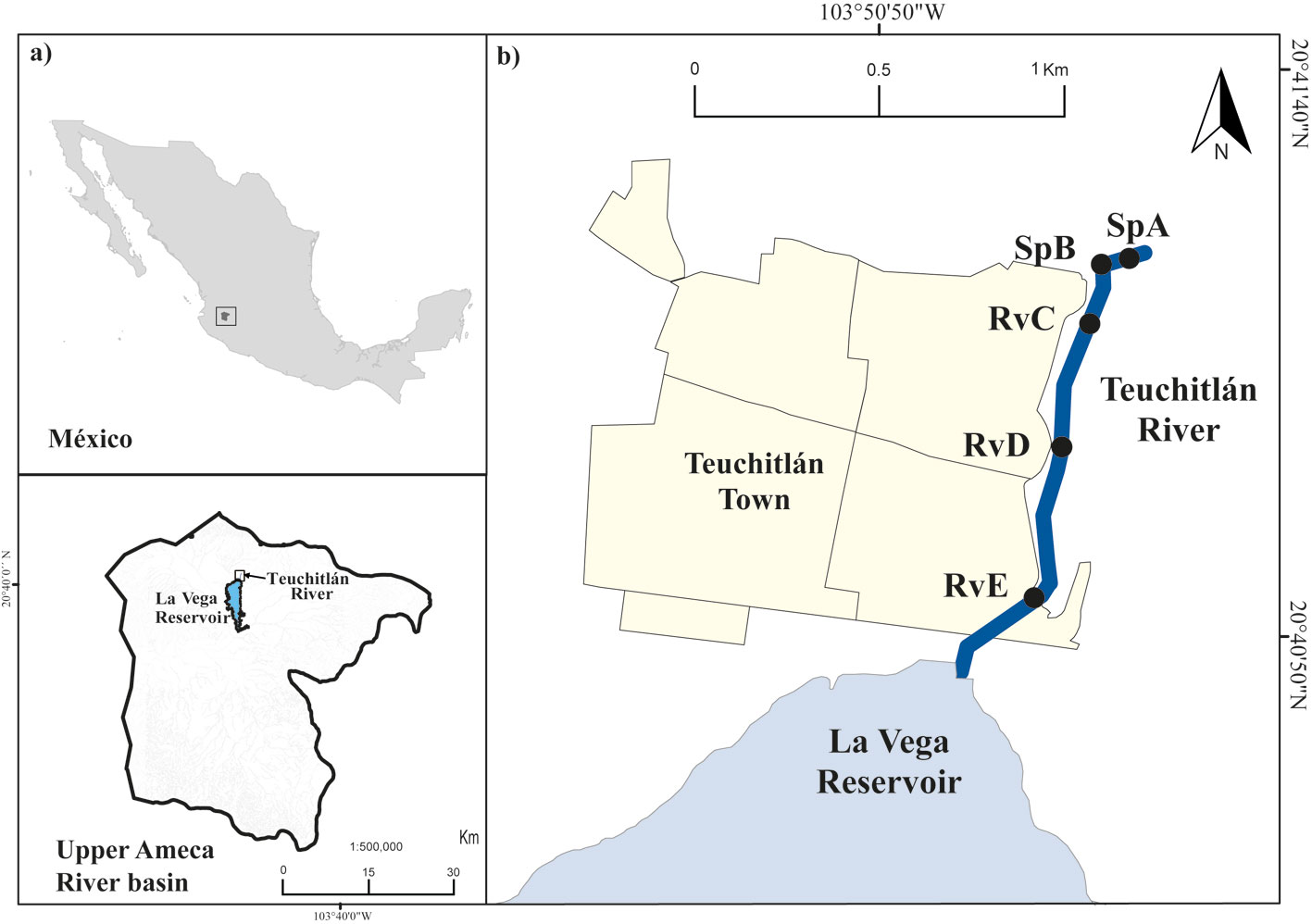
The Teuchitlán River is an exorheic system at the headwaters of the Ameca River basin, in Jalisco State in west-central Mexico (Fig. 1). The Teuchitlán River is a first-order river of 1.5 km in length from its source at the springs of El Rincón to its mouth at La Vega Reservoir with an average width of 15.9 m (López-López et al., 2004). According to the Köppen climate classification, modified by García (1988), the climate in the region is subtropical, classified as semi-warm (A)Ca (the warmest of the wet-temperate climates). The town of Teuchitlán has a human population of ~ 3,500 and is located along one bank of the river (INEGI, 2010).
Visual characterization of the river was performed during a prospective field trip for the identification of geomorphic units. Two main habitats were determined: springs (Sp), located in the upstream area, and river channel (Rv), located at the middle and end of the lotic system, near the mouth of the river at La Vega Reservoir.
Five sampling sites were chosen along the river: 2 of them in the spring habitats (sites SpA and SpB) and 3 in the river channel (sites RvC, RvD and RvE). In the visual characterization, human impacts on the physical river environment were found to be diverse and caused mainly by the partial concreting of the riverbank in the river channel and springs habitats. The springs at the river source are used for swimming and the river downstream of the springs is used for cattle watering. Cover of floating vegetation in the form of the non-native Pistia stratiotes is presented among the first section of the river channel. The stream receives discharges of untreated domestic sewage along its length, although this is more evident downstream (Herrerías-Diego et al., 2019) (Fig. 1). Accordingly, these sites were selected to reflect differences in the river gradient and these different human impacts.
The substrate of the riverbed was characterized in reference to Bunte and Abt (2001), collecting particles in a traverse from bank-to-bank in order to cover the entire site. The first particle reached by hand was measured at its longest dimension with calipers (PRETUL® model 21454, precision 0.01 mm). One hundred particles were measured per site. Physical and chemical parameters of the water were recorded prior to fish sampling and evaluated following the criteria of the American Public Health Association, American Water Works Association, and the Water Environment Federation (Rice et al., 1995). These parameters included temperature (°C), transparency (cm), pH, conductivity (µS/cm), dissolved oxygen (mg L-1), turbidity (NTU), nitrites (mg L-1), nitrates (mg L-1), sulfates (mg L-1), chlorides (mg L-1), and total dissolved solids (mg L-1).
Fish samples were collected bimonthly from January 2015 to November 2016. All samples were taken between 10:00 and 16:00 h. Three seasons were determined according to climatic variations (Jiménez-Román, 1994). The wet season extended from July to October, which presented the highest average precipitation at 260 mm. The dry season was sub-divided into the warm dry season from February to June (max. temp. 25.3 °C) and the cold dry season from November to January (min. temp. 16.7 °C in January) (De La Mora-Orozco et al., 2014; Jiménez-Román, 1994).
Fish were collected using a seine net (4.5 m in length, 2.3 m in height and with a mesh size of 1.35 mm) and by electrofishing (DC-backpack electrofisher model ABP-3, ETS Electrofishing Systems LLC, average power 200 watts, peak voltage ~ 250 V, peak current ~ 10 amps, pulse energy capability of 30 joules, 12 V acid battery, 18 amps).
Through prospective sampling, we determined that both fishing methods provided a representative sample of the fish assemblage, capturing individuals from 9.35 mm to 160.38 mm in standard length; i.e., within the range of the maximum known standard length of the target fish species (Miller et al., 2009). Their combined use is recommended for wadeable tropical streams and rivers (Rabeni et al., 2009). According to the assessment of sampling effort (Herrerías-Diego et al., 2019), seine netting was conducted twice in each sampling episode and was deployed to cover an area of approximately 8.86 m2. Electrofishing (effective area of the pulse ~ 0.78 m2) was conducted in an upstream direction, by slowly moving from one bank to the other in a zig-zag pattern. The backpack electrofishing covered a fishing area of ~ 30.86 m2 per site. A separation of 250 m between fish gear was defined at each site to avoid overlapping of net sets and electrofishing.
The captured fish were transported alive to the field station and maintained in aerated tanks for data collection. All field sampling techniques performed and laboratory fish handling protocols followed in this study were reviewed and approved by the Mexican Ministry of Environmental and Natural Resources (Semarnat-SGPA/DGVS/001774). The fish specimens were anesthetized using tricaine mesylate (MS-222), according to the Official Mexican Norm NOM-051-ZOO-1995 and NOM-033-SAG/ZOO-2014 for humane treatment in the transportation of animals.
Specimens were identified using the keys of Miller et al. (2009) and for the genus Oreochromis, the keys of Arredondo and Guzmán (1986). The specimens were separated according to species and capture method; counted, measured to the nearest 0.1 mm (standard length) with a digital caliper (MITUTOYO SERIES 505-637-50 precision 0.01 mm), and weighed with an electronic balance (OHAUS Scout® Pro model SP402 precision 0.01 g). The fish were released at the sites from where they had been collected. A small number of specimens died from overdoses of tricaine mesylate and were deposited in the ichthyological collection at the Universidad Michoacana de San Nicolás de Hidalgo.
To evaluate differences among sites and seasons, multi-factorial analyses of variance (ANOVA) were used for parameters of habitat, fish abundance (individuals/m2), biomass (g/m2) and diversity. The data were log-transformed (x+1) in order to comply with the assumptions of normality (Kolmogorov Smirnov) (Zar, 1999) and heteroscedasticity (Sokal & Rohlf, 1995) and assessed prior to the analysis of variance. The Tukey-Kramer honest significant difference (HSD) post hoc test (Zar, 1999) was used when the ANOVA showed significant differences. Analyses were performed using JMP 6 software (© SAS Institute Inc, Jones & Sall, 2011).
Rank abundance plots for fish density and biomass were used to compare the abundance of species with their spatial and seasonal variation. The relative abundance and biomass values of each species were log10 transformed and ordered from most to least abundant (Feisinger, 2001). The number of species (richness) in the rank abundance was used to compare the composition of the assemblages and their spatio-temporal variation with a multi-factor analysis of variance (ANOVA), as described above. The diversity of assemblages was estimated using the “true diversity index” corrections proposed by Jost (2006). The non-parametric estimator Chao1 was used to represent the diversity of order 0 (species richness). The diversity of order 1 (abundant species) was estimated with the exponential of the Shannon index (1D = Exp (H´), in which H’ = -Σ pi x ln (pi), s = number of species and pi = proportion of species i). The diversity of order 2 (dominant species) was estimated with the inverse of the Simpson index (2D = 1/D; were D = Σ = pi2, in which pi is the proportion of species i). The results were reported as the effective number of species (Hill, 1973; Magurran, 1988). The true beta diversity was calculated to estimate the variation in diversity among assemblages (Baselga, 2010; Gregorius, 2016; Whitaker, 1960). As proposed by Jost (2007), the gamma component of the diversity was converted to “true diversity” and divided by the diversity of order 1 (1Dβ = 1Dγ/1Dα).
Multidimensional Scaling (MDS) was used to explore the relationship of fish species abundance with the physicochemical water variables (Gower, 1966). We used principal component analysis (PCA) and correlation analysis to select the habitat characterization variables relevant for the spatio-temporal variation. Species with density and biomass of less than 1% were excluded from the MDS, since rare species have a low influence on the statistical analysis and can instead be presented as extreme values in the ordination analysis (Gauch, 1982). The procedure was performed in R software using the Stats package v 3.6.2 (R core team, 2013).
Results
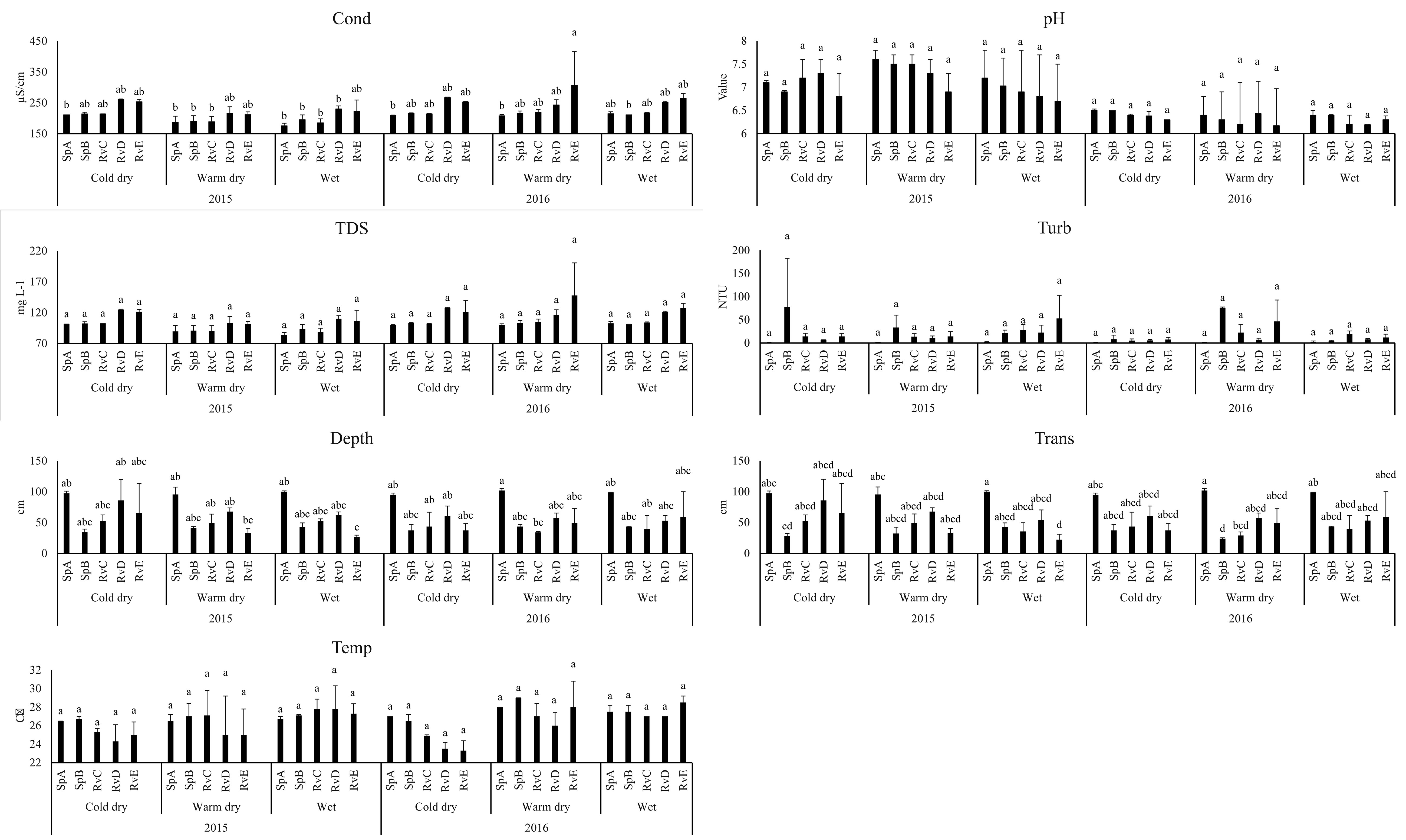
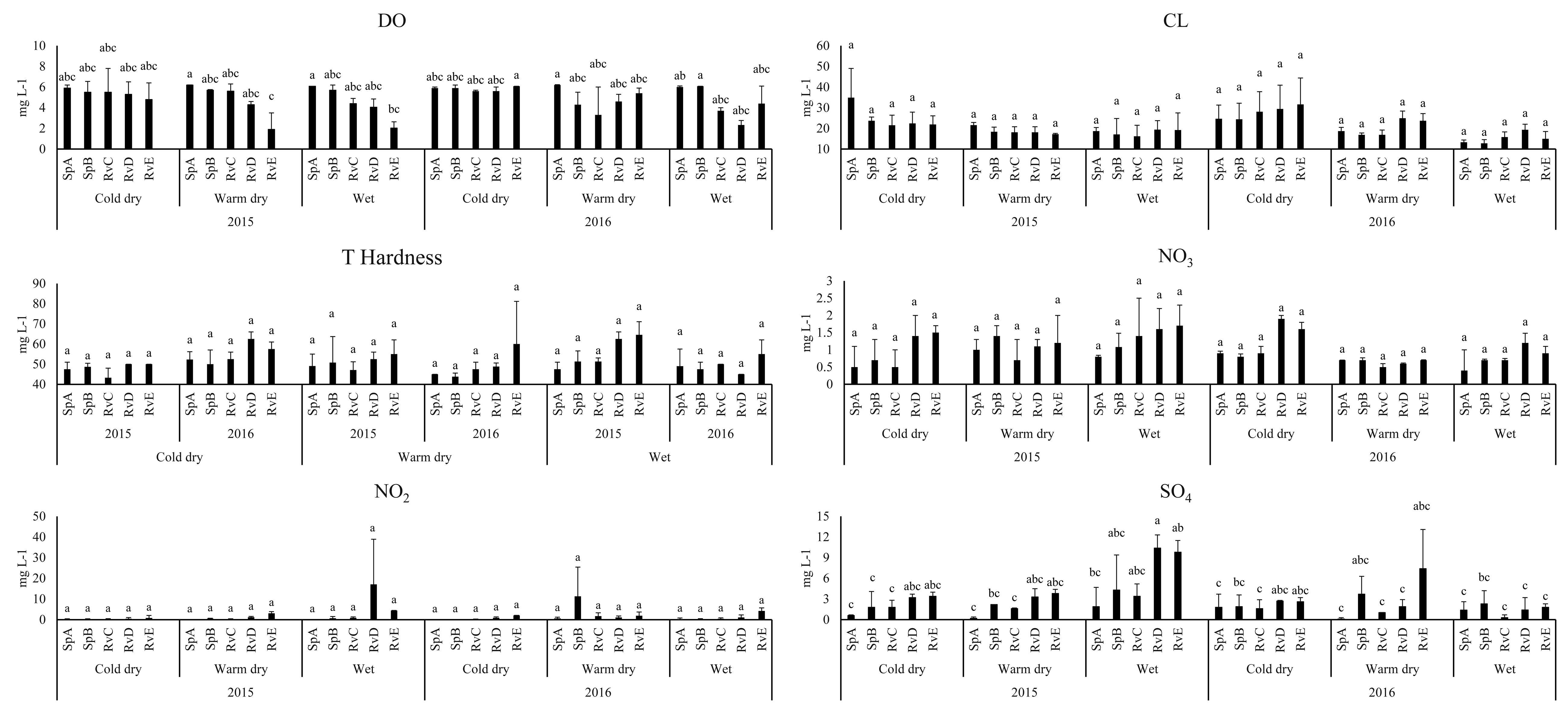
Six physicochemical water parameters differed spatially (p < 0.01): dissolved oxygen, sulfates, total dissolved solids, conductivity, depth and transparency. Dissolved oxygen presented a longitudinal gradient being higher in the spring site SpA (6.2 ± 0.1 g L) and decreased significantly downstream, reaching a minimum in the sites near La Vega dam reservoir (1.9 ± 1.6 g L). Sulfates and conductivity presented a longitudinal gradient and were lower in SpA (SO4 = 0.2 ± 0.2 mg L-1), SpB (SO4 = 1.8 ± 2.3 mg L-1 , Cond = 1.8 ± 1.02 µS/cm) and RvC (SO4 = 1.8 ± 1.02 mg L-1 , Cond = 185.4 ± 12.7 µS/cm), while the sites with the highest values were RvD (SO4 = 10.4 ± 1.9 mg L-1 , Cond = 266.5 ± 2.1 µS/cm) and RvE (SO4 = 9.8 ± 1.7 mg L-1 , Cond = 307 ± 108.9 µS/cm). The deepest site (101.8 ± 3.2 cm) was SpA, which also presented the greatest transparency (101.8 ± 3.2 cm). The shallowest site was RvE (33 ± 7.07 cm) (Fig. 2).
The riverbed substrate mainly consisted of particles of small size (mean diameter < 0.062 mm) in the sites SpB, RvC, RvD and RvE, dominated by clay and silt. The mean particle diameter only exceeded > 1 mm in SpA, because little rocks and boulders were present at this site. The water flow rate was 0.1-0.5 m/s.
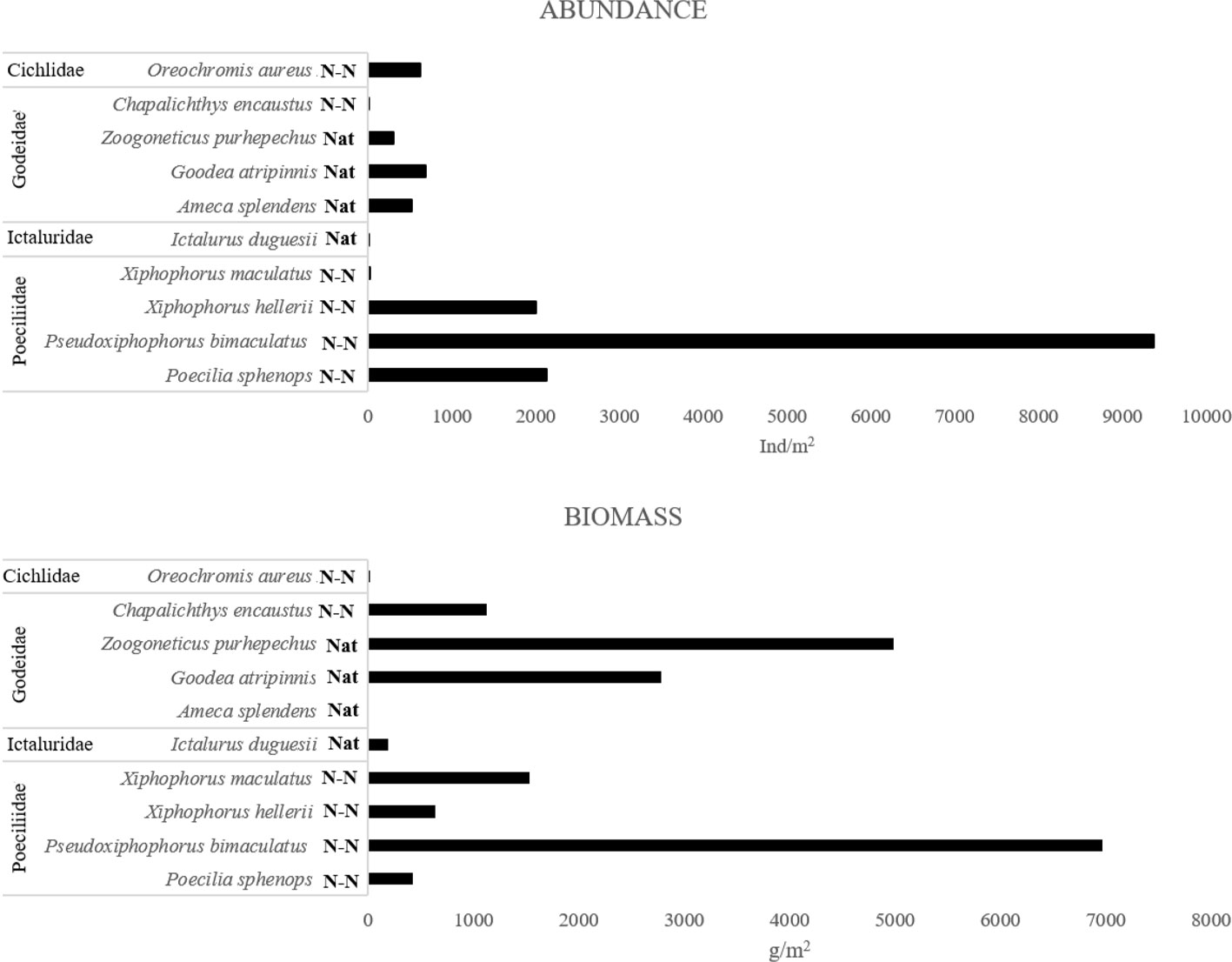
A total of 15,675 specimens were obtained, gathering a total weight of 18,648 g. Four families, 9 genera, and 10 species were identified (Fig. 3). The families with the greatest number of species were Goodeidae (4) and Poeciliidae (4).
The species with the overall greatest abundance were the non-native poecilids Pseudoxiphophorus bimaculatus (59.78%), Poecilia sphenops (13.61%) and Xiphophorus hellerii (12.8). Together, these non-native species represented 86.19% of the fish abundance. The highest biomass was found for the non-natives Oreochromis aureus (37.34%), P. bimaculatus (26.76%), and P. sphenops (14.92%), which accounted for 78.98% of the overall fish biomass.
Species density presented spatial (p < 0.0001) and seasonal (p = 0.04) differences. The site with the highest fish density was RvD (2.69 ± 0.25 ind/m2), while the lowest was observed in SpA (1.38 ± 0.25 ind/m2). The highest density was recorded during the warm dry season (2.65 ± 0.19 ind/m2). Species showed significant differences in density (p < 0.0001), with the non-native P. bimaculatus presenting the highest density (12.51 ± 0.35 ind/m2) and found to be the most abundant in all sites.
Fish biomass differed significantly among sites (p = 0.0002), but no seasonal differences were observed (p = 0.65). The highest biomass was obtained at RvE (3.09 ± 0.45 g/m2), at this site, the non-native O. aureus presented the highest biomass (20.55 ± 1.64 g/m2) at this site. Overall biomass differed among species (p < 0.0001); the highest biomass presented by the non-native P. bimaculatus (5.64 ± 0.64 g/m2).
The rank abundance plots (rank-density and rank-biomass) did not show differences in richness among climatic seasons (p = 0.99), but differed spatially (p = 0.002). The species assemblage varied with season, but did not differ significantly in terms of the relative abundance of species (p < 0.0001). According to the rank-density plots, the non-native P. bimaculatus was dominant in all sites (Fig. 4). In the rank biomass plots, the non-native P. bimaculatus was dominant in SpA, SpB, RvC and RvD, while the non-native O. aureus was dominant in RvE (Fig. 4).
The highest species richness (q = 8) was found in SpA and SpB during the cold dry season of both 2015 and 2016. During the warm dry season of 2016, the highest species richness (q = 8) was observed in RvD. Site SpA also presented 8 species during the warm dry season of both 2015 and 2016. While species richness reached 8, the first-order true diversity value indicated that the number of effective species fluctuated between 2 and 5 (Fig. 5). The number of effective assemblages was close to (1Dβaverage = 1.17 effective assemblages) in all seasons. The maximum value (1Dβ = 1.21 effective assemblages) was found during the cold dry season of 2015 and the wet season of both 2015 and 2016. The minimum (1Dβ = 1.02 effective assemblages) value occurred during the warm dry season of 2015.
The results of MDS analysis showed a spatial tendency for fish density, we did not find temporal tendency of the data. We found 3 zones according to dissolved oxygen, chlorides, total hardness, nitrites, nitrates, sulfates, conductivity and transparency differentiation. The SpA site was a group that differed from the rest of the river by dissolved oxygen, transparency and native fish abundance. The sites SpB and RvC conformed a second group, and the third group was sites RvD and RvE. The sites RvD and RvE during wet and dry season of 2015 were in the second group (Fig. 6). The relationship between fish species, absolute density and habitat characteristics was significant (Monte Carlo test p = 0.03). The relative distribution of fish species among the sites showed the contrast between the springs (sites SpA, the sites SpB and RvC) and the river mouth (sites RvD and RvE), with the higher density of the non-natives P. sphenops and Oreochromis aureus in RvE, and the higher density of the native species G. atripinnis, and Z. purhepechus in SpA (Fig. 6).
Discussion
The Teuchitlán River can be considered a model site where it can be evaluated the influence of anthropogenic disturbance on the dynamics of fish assemblages due to the co-occurrence of high native fish diversity (characterized by a high number of endemic species), the environmental degradation and the introduction of non-native species. We found that non-native fish species were the abundant-dominant species at Teuchitlán River and represented more than 50% of the fish assemblage in all sites. This abundance of non-native species and the extirpation of native ichthyofauna reflect the high degree of human impact on the Teuchitlán River over the past 60 years or more, leading to the current semi-replacement of native species. In the present study, we found 10 species, 4 native and 6 introduced, and can, therefore, corroborate the negative tendency in native species abundance and the increase in non-native richness since the last survey conducted by López-López and Paulo-Maya (2001).
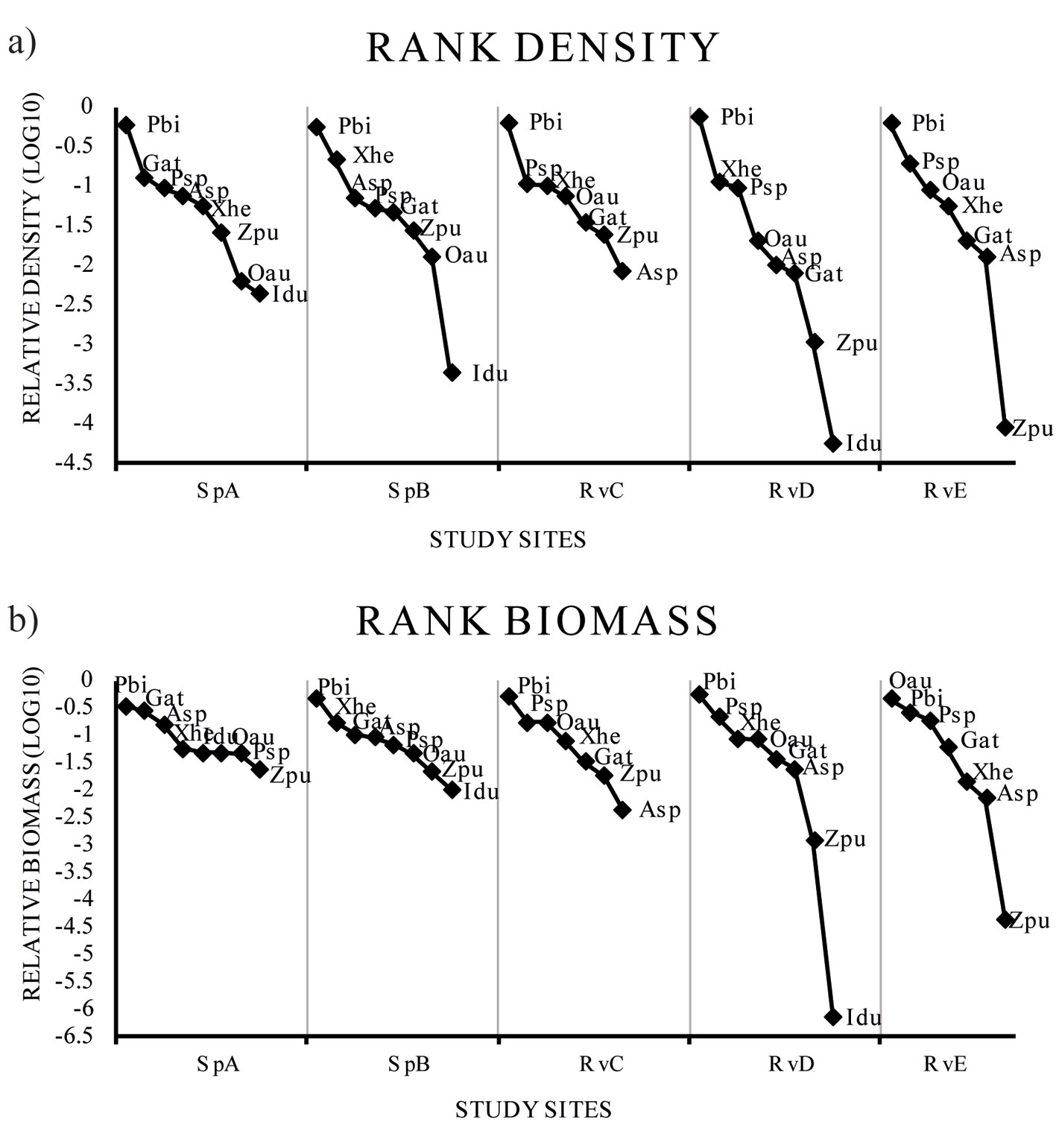
Our results show that the non-native poecilid P. bimaculatus is the most abundant species of the Teuchitlán River fish assemblage (Fig. 4). This species had not been reported previously at this site and it is, thus, considered a recent introduction (Kingston, 1978; López-López & Paulo-Maya, 2001; Miller & Fitzsimons, 1971; Webb & Miller, 1998). Previous studies have reported that P. bimaculatus presents high trophic plasticity, highly adaptable reproductive traits, and tolerance to environmental degradation (Mercado-Silva et al., 2002; Olinger et al., 2016; Trujillo-Jiménez & Toledo-Beto, 2007). We found a high abundance and biomass of P. bimaculatus in all of the sites (Fig. 4), even under different conditions of environmental variables and habitat characteristics. For example, in the SpA site the characteristics being higher in oxygen, lower in dissolved solid and nitrogenous compound, and in the RvE site at the river mouth contrasting conditions of lower oxygen and more nitrogenous and dissolved compounds. This denotes a high tolerance of the species to the human perturbation that is evident in the river.
The specific effects of non-native species on the native assemblage at the Teuchitlán River system are unknown, but decreased abundance of native goodeids has been associated with high abundance of exotic poecilid species (Kingston, 1978), including a negative relationship between P. bimaculatus abundance and the native G. atripinnis in other central Mexican freshwater systems (Ramírez-Carrillo & Macías-García, 2015). Kingston (1978), and Webb and Miller (1998) stated that Xiphophorus maculatus was a severe threat to native fishes at the Teuchitlán River system due to the possibility of competition for food resources and reported the species as abundant, although it was not reported in the study conducted by López-López and Paulo-Maya (2001); in the present study was captured at low numbers.
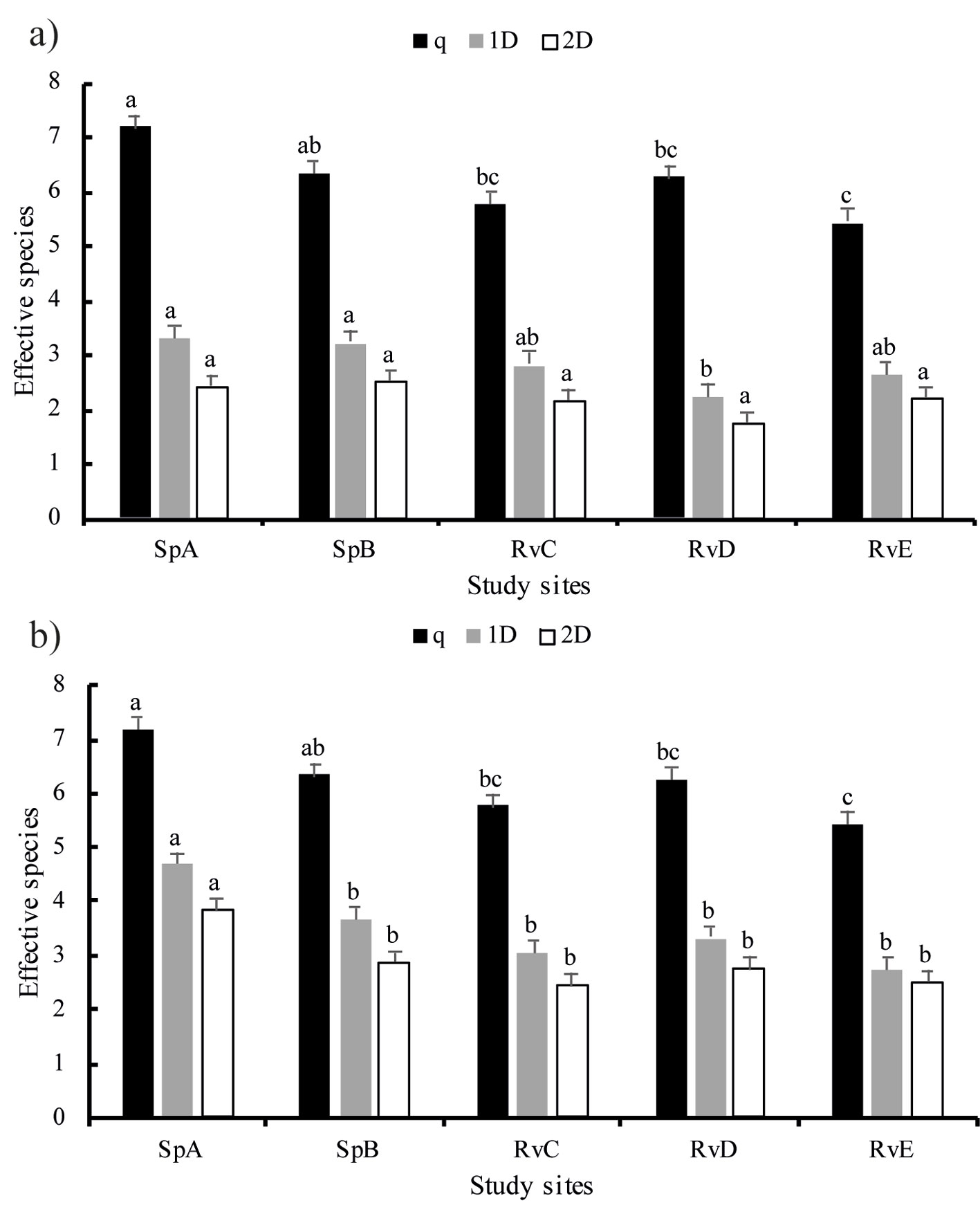
The diversity of results showed an effect of non-native species over the assemblage structure, since the effective species number was close to 2 and 1, indicating that the assemblage tends to be moving to a monospecific stage dominated by P. bimaculatus, mainly in the RvC, RvD and RvE river sites (Fig. 5). The beta diversity analysis indicated that the number of effective communities is close to 1, and therefore the species turnover is low, indicating a trend among the assemblages toward biotic homogenization with the exotics P. bimaculatus and P. sphenops widely distributed among sites (Lawson & Johnston, 2015; Olden & Poff, 2003; Olden et al., 2004; Scott & Helfman, 2001).
We found a relationship between fish species abundance and the physicochemical water variables with a spatial tendency of river zonation (Fig. 6). The upstream spring sites showed a short water residence period, which prevent the accumulation of hydrolyzable organic material and dilute the concentration of ionized compounds decreasing oxidation rates and fostering optimum concentrations of dissolved oxygen (Guerrero-Naranjo, 2017). However, in the downstream sites (Fig. 2), the nitrates and total dissolved solids were higher as a result of accumulation from upstream and the release of untreated domestic sewage producing a concentration of hydrolysable organic matter and increased sulfates, conductivity and dissolved solids, as well as a decrease in oxygen content (Guerrero-Naranjo, 2017). Moreover, the concentration of nitrites and nitrates was high at the end of river (NO2– up to 17.1 ± 21.8 mg L-1, NO3– up to 1.9 ± 0.1 mg L-1). Concentrations of nitrogenous compounds in pristine lotic systems have been reported at 0.001 mg L-1 NO2 and 0.015 mg L-1 NO3 (Allan & Castillo, 2007), and the magnification of these compounds in freshwater systems has been reported as a result of anthropogenic sources such as sewage and agricultural fertilizers (Weigelhofer et al., 2018). Therefore, the decline of water quality, including the enrichment of nitrogenous compounds in a downstream gradient, is caused mainly by the human impact on the river.
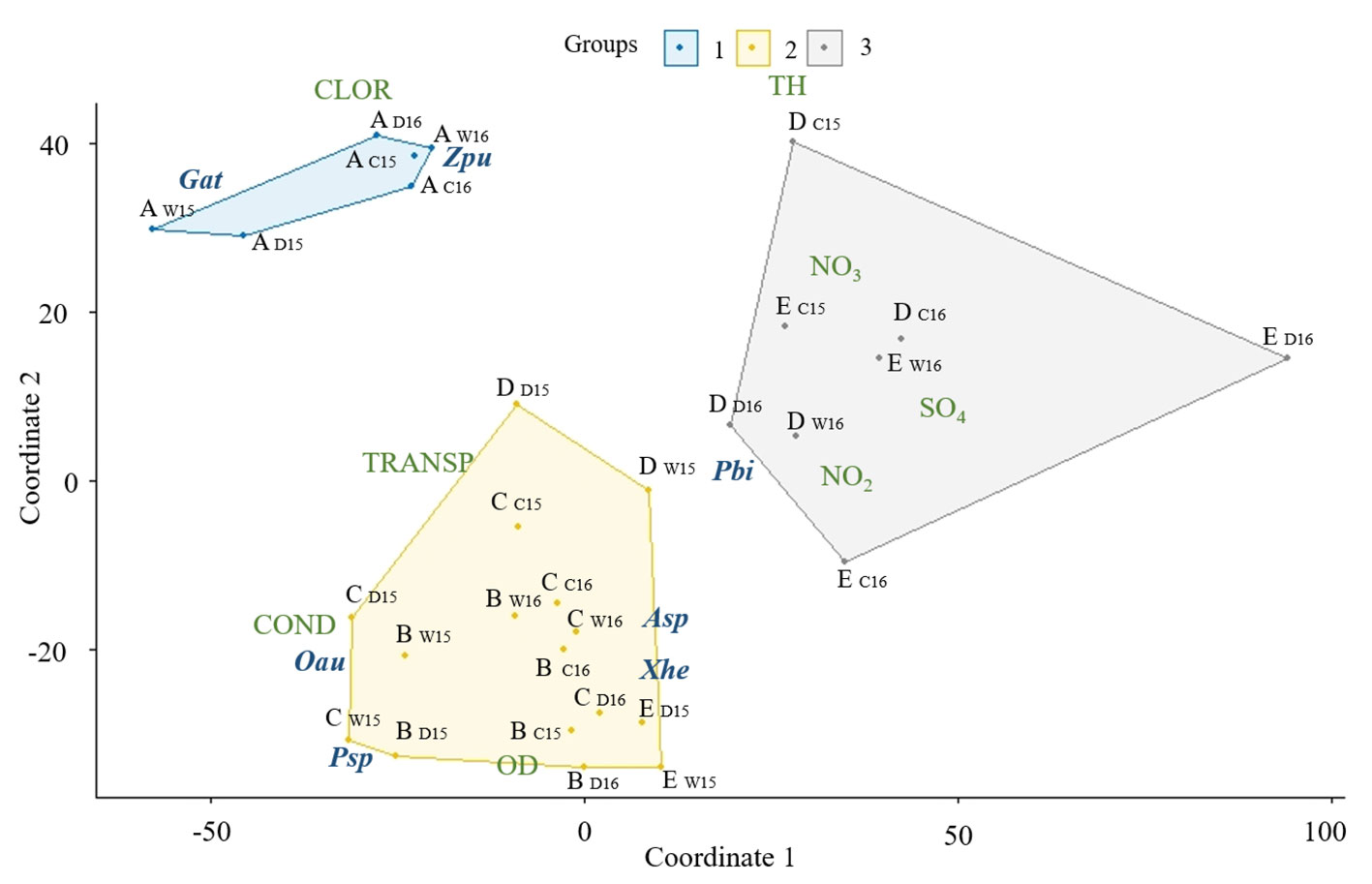
According to the nitrogenous compound we found, a nitrates concentration in all sites is acceptable for human health and aquatic life criteria (< 10 mg/L). However, the dissolved oxygen in the river mouth sites could be lower than the acceptable by the water quality criteria (< 3 mg/L per day). As a result of this, the Teuchtitlán River is a system with a variable water quality, i. e. with water parameters from acceptable to low polluted in a downstream gradient (APHA, 2017). We found that the importance of the Teuchitlán River native fishes decreased downstream, probably reflecting the response of the fish assemblage to this environmental perturbation. Some of the native fish species are sensitive to habitat degradation and these species could be stressed in environments with poor quality habitat conditions (Mercado-Silva et al., 2002). This could be related to the reduction in the fish populations (Kingston, 1978; Soto-Galera et al., 1999). The native goodeidae family presented variation on its tolerance to pollutants such as nitrogenous compounds (De La Vega-Salazar, 2006). Goodea atripinnis is a relatively tolerant species in the Teuchitlán River that can withstand nitrite levels up to 0.24 mg L-1, while Skiffia multipunctata (Pellegrin, 1901), a species related to the extinct Skiffia francesae, suffers physiological damage at a concentration of 0.001 mg L-1 (Rueda-Jasso et al., 2017). The concentration of nitrites in the Teuchitlán River is considered high and, in some sites, the level (up to 17.1 ± 21.8 mg L-1) exceeds the tolerance of native species (Tejera-Vera et al., 2007). Accordingly, the native species such as I. duguesii, Z. purhepechus, and A. splendens presented higher abundance in the spring sites, which have lower concentrations of nitrogenous pollutants, total dissolved solids and more dissolved oxygen. However, non-native species were dominant in the fish assemblages at all of the river sites (up to 50% of the total assemblage), regardless of local habitat characteristics (Fig. 6), and O. aureus presented high abundance and biomass downstream in the presence of high concentrations of nitrites, nitrates and sulfates (Fig. 2). Some non-native fish species, such as O. aureus, showed a mechanism of tolerance to nitrogenous compound toxicity and higher tolerance to other environmental stressors enabling them to survive better than the native fish (Karatayev et al., 2009; Leuven et al., 2011; Palachek & Tomasso, 1984). The process of human impact in the river, seen as the degradation of water quality, can therefore partially explain the reduction in the native sensitive fish populations and, possibly, plays an important role in the change of fish assemblage, acting to limit native species abundance and distribution.
Our findings support the fact that the native ichthyofauna at Teuchitlán River has largely been replaced by non-native species. However, this decline of the native ichthyofauna seems to be due not only to the interaction with the non-native species, but also to a combination of other factors, such as environmental degradation, a phenomenon that occurs in other basins of central Mexico (Ramírez-Herrejón et al., 2015). This represents a fundamental contribution to our understanding of the role of non-native freshwater fish species in the community dynamics of lotic ecosystems, in a region that has had few studies on aquatic fauna and gives direction to future management plans and conservation efforts in neotropical freshwater systems (Simberloff, 2014).
Acknowledgments
The first author thanks the Consejo Nacional de Ciencia y Tecnología (Conacyt) for the doctoral fellowship No. 509825. This work was funded by Chester Zoo, the Mohammed Bin Zayed Species Conservation Fund, L’association Beauval Nature Pour la Conservation et la Recherche, the Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio), Haus des Meeres and Poecilia Scandinavia. We also thank to the División de Estudios de Posgrado of Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, for financial support for the English editing services. The authors thank Javier Ponce for valuable comments on the manuscript. To Luis Martín Mar Silva, Oscar Gabriel Ávila Morales, Arely Ramírez García, Miguel Aurelio Piñón Flores, Alejandro Chávez García, Ricardo Raúl Chávez Jiménez, and Pedro de Jesús Martínez Morales, for assistance in fish collection and Guillermo Alejandro Guerrero Naranjo for sampling and laboratory analysis of the habitat parameters.
References
Allan, J. D., & Castillo, M. M. (2007). Stream ecology. The structure and function of running waters. Dordrecht, Netherlands: Springer.
APHA-AWWA-WEF (2017). Standard methods for examination of water and wastewater, 23nd Ed. Washington D.C.: American Public Health Association.
Arredondo, J. L., & Guzmán, M. A. (1986). Situación taxonómica actual de la tribu tilapinni (Pisces: Cichlidae); con énfasis en las especies introducidas en México. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología, 2, 555–572.
Arthington, A. H., Dulvy, N. K., Gladstone, W., & Winfield, I. J. (2016). Fish conservation in freshwater and marine realms: status, threats and management. Aquatic Conservation: Marine and Freshwater Ecosystems, 26, 838–857. https://doi.org/10.1002/aqc.2712
Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography, 19, 134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Bunte, K., & Abt, S. R. (2001). Sampling surface and subsurface particle-size distributions in wadable gravel- and cobble-bed streams for analyses in sediment transport. Hydraulics, and streambed monitoring. Fort Collins, Colorado: United States Department of Agriculture, Forest Service.
Collen, B., Whitton, F., Dyer, E. E., Baillie, J. E., Cumberlidge, N., Darwall, W. R. et al. (2014). Global freshwater species congruence. Global Ecology and Biogeography, 23, 40–51. https://doi.org/10.1111/geb.12096
De La Mora-Orozco, C., Flores-López, H. E., Ariel-Ruiz-Corral, J., Chávez-Durán, A. A., & Figueroa-Montaño, A. (2014). Impacto del cambio climático en las tendencias de la evaporación en la presa La Vega, Teuchitlán, Jalisco, México. Revista Mexicana de Ciencias Agrícolas, spe10, 1993–2005.
De La Mora-Orozco, C., Flores-López, H. E., & Durán-Chávez, A. A. (2013). Calidad del agua del embalse de la presa La Vega y su impacto en las tierras agrícolas bajo riego. México D.F.: Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias.
De La Vega-Salazar, M. Y. (2006). Estado de conservación de los peces de la familia Goodeidae (Cyprinodontiformes) en la mesa central de México. Revista de Biología Tropical, 54, 163–177. https://doi.org/10.15517/RBT.V54I1.13989
De La Vega-Salazar, M. Y., Ávila-Luna, E. G., & Macías-García, C. (2003a). Threatened fishes of the world: Zoogoneticus tequila Webb & Miller 1998 (Goodeidae). Environmental Biology of Fishes, 68, 14–14. https://doi.org/10.1023/A:1026040727808
De La Vega-Salazar, M. Y., Ávila-Luna, E. G., & Macías-García, C. (2003b). Ecological evaluation of local extinction: the case of two genera of endemic Mexican fish, Zoogoneticus and Skiffia. Biodiversity & Conservation, 12, 2043–56. https://doi.org/10.1023/A:1024155731112
Domínguez-Domínguez, O., Doadrio, I., & Pérez-Ponce de León, G. (2006). Historical biogeography of some river basins in central Mexico evidenced by their goodeine freshwater fishes: a preliminary hypothesis using secondary Brooks parsimony analysis. Journal of Biogeography, 33, 1437–1447. https://doi.org/10.1111/j.1365-2699.2006.01526.x
Domínguez-Domínguez, O., Pérez-Rodríguez, R., & Doadrio, I. (2008). Morphological and genetic comparative analyses of populations of Zoogoneticus quitzeoensis (Cyprinodontiformes: Goodeidae) from Central Mexico, with description of a new species. Revista Mexicana de Biodiversidad, 79, 373–383. http://dx.doi.org/10.22201/ib.20078706e.2008.002.563
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Lévêque, C. et al. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews, 81, 163–182. https://doi.org/10.1017/S1464793105006950
Dzul-Caamal, R., Olivares-Rubio, H. F., Medina-Segura, C. G., & Vega-López, A. (2013). Endangered Mexican fish under special protection: Diagnosis of habitat fragmentation, protection, and future-a review. In M. E. Lucas-Borja (Ed.), Endangered species: habitat, protection and ecological significance (pp. 109–130). New York: Nova Science Publishers.
Favari-Perozzi, L., López-López, E., & Madrigal-Ortiz, M. (2003). The effect of water from de la Vega reservoir on lipoperoxidation and acetylcholinesterase levels in liver and muscle of Xiphophorus helleri. Revista Internacional de Contaminación Ambiental, 19, 145–155.
Feisinger, P. (2001). Designing field studies for biodiversity conservation. Washington D.C.: Island Press.
García, E. (1988). Modificaciones al sistema de clasificación climática de Köeppen. México D.F.: Instituto de Geografía, Universidad Nacional Autónoma de México.
Gauch, H. G. (1982). Multivariate analysis in assemblage ecology. New York: Cambridge University Press.
Gower, J. C. (1966). Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika, 53, 325–338. https://doi.org/10.1093/biomet/53.3-4.325
Gregorius, H. R. (2016). Effective numbers in the partitioning of biological diversity. Journal of Theoretical Biology, 409, 133–1347. https://doi.org/10.1016/j.jtbi.2016.08.037
Guerrero-Naranjo, G. A. (2017). Physicochemical and microbiological characterization of the upper section of the Teuchitlán River basin, Jalisco, Mexico (Tesis). Universidad Michoacana de San Nicolas de Hidalgo. Morelia, Michoacán, México.
Herrerías-Diego, Y., Domínguez-Domínguez, O., Medina-Nava, M., Ávila, O., & Mar-Silva, V. (2019). Comparación de la composición y abundancia de la comunidad íctica del río Teuchitlán, Jalisco, México empleando tres artes de pesca. In C. P. Ornelas-García, F. Álvarez, & A. Wegier (Eds.), Antropización: primer análisis integral (pp. 265–282). Ciudad de México: IB-UNAM/ Conacyt.
Hill, M. O. (1973). Diversity and evenness: a unifying notation and its consequences. Ecology, 54, 427–432. https://doi.org/10.2307/1934352
INEGI (Instituto Nacional de Estadística y Geografía). (2010). Censo de población y vivienda 2010. México D.F.: Instituto Nacional de Estadística y Geografía. Retrieved on December 28th, 2017 from: https://www.inegi.org.mx/programas/ccpv/2010/
IUCN (International Union for Conservation of Nature). (2017). The IUCN Red List of Threatened Species. The International Union for Conservation of Nature. Recuperado el 28 de diciembre, 2017 de: https://www.iucnredlist.org/
Jiménez-Román, A. (1994). Estudio comparativo de la precipitación y el escurrimiento en la cuenca del río Ameca, México. Revista Geográfica, 119, 59–79. http://www.jstor.org/stable/40992684
Jones, B., & Sall, J. (2011). JMP statistical discovery software. Wiley Interdisciplinary Reviews: Computational Statistics, 3, 188-194. https://doi.org/10.1002/wics.162
Jost, L. (2006). Entropy and diversity. Oikos, 113, 363–374. https://doi.org/10.1111/j.2006.0030-1299.14714.x
Jost, L. (2007). Partitioning diversity into independent alpha and beta components. Ecology, 88, 2427–2439. https://doi.org/10.1890/06-1736.1
Karatayev, A., Burlakova, L., Padilla, D., Mastitsky, S., & Olenin, S. (2009). Invaders are not a random selection of species. Biological Invasions, 11, 2009–2019. https://doi.org/10.1007/s10530-009-9498-0
Kingston, D. I. (1978). Skiffia francesae, a new species of goodeid fish from Western Mexico. Copeia, 1978, 503–508. https://doi.org/10.2307/1443618
Lawson, K. M., & Johnston, C. E. (2015). The role of flow dependency and water availability in fish assemblage homogenization in tributaries of the Chattahoochee River, Alabama. Ecology of Freshwater Fishes, 25, 631–641. https://doi.org/10.1111/eff.12241
Leuven, R. S. E. W., Hendriks, A. J., Huijbregts, M. A. J., Lenders, H. J. R., Matthews, J., & Van der Velde, G. (2011). Differences in sensitivity of native and exotic fish species to changes in river temperature. Current Zoology, 57, 852–862. https://doi.org/10.1093/czoolo/57.6.852
López-López, E., & Paulo-Maya, J. (2001) Changes in the fish assemblages in the upper río Ameca, México. Journal of Freshwater Ecology, 16, 179–187. https://doi.org/10.1080/02705060.2001.9663803
López-López, E., Paulo-Maya, J., Carvajal, A. L., Ortiz-Ordóñez, E., Uría-Galicia, E., & Mendoza-Reynosa, E. (2004). Populations of the Butterfly Goodeid (Ameca splendens) in the Upper Río Ameca Basin, Mexico. Journal of Freshwater Ecology, 19, 575–580. https://doi.org/10.1080/02705060.2004.9664737
Magurran, A. E. (1988). Ecological diversity and its measurement. New Jersey: Princeton University Press.
Mar-Silva, V., Mar-Silva, L. M., Herrerías-Diego, Y., Medina-
Nava, M., & Domínguez-Domínguez, O. (2019). Trans-
locación de Chapalichthys encaustus (Actinopterygii: Goodeidae) en el río Teuchitlán, Jalisco Mexico. Hidrobiológica, 29, 45–47. https://doi.org/10.24275/uam/izt/
dcbs/hidro/2019v29n1/Mar
Mercado-Silva, N., Lyons, J. D., Salgado-Maldonado, G., & Medina-Nava, M. (2002). Validation of a fish-based index of biotic integrity for streams and rivers of central Mexico. Reviews in Fish Biology and Fisheries, 12, 179–191. https://doi.org/10.1023/A:1025099711746
Miller, R. R., & Fitzsimons, J. M. (1971). Ameca splendens, a new genus and species of goodeid fish from western Mexico, with remarks on the classification of the Goodeidae. Copeia, 1971, 1–13. https://doi.org/10.2307/1441593
Miller, R. R., Minckley, W. L., & Norris, S. M. (2009). Freshwater fishes of Mexico. Chicago: University of Chicago Press.
Miller, R. R., & Smith, L. M. (1986). Origin and geography of the fishes of central Mexico. In C. R. Hocutt, E. O. Wiley (Eds.), Zoogeography of North American Freshwater Fishes (pp. 487–517). New York: John Wiley & Sons.
Olden, J. D., & Poff, N. L. (2003). Toward a mechanistic understanding and prediction of biotic homogenization. The American Naturalist, 162, 442–460. https://doi.org/10.1086/378212
Olden, J. D., Poff, N. L., Douglas, M. R., Douglas, M. E., & Fausch, K. D. (2004). Ecological and evolutionary consequences of biotic homogenization. Trends in Ecology & Evolution, 19, 18–24. https://doi.org/10.1016/j.tree.2003.09.010
Olinger, C., Peoples, T. B. K., & Frimpong, E. A. (2016). Reproductive life history of Heterandria bimaculata (Heckel, 1848) (Poeciliinae: Poeciliidae) in the Honduran interior highlands: trait variation along an elevational gradient. Neotropical Ichthyology, 14, e150050. https://doi.org/10.1590/1982-0224-20150050
Palachek, R. M., & Tomasso, J. R. (1984). Toxicity of Nitrite to Channel Catfish (Ictalurus punctatus), Tilapia (Tilapia aurea), and Largemouth Bass (Micropterus salmoides): evidence for a Nitrite exclusion mechanism. Canadian Journal of Fisheries and Aquatic Sciences, 41, 1739–1744. https://do.org/10.1139/f84-214
Pelicice, F. M., Azevedo-Santos, V. M., Vitule, J. R. S., Orsi, M. L., Lima-Junior, D. P., Magalhães, A. L. B. et al. (2017). Neotropical freshwater fishes imperilled by unsustainable policies. Fish and Fisheries, 18, 1119–1133. https://doi.org/10.1111/faf.12228
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/
Rabeni, C. F., Lyons, J., Mercado-Silva, N., & Peterson, J. T. (2009). Warmwater fish in wadeable streams. In S. A. Bonar, W. A. Hubert, & D. W. Willis (Eds.), Standard methods for sampling North American freshwater fishes (pp. 43–56). Bethesda, Maryland: American Fisheries Society.
Ramírez-Carrillo, E., & Macías-García, C. (2015). Limited options for native goodeid fish simultaneously confronted to climate change and biological invasions. Biological Invasions, 17, 245–256. https://doi.org/10.1007/s10530-014-0723-0
Ramírez-García, A., Ramírez-Herrejón, J. P., Medina-Nava, M., Hernández-Morales, R., & Domínguez-Domínguez, O. (2017). Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the Teuchitlán River, México. Journal of Applied Ichthyology, 34, 81–90. https://doi.org/10.1111/jai.13543
Ramírez-Herrejón, J. P., Mercado-Silva, N., Balart, E. F., Moncayo-Estrada, R., Mar-Silva, V., & Caraveo-Patiño J. (2015). Environmental degradation in a eutrophic shallow lake is not simply due to abundance of non-native Cyprinus carpio. Environmental Management, 56, 603–617. https://doi.org/10.1007/s00267-015-0524-y
Reis, R. E., Kullander, S. O., & Ferraris Jr, C. J. (2003). Check list of the freshwater fishes of South and Central America. Porto Alegre: EDIPUCRS.
Rice, E. W., Baird, R. B., Eaton, A. D., & Clesceri, L. S. (1995). Standard methods for examination of water and wastewater. Washington D.C.: American Public Health Association/ American Water Works Association/ Water Environment Federation.
Rueda-Jasso, R. A., De los Santos-Bailón, A., & Campos-Mendoza, A. (2017). Nitrite toxicity in juvenile Goodeinae fishes Skiffia multipunctata (Pellegrin, 1901) and Goodea atripinnis (Jordan, 1880). Journal of Applied Ichthyology, 33, 300–305. https://doi.org/10.1111/jai.13292
Scott, M. C., & Helfman, G. S. (2001). Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries, 26, 6–15. https://doi.org/10.1577/
1548-8446(2001)026<0006:NIHATM>2.0.CO;2
Semadet (Secretaría de Medio Ambiente y Desarrollo Territorial) Jalisco. (2014). Programa de conservación y manejo presa de la Vega. Guadalajara: Secretaría de Medio Ambiente y Desarrollo Territorial del Estado de Jalisco.
Simberloff, D. (2014). Prefacio. In R. Mendoza-Alfaro, P. Koleff-Osorio (Eds.), Especies acuáticas invasoras en México (pp. 7–9). México D.F.: Comisión Nacional para el uso y conocimiento de la Biodiversidad.
Sokal, R. R., & Rohlf, F. J. (1995). Biometry: the principles and practice of statistics in biological research. Third edition. New York: W.H. Freeman.
Soto-Galera, E., Paulo-Maya, J., López-López, E., & Serna-Hernández, J. A. (1999). Change in fish fauna as indication of aquatic ecosystem condition in Río Grande de Morelia-Lago de Cuitzeo basin, Mexico. Environmental Management, 24, 133–140. https://doi.org/10.1007/s002679900221
Tejera-Vera, R., López-López, E., & Sedeño-Díaz, J. E. (2007). Biomarkers and bioindicators of the health condition of Ameca splendens and Goodea atripinnis (Pisces: Goodeidae) in the Ameca River, Mexico. Environment International, 33, 521–531. https://doi.org/10.1016/j.envint.2006.11.018
Trujillo-Jiménez, P., & Toledo-Beto, H. (2007). Alimentación de los peces dulceacuícolas tropicales Heterandria bimaculata y Poecilia sphenops (Cyprinidontiformes: Poeciliidae). Revista de Biología Tropical, 55, 603–15.
Webb, S. A., & Miller, R. R. (1998). Zoogoneticus tequila, a new goodeid fish (Cyprinodontiformes) from the Ameca drainage of Mexico, and a rediagnosis of the genus. Occasional Papers of the Museum of Zoology, University of Michigan, 725, 1–23.
Weigelhofer, G., Hein, T., & Bondar-Kunze, E. (2018). Phosphorus and Nitrogen dynamics in riverine systems: human impacts and management options. In S. Schmutz, & J. Sendzimir (Eds.), Riverine ecosystem management. Aquatic Ecology Series, Vol 8 (pp. 187–202). Cham, Switzerland: Springer.
Whittaker, R. H. 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs, 30, 279–338. https://doi.org/10.2307/1943563
Zar, J. H. (1999). Biostatistical analysis. New Jersey: Prentice Hall.