New records of subtidal benthic marine algae from the state of Veracruz, southern Gulf of Mexico
José Luis Godínez-Ortega a, *, Pedro Ramírez-García a, Alejandro Granados-Barba b, Michael J. Wynne c
a Instituto de Biología, Departamento de Botánica, Universidad Nacional Autónoma de México, Apartado postal 70-233, Ciudad Universitaria, 04510 Ciudad de México, México
b Instituto de Ciencias Marinas y Pesquerías, Universidad Veracruzana, Calle Hidalgo 617, Col. Río Jamapa, 94290 Boca del Río, Veracruz, Mexico
c Department of Ecology and Evolutionary Biology and Herbarium, University of Michigan, Ann Arbor, MI 48108, USA
*Corresponding author: jlgo@unam.mx (J.L. Godínez-Ortega)
Abstract
New records of subtidal benthic marine algae from the State of Veracruz, the southern Gulf of Mexico, are presented, which include 7 species and 2 forms. The material was collected from 6 sampling forays at 4 locations between October 2013 and September 2017. The collection sites included the “Ana Elena” and “Riva Palacio” shipwrecks and some poorly explored reefs in the State of Veracruz, Mexico. The morphological characteristics of Hypoglossum simulans, Halymenia hancockii, Dictyota humifusa, Caulerpa sertularioides f. longiseta, Avrainvillea levis f. translucens, Udotea caribaea, U. dixonii, U. unistratea, and Anadyomene saldanhae are described. Information about the habitats and geographic distributions of these taxa is provided.
Keywords:
Shipwrecks; Reef system; Veracruz; Atlantic; Southern Gulf of Mexico
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Nuevos registros de algas marinas bentónicas submareales del estado de Veracruz, sur del golfo de México
Resumen
Se presentan nuevos registros de algas marinas bentónicas submareales del estado de Veracruz, sur del golfo de México, que incluyen 7 especies y 2 formas. El material fue recolectado en 6 muestreos de 4 localidades, entre octubre de 2013 y septiembre de 2017. Entre los sitios de recolección se encuentran los pecios “Ana Elena” y “Riva Palacio”, así como algunos arrecifes poco explorados en el estado de Veracruz, México. Se describen las características morfológicas de Hypoglossum simulans, Halymenia hancockii, Dictyota humifusa, Caulerpa sertularioides f. longiseta, Avrainvillea levis f. translucens, Udotea caribaea, U. dixonii, U. unistratea y Anadyomene saldanhae. Se proporciona información sobre los hábitats y la distribución geográfica de estos taxones.
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Pecios; Sistema arrecifal; Veracruz, Atlántico; Sur del golfo de México
Introduction
The past few decades have witnessed a heightened degree of phycological activity in the tropical and subtropical Western Atlantic and in the Gulf of Mexico, including the Caribbean coast of Mexico (Fredericq et al., 2009; Mateo-Cid et al., 2006; Ortega et al., 2001; Wynne, 2017). Numerous new records have been reported from Mexico, which is evidence that poorly known areas of the coastline are being explored and inventoried. Benthic marine algae of the southern Gulf of Mexico are described in intertidal and subtidal marine habitats from Yucatán to Tamaulipas (Ciudad Madero), including Campeche, Tabasco, and Veracruz according to the “Ecorregiones marinas de América del Norte” (Wilkinson et al., 2009). The taxonomic diversity of Veracruz is rich and varied. This means that Veracruz includes 44% of the algal biodiversity of the Mexican coasts of the western Atlantic (Ortega et al., 2001): 284 species, 55.3% corresponding to Rhodophytan algae, 14.1% to Phaeophycean algae and 30.6% to Chlorophytan algae (Galicia-García & Morales-García, 2007). However, the largest exploration in Veracruz has been in the intertidal zone. In this paper, we explore the subtidal zone that has had relatively little explored, namely, the shipwrecks “Ana Elena” and the “Riva Palacio”. “Ana Elena” was a fishing boat that ran aground on the Anegada de Afuera Reef at a depth of 12 m, an event that occurred almost 60 years ago. The “Riva Palacio” was a ship that sank 10 years ago and now is an artificial reef at a depth of 35 m. Along with sub-tidal regions of non-emergent reefs: Segunda Laja (5 m deep) near Chachalacas to the north of the Veracruz Reef System [SAV] and the Pantepec Reef (27 m depth) north of the Tuxpan River estuary in the Lobos-Tuxpan Reef System [SAL] (Fig. 1).
Material and methods
Specimens were collected from 6 sampling forays at 4 locations between October 2013 and September 2017, using scuba diving (SCUBA). The samples were preserved in a 4% formalin solution neutralized in seawater, while other samples were preserved as herbarium specimens. For morpho-anatomical observations 20 µm-thick sections were made with a standard microtome. Small portions were stained with a solution of HCl-acidified 1% aniline blue and then mounted in a solution of Karo® corn syrup /water 70:30 with a trace of phenol added to prevent fungal growth (Tsuda & Abbott, 1985). Only the antheridial sorus of D. humifusa was mounted directly on EM metal stubs and dried. The mounted samples were then coated with gold and observed using SEM (Hitachi SU1510). Appropriate literature was used to identify the material (De Clerck, 2003; Horta et al., 2003; Littler & Littler, 1990, 1991, 1992, 2000; Oliveira-Filho, 1969; Santos & Nunes, 2015; Taylor, 1960; Womersley & Shepley, 1982; Wynne et al., 1989). All specimens are deposited in the Herbarium of the Universidad Nacional Autónoma de México (MEXU).
Descriptions
For Veracruz, including the SAV, we report here 7 species and 2 forms of seaweeds assigned to 7 genera, 7 families, 5 orders and 3 classes of Rhodophyta, Ochrophyta, and Chlorophyta. In the section of the genus, previous records of Veracruz are included, except in the genus Hypoglossum in that there are no previous species for Veracruz, so those of the southern Gulf of Mexico are included.
Phylum Rhodophyta
Hypoglossum Kützing
Species previously reported from the southern Gulf of Mexico —Campeche Banks, Yucatán— (Mateo-Cid et al., 2013): Hypoglossum hypoglossoides (Stackhouse) Collins & Hervey, Hypoglossum subsimplex M.J. Wynne, Hypoglossum tenuifolium (Harvey) J. Agardh.
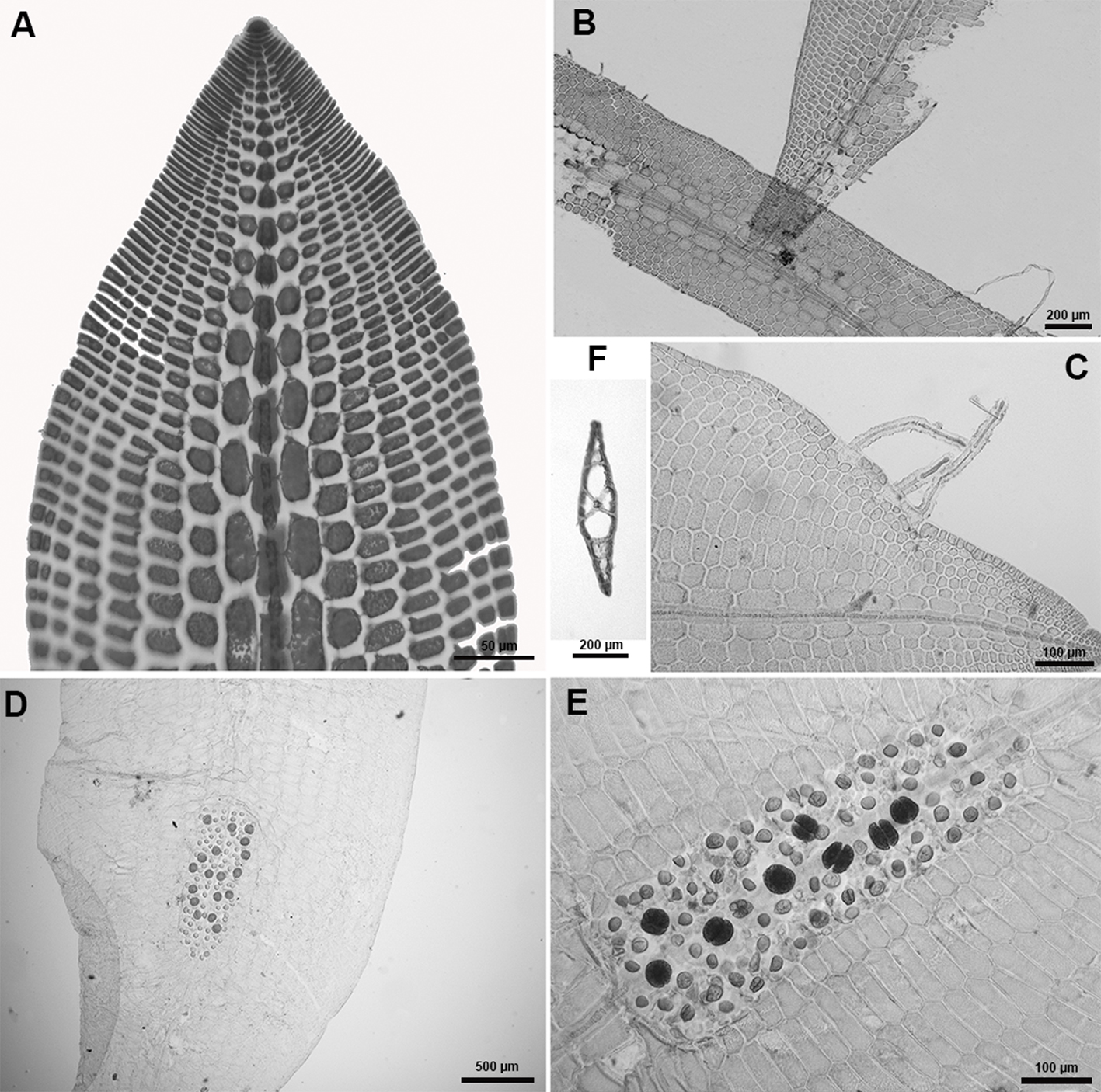
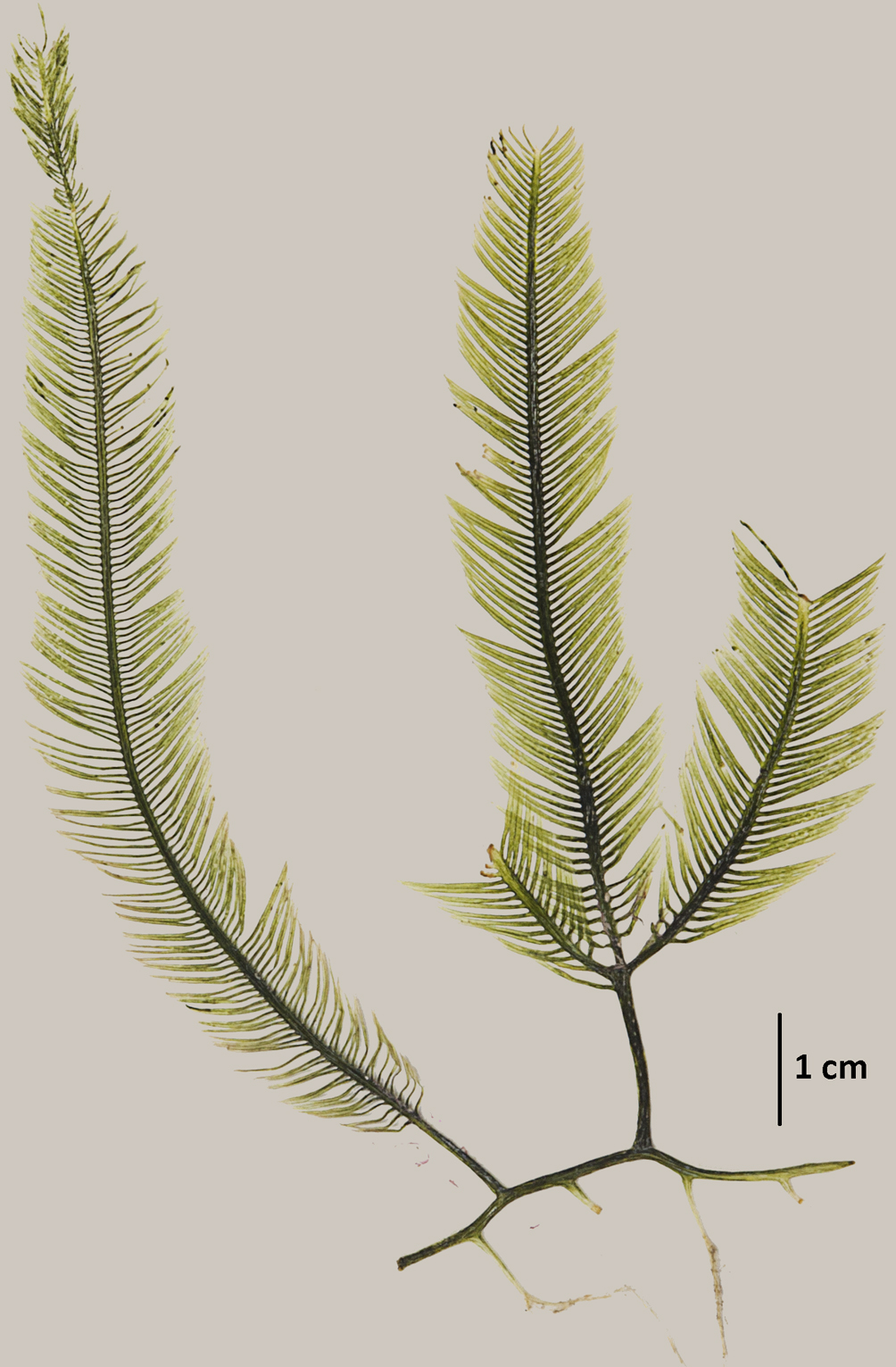
Hypoglossum simulans M.J. Wynne, I.R. Price & D.L. Ballantine, 1989: 31, figs 12-26.
Figure 2.
Taxonomic summary
Type locality: Îlet de Pigeon, Malendure, west side of Basse-Terre, Guadeloupe, West Indies.
Holotype: MICH.
Description: laminar thallus, mostly prostrate, delicate, pale pink, 1-3 cm high. Blades are linear or oval, 1-2 cm long and 1-2.5 mm wide, monostromatic except for the midline; irregular branching arising from the central axial cell row; blade margins smooth and undulate with marginal rhizoids; each segment from the divisions of the apical cell (primary-cell row) produces 4 pericentral cells, of which the pair of lateral pericentral cells continue to grow out producing second-order cell rows, some of which produce abaxially the third-order cells rows; thus all these divisons forming the blade; the lateral pericentral cells 40-65 µm wide and 140-175 µm long; blades with pointed apices and a prominent apical cell; not all cells of the second-order rows producing cells of the third order; absence of cells from intercalary divisions (apical organization type 2; Wynne et al., 1989); formation of rows of hexagonal cells extending towards the thallus margin; isodiametric cells near the margin. Rhizoids are attached to the substrate by small discs or pads and blade margins also forming multicellular rhizoids. Tetrasporangial sori occupying the central region of the blade (the midline and adjecent lateral areas), thus producing an elongate sorus (275-374 × 760-856 µm); tetrasporangia spherical, 40-60 µm in diameter.
References: Wynne et al. (1989); Wynne (2014).
Specimens examined: Veracruz: “Ana Elena” shipwreck, Anegada de Afuera Reef (19°10’07.76” N, 95°51’39.12” W), 12 m deep, Coll. J. L. Godínez Ortega, March 21, 2014 (NI-1779), May 27, 2014 (NI-1683, MEXU=2287) and February 21, 2015 (NI-1781).
Habitat: epiphytic on Dictyota humifusa Hörnig, Schnetter & Coppejans, Jania pumila J.V. Lamouroux and Lobophora variegata (J.V. Lamouroux) Womersley ex E. Oliveira, at depth of 12 m.
Distribution in western Atlantic: Bahamas, Guadeloupe (Guiry & Guiry, 2019), State of Veracruz, Gulf of Mexico.
Remarks
We dismiss Hypoglossum tenuifolium since its habit is of an erect form, contrary to the decumbent form of H. simulans. Our material also cannot be H. subsimplex because the arrangement of the apical blade in that species is of type “1” (all the cells of second-order rows produce rows of the third order), the sorus not discrete in length but elongated by some distance and the tetrasporangia at each side of the midline; and the tetrasporangia do not come from lateral pericentral cells, as in the case of H. simulans. Hypoglossum simulans was previously mis-identified as H. hypoglossoides by Godínez-Ortega et al. (2015). Thus, in this study we correct the identification of the same material collected from the “Ana Elena” shipwreck, where figure 2 in Godínez-Ortega et al. (2015) shows that not all cells of the second-order rows (“2”) produce third-order rows. That feature points to a species identification other than H. hypoglossoides. The production of marginal rhizoids in abundance in fig 3A (in Godínez-Ortega et al., 2015), the tetrasporangial sorus occupying the central region of the blade invading the central axis and the lack of cortex along the midline are all evidence that this material is to be determined as H. simulans instead of H. hypoglossoides, which has a well-developed midrib with abundant cortication, a feature that is not observed in figure 2A, and the sori are strongly corticated on both sides of the central axis, arranged in the upper regions of the blades, and the tetrasporangia do not invade the central axis, contrary to what is shown in figure 2C.
Halymenia C. Agardh, nom. cons.
Species previously reported from Veracruz (Galicia-García & Morales-García, 2007; Ortega et al., 2001): Halymenia echinophysa Collins & M. Howe, Halymenia elongata C. Agardh, Halymenia pseudofloresii Collins & M. Howe, Halymenia rosea M. Howe & W.R. Taylor.
Halymenia hancockii W.R. Taylor, 1942: 98, pl. 3: fig. 6; pl. 14.
Figures 3-5
Taxonomic summary
Type locality: Galera Point, Colombia.
Holotype: UC; isotype: MICH.
Description: blades small, pink to red, 0.7-9 cm tall, to 20 mm wide, lanceolate to oval blades and sometimes bifurcate, entire margins, rounded apices; thallus 52-65μm thick. Medulla of thallus composed of few longitudinal lax filaments, 3-7 μm in diameter, connected to cortical cells of 3 or 4 arms, without stellate ganglia; single-layered cortex, cells 6-8 μm in diameter and 4-7 μm in width, angular-rounded, almost square and compact. Stipe short to 3 mm long and 153-300 μm in diameter, discoid holdfasts. Tetrasporangia are ovoid, cruciately divided, 6-8 μm in diameter, 4-7 μm high, scattered among cortical cells. Cystocarp rounded, 212-663 μm in width and 162-227 in height, swollen on the surface of the leaf, irregular or rounded carpospores, 8-10 μm in diameter.
References: Dawes and Mathieson (2008); Schneider (1975); Schneider and Searles (1991).
Specimens examined: State of Veracruz: near C50 “Riva Palacio” shipwreck (19°12’38.5” N, 96°03’13.0” W), 27 m deep, Coll. P. Ramírez García, September 26, 2015 (NI-2388), September 28, 29, 2016 (NI-2738, 2733, 2734, 2735), October 28, 2016 (NI-2635, 2637, 2638, 2736, 2737, July 28, 2017 (NI-2739), September 27, 2017 (NI-2725).
Habitat: on rock and nylon rope at a depth of 27 m.
Distribution in western Atlantic: Bermuda, Florida, Georgia, North Carolina, South Carolina, Cuba, Colombia (Guiry & Guiry, 2019); State of Veracruz, Gulf of Mexico.
Remarks
This alga was described on the basis of 2 collections (at 22 and 24 m) from Colombia, Galera Point being the type locality (Taylor, 1942). It was later reported from 17-55 m deep, from the southeastern United States (Schneider & Searles, 1991), Bermuda (Schneider, 2003), and Cuba (Suárez et al., 2015); however, it was not known in the southern Gulf of Mexico and particularly on the coast of Veracruz. An evident characteristic that separates H. hancockii from the species reported in the southern Gulf of Mexico is the absence of ganglionic cells in the medulla and its small size 0.7-9 cm, in addition to the presence of a single layer of cells in the cortex. There are also differences in the shape of the algae under study (lanceolate, oval), which differs with Halymenia echinophysa (lobed), H. elongata (cylindrical), H. pseudofloresii (foliose), H. rosea (oval to rounded); H. rosea is similar to H. hancockii in size, shape and margin but different due to the presence of ganglionic cells and the cortex of 1-3 layers of cells. Blades of Halymenia hancockii are attached to hard substrates but also to the shells of living populations of the snail Strombus alatus, a habitat not reported for H. hancockii. It continues to be a rare taxon with small populations and minor size unlike other species of this genus (Schneider et al., 2010). Schneider et al. (2010) pointed out with molecular bases that Halymenia pseudofloresii is the species distributed on the coasts of the western Atlantic (Bermuda), and H. floresii seems to be restricted to coasts of Europe (Wynne, 2017).
Dictyota J.V. Lamouroux, nom. cons.
Species previously reported from Veracruz (Fredericq et al., 2009; Galicia-García & Morales-García, 2007; Guiry & Guiry, 2019; Ortega et al., 2001): Dictyota bartayresiana J.V. Lamouroux, Dictyota ciliolata Sonder ex Kützing, Dictyota crenulata J. Agardh, Dictyota guineënsis (Kützing) P. Crouan & H. Crouan, Dictyota implexa (Desfontaines) J.V. Lamouroux, Dictyota menstrualis (Hoyt) Schnetter, Hörning & Weber-Peukert, Dictyota mertensii (C. Martius) Kützing, Dictyota pulchella Hörnig & Schnetter.
Dictyota humifusa Hörnig, Schnetter & Coppejans in Hörnig et al. 1992: 57, fig. 6.
Figures 6, 7
Taxonomic summary
Type locality: Punta Chengue, Santa Marta, Departamento del Magdalena, Colombia.
Holotype: COL.
Description: thallus consisting of delicate blades and stipe, prostrate, creeping, forming a densely interwoven clump, to 2.0 cm high; thallus light brown, commonly blue-iridescent throughout, although the iridescence is not banded; branching irregular to somewhat dichotomous, angle of branches 70-90°. Blades strap-shaped, 0.7-2-1 mm wide, 68-85 µm thick; apices broad and rounded. The species possesses a single-layered medulla, composed of square to rectangular cells, 47-59 µm thick × 60-68 µm, in parallel rows. Surface hairs tufted, scattered, seldom present. Adherent to the substrate by marginal rhizoids, without evident attachment discs. Antheridial sori scattered without invading the apices with 8-10 antheridia with stalk cells. Sporangia were not observed.
References: Hörnig et al. (1992); Littler and Littler (2000).
Specimens examined: State of Veracruz: “Ana Elena” shipwreck, Anegada de Afuera Reef (19°10’07.76” N, 95°51’39.12” W), 12 m deep, Coll. J. L. Godínez Ortega, March 21, 2014 (NI-2093).
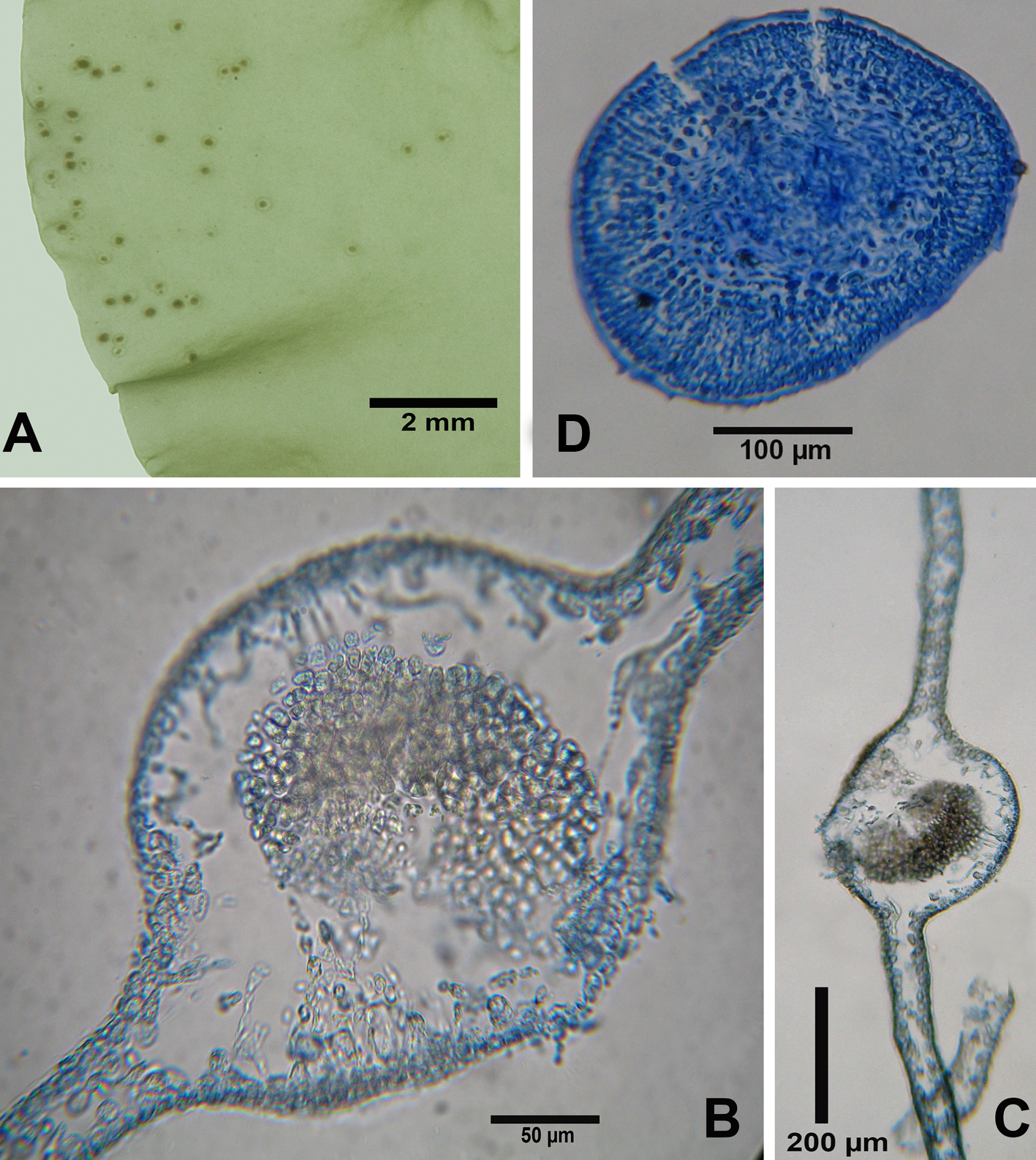
Habitat: On a ship hull at a depth of 12 m.
Distribution in western Atlantic: Florida, Bermuda, Belize, southern Caribbean, western Caribbean (Littler & Littler, 2000, Guiry & Guiry, 2019); State of Veracruz, Gulf of Mexico.
Remarks
Dictyota humifusa is separated from all species reported for Veracruz by its prostrate form and irregular branching, contrary to the erect form and the regularly dichotomous branching of the other species recorded from this State. Thalli of D. bartayresiana can occur in patches that are fixed to the substrate anywhere; however, our material cannot be that species because of its large size (10-15 cm), and D. humifusa just reaching 2 cm in length. It also does not resemble D. ciliolata or D. crenulata; those species have dentate margins, an absence of superficial proliferations and regular dichotomous ramification. Dictyota guineënsis is separated from D. humifusa by its medulla composed of 2 layers of cells. Although D. mertensii, D. menstrualis and D. pulchella display iridescence just like D. humifusa, those species cannot be considered because of their larger thallus sizes and regular branching. Dictyota implexa differs from D. humifusa by its regularly dichotomous branching, its twisted branches and a branch that narrows towards the apex. Although D. humifusa has been confused with D. friabilis Setchell because of its size, smooth margin without proliferations and its decumbent growth form, it is different from the studied species due to its irregular branching and absence of hairs. It was Bula-Meyer’s (1994) opinion that these 2 species were conspecific; Littler and Littler (2000, as D. pfaffii Schnetter) and De Clerck (2003), however, maintained D. friabilis as taxonomically distinct from D. humifusa.
Phylum Chlorophyta
Caulerpa J.V. Lamouroux
Species previously reported from Veracruz (Fredericq et al., 2009; Galicia-García & Morales-García, 2007; Guiry & Guiry, 2019; Ortega et al., 2001): Caulerpa ambigua Okamura, Caulerpa chemnitzia (Esper) J. V. Lamouroux, Caulerpa cupressoides (Vahl) C. Agardh, Caulerpa mexicana Sonder ex Kützing, Caulerpa microphysa (Weber Bosse) Feldmann, Caulerpa prolifera (Forsskål) J.V. Lamouroux, Caulerpa racemosa (Forsskål) J. Agardh, Caulerpa sertularioides (S.G. Gmelin) M. Howe, Caulerpa sertularioides f. brevipes (J. Agardh) Svedelius, Caulerpa sertularioides f. farlowii (Weber Bosse) Børgesen, Caulerpa sertularioides f. longiseta (Bory de Saint-Vincent) Svedelius, Caulerpa verticillata J. Agardh, Caulerpa webbiana Montagne.
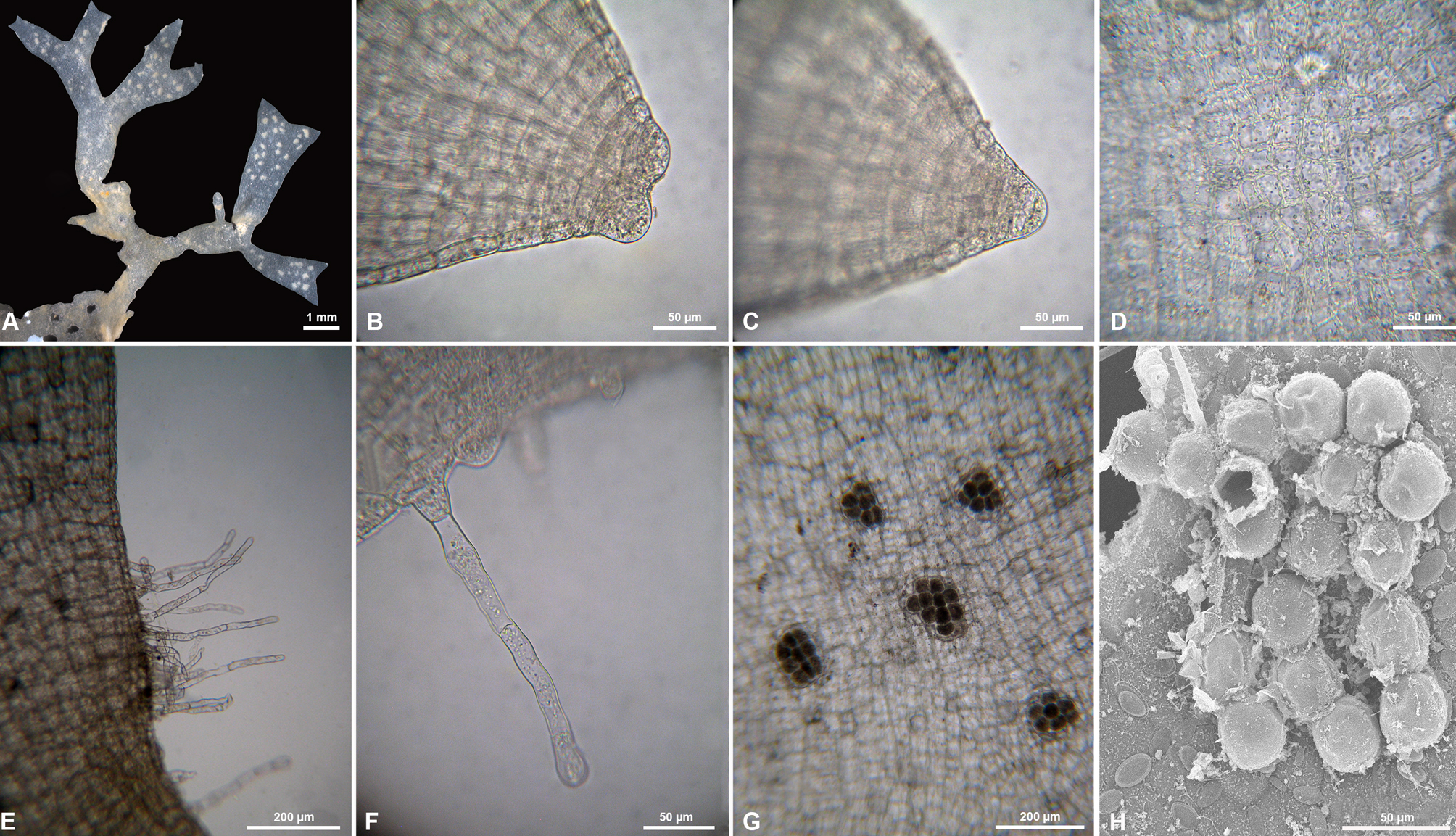
Caulerpa sertularioides f. longiseta (Bory de Saint-Vincent) Svedelius, 1906: 114-115, fig. 10.
Figure 8
Taxonomic summary
Type locality: not specified.
Description: frond erect to 21 cm high, feather-like, 20-28 mm wide with a naked stalk 2-4 cm long, frequently forked, distichous. Branchlet opposite, cylindrical, 230-400 µm diam, 7-14 mm long, upcurved or straight; bluntly pointed apex. Central axes flattened above, cylindrical below, 1-2 mm diam. Long stolons, 1.8-2.0 mm diam; rhizoids crawls, 300-400 µm diam. The stolon branches into fine rhizoids.
References: Littler and Littler (2000); Svedelius (1906); Taylor (1960).
Specimens examined: state of Veracruz: near C50 “Riva Palacio” shipwreck (19°12’38.5’’ N, 96°03’13.0’’ W), 27 m deep. Coll. David Carmona, September 26, 2015 (NI-2387); Coll. P. Ramírez García, 14 April, 2016 (NI-2726); Pantepec Reef (40°42’46.021 N, 74°0’21.388’’ W), 17 m deep. Coll. J. L. Godínez Ortega, September 29, 2015 (NI-2337, 2408).
Habitat: on fine sand at a depth of 27 m.
Distribution in western Atlantic: Caribbean, Cuba, Martinique, Brazil, Venezuela (Guiry & Guiry, 2019); Veracruz, Gulf of Mexico.
Remarks
Caulerpa sertularioides is separated from the 10 species reported for Veracruz by its unmistakable feather-like arrangement. However, the 3 forms of C. sertularioides have different characteristics. Blades of forma brevipes are 1-5 cm long and sessile, with opposite branchlets (pinnules) and rarely forked branches. Blades of forma farlowii are 5-10 cm long with radially arranged and somewhat curved branchlets, whereas C. sertularioides f. longiseta has branchlets opposite, cylindrical, needle-shaped (2-5 mm long) straight or slightly curved and apices blunty pointed. Caulerpa sertularioides f. longiseta was reported by Taylor (1960) from Mexico and other regions of America on the basis of blades with a naked stipe 1-3 cm long, and the whole thallus reaching 18 cm in height, not infrequently forked, distichous or occasionally tristichous. Similarly, Littler and Littler (2000) maintained this distinction for this forma but pointed out that the dichotomies can be to 2-4 orders. Caulerpa sertularioides f. longiseta is a taxon reported for the southern Gulf of Mexico (Espinoza-Avalos et al., 2015), even for Veracruz (Galicia-García & Morales-García, 2007); however, its large size in Veracruz stands out, as well as growing in subtidal waters (21 m deep). There is a previous record of a specimen found in shallow water from the Campeche littoral (Ortega, 1995), and its size was smaller (only 7 cm), with a naked stipe and a single dichotomy.
Avrainvillea Decaisne
Species previously reported from Veracruz (Fredericq et al., 2009; Galicia-García & Morales-García, 2007; Guiry & Guiry, 2019; Ortega et al., 2001): Avrainvillea longicaulis (Kützing) G. Murray & Boodle, Avrainvillea nigricans Decaisne.
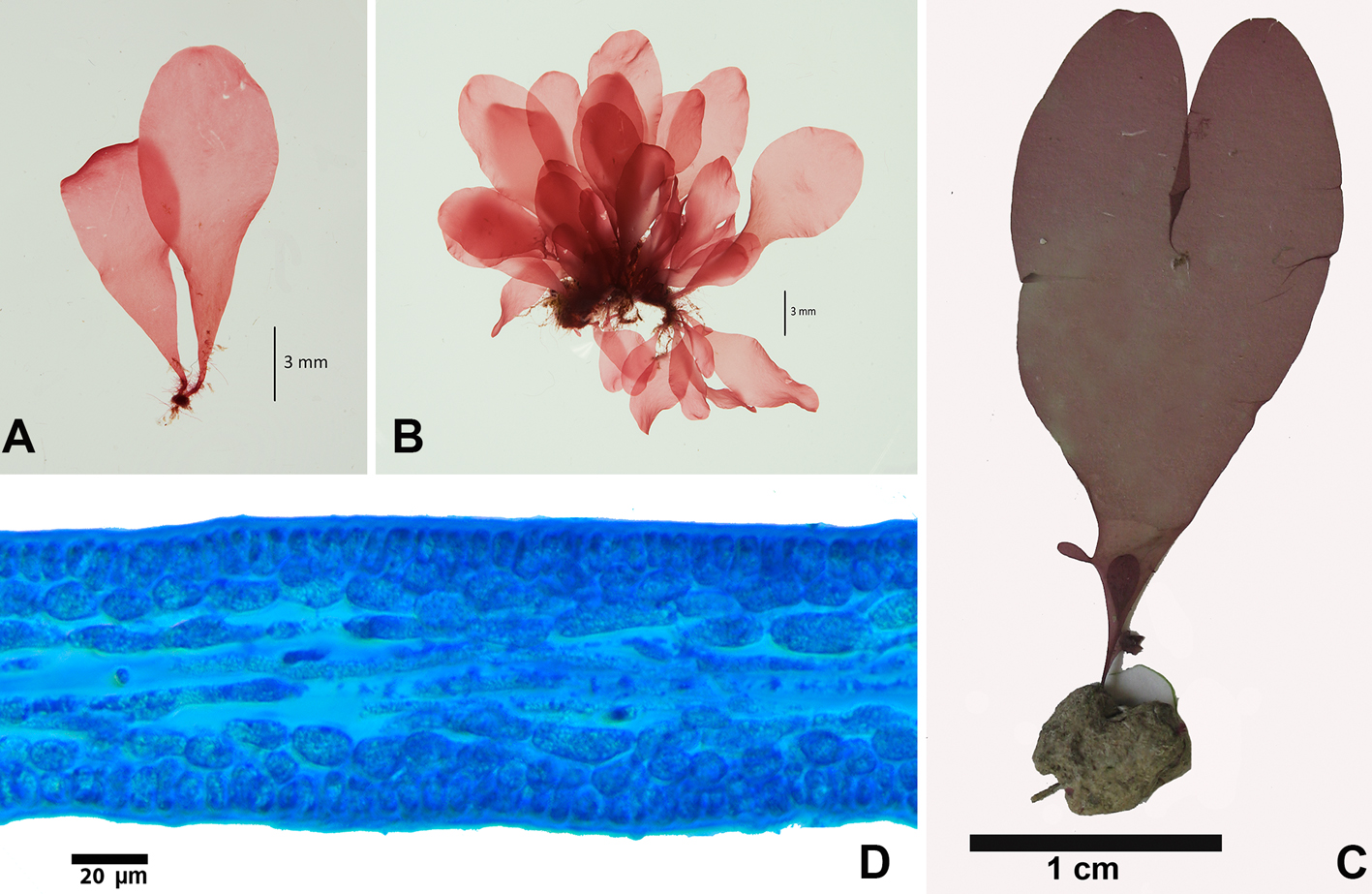
Avrainvillea levis f. translucens D.S. Littler & M.M. Littler, 1992: 394, fig. 11.
Figure 9
Taxonomic summary
Type locality: Great Blue Hole, Lighthouse Reef Atoll, Belize.
Holotype: US.
Description: thallus robust, solitary, erect, seldom branched, to 6 cm high. Translucent margins gray-green to yellow-green. Blade is flabellar to reniform, to 3.5 cm wide, 4 cm long and 1.4 mm thick, flat, smooth, with a fibrous texture, loosely woven, smooth; margins smoothly rounded, base shallowly lobed; with absent or indefinite zonation; medullary siphons to 23 µm diam., with rounded apices, slightly moniliform to straight, intermixed with fine colorless siphons, 7-9 µm diam., slightly moniliform, rarely cylindrical, with a diameter of 7-9 μm; cortex lacking. Stipe is cylindrical to slightly flattened to 5 mm diam., to 1.8 cm long, siphons of the stipe are slightly moniliform to straight, 20 µm diam.; surface siphons 7 µm diam., slightly moniliform to cylindrical. Reproductive specimens were not observed.
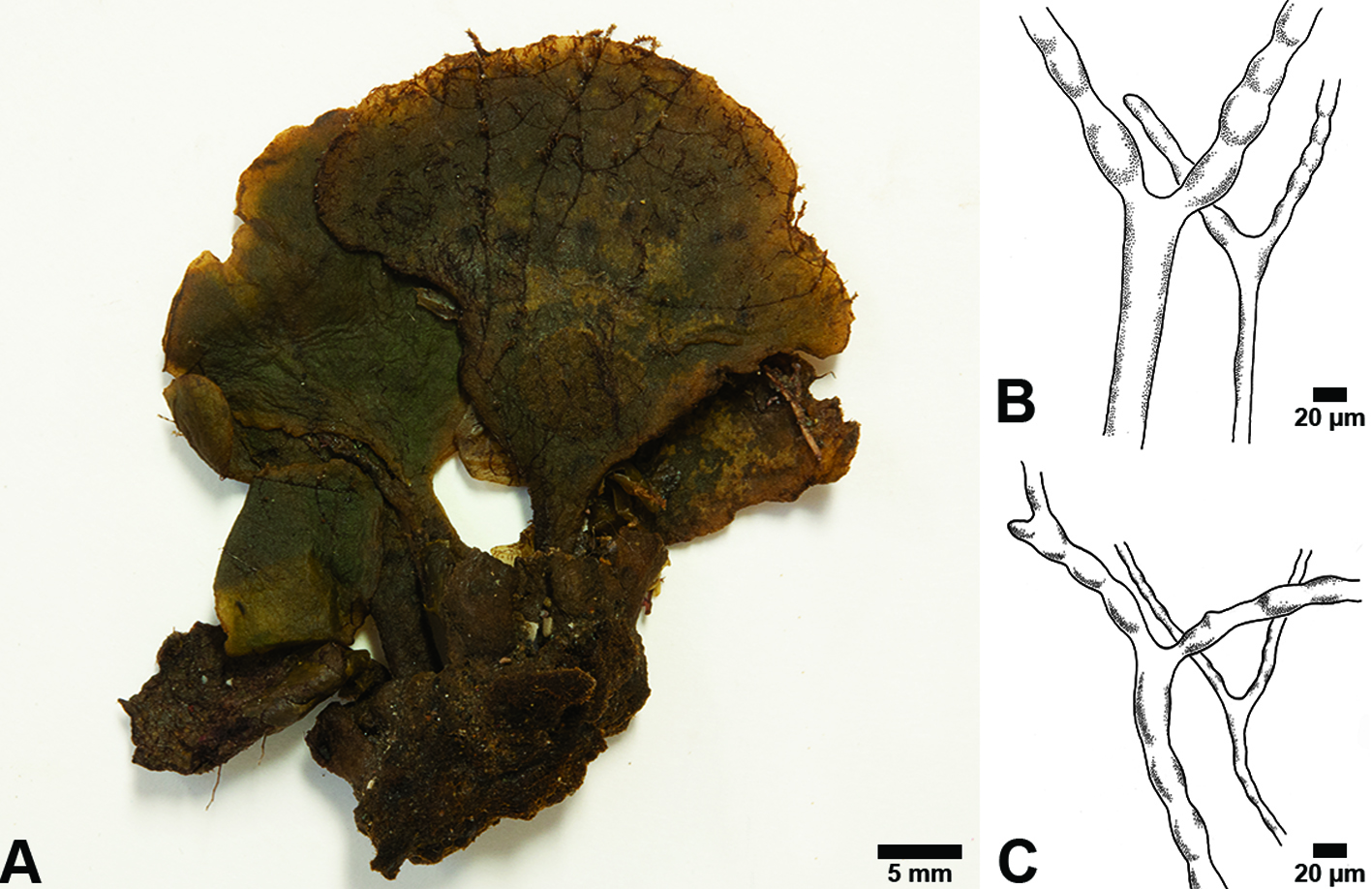
References: Littler and Littler (2000), Santos and Nunes (2015).
Specimens examined: state of Veracruz: Segunda laja, near Chachalacas (19°29’9.913” N, 96°18’33.271” W). Coll. J. L. Godínez Ortega, 17 April 2016 (NI-2732).
Habitat: on sand, at a depth of 5 m., with Dictyopteris and Centroceras as epiphytes.
Distribution in western Atlantic: Bahamas, Western Caribbean (Littler & Littler, 2000), State of Veracruz, Gulf of Mexico.
Remarks
The samples studied are in agreement with the description of this forma of the species by Littler and Littler (1992, 2000), although the specimens studied by those authors showed considerably larger sizes compared to those in this study. Our material cannot be A. nigricans or A. longicaulis because of the large size of those species (30 cm and 14 cm, respectively); however, A. levis f. translucens (6 cm high) is more similar to A. longicaulis by its moniliform siphons and lack of cortex in the distal region, but that species has siphons that reach up to 70 μm in diameter, unlike forma translucens with siphons to 23 μm in diameter. In the opinion of Santos and Nunes (2015) A. levis M. Howe has been confused with A. elliottii A. Gepp & E.S. Gepp (Florida), although in that species the presence of a blade with well-defined zonation that have a leathery texture, small appressorium to secure it to the substrate and a thicker blade distinguishes it from A. levis with its very thin blade lacking zonation and with a large appressorium.
Udotea J.V. Lamouroux
Species previously reported from Veracruz (Galicia-García & Morales-García, 2007; Guiry & Guiry, 2019; Ortega et al., 2001): Udotea cyathiformis Decaisne, Udotea flabellum (J. Ellis & Solander) M. Howe, Udotea spinulosa M. Howe.
Udotea caribaea D.S. Littler & M.M. Littler, 1990: 211, fig. 2.
Figure 10

Taxonomic summary
Type locality: northwestern Tobacco Range, Belize.
Holotype: US.
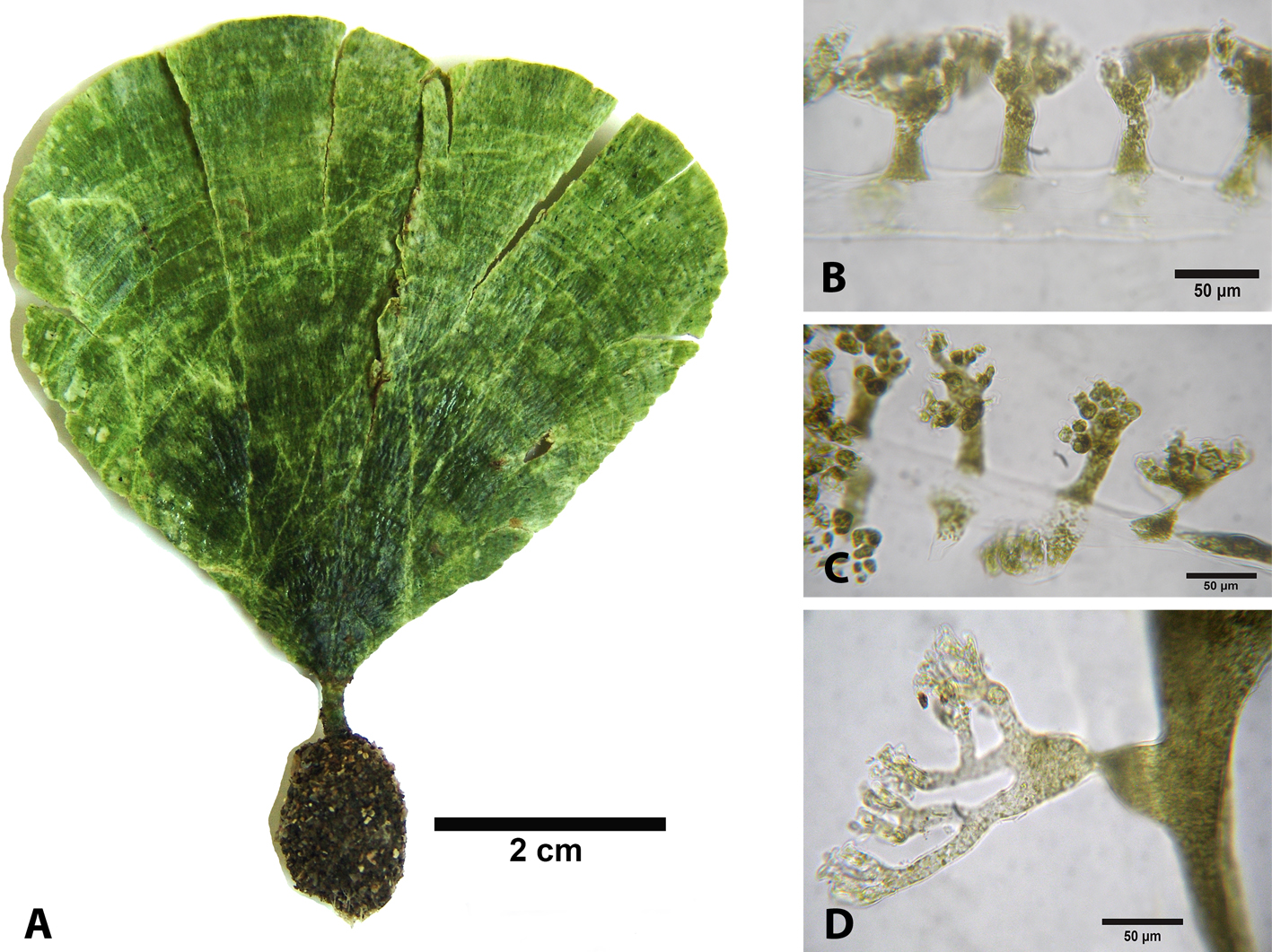
Description: thallus consisting of delicate blades, white green, slightly calcified to 8 cm in height. Blade to 6 cm high and 7 cm wide, zonation present, to 1 mm thick; ecorticated, siphons without lateral appendages of 39-54 μm diam., sometimes constricted above the dichotomies. Cylindrical stipe, to 8 mm high and 2 mm wide; there is a difference between the stipe and the blade. Stipe siphons of 43-84 μm diam., di-trichotomic lateral appendages with short obtuse apices to 10 μm in diameter. Tangled rhizoids. Reproduction not observed.
References: Acosta-Calderón et al. (2018); Collado-Vides et al. (2009); Littler and Littler (1990, 2000).
Specimens examined: state of Veracruz: near C50 “Riva Palacio” shipwreck (19°12’38.5” N, 96°03’13.0” W), 28 m deep. Coll. P. Ramírez García, September 27, 2017 (NI-2724A).
Habitat: on fine sand at a depth of 28 m.
Distribution in western Atlantic: Florida, Bermuda, Belize, Panama, southern Caribbean, western Caribbean, Brazil (Guiry & Guiry, 2019; Littler & Littler, 2000); Veracruz, Gulf of Mexico.
Remarks
Udotea caribaea is separated from U. cyathiformis by its cup-shaped thalli and from U. flabellum by the presence of cortex and coriaceous texture. Udotea spinulosa contrasts with U. caribaea by presenting continuous lateral siphons. In U. caribaea the shape of the blade and the siphons coincide with that reported by Littler and Littler (2000); however, there is a clear demarcation between the stipe and the leaf, in addition to not being totally calcified.
Udotea dixonii D.S. Littler & M.M. Littler, 1990: 220, fig. 8.
Figure 11
Taxonomic summary
Type locality: on the east side of Curlew Cay, Belize.
Holotype: US.
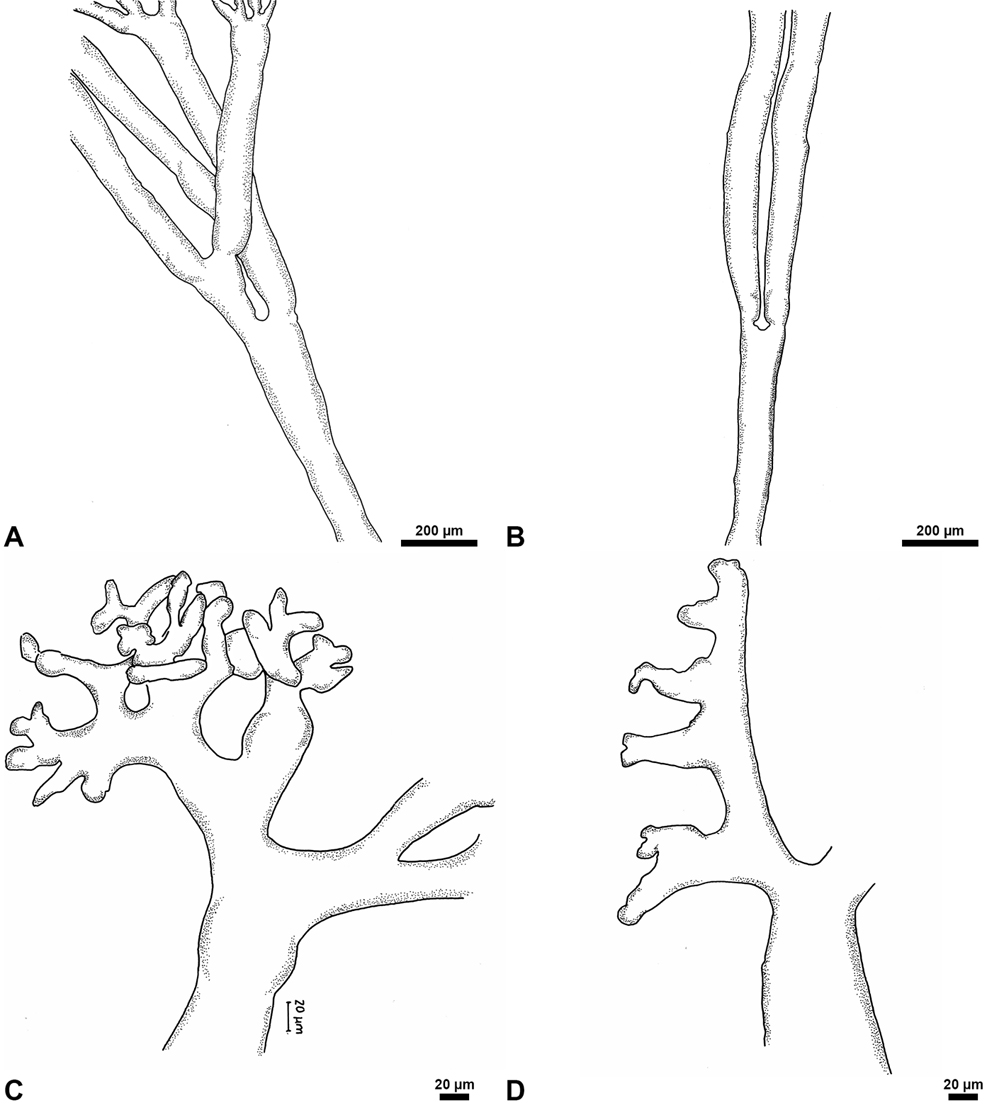
Description: thallus consisting of a coriaceus fan-shaped blade, green, calcified to 8 cm high, occasionally solitary and to 1 mm in thickness. Blade to 6 cm high and 6 cm wide, zonation present, to 2 mm thick; corticated, siphons of 19-42 μm diam., opposite lateral appendages in 2 vertical rows with swollen knobs apices of short pedicels. Cylindrical stipe, to 5 mm high and 3 mm wide; there is a difference between the stipe and the blade. Stipe siphons of 47-80 μm diam., di-trichotomous lateral appendages with short, fingerlike, flattened apices to 10 μm in diameter. Tangled rhizoids. Reproduction not observed.
Reference: Acosta-Calderón et al. (2018); Collado-Vides et al. (2009); Littler and Littler (2000).
Specimens examined: State of Veracruz: near C50 “Riva Palacio” shipwreck (19°12’38.5” N, 96°03’13.0” W), 28 m deep. Coll. P. Ramírez García, September 27, 2017 (NI-2720).
Habitat: on fine sand at a depth of 28 m.
Distribution in western Atlantic: Florida, Belize, Panama, southern Caribbean, western Caribbean, Brazil (Guiry & Guiry, 2019; Littler & Littler, 2000); Veracruz, Gulf of Mexico.
Remarks
Udotea caribaea is separated from U. cyathiformis by its cup-shaped thalli and from U. flabellum by the presence of cortex and coriaceous texture. Udotea spinulosa contrasts with U. caribaea by presenting continuous lateral siphons. In U. caribaea the shape of the blade and the siphons coincide with that reported by Littler and Littler (2000); however, there is a clear demarcation between the stipe and the leaf, in addition to not being totally calcified.
Udotea dixonii D.S. Littler & M.M. Littler, 1990: 220, fig. 8.
Figure 11
Taxonomic summary
Type locality: on the east side of Curlew Cay, Belize.
Holotype: US.
Description: thallus consisting of a coriaceus fan-shaped blade, green, calcified to 8 cm high, occasionally solitary and to 1 mm in thickness. Blade to 6 cm high and 6 cm wide, zonation present, to 2 mm thick; corticated, siphons of 19-42 μm diam., opposite lateral appendages in 2 vertical rows with swollen knobs apices of short pedicels. Cylindrical stipe, to 5 mm high and 3 mm wide; there is a difference between the stipe and the blade. Stipe siphons of 47-80 μm diam., di-trichotomous lateral appendages with short, fingerlike, flattened apices to 10 μm in diameter. Tangled rhizoids. Reproduction not observed.
Reference: Acosta-Calderón et al. (2018); Collado-Vides et al. (2009); Littler and Littler (2000).
Specimens examined: State of Veracruz: near C50 “Riva Palacio” shipwreck (19°12’38.5” N, 96°03’13.0” W), 28 m deep. Coll. P. Ramírez García, September 27, 2017 (NI-2720).
Habitat: on fine sand at a depth of 28 m.
Distribution in western Atlantic: Florida, Belize, Panama, southern Caribbean, western Caribbean, Brazil (Guiry & Guiry, 2019; Littler & Littler, 2000); Veracruz, Gulf of Mexico.
Remarks
The shape of the thallus and siphons (blade and stipe) coincides with that reported for this species by Littler and Littler (1990; 2000). The lateral appendages of the blade are swollen (Fig. 11B) and in some zones (Fig. 11C) could correspond with the reproductive structures, still unknown for this species. These swollen appendages in U. dixoni differ from those in U. cyathiformis, U. flabellum and U. spinulosa.
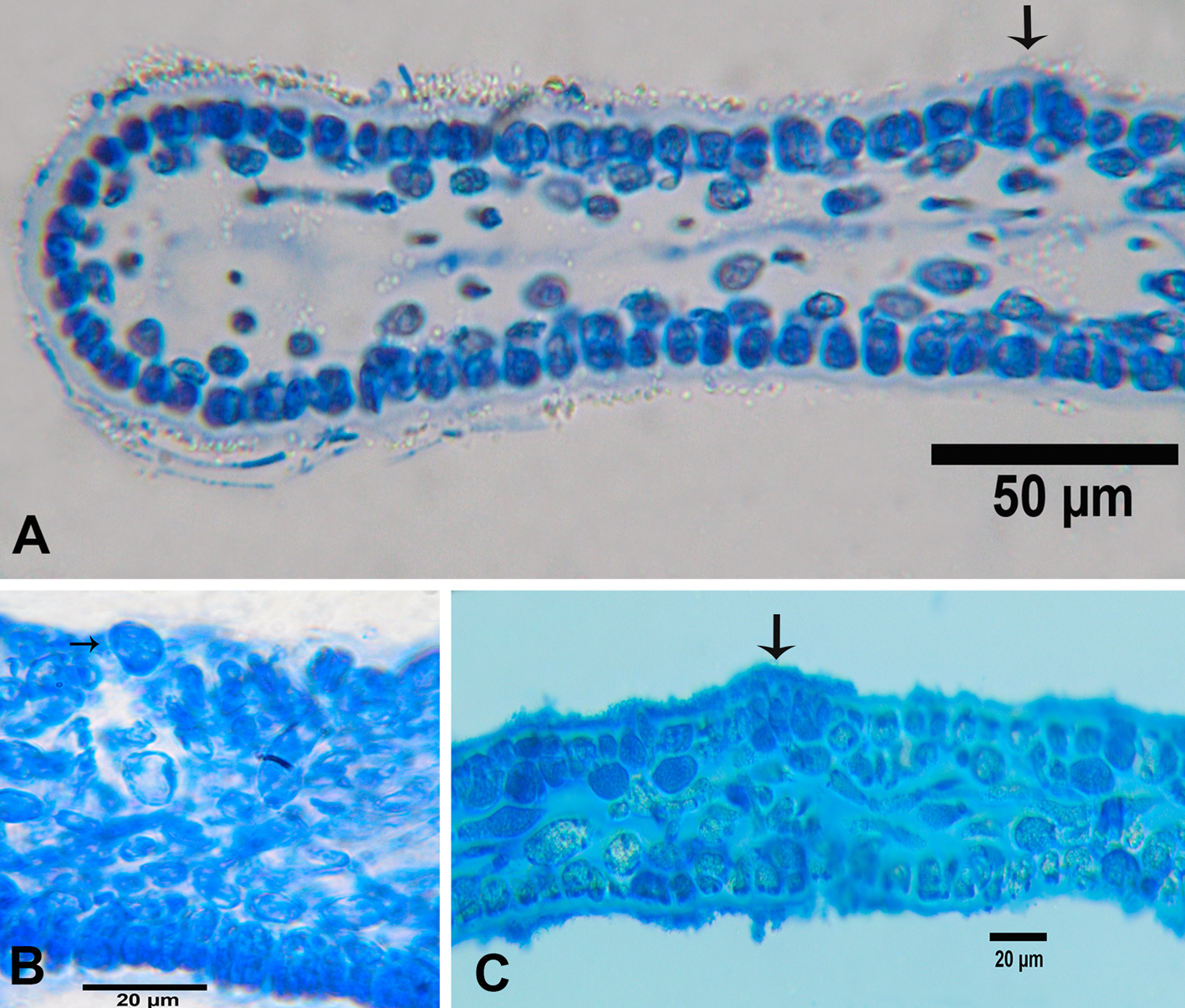
Udotea unistratea D.S. Littler & M.M. Littler, 1990: 240, fig. 21.
Figure 12, 13
Taxonomic summary
Type locality: on the east side of Carrie Bow Cay, Belize.
Holotype: US.
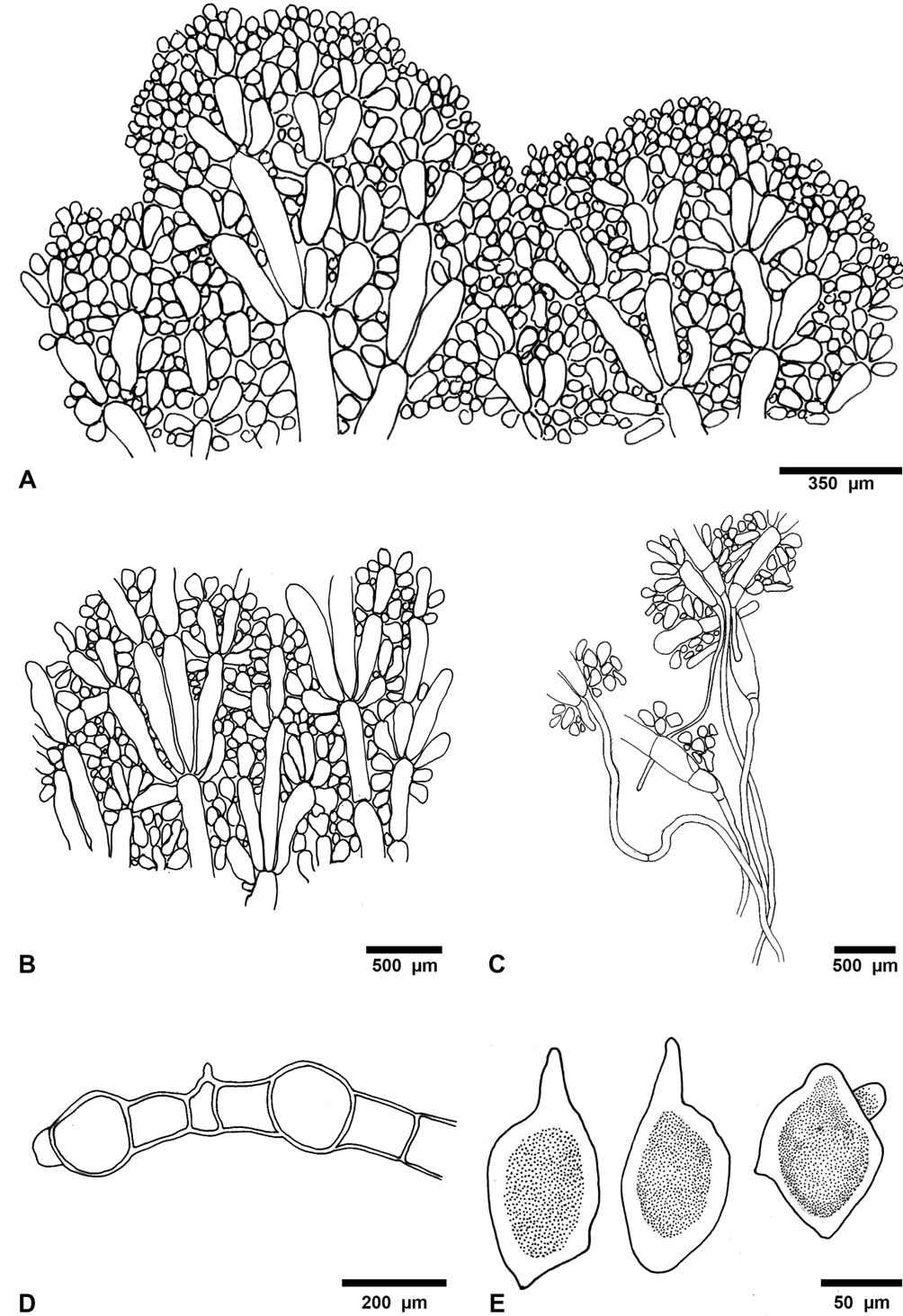
Description: small thallus to 5 cm high, fan-shaped, lightly calcified, with a delicate appearance, blue-green to olive-green. Blade to 3 cm wide, 2 cm long, 0.1-0.4 mm thick; cortication absent; blade consisting of a single layer of siphons, generally coarse, zonation faint; the siphons of the blade are long, 82-155 μm in diameter, with constrictions above the dichotomies, lacking lateral appendages, strictly aligned or parallel to one another, dichotomies occurring equidistant from stipe; base of blade fibrous. Stipe to 0.1-1 cm diam., 1-5 mm long, the siphons of the stipe are 26-50 μm in diameter; the appendages are subdichotomously branched, and end in short digitiform lobes, with blunt apices. Rhizoids fine, fibrous.
Reference: Littler and Littler (1990, 2000).
Specimens examined: State of Veracruz: “Ana Elena” shipwreck, Anegada de Afuera Reef (19°10’7.20” N, 95°51’39.0” W), 12 m deep, Coll.: J. L Godínez Ortega, 4 October 2013 (NI-1189).
Habitat: rare in sand pockets on deep vertical walls; at a depth of 8-12 m.
Distribution in western Atlantic: Florida, Belize, Bahamas, Caribbean, Cuba, Jamaica, Martinique, Puerto Rico, Virgin Islands (Guiry & Guiry, 2019), Veracruz, Gulf of Mexico.

Remarks
There are strong differences with the species cited for Veracruz, mainly due to the absence of appendages in blade siphons, except with U. cyathiformis, but that species has a cup-shaped thallus. There is a similarity with U. caribaea for presenting siphons on the blade without appendages with constrictions above the dichotomies, but U. unistratea contrasts with this species by the siphons in the stalk, with short digitiform lobes. This species has been reported at Santa Rosalía Lighthouse, Campeche, from shallow waters (Callejas-Jiménez et al., 2005) and the Campeche Bank between 30 and 49 m depth (Mateo-Cid et al., 2013, Mendoza-González et al., 2013). Littler and Littler (2000) reported it on vertical walls between 24-46 m depth, which is more consistent with the Veracruz record.
Anadyomene J.V.Lamouroux, nom. cons.
Species previously reported from Veracruz (Galicia-García & Morales-García, 2007; Ortega et al., 2001): Anadyomene stellata (Wulfen) C. Agardh.
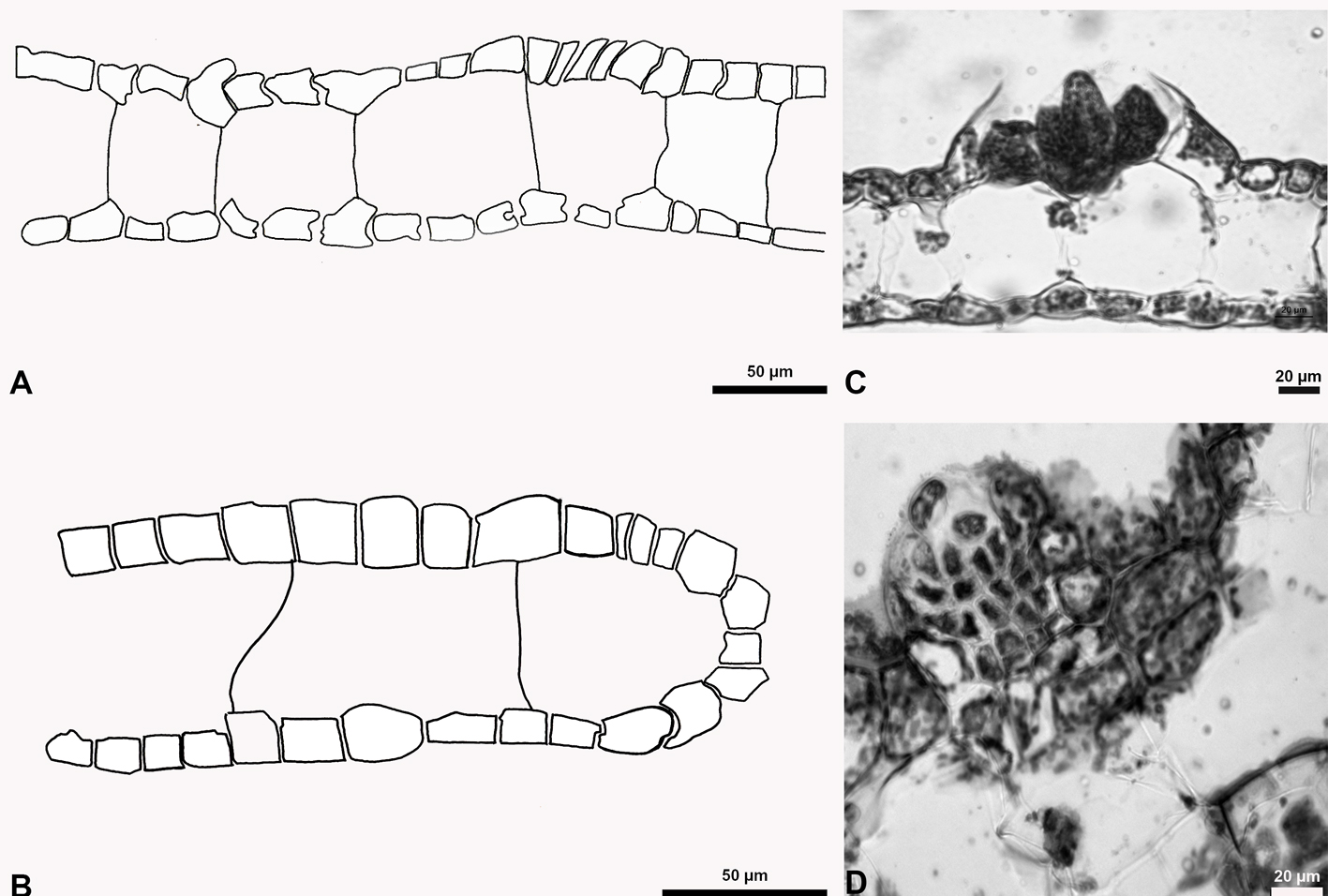
Anadyomene saldanhae A.B. Joly & E.C. Oliveira, 1969: 30, figs 1-3.
Figures 14, 15

Taxonomic summary
Type locality: Banco Dogareza, off the coast of Victoria, Espirito Santo State, Brazil.
Holotype: SPF.
Description: thallus laminate, prostrate, delicate, non-perforate, bright green, 1-2 cm high. Blades are fan-shaped, 5-6 mm long and 4-5 mm wide, with a single layer of cells; blades firm margins smoothly rounded; marginal cells small, ovoid; veins (swollen cells) barely visible, proliferous peripherally from the base, branching polychotomous at distal segment; length of the middle blade cells (0.38 × 0.11 mm) in width (0.21-0.35 mm) ratio 4:1. Interstitial cells small, ovoid, in a random arrangement. Other interstitial cells showing foot-like tentacula typically wedged between adjacent cells. Rhizoids not forming stipes, lightly entangled, originating from basal veins, generally suspended free or adhering to the substratum.
References: Alves et al. (2011); Bula-Meyer (1982); Joly and Oliveira-Filho (1969); Littler and Littler (1991, 2000).
Specimens examined: State of Veracruz: “Ana Elena” shipwreck, Anegada de Afuera Reef (19°10’07.76” N, 95°51’39.12” O), 12 m deep, Coll. J. L. Godínez Ortega, March 21, 2014 (NI-1779), May 27, 2014 (NI-1683, MEXU=2287) and February 21, 2015 (NI-1781).
Habitat: epiphitic on Dictyota humifusa, Jania pumila and Lobophora variegata, at a depth of 12 m.
Distribution in western Atlantic: Bermuda, Florida, North Carolina, Belize, Bahamas, Caribbean, Cuba, Martinique, Brazil (Guiry & Guiry, 2019); Veracruz, Gulf of Mexico.
Remarks
Anadyomene stellata is separated from A. saldanhae by its size (10 cm) growing in shallow areas, erect aspect, interstitial cells arranged in parallel, interstitial cells H-shaped with each end having blunt forks straddling adjacent rhizoids (rarely with tentacula). Vázquez-Machorro et al. (2016) reported A. saldanhae as a new SAV record; however, it was not described or illustrated. Thus, we include this species in order to present its description and illustration. The material analyzed and identified as Anadyomene saldanhae from the State of Veracruz matches the type material and the descriptions and illustrations provided by Bucher et al. (1990), Littler and Littler (1991, 1997, 2000) and Schneider and Searles (1991).
Discussion
Nine new records are reported for Veracruz: 2 taxa of red algae, 1 taxon of brown algae and 6 taxa of green algae. These additions complement and enrich the marine algal flora of Veracruz, in particular, the Veracruz Reef System [SAV]. The 9 records increase the flora of the Gulf of Mexico by 2% (Fredericq et al., 2009; Pedroche & Sentíes, 2003). Hypoglossum simulans and Halymenia hancockii are rare and infrequent; so their record is interesting in Veracruz (within the southern Gulf of Mexico; Schneider & Searles, 1991). Hypoglossum simulans was originally reported from the Antilles, and it is interesting to find it in the Veracruz region. These records from the Gulf of Mexico are important in order to complete their distribution in subtropical and tropical waters. Based on the great marine regions, the southern Gulf of Mexico is separated from the Caribbean. So the presence of a Caribbean plant indicates that its geographical distribution is wider (Wilkinson et al., 2009). The presence of a male plant of D. humifusa (with anteridial sori) had not been reported in previous studies of the area neither in the monograph of the genus Dictyota from the Indian Ocean (De Clerck, 2003; Littler & Littler, 2000). Thus, this study complements the reproductive knowledge of the species. The genus Caulerpa in Veracruz includes 50% of the species reported for the Gulf of Mexico (18 species; Fredericq et al., 2009). Caulerpa sertularioides is a species that is widely distributed in tropical waters, although its forma longiseta is larger in size and widely branched and found only in waters greater than 20 m deep (near the C-50 and Pantepec Reef). Fernández-García et al. (2012) noted that thalli in shallow areas with high solar radiation tended to be smaller with thicker stolons, whereas at greater depths and with fine sediments, the thalli are larger and the stolons fine and more often branched. This adaptation appears to allow the alga to bury itself more firmly in the sediments and to more easily obtain nutrients. Avrainvillea and Udotea in Mexico are mainly distributed in the Caribbean (Acosta-Calderón et al., 2018; Ortega et al., 2001); however, there are now 6 species of Udotea recognized in Veracruz, duplicating those previous records. The genus Avrainvillea in Mexico is mainly distributed in the Caribbean (12 species; Littler & Littler, 2000); the presence of this genus in the Gulf of Mexico is infrequent (12%, A. levis, A. longicaulis, A. nigricans; Fredericq et al., 2009) with its presence recorded so far only from Veracruz in shallow waters (A. longicaulis, A. nigricans). Littler and Littler (2000) pointed out this forma as rare and restricted to deep water (5-60 m). Avrainvillea levis forma translucens has been recorded only a few times in the western Atlantic. The genus Udotea in Veracruz includes 21% of the species reported for the Gulf of Mexico (14 species; Fredericq et al., 2009). Udotea caribaea, U. dixonii and U. unistratea are reported from depths between 15-54 m depth (Littler & Littler, 2000), depths that coincide with those found in Veracruz (18-28 m). Acosta-Calderón et al. (2018) pointed out that Campeche is the boundary of the distribution of Udotea in the Caribbean because the Yucatán Peninsula is the physical barrier between the Caribbean and the Gulf of Mexico. So the 3 species found in Veracruz are interesting findings in subtidal waters that expand their ranges. Acosta-Calderón et al. (2018) pointed out that U. unistratea has affinities for depth greater than 7 m. Littler and Littler (1990) reported it at 46 m and Ballantine et al. (2011) at 70 m. Anadyomene saldanhae constitutes a new record for SAV as a geographical distribution of species with affinities with tropical regions and an amphi-Atlantic distribution (Cape Verde and Caribbean Sea; Collado-Vides et al., 2013). Previously, it had been reported only from Florida and the Caribbean Sea (Littler & Littler, 2000). Collado-Vides et al. (2013) reported that A. saldanhae from St. Croix (Virgin Islands, Caribbean Sea) is closely related to A. stellata from Florida. Anadyomene saldanhae complements the geographical distribution of species with affinities to tropical regions.
Considering the scarcity of studies on shipwrecks and some poorly explored reefs, and the number of new records, we assume that the reported algal diversity of this region is still underestimated. Therefore, to have a broader knowledge of this group of organisms, as well as its species distribution, further floristic exploration in the Gulf of Mexico is called for. Molecular studies are also desirable to compare our populations with populations of these species occurring elsewhere. Because fishing, diving, and tourism are intensifying in the area, consideration should be given to conservation efforts.
Acknowledgments
To Susana Guzmán for her advice on digital stereoscopic microscopy; to Carmen Loyola the photography of figures 3, 8 and 9, and to Elvia Esparza for the drawings. This work was done within the framework of the Red para el Análisis y Síntesis de la Zona Costera Veracruzana, Golfo de México (RASZCOV), supported by the Programa para el Desarrollo Profesional Docente (PRODEP-SEP).
References
Acosta-Calderón, J. A., Hernández-Rodríguez, C., Mendoza-González, A. C., & Mateo-Cid, L. E. (2018) Diversity and distribution of Udotea genus J.V. Lamouroux (Chlorophyta, Udoteaceae) in the Yucatán Peninsula littoral, Mexico. Phytotaxa, 345, 179–218.
Alves, A. M., Souza-Gestinari, L. M., & Nascimento-Moura, C. W. D. (2011). Morphology and taxonomy of Anadyomene species (Cladophorales, Chlorophyta) from Bahia, Brazil. Botanica Marina, 54, 135–145.
Ballantine, D., Athanasiadis, A., & Ruiz, H. (2011) Notes on the benthic marine algae of Puerto Rico. X. Additions to the flora. Botanica Marina, 54, 293–302.
Bucher, K. E., Norris, J. N., Littler, M. M., & Littler, D. S. (1990). Marine algae new to Florida, including Trichosolen molassensis sp. nov. (Chlorophyta) and Diplothamnion jolyi var. ecellulare var. nov. (Rhodophyta). Cryptogamic Botany, 1, 295–307.
Bula-Meyer, G. (1982) Adiciones a las clorofíceas marinas del Caribe colombiano. I. Anales Instituto de Investigaciones Marinas. Punta de Betín, 12, 117–136.
Bula-Meyer, G. (1994). Notas sobre Dictyota pfaffii y D. humifusa (Dictyotales, Phaeophyta). Anales Instituto de Investigaciones Marinas. Punta de Betín, 23, 177–181.
Callejas-Jiménez, M. E., Sentíes G., A., & Dreckmann, K. M. (2005). Macroalgas de Puerto Real, Faro Santa Rosalía y Playa Preciosa, Campeche, México, con algunas consideraciones florísticas y ecológicas para el estado. Hidrobiológica, 15, 89–96.
Collado-Vides, L., Ávila, C., Blair, S., Leliaert, F., Rodríguez, D., Thyberg, T. et al. (2013). A persistent bloom of Anadyomene J.V. Lamouroux (Anadyomenaceae, Chlorophyta) in Biscayne Bay, Florida. Aquatic Botany, 111, 95–103.
Collado-Vides, L., Suárez, A. M., & Cabrera, R. (2009) Una revisión taxonómica del género Udotea en el caribe mexicano y cubano. Revista de Investigaciones Marinas, 30, 145–161.
Dawes, C. J., & Mathieson, A. C. (2008). The seaweeds of Florida. Gainesville: University Press of Florida.
De Clerck, O. (2003). The genus Dictyota in the Indian Ocean. Opera Botanica Belgica, 13, 1–205.
Espinoza-Ávalos, J., Aguilar-Rosas, L. E., Aguilar-Rosas, R., Gómez-Poot, J. M., & Raigoza-Figueras, R. (2015). Presencia de Caulerpaceae (Chlorophyta) en la Península de Yucatán, México. Botanical Sciences, 93, 845–854.
Fernández-García, C., Cortés, J., Alvarado, J.J., & Nivia-Ruiz, J. (2012). Physical factors contributing to the benthic dominance of the alga Caulerpa sertularioides (Caulerpaceae, Chlorophyta) in the upwelling Bahía Culebra, North Pacific of Costa Rica. Revista de Biología Tropical, 60, Suppl. 2, 93–107.
Fredericq, S., Cho, T. O., Earle, S. E., Gurgel, C. F., Krayesky, D. M., Mateo-Cid, L. E. et al. (2009). Seaweeds of the Gulf of Mexico. In D. L. Felder, & D. K. Camp (Eds.), Gulf of Mexico: its origins, waters and biota. Vol. 1. Biodiversity (pp. 187–259). College, Texas: Texas A & M University Press.
Galicia-García, C., & Morales-García, A. (2007) Investigaciones sobre macroalgas realizadas en el sistema arrecifal veracruzano. In A. Granados-Barba, L. G. Abarca-Arenas y J. M. Vargas-Hernández (Eds.), Investigaciones científicas en el Sistema Arrecifal Veracruzano (pp. 141–160). Campeche: Universidad Autónoma de Campeche.
Godínez-Ortega, J. L., Ramírez-García, P., Granados-Barba, A., & Vázquez-Machorro, A. (2015). Hypoglossum hypoglossoides (Delesseriaceae: Rhodophyta): un registro nuevo para la costa atlántica de México. Revista Mexicana de Biodiversidad, 86, 1078–1082.
Guiry, M. D., & Guiry, G. M. (2019). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Retrieved on 14 January 2019, from: http://www.algaebase.org
Hörnig, I., Schnetter, R., & Prud’ homme van Reine, W. F. (1992). The genus Dictyota (Phaeophyceae) in the North Atlantic. 1. A new generic concept and new species. Nova Hedwigia, 54, 45–62.
Horta, P., Yokoya, N., Guimarães, S., Bacci, D., & Oliveira, E. (2003). Morphology, reproduction and development of Hypoglossum hypoglossoides (Stackhouse) Collins and Hervey (Ceramiales, Rhodophyta) from the south and southeastern Brazilian coast. Revista Brasileira de Botânica, 26, 453–460.
Joly, A. B., & de Oliveira-Filho, E. C. (1969). Notes on Brazilian algae II. A new Anadyomene of the deep water flora. Phykos, 7, 27–31.
Littler, D. S., & Littler, M. M. (1990). Systematics of Udotea species (Bryopsidales, Chlorophyta) in the tropical western Atlantic. Phycologia, 29, 206–252.
Littler, D. S., & Littler, M. M. (1991). Systematics of Anadyomene species (Anadyomenaceae, Chlorophyta) in the tropical western Atlantic. Journal of Phycology, 27, 101–118.
Littler, D. S., & Littler, M. M. (1992). Systematics of Avrainvillea (Bryopsidales, Chlorophyta) in the tropical western Atlantic. Phycologia, 31, 375–418.
Littler, D. S., & Littler, M. M. (1997). An illustrated flora of the Pelican Cays, Belize. Bulletin of the Biological Society of Washington, 9, 1–149.
Littler, D. S., & Littler, M. M. (2000). Caribbean reef plants: an identification guide to the reef plants of the Caribbean, Bahamas, Florida and Gulf of Mexico. Washington D.C.: OffShore Graphics Inc.
Mateo-Cid, L. E., Mendoza-González, A. C., Ávila-Ortiz A. G., & Díaz-Martínez, S. (2013). Algas marinas bentónicas del litoral de Campeche, México. Acta Botánica Mexicana, 104, 53–92.
Mateo-Cid, L. E., Mendoza-Gonzáles, A. C., & Searles, R. B. (2006). A checklist and seasonal account of the deepwater Rhodophyta around Cozumel Island on the Caribbean coast of Mexico. Caribbean Journal of Science, 42, 39–52.
Mendoza-González, C., Mateo-Cid, L. E., & López Garrido, P. H. (2013). Algas marinas bentónicas asociadas a pecios y otras estructuras submareales de Campeche, México. Acta Botánica Venezuelica, 36, 119–140.
Oliveira-Filho, E. C. de. (1969). Algas marinhas do sul do estado do Espirito Santo (Brasil). I.- Ceramiales. Boletim da Faculdade de Filosofia, Ciências e Letras da Universidade de São Paulo, Série Botânica, 26, 1–297.
Ortega, M. M. (1995). Observaciones del fitobentos de la laguna de Términos, Campeche, México. Anales del Instituto de Biología, Univerisdad Nacional Autónoma de México. Serie Botánica, 66, 1–36.
Ortega, M. M., Godínez, J. L., & Garduño-Solórzano, G. (2001). Catálogo de algas bénticas de las costas mexicanas del Golfo de México y Mar Caribe. México D.F.: Instituto de Biología, Universidad Nacional Autónoma de México (Cuadernos 34).
Ortiz-Lozano, L., Pérez-España, H., Granados-Barba, A., González-Gándara, C., Gutiérrez-Velázquez, A., & Martos, J. (2013). The Reef Corridor of the Southwest Gulf of Mexico: Challenges for its management and conservation. Ocean & Coastal Management, 86, 22–32.
Pedroche, F. F., & Sentíes, A. (2003) Ficología marina mexicana. Diversidad y Problemática actual. Hidrobiológica, 13, 23–32.
Santos, G. N., & Nunes, J. M. C. (2015). True identity of Avrainvillea and Rhipilia (Bryopsidales, Chlorophyta) from the coast of Bahia, Brazil. Phytotaxa, 213, 71–86.
Schneider, C. W. (1975). North Carolina marine algae. V. Additions to the flora of Onslow Bay, including the reassignment of Fauchea peltata Taylor to Weberella schmitz. British Phycological Journal, 10, 129–138.
Schneider, C. W. (2003). An annotated checklist and bibliography of the marine macroalgae of the Bermuda Islands. Nova Hedwigia, 76, 275–361.
Schneider, C. W., Lane, C. E., & Saunders, G.W. (2010). Notes on the marine algae of the Bermudas. 11. More additions to the benthic flora and a phylogenetic assessment of Halymenia pseudofloresii (Halymeniales, Rhodophyta) from its type locality. Phycologia, 49, 154–168.
Schneider, C. W., & Searles, R. B. (1991). Seaweeds of the southeastern United States. Cape Hatteras to Cape Canaveral. Durham and London: Duke University Press.
Suárez, A. M., Martínez-Daranas, B., & Alfonso, Y. (2015). Macroalgas marinas de Cuba. La Habana: UH [Universidad de La Habana] Editorial.
Svedelius, N. (1906). Reports on the marine algae of Ceylon. No. 1. Ecological and systematic studies of the Ceylon species of Caulerpa. Reports of the Ceylon Marine Biological Laboratory, 2, 81–144.
Taylor, W. R. (1942). Caribbean marine algae of the Allan Hancock Expedition, 1939. Allan Hancock Atlantic Expedition Report, 2, 1–193.
Taylor, W. R. (1960). Marine algae of the eastern tropical and subtropical coasts of the Americas. Ann Arbor: The University of Michigan Press.
Tsuda, R. T., & Abbott, I. A. (1985). Collection, handling, preservation, and logistics. In M. M. Littler, & D. S. Littler (Eds.), Handbook of phycological methods. Ecological field methods: macroalgae (pp. 67–86). Cambridge: Cambridge University Press.
Vázquez-Machorro, A., Godínez-Ortega, J. L., Granados-Barba, A., & Ramírez-García, P. (2016). Estructura y composición de la macroflora dominante del pecio “Ana Elena”, Sistema Arrecifal Veracruzano, Golfo de México. Hidrobiológica, 26, 257–265.
Wilkinson, T., Wiken, E., Bezaury-Creel, J., Hourigan, T., Agardy, T., Herrmann, H. et al. (2009). Ecorregiones marinas de América del Norte. Montreal: Comisión para la Cooperación Ambiental.
Womersley, H. B. S., & Shepley, E. A. (1982). Southern Australian species of Hypoglossum (Deleseriaceae, Rhodophyta). Australian Journal of Botany, 30, 321–346.
Wynne, M. J. (2014). The red algal families Delesseriaceae and Sarcomeniaceae. Königstein: Koeltz Scientific Books.
Wynne, M. J. (2017). A checklist of benthic marine algae of the tropical and subtropical western Atlantic: fourth revision. Nova Hedwigia Beihefte, 145, 1–202.
Wynne, M. J., Price, I. R., & Ballantine, D. L. (1989). Distinctions between Hypoglossum barbatum Okamura, H. minimum Yamada and H. simulans sp. nov. (Delesseriaceae, Rhodophyta). Phycologia, 28, 28–38.



