Guillermo Horta-Puga a, *, Aura Aletse Morales-Aranda a, José Luis Tello-Musi b
a Universidad Nacional Autónoma de México, Unidad de Biotecnología y Prototipos, Facultad de Estudios Superiores Iztacala, Av. de los Barrios Núm. 1, Los Reyes Iztacala, 54090 Tlalnepantla, Estado de México, Mexico
b Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Laboratorio de Zoología, Av. de los Barrios Núm. 1, Los Reyes Iztacala, 54090 Tlalnepantla, Estado de México, Mexico
*Corresponding author: horta@unam.mx (G. Horta-Puga)
Received: 31 August 2021; accepted: 7 April 2022
Abstract
Reef fish species richness of the Veracruz Reef System National Park (VRSNP) in the SW Gulf of Mexico is well known. However, the knowledge of the assemblage structure and its spatial variability in the reef ecosystem is quite limited. For that purpose, 5 field surveys (2012-2015) were performed, using the stationary visual census method, at 10 selected reefs. The most important findings were: 116 reef species were recorded. Average total reef fish density (2.31 Ind/m2) is similar to the records for the Caribbean reefs in the 20th century. The top 5 most abundant species were: Chromis multilineata, Ocyurus chrysurus, Abudefduf saxatilis, Stegastes leucostictus, and Elacatinus jarocho. We found evidence of a spatial distribution pattern with 3 well-defined groups of reefs: (1) those near the city of Veracruz, (2) those near the outlet of the Jamapa River, and (3) those farther from the city. Higher fish densities are associated to both high hermatypic coral and low crustose coralline algae bottom covers. The assemblage structure of reef fishes is different at distinct geomorphological reef zones. As expected, with some differences in the species abundance order, the assemblage structure of reef fishes is similar at all coral reefs in the Gulf of Mexico.
Keywords: Veracruz; Reef ecology; Community ecology
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Ensamble de peces de arrecife de un sistema arrecifal coralino en el suroeste del golfo de México
Resumen
La riqueza de peces arrecifales es bien conocida en el Parque Nacional Sistema Arrecifal Veracruzano (PNSAV), SE del golfo de México. Sin embargo, el conocimiento de la estructura del ensamble y su variabilidad espacial es limitada. Con este propósito, 5 campañas de muestreo (2012-2015) se llevaron a cabo usando el método del censo visual estacionario, en 10 arrecifes seleccionados. Los hallazgos más importantes fueron: 116 especies registradas. La densidad promedio total (2.31 Ind/m2) es similar a la que se registró en los arrecifes del Caribe en el siglo XX. Las 5 especies más abundantes fueron: Chromis multilineata, Ocyurus chrysurus, Abudefduf saxatilis, Stegastes leucostictus y Elacatinus jarocho. Encontramos evidencia de un patron de distribución espacial con 3 grupos de arrecifes: (1) los que están cerca de la ciudad de Veracruz, (2) los que están cerca de la desembocadura del río Jamapa y (3) los que están alejados de la ciudad. Las densidades altas están asociadas a coberturas altas de corales hermatípicos y bajas de algas coralinas encostrantes. La estructura del ensamble difiere entre zonas geomorfológicas arrecifales. Con diferencias en el orden de abundancia, la estructura del ensamble de peces arrecifales es similar en los arrecifes del golfo de México.
Palabras clave: Veracruz; Ecología de arrecifes; Ecología de comunidades
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The southwestern Gulf of Mexico (SWGM) is a tropical area situated 18º-26º N, 92º-97º W (Felder et al., 2009; Mendelssohn et al., 2017). It is bordered by sandy beaches and has a narrow continental shelf (< 100 km wide), that is an extension of the alluvial plain (west to the Sierra Madre Mountain range) in the adjacent land (Bryant et al.,1991). The bottom of the SWGM shelf is composed mainly by fine terrigenous siliciclastic sediments sourced from the continent by several rivers (Carrillo et al., 2007; Mendelssohn et al., 2017). Two coastal areas with rocky bottoms are also present in the SWGM, one between Villa Rica and La Mancha (19º36-41’ N), and the other between Roca Partida and Coatzacoalcos (18º09-42’ N) (González-Gándara, 2020; Murawski et al., 2018; Ortiz-Lozano et al., 2013). Additionally, 3 large coral reef systems are present in the SWGM, the Tuxpan Reef System (21º00-33’ N), the Veracruz Reef System (19º03-15’ N), and the Tuxtlas Reef System (Carricart-Ganivet & Horta-Puga, 1993; Ortiz-Lozano et al., 2013; Tunnell, 2007). High productivity in the nearshore region is due to wind-driven nutrient upwelling and freshwater input from rivers, especially in the rainy season, when chlorophyll-a concentrations in surface seawater are higher (Salmerón-García et al., 2010). Notwithstanding the high diversity of habitats and environmental conditions, the SWGM has the lowest recorded fish species richness in the Gulf of Mexico, with only 900 recorded species, compared to 1,100 species recorded for the northwestern region, and the 1,300 species for the eastern region, from a total of 1,541 species (McEachran, 2009). For the Gulf of Mexico, Robertson and Cramer (2014) also reported the presence of 1,102 species of shallow-water fishes, and 405 out of them were reef fishes. The SWGM also is an important fishing area, mainly for local/regional human consumption (Beaver & Chávez, 2007). Although catch statistics are not reliable, it is calculated that the average annual production of fishery products was ~200×103 ton/year in the 2015-2020 period, including shrimps and octopuses (PD, 2021). As in any tropical zone around the world, more than 290 species of fishes are exploited (Arenas-Fuentes & Jiménez-Badillo, 2004; CNP, 2018; Vargas-Hernández, Badillo et al., 2002). The most important fished species include tunas, groupers, snappers, catfishes, mullets, snooks, jacks, mackerels, sardines, rays, and sharks, and some of them are considered overfished or in the maximum sustainable limit (CNP, 2018; Díaz-de-León et al., 2004). Due to the importance of the SWGM, there are various studies on the assemblage structure of marine shallow-water fishes, which include: 1) ichthyoplankton and pelagic larval fish assemblages (Flores-Coto et al., 2014; Sanvicente-Añorve et al., 1998); 2) economically and ecologically important demersal and/or benthic species (Amezcua-Linares et al., 2014; Murawski et al., 2018); 3) rocky-bottom fishes (González-Gándara, 2020a, b); and reef fishes (Olán-González et al., 2020; Pérez-España et al., 2015; Rangel-Ávalos et al., 2008). The lasts 3 studies focused on the Veracruz Reef System National Park (VRSNP).
The VRSNP is the largest reef ecosystem in the SWGM (Carricart-Ganivet & Horta-Puga, 1993; Ortiz-Lozano et al., 2013; Tunnell, 2007). As it lies off the Port of Veracruz, one of the most populated cities in the SWGM, and is directly influenced by the discharge of the Jamapa River which drains a large continental area, this reef system also is considered a long-time highly impacted reef setting (Gil-Agudelo et al., 2020; Horta-Puga, 2007; Horta-Puga et al., 2019; Ortiz-Lozano, 2012). Consequently, the hermatypic coral community in the VRSNP has been declining at least since the 1960s (Chávez et al., 2007; Horta-Puga et al., 2015), and benthic macroalgae cover now is higher than coral cover (Horta-Puga et al., 2020). However, each reef has been subjected to a set of different environmental conditions, due to their location and distance from the main sources of anthropogenic impact (Gil-Agudelo et al., 2020; Horta-Puga, 2007; Horta-Puga et al., 2019; Ortiz-Lozano, 2012).
Horta-Puga et al. (2020), used the relationship between the bottom cover of the main photosynthetic reef organisms (hermatypic corals and macroalgae) to classify the degree of impact of 10 selected reefs, and for northern and southern groups of reefs of the VRSNP. They ranked them as degraded, moderately conserved, and conserved. The reefs of the northern group, and those located near the port, presented low coral and high macroalgae covers, so they were classified as degraded. The southern group of reefs and some offshore reefs were ranked as moderately conserved, as they presented a better condition. Lastly, 2 reefs (Isla de Enmedio and Chopas), with the highest coral and the lowest macroalgae covers, were allocated as conserved. Hence, as the environmental and ecological conditions of the benthic communities are different for each single reef or group of reefs, so, it is also possible that other reef communities could be similarly affected, like reef fishes.
The impact/influence from various environmental and ecological factors on reef fish assemblages also have been recorded elsewhere. Reefs near densely human-populated areas have lower fish biomass densities, and lower abundances of larger-bodied and upper trophic level fishes (Friedlander & DeMartini, 2002; Sandin et al., 2008; Stallings, 2009; Williams et al., 2015). Reefs located in high oceanic productivity areas (chlorophyll-a concentration), usually have higher reef fish densities (Williams et al., 2015). High coral cover and/or reef bottom tridimensional complexity also is associated to high reef fish abundance (Álvarez-Filip et al., 2015; Coker et al., 2014; Graham & Nash, 2013). Finally, reefs farther from river influence have higher reef fish densities and a higher trophic diversity (Beger & Possingham, 2008; Mallela et al., 2007; Neves et al., 2016).
The ichthyofauna of the VRSNP is well known, 472 species of reef and pelagic fishes have been recorded (Del-Moral et al., 2013; Robertson et al., 2019). However, the knowledge on the assemblage structure of reef fishes is scarce, with few studies that just averaged abundances for the whole reef system, which recorded Chromis multilineata, Halichoeres burekae, Coryphopterus personatus, Haemulon aurolineatum, Stegastes adustus, and Abudefduf saxatilis as the most abundant species. Additionally, higher fish densities have been reported for the reefs of the northern group, for the 10-15 m range depth, and for the 2009 year in the 2006-2014 period (González-Gándara & Chávez, 2020; Olán-González et al., 2020; Pérez-España et al., 2015; Rangel-Ávalos et al., 2008). However, none of these studies addressed for differences in the reef fish assemblage structure among reefs, nor they found a relationship with any ecological or environmental factor.
Thus, this study aimed to describe the species richness, abundance, and the assemblage structure of reef fishes at 10 selected reefs of the VRSNP, and their spatial distribution pattern associated to reef location (distance from shore and/or fluvial discharge; north vs. south reef groups), and to ecological (coral and crustose coralline algae cover; degraded, moderately conserved, and conserved reefs), and climate (dry and rainy seasons) drivers, as has been proven for the benthic macroalgae community (Horta-Puga et al., 2020). In order to know how different or similar they are, we also compared the reef fish assemblage structure with previous studies in the VRSNP, and with those from other reef settings in the Gulf of Mexico.
Materials and methods
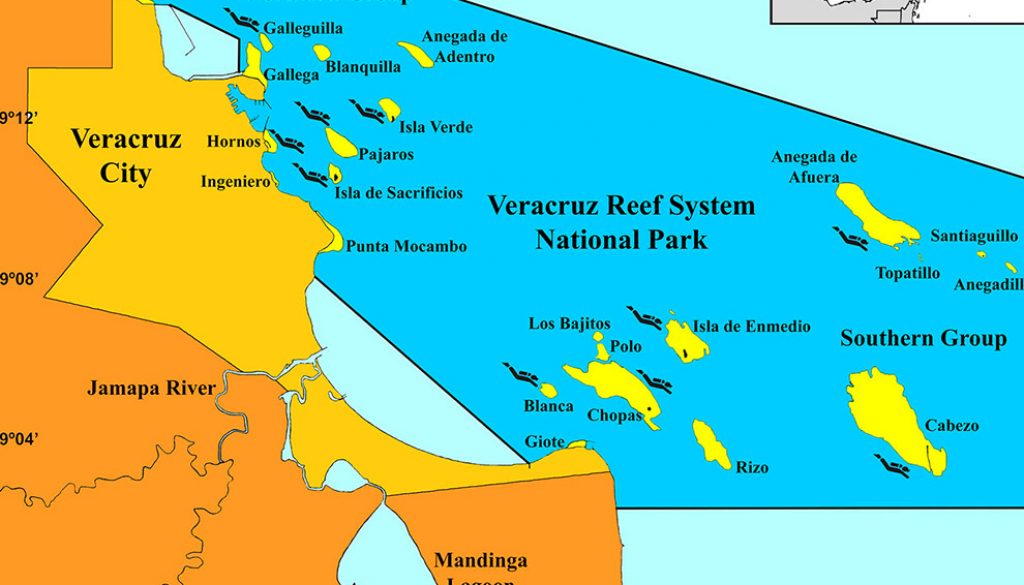
The VRSNP (19º03’-19º16’ N, 95º47’-96º14’ W; Fig. 1) is composed by > 50 patch, fringing and platform type coral reefs that have formed during the Holocene, from depths up to 45 m (Mayorga-Martínez et al., 2021; Ortiz-Lozano et al., 2018; Tunnell, 2007). The reefs are geostructures with well-defined geomorphological zones including the fore reef (windward slope), the reef crest, the reef flat, and the back reef (leeward slope). Because of freshwater discharge from the Jamapa River, the VRSNP is divided into the northern and southern reef groups. Also, due to fluvial influence, seawater has a high load of suspended solids, which contribute to reduce the visibility and to increase the dissolved nutrient concentrations (Carriquiry & Horta-Puga, 2010; Horta-Puga, 2017; Horta-Puga et al., 2020). In the SWGM, there are 3 clearly defined climate seasons: rainy (June to October), northern cold fronts (October to April) and dry (March-June) seasons, overlapping 1-2 months (Avendaño-Álvarez et al., 2017; Carrillo et al., 2007; Gutiérrez-de-Velasco & Winant, 1996). These harsh conditions for the development of the coral reef ecosystem have caused a shift toward the abundance of sediment-resistant coral species, an important difference compared with other coral reefs in the tropical Western Atlantic (Horta-Puga, 2003; Horta-Puga et al., 2015; Tunnell, 1988).

This study was part of a large monitoring program of the VRSNP, during the 2009-2015 period (CONABIO GM005: http://www.conabio.gob.mx/institucion/cgi-bin/datos.cgi?Letras=GM&Numero=5). The main goal of the project was to determine the general condition of the reef system, as well as the condition of various single reefs, which was based on the community structure, abundance, and health of hermatypic corals (Carricart-Ganivet et al., 2011; Horta-Puga et al., 2015), and the abundance of the main benthic macroalgae morpho-functional groups (Horta-Puga et al., 2020). As the VRSNP is a large ecosystem, 10 single coral reefs were selected (Table 1), 5 from the northern group (Galleguilla, Hornos, Isla de Sacrificios, Isla Verde, and Pájaros); and 5 from the southern group (Anegada de Afuera, Blanca, Cabezo, Chopas, and Isla de Enmedio). The reefs were selected to include the wide spectrum of environmental variability associated with the different distance and precise location from the 2 main sources of impact, the Port of Veracruz and the the Jamapa River (Horta-Puga, 2007; Horta-Puga et al., 2019; Ortiz-Lozano, 2012). Another important goal of the monitoring program was to provide information, in a timely manner, to the authorities of the VRSNP about the ecological condition of each reef and the reef system in general, for conservation and management purposes. So, the reefs were surveyed twice a year, in the 2 more contrasting weather seasons, dry (March-April) and rainy (September-October), for the early detection of any significant change. The monitoring program comprised 12 semiannual surveys, but the study of the reef fish assemblages was performed only in the lasts 5 surveys: October/2012 (FS01), March/2013 (FS02), September/2013 (FS03), March/2014 (FS04), and March/2015 (FS05). In all surveys at each reef, the species richness and abundance of reef fishes were determined in the back reef (leeward slope), at 9-12 m depths, which is considered the most conserved zone, with the highest hermatypic coral cover and species richness in the VRSNP (Horta-Puga, 2003; Horta-Puga et al., 2015, 2020). The Hornos and Blanca reefs do not have a leeward slope, so, they were surveyed at the most protected area from surge of the windward slope, that is the most similar to the leeward side. Also, Hornos was surveyed at 4-7 m depths, because it is a shallow fringing-type reef, wherein depths of the windward slope do not exceed 7 m.
Table 1
Reefs surveyed in this study, and sampling effort information.
| Reef | RT | Depth (m) | Lat N | Long W | C | F |
| Anegada de Afuera | Platform | 9-12 | 19°09’39.1” | 95°51’57.6” | 60 | 6,744 |
| Blanca | Platform | 9-12 | 19°05’25.3” | 96°00’11.7” | 60 | 3,036 |
| Cabezo | Platform | 9-12 | 19°03’03.4” | 95°49’45.9” | 60 | 6,382 |
| Chopas | Platform | 9-12 | 19°05’50.7” | 95°57’53.8” | 60 | 3,623 |
| Isla de Enmedio | Platform | 9-12 | 19°06’51.0” | 95°56’48.9” | 60 | 5,166 |
| Galleguilla | Platform | 9-12 | 19°14’01.4” | 96°07’32.0” | 60 | 2,332 |
| Hornos1 | Fringing | 4-7 | 19°11’39.5” | 96°07’14.4” | 48 | 1,454 |
| Isla de Sacrificios2 | Platform | 9-12 | 19°10’44.2” | 96°05’43.5” | 48 | 3,518 |
| Isla Verde1 | Platform | 9-12 | 19°12’03.0” | 96°04’14.1” | 48 | 3,316 |
| Pájaros | Platform | 9-12 | 19°11’40.8” | 96°05’47.1” | 60 | 1,570 |
RT = Reef type; C = total number of censuses per reef; F = total number of fishes recorded per reef.
1 = Survey FS04 missing; 2 = survey FS02 missing.
Table 2
Reef fish species richness (number of total observed species) per survey, season, reefs and group of reefs.
| R/G | FS01 | FS02 | FS03 | FS04 | FS05 | Rainy | Dry | Total |
| AF | 36 | 34 | 36 | 33 | 30 | 51 | 46 | 63 |
| BL | 24 | 29 | 22 | 21 | 32 | 32 | 44 | 51 |
| CA | 36 | 25 | 27 | 40 | 43 | 46 | 61 | 67 |
| CH | 33 | 26 | 28 | 26 | 30 | 42 | 46 | 60 |
| IE | 29 | 23 | 32 | 32 | 35 | 46 | 48 | 65 |
| GL | 32 | 26 | 20 | 25 | 19 | 35 | 36 | 47 |
| HO | 27 | 20 | 16 | 18 | 30 | 27 | 43 | |
| IS | 34 | 25 | 29 | 31 | 42 | 42 | 54 | |
| IV | 39 | 22 | 33 | 33 | 49 | 37 | 58 | |
| PJ | 31 | 17 | 26 | 22 | 23 | 39 | 35 | 51 |
| SG | 67 | 48 | 51 | 59 | 60 | 81 | 82 | 102 |
| NG | 60 | 41 | 47 | 48 | 50 | 73 | 72 | 92 |
| DE | 53 | 37 | 38 | 48 | 46 | 61 | 68 | 83 |
| MC | 61 | 47 | 47 | 52 | 55 | 70 | 75 | 86 |
| CO | 48 | 29 | 39 | 39 | 43 | 61 | 58 | 81 |
| VRS | 76 | 59 | 61 | 72 | 68 | 94 | 97 | 116 |
R/G = Reef or group of reefs; RS = rainy season; DS = dry season; FSN/survey = average fish species number per survey; AF = Anegada de Afuera; Bl = Blanca; Ca = Cabezo; CH = Chopas; IE = Isla de En medio; GL = Galleguilla; HO = Hornos; IS = Isla de Sacrificios; IV = Isla Verde; PJ = Pájaros; SG = southern group; NG = northern group; DE = degraded; MC = moderately conserved; CO = conserved; VRS = Veracruz Reef System.
Horta-Puga et al. (2020) used the ratio between the relative cover (%) of the reef building community (RB = corals + crustose corallines) and the fleshy macroalgae community (FM = turf + frondose), to determine the reef condition as has been used in other studies (Smith et al., 2016). They found that the VRSNP is divided into 3 groups of reefs: 1) the degraded, nearshore reefs, dominated by a benthic non-reef-building community, which included the reefs Hornos, Galleguilla, Isla de Sacrificios, and Pájaros, and the northern group (RB/FM < 1); 2) the moderately conserved, offshore reefs, like Anegada de Afuera, Blanca, Cabezo, and Isla Verde, and the southern group (RB/FM= 1-1.5); and 3) the conserved reefs, those dominated by a benthic reef-building community, as Chopas an Isla de Enmedio (RB/FM > 2). In the analyses of results, we used this classification, searching for differences in the general abundance of the reef fish assemblage. Also, the general average cover of crustose coralline algae and hermatypic corals reported by Horta-Puga et al. (2020), were used to determine their influence on the abundance of reef fishes.
Table 3
Average total reef fish density (Ind/m2 ± s) by reef survey, season, reefs and groups of reefs
| R/G | FS01 | FS02 | FS03 | FS04 | FS05 | RS | DS | TOTAL |
| AF | 3.1±2.0 | 3.7±1.2 | 4.5±1.4 | 3.8±1.0 | 4.8±1.7 | 3.8±0.9 | 4.1±0.6 | 4.0±0.6 |
| BL | 1.7±1.6 | 1.9±0.5 | 3.2±1.2 | 0.8±0.3 | 1.3±1.3 | 2.5±1.1 | 1.3±0.5 | 1.8±0.9 |
| CA | 4.5±3.2 | 2.5±1.1 | 2.4±0.6 | 4.8±2.3 | 4.6±3.3 | 3.5±1.5 | 4.0±1.3 | 3.8±1.2 |
| CH | 1.8±1.3 | 1.2±0.9 | 3.3±1.1 | 1.8±0.9 | 2.6±1.0 | 2.5±1.1 | 1.9±0.7 | 2.1±0.8 |
| IE | 1.7±1.6 | 2.7±1.0 | 4.8±2.8 | 2.6±0.7 | 3.4±2.0 | 3.3±2.1 | 2.9±0.4 | 3.0±1.1 |
| GL | 2.0±1.8 | 1.8±1.0 | 1.9±1.2 | 0.7±0.3 | 0.5±0.2 | 1.9±0.1 | 1.0±0.7 | 1.4±0.7 |
| HO | 0.9±0.6 | 1.5±1.0 | 0.7±0.3 | 1.1±0.7 | 0.8±0.1 | 1.3±0.3 | 1.1±0.4 | |
| IS | 2.4±1.2 | 0.8±0.9 | 0.9±0.4 | 6.2±2.7 | 1.6±1.1 | 3.6±3.7 | 2.6±2.5 | |
| IV | 3.9±2.1 | 1.4±0.6 | 2.7±1.5 | 1.8±1.2 | 3.3±0.8 | 1.6±0.3 | 2.4±1.1 | |
| PJ | 1.8±1.1 | 0.3±0.2 | 1.1±0.7 | 0.9±0.8 | 0.6±0.3 | 1.4±0.5 | 0.6±0.3 | 0.9±0.6 |
| SG | 2.6±1.2 | 2.4±0.9 | 3.6±1.0 | 2.8±1.6 | 3.4±1.4 | 3.1±0.6 | 2.8±1.2 | 2.9±1.0 |
| NG | 2.2±1.1 | 1.2±0.7 | 1.4±0.8 | 0.9±0.1 | 2.0±2.4 | 1.8±0.9 | 1.6±1.1 | 1.7±0.8 |
| DE | 1.8±0.6 | 1.2±0.8 | 1.1±0.5 | 0.9±0.1 | 2.1±2.7 | 1.5±0.5 | 1.6±1.3 | 1.5±0.8 |
| MC | 3.3±1.2 | 2.4±1.0 | 3.2±0.9 | 3.1±2.1 | 3.1±1.8 | 3.3±0.6 | 2.7±1.5 | 3.0±1.0 |
| CO | 1.8±0.0 | 1.9±1.1 | 4.0±1.0 | 2.2±0.6 | 3.0±0.6 | 2.9±0.5 | 2.4±0.7 | 2.6±0.6 |
| VRS | 2.4±1.1 | 1.9±1.0 | 2.5±1.4 | 2.1±1.5 | 2.7±2.0 | 2.5±1.0 | 2.2±1.3 | 2.3±1.1 |
R/G = Reef or group of reefs; RS = rainy season; DS = dry season; AF = Anegada de Afuera; Bl = Blanca; Ca = Cabezo; CH = Chopas; IE = Isla de Enmedio; GL = Galleguilla; HO = Hornos; IS = Isla de Sacrificios; IV = Isla Verde; PJ = Pájaros; SG = southern group; NG = northern group; DE = degraded; MC = moderately conserved; CO = conserved; VRS = Veracruz Reef System.
A slightly modified version of the stationary visual census method of Bohnsack and Bannerot (1986) was used, which has proved to be an efficient and a reliable technique for the evaluation of the reef fish assemblage (Caldwell et al., 2016; Samoilys & Carlos, 2000). In the VRSNP seawater is turbid, due to a high load of suspended solids from river discharge, so visibility is usually < 10 m (Horta-Puga, 2007; Horta-Puga et al., 2015, 2020). Thus, to standardize the visual stationary censuses, the field of view was restricted to a radius of 3 m, and each census was performed in an imaginary cylinder 6 m in height, with a 3 m radius, with a bottom area of ~28.3 m2. Twelve censuses were performed by reef site (1 day/reef; 2 dives/day; 6 censuses/dive; average diving time = 50 min), during daylight, between 10.00 to 15.00 h, usually in the same day for each reef site. Once in the selected location, the surveyor took position haphazardly ~3 m from the reef bottom, looking around, up and down, and slowly approaching the bottom for observing and recording, as long as possible, all reef fishes. Each census lasted 5 minutes, and all observed fishes were counted, and identified to species. Due to the nature of the used method, the small cryptic reef fishes that live hidden in crevices and holes among corals (Atta et al., 2019), could not be properly assessed. Most of the observed reef fishes were identified to species in the field. In case of doubt about species identity, after each census some more time was spent on site, to look for those species. Once they were found a drawing of the contour of the organism was made, and its coloration pattern was described, both data were annotated in the underwater data sheets. Also, whenever possible, photographs of that fish species were taken to facilitate their later identification. This information was compared with species descriptions and photographs in field guides and books (Claro, 1994; Humann & Deloach, 2014), and FishBase (https://www.fishbase.se/search.php). The species list was systematically ordered following the classification proposed by Nelson et al. (2016). Reef fish assemblage data for other coral reef areas from the Gulf of Mexico and the Caribbean, that were used for taxonomic and ecological comparisons with our results, were obtained from the available published literature.
Abundance (density) is reported as number of individuals per square meter (Ind/m2). Species richness, total reef fish density, and species density are presented for individual reefs, reef groups (northern and southern; and degraded, moderately conserved, and conserved: sensu Horta-Puga et al., 2020), and climate seasons (dry and rainy), and for the whole VRSNP. All this information was used to determine the differences/similarities among sites and climate seasons, the reef fish assemblage spatial distribution pattern, the relationship with some components of the reef bottom, and for comparison with other reefs.
The Mann-Whitney test was used to look for differences in fish density between reef groups, and the paired t-test for differences between seasons. The relationship between corals and crustose coralline algae covers (Horta-Puga et al., 2020), and species richness with reef fish density, were obtained with the Pearson linear correlation analysis. Data used for the correlation analyses, species richness, average density and cover for each reef, group of reefs (north, south, degraded, moderately conserved, conserved), and the whole VRSNP, are showed in Supplementary material (Table S3). In order to determine the spatial distribution pattern of the reef fish assemblage, and the species that drove to that pattern in the VRSNP, the total average density of each species for each reef (Supplementary material: Tables S2A-I), were analyzed with the principal components multivariate analysis (PCA). All averages included the standard deviation. The free software package PAST (v4.02) was used to perform all statistical analyses (Hammer et al., 2001).
Results
During the field work a total of 564 underwater visual stationary censuses were performed, and 37,141 fishes were seen and recorded. It is important to mention that the field survey of the 2014 rainy season could not be accomplished because of logistical issues. In addition, bad weather conditions also hindered the surveys of the reefs Isla de Sacrificios (FS02), and Hornos and Isla Verde (FS04).
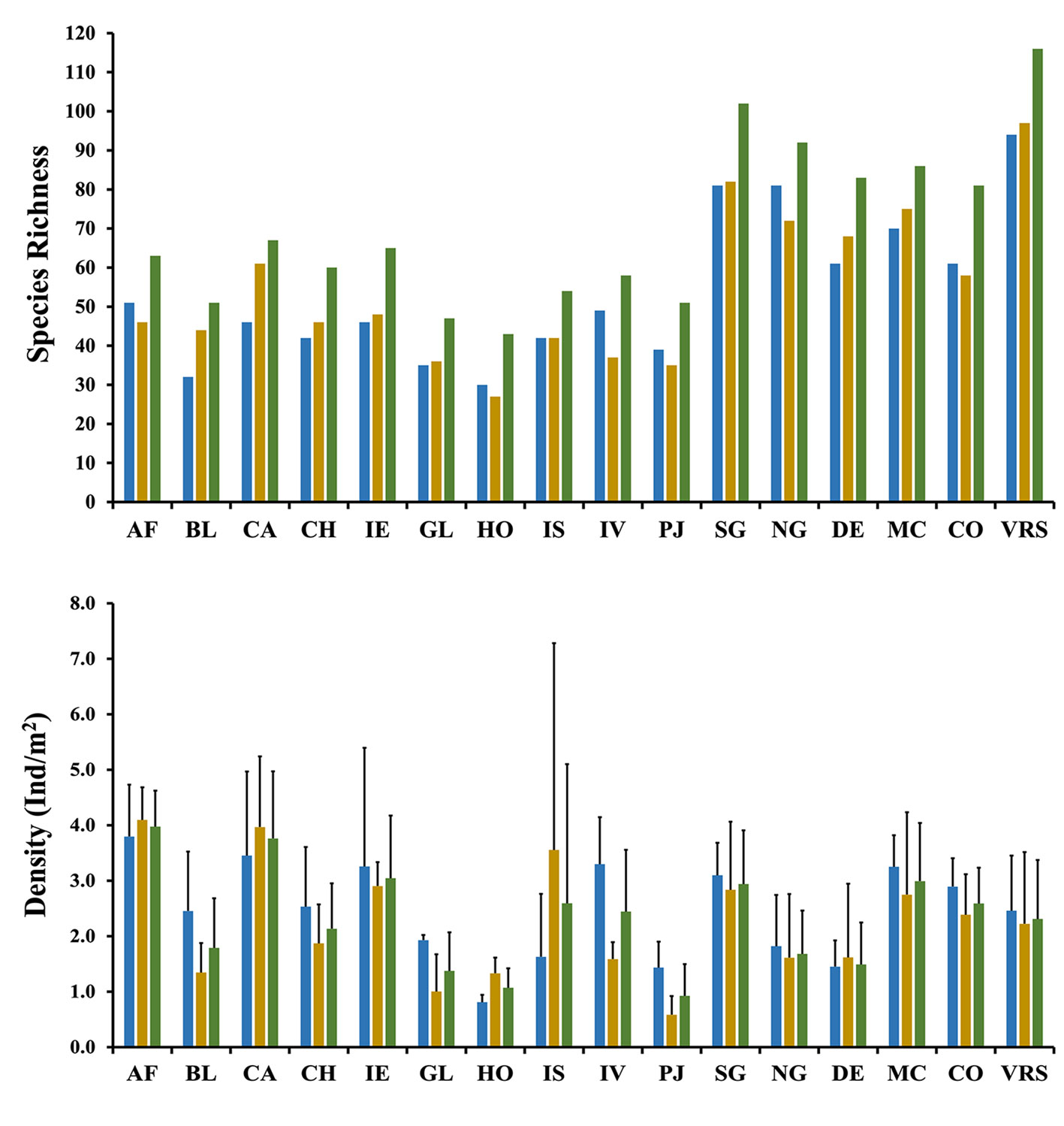
All the fish species recorded here were bony fishes (superclass Gnathostomata, class Actinopterygii). A total of 116 species, belonging to 61 genera and 34 families, were observed (Supplementary material: Table S1). The families with the higher species richness were Serranidae (15 spp.), Haemulidae and Pomacentridae (10 spp.), Lutjanidae and Scaridae (9 spp.) and Labridae (7 spp.). The reef with the highest total species richness was Cabezo (67 spp.), and Hornos had the lowest (43 spp.) (Table 2, Fig. 2a). A total of 102 spp. were recorded in the southern group, for only 92 in the northern group. The total number of recorded species was similar among the degraded reefs (83 spp.), the moderately conserved reefs (86 spp.), and the conserved reefs (81 spp.); and between the rainy (94 spp.) and dry seasons (97 spp.). However, some reefs presented differences, with a higher number of recorded fish species, like Cabezo and Blanca in the dry season, and Isla Verde in the rainy season, with a difference of > 25% more species.

Average density exhibited a high variability, the lowest value was recorded in the FS03 at Hornos with 0.7±0.3 Ind/m2 (Table 3). The highest density was 6.2±2.7 Ind/m2, recorded in the FS05 at Isla de Sacrificios. General average density in the VRSNP was 2.3±1.1 Ind/m2. The reefs with the highest average total density were Anegada de Afuera (4.0±0.6 Ind/m2) and Cabezo (3.8±1.2 Ind/m2) (Fig. 2b). Pájaros had the lowest (0.9±0.6 Ind/m2). The reefs with high fish densities also had high species numbers (Pearson linear correlation, r = 0.869, p = 0.001). The southern group presented a higher fish density (2.9±1.0 Ind/m2) than the northern group (1.7±0.8 Ind/m2), but the difference was not significant (Mann-Whitney test, p = 0.094). The moderately conserved reefs (3.0±1.0 Ind/m2), and the conserved reefs (2.6±0.6 Ind/m2), presented higher average densities than the degraded reefs (1.5±0.8 Ind/m2). Concerning the temporal variability, in the first 4 surveys general average densities were higher during the rainy than the dry seasons, except for the last dry season (FS05), which had the highest abundance (2.7±2.0 Ind/m2). However, no statistical differences were found between the dry (2.2±1.3 Ind/m2) and rainy (2.5±1.0 Ind/m2) seasons (paired t-test, p = 0.5149). Average density for each species by surveys, seasons and reefs are presented in Supplementary materials: Tables S2A-I.
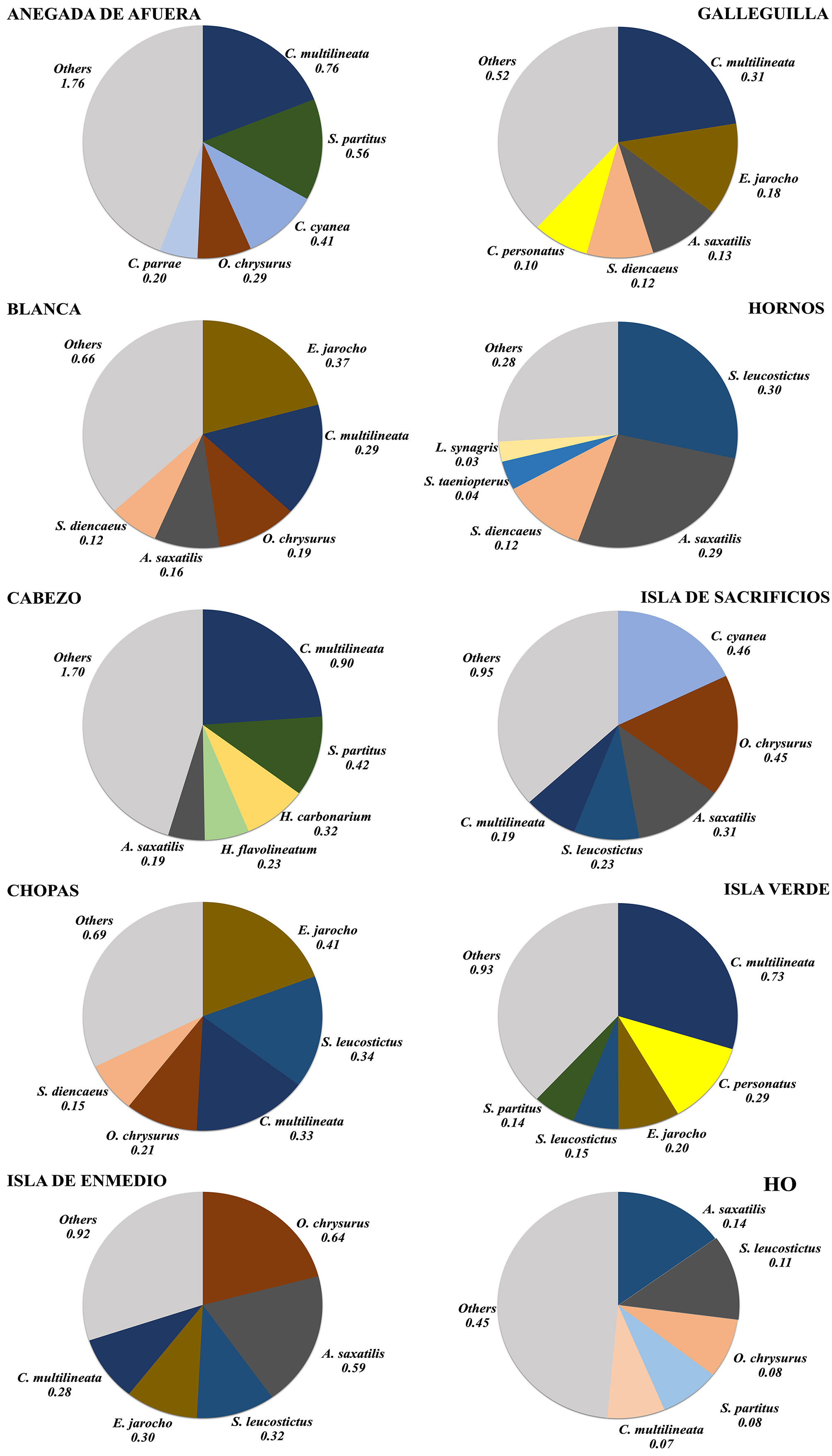
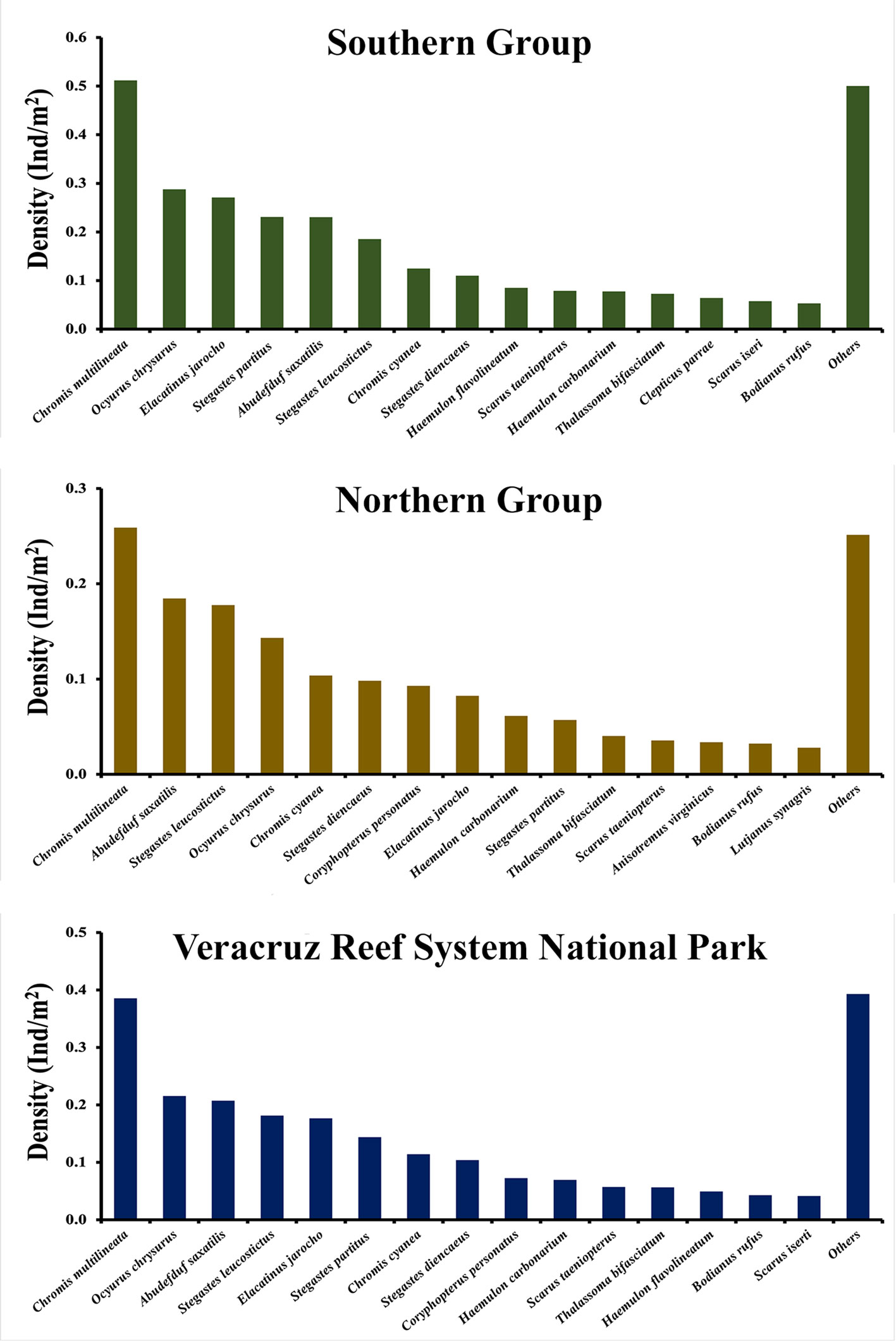
Figures 3 and 4 show the general average density of the 15 most abundant species, in the northern and southern reef groups, the whole VRSNP, and for those reefs with a different degree of conservation. Figure 5 shows the 5 most abundant fishes by reef. At each reef or group of reefs, the 5 most abundant species accounted for ≥ 50% of the total abundance, although they were not the same for all reefs. The top 5 most abundant species in the whole VRSNP were: C. multilineata, O. chrysurus, A. saxatilis, S. leucostictus, and E. jarocho. Any of them was the most abundant species at any reef or group of reefs, except at Isla de Sacrificios wherein C. cyanea was the most abundant. Thus, in general, the reef fish assemblage of the VRSNP is characterized by pomacentrids (Chromis multilineata, C. cyanea, Abudefduf saxatilis, Stegastes leucostictus, S. partitus, and S. diencaeus), lutjanids (O. crysurus), gobids (Elacatinus jarocho, and Coryphopterus personatus), haemulids (Haemulon carbonarium, and H. flavolineatum), labrids (Thalassoma bifasciatum, and Bodianus rufus), and scarids (Scarus taeniopterus, and S. iseri). Assemblage structure between the rainy and dry seasons at each reef also presented no important differences, as broadly the same species were dominant at both seasons (Supplementary material,
Tables 2A-I).
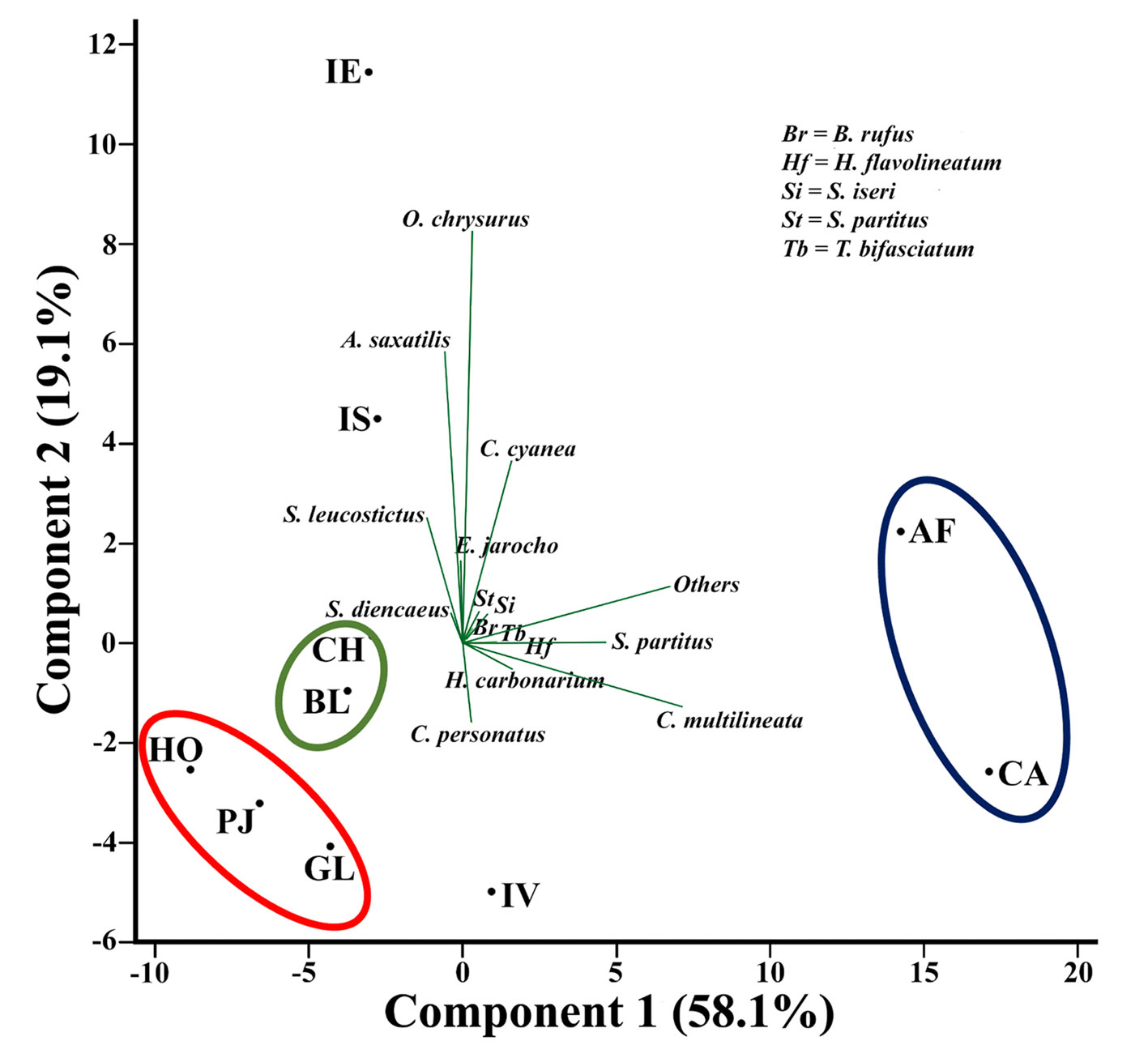
In order to determine the spatial distribution pattern of reef fishes in the VRSNP, the general average assemblage structure for each reef was used to perform a principal component analysis statistical procedure (Fig. 6). As can be seen, reefs located near each other in the biplot diagram seem to form distinct groups: 1) Hornos, Galleguilla and Pájaros, that are near the Port of Veracruz; 2) Blanca and Chopas, positioned near the outlet of the Jamapa River; and 3) Anegada de Afuera and Cabezo, which are the farthest reefs from the Port of Veracruz. The reefs Isla Verde, Isla de Enmedio and Isla de Sacrificios are not part of any group.
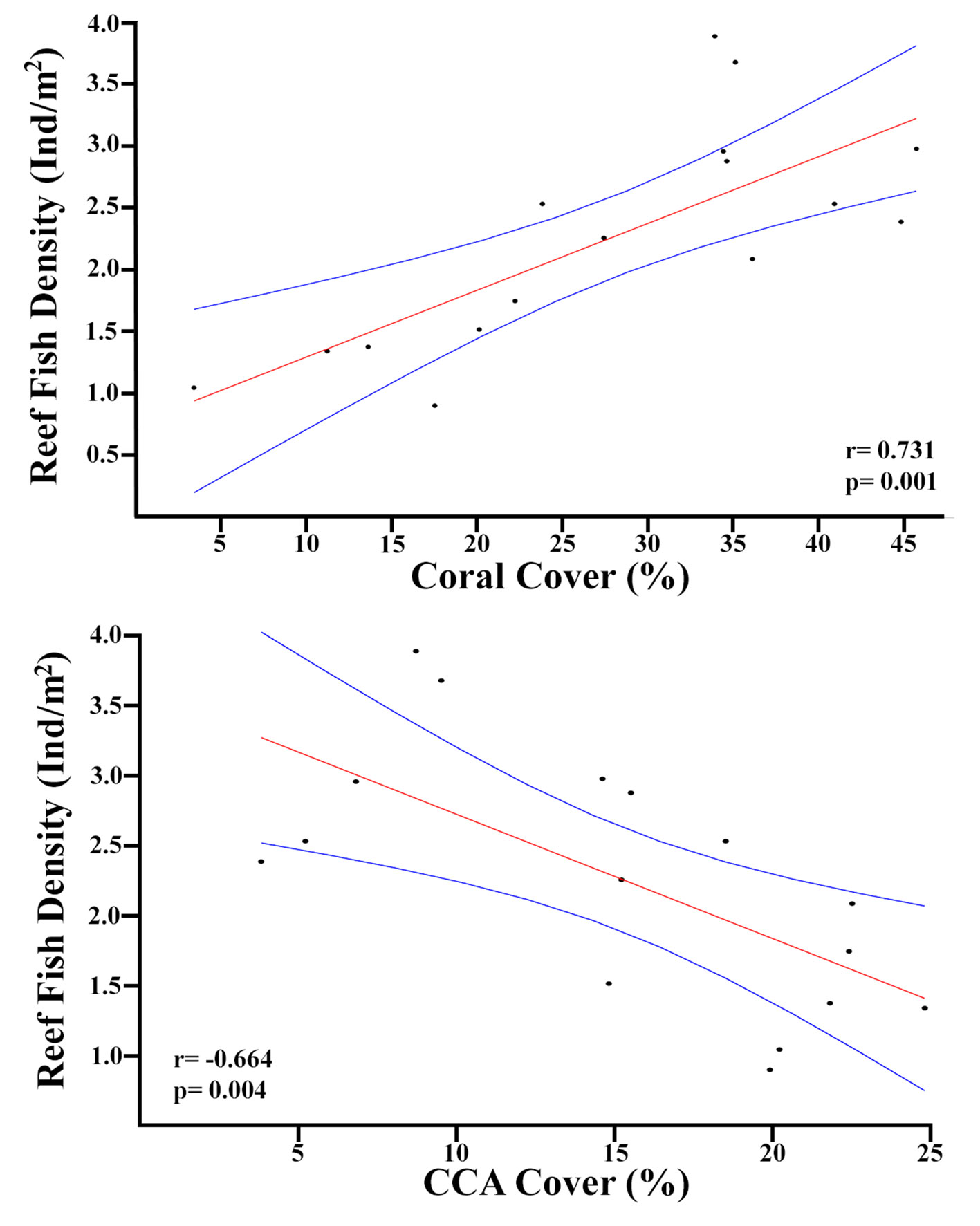
High and significant correlations were found between the reef fish abundance and the bottom cover of hermatypic corals (r = 0.731, p = 0.001), and crustose coralline algae (r =-0.664, p = 0.004) (Fig. 7). So, high fish densities are associated to both high hermatypic coral and low crustose coralline algae covers.
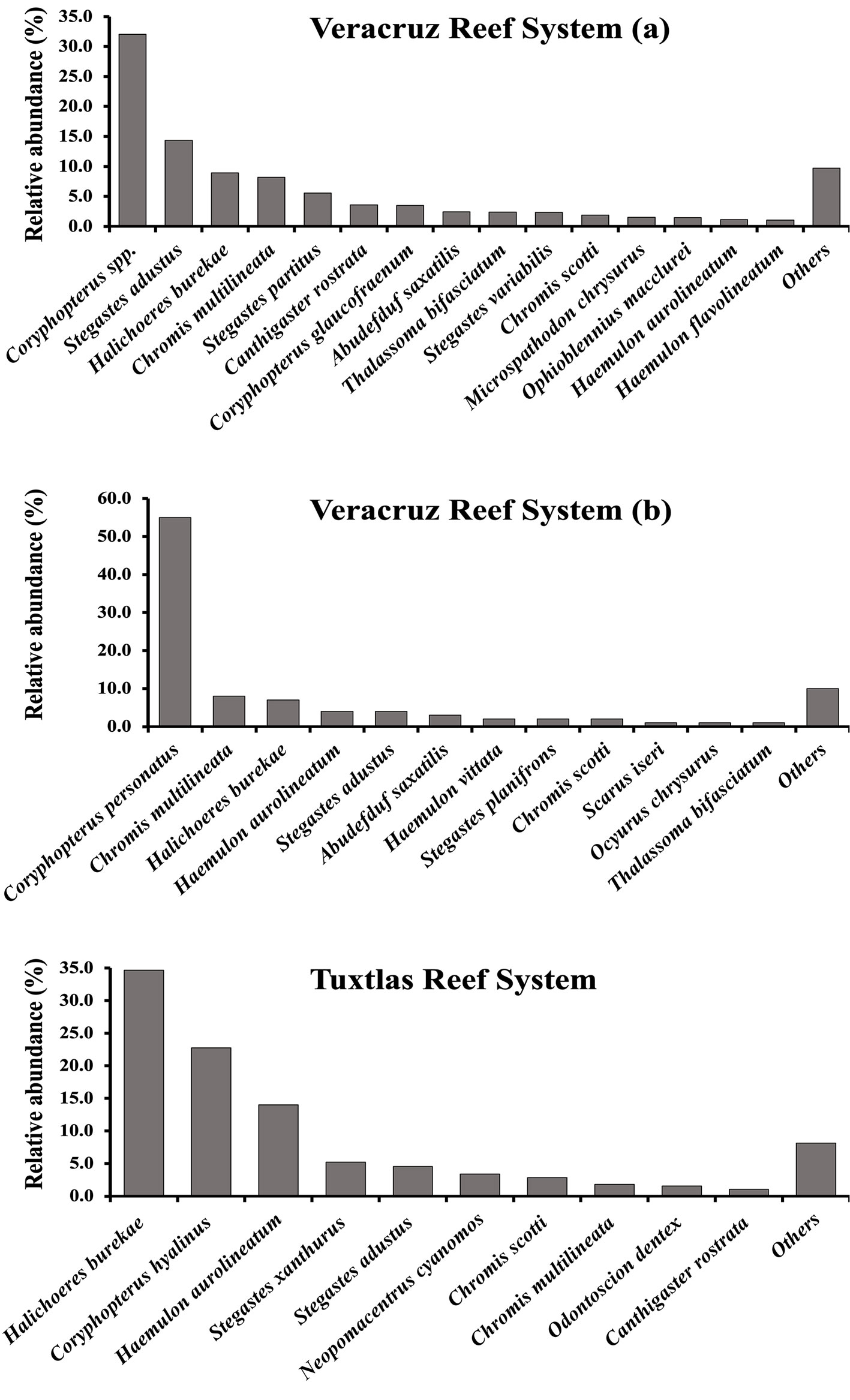
The general reef fish assemblage structure of the VRSNP, was compared with previous studies from the SWGM (Fig. 8), and from other reef settings in the Gulf of Mexico (Fig. 9). As can be seen in the figures, there is a high resemblance among reef sites, as roughly the same species are included among the fifteen most abundant, with usually a marked dominance of 3 or 4 species.

Discussion
In the Gulf of Mexico 1,541 species of fishes have been recorded, which are ~65% of the ~2,400 species recorded for the Greater Caribbean Region. However, in the SWGM, from Cabo Rojo, Veracruz (21º33´ N, 97º19´ W) to Cabo Catoche, Yucatán (21º36´ N, 87º06´ W), fish species richness is lower with about 900 species (McEachran, 2009). González-Gándara and Chávez (2020) reported 509 reef fish species for the coral reefs of the Veracruz state continental shelf. The ichthyofauna of the VRSNP is also well known with 472 species (Ayala-Rodríguez et al., 2016; Del-Moral et al., 2013; Robertson et al., 2019; Tello-Musi et al., 2018; Vargas-Hernández, Nava-Martínez et al., 2002). It is remarkable that sharks, skates, or rays were not recorded during our field work. Compared with other reef areas in the SWGM and the Campeche Bank, the total number of known species in the VRSNP is higher than the 282 species reported for the Tuxpan Reef System (González-Gándara et al., 2013), 162 species for Cayo Arcas (Robertson et al., 2016), and 294 species in Alacranes Reef (González-Gándara & Arias-González, 2001a, b). During the surveys individuals of 3 species not previously recorded for the VRSNP were observed: Melichthys niger (Bloch, 1786), Serranus baldwini (Evermann & Marsh, 1899), and Serranus tabacarius (Cuvier, 1829). Unfortunately, as we do not have photographs and/or collected specimens, in order to consider them as new records it is necessary to get physical evidence in the future. The high recorded species richness for the VRSNP could be explained because it is the largest reef ecosystem in the southern Gulf of México (< 50 reefs), which provides a high environmental/ecological heterogeneity that could be exploited by several reef fish species. Another plausible reason is because in the VRSNP more fish surveys have been carried out than in other areas (Del-Moral et al., 2013; González-Gándara & Chávez, 2020; Robertson et al., 2019). Compared to similar ecological studies (assemblage structure) in other coral reefs in the Gulf of Mexico, the number of species recorded here (116) is slightly higher than the 91 species for the Tuxtlas Reef System (González-Gándara, 2020a), and 79 species in the VRSNP (Olán-González et al., 2020). However, is lower than the 144 (Pérez-España et al., 2015) and 155 species (Rangel-Ávalos et al., 2008), previously reported for the VRSNP, and the 129 species reported in the Flower Garden Banks (Buckel et al., 2014).
Table 4
Total reef fish abundance (Ind/m2) at some reef localities in the Caribbean and the Gulf of MexicoA.
| Reference | Locality | SY | # sites | D |
| Mejía & Garzón-Ferreira (2000) | San Andrés, Providencia, Colombia | 1994-5 | 4 | 2.51 |
| Núñez-Lara et al. (2003) | N Sian Kaan, Mexico | 1999 | 3 | ~2.60B |
| Núñez-Lara et al. (2003) | S Sian Kaan, Mexico | 1999 | 2 | ~2.34B |
| Núñez-Lara et al. (2003) | S Caribbean, Mexico | 1999 | 3 | ~2.70B |
| Claro et al. (2007) | Sabana-Camagüey, Cuba | 2000 | 12 | 1.04 |
| Menza et al. (2006) | Buck Island, US Virgin Islands | 2000-5 | 4 | 1.58 |
| Menza et al. (2006) | St. John, US Virgin Islands | 2000-5 | 8 | 1.73 |
| Pittman et al. (2010) | La Parguera, Puerto Rico | 2001-7 | 10 | 2.10 |
| Toller et al. (2010) | Saba Bank, Netherland Antilles | 2007 | 5 | 0.16 |
| Del Mónaco et al. (2010) | Isla Tortuga, Venezuela | 2008 | 5 | 1.33 |
| López-Ordaz & Rodríguez-Quintal (2010) | Morrocoy National Park, Venezuela | 2009 | 1 | 1.11 |
| Buckel et al. (2014) | Flower Garden Banks, US, GoM | 2010-2 | 225 | 3.73 |
| Olán-González et al. (2020) | Puerto Morelos, Mexico | 2016 | 10 | 0.74 |
| Olán-González et al. (2020) | VRSNP, Mexico, GoM | 2016 | 11 | 4.64 |
| This study | VRSNP, Mexico, GoM | 2012-5 | 10 | 2.31 |
SY = Sampling year; D = density; GoM = Gulf of Mexico. A= The list is not exhaustive. B= Data were extracted from a graph (Fig. 3), using the free online software Graphreader (https://www.graphreader.com).
Average total reef fish density in the VRSNP was 2.3±1.1 Ind/m2, which is similar to the records for the Caribbean before the year 2000 (Table 4), which are also higher than more recent records (Álvarez-Filip et al., 2015; Paddack et al., 2009). Olán-González et al. (2020) reported for the VRSNP a total average density of 4.64 Ind/m2, which is the highest on record, even higher than the 3.73 Ind/m2 recorded in the Flower Garden Banks, in the northwestern Gulf of Mexico (Buckel et al., 2014), one of the most conserved coral reefs in the tropical Atlantic (Gil-Agudelo et al., 2020; Johnston et al., 2016). The same was reported for the hermatypic coral community in the VRSNP, as coral cover in general is higher compared to other coral reefs in the western tropical Atlantic (Horta-Puga et al., 2015). So, these records suggest that the VRSNP could be considered as a resilient ecosystem, despite that fishing and several other environmental pressures have been contributing to reduce the environmental quality of the reef ecosystem in the last decades (Horta-Puga, 2007; Horta-Puga et al., 2019; Ortiz-Lozano, 2012). However, to be conclusive, this issue should be properly addressed in the future.
The reef fish assemblage at each reef and group of reefs in the VRSNP, was dominated by few species (5 spp.), which constitute > 50% of the total abundance (Figs. 3-5). However, we found differences in the order of dominance and the total abundance among reefs, so we tried to determine if there was a spatial distribution pattern in the assemblage structure of reef fishes (Fig. 7). Three groups of reefs are formed by its affinities. The reefs Hornos, Pájaros and Galleguilla form the first group. They are located near the Port of Veracruz and have in common the lowest fish density (< 1.4 Ind/m2) and species richness (≤ 51 spp.) in the VRSNP. However, their communities are dominated by a different suite of species, and no loading in the PCA could explain that. All these reefs were classified as degraded by Horta-Puga et al. (2020), because they are highly impacted, and our results support that assumption. The reefs Blanca and Chopas form another group. These reefs share Elacatinus jarocho as the most abundant species and have intermediate average density values (1.8-2.1 Ind/m2). Their resemblance, and differences with other reefs is explained by a high continental influence by the discharge of the Jamapa River, as its fluvial plume extends to their position. The continental influence also could be boosted by a cyclonic eddy that forms to the NE of these reefs, with higher nutrient concentrations and lower seawater temperatures (Salas-Monreal et al., 2009). Blanca Reef was classified as moderately degraded, and Chopas as conserved (Horta-Puga et al., 2020), that is slightly different to what we found here. The last group comprises Anegada de Afuera and Cabezo, which are the farthest from the shoreline and the Port of Veracruz. They are characterized by the dominance of Chromis multilineata and Stegastes partitus, and the highest reef fish abundance (> 3.8 Ind/m2) and species richness (> 63 spp.) in the VRSNP, as also was observed by Pérez-España et al. (2015). Horta-Puga et al. (2020) classified them as moderately conserved, due to lowest human influence because of their location, and our results are in concordance with this classification. Isla Verde Reef, classified as moderately conserved (Horta-Puga et al., 2020), is also far from the coast, and its assemblage is also dominated by C. multilineata, like Anegada de Afuera and Cabezo Reefs. However, the abundance of Coryphopterus personatus, and a lower species richness (58 spp.), and abundance (2.4±1.1 Ind/m2), compared to Cabezo and Anegada de Afuera, preclude its inclusion with the other 2 reefs to form a distinctive group. The remaining 2 reefs, Isla de Enmedio and Isla de Sacrificios, are characterized by the high abundances of Ocyurus chrysurus, and Abudefduf saxatilis, although the order of dominance is different at both reefs. As they are in different reef groups (northern and southern) and were ranked distinctly as moderately conserved (Isla de Sacrificios), and conserved (Isla de Enmedio), that could explain their differences rather than their similarities, so, they do not form a distinct group. In summary, based on the assemblage structure of reef fishes in the VRSNP, we found evidence of spatial distribution pattern with 3 well-defined groups of reefs, which more or less resemble the classification based on the cover of the photosynthetic benthic communities (Horta-Puga et al., 2020). Considering that the response of the different communities (corals, macroalgae and fishes) is similar, that suggests that their abundance is influenced by the same environmental drivers.
A high reef bottom tridimensional complexity is essential to maintain a high diversity of ecological niches and species that inhabit tropical coral reef ecosystems (Graham & Nash, 2013; Jones & Sims, 1998). It is well known that reef fish abundance is higher at reefs with a high reef bottom rugosity (Coker et al., 2014; Graham & Nash, 2013). Furthermore, reef bottom complexity is positively associated to coral cover, and areas with a high alive hermatypic coral cover also have higher rugosities (Álvarez-Filip et al., 2009, 2011, 2013). During our surveys we also determined the cover of hermatypic corals and crustose coralline algae, the main reef-builders in the VRSNP (Horta-Puga et al., 2020). So, we analyzed if their cover was correlated to reef fish density. The correlation between reef fish density and coral cover by reefs and reef groups was high and significant (r = 0.731, p = 0.001; Fig. 7). Therefore, as expected the reefs with a high coral cover also have higher densities of reef fishes, as also was found in the West Flower Garden Banks in the NW Gulf of Mexico (Wetmore et al., 2020). However, species richness showed no correlation with coral cover (r = 0.268, p = 0.314). Olán-González et al. (2020) found no significant correlation between reef fish species richness or density with coral cover in the VRSNP. Another important finding of our study was that there is a negative correlation between fish density and crustose coralline algae cover (r = -0.664, p = 0.004; Fig. 7). The calcifying rhodophyte non-geniculate crustose coralline algae are very important for the construction of the reef framework (Teichert et al., 2020). However, as they usually grow as a flat crust over the substratum, or form rhodoliths, they do not contribute significantly to increase reef tridimensional complexity (Basso, 2012; Weiss & Martindale, 2017). So, this could explain why those areas with a high cover of crustose corallines algae tend to flatten the reef bottom, with a concomitant decrease in the abundance of fishes. The correlation between the abundance of hermatypic corals and reef fishes, is additional evidence to support that their abundance might be influenced by the same
environmental drivers.
The SWGM has been subjected at least during the Holocene (Emery, 1963; Lidell, 2007; Morelock & Koenig, 1967) to a high influence of fluvial discharge from several rivers (Carrillo et al., 2007; Gil-Agudelo et al., 2020; Horta-Puga et al., 2015). So, the numerous reefs located in this coastal area (Tuxpan, Veracruz and Tuxtlas Reef Systems), usually have turbid waters and their communities are adapted to that set of conditions (Horta-Puga, 2007; Horta-Puga et al., 2015; Jordán-Garza et al., 2017). Thus, we expected to find roughly the same assemblage structure in all reef systems of the SWGM. In general, the reef fish assemblages previously reported from the VRSNP (Olán et al., 2020; Pérez-España et al., 2015), and from the Tuxtlas Reef System are similar as expected (González-Gándara, 2020b) (Fig. 8). These studies reported Coryphopterus spp., Halichoeres burekae, Chromis multilineata, Stegastes adustus, and Haemulon aurolineatum, as the most abundant species. However, an important difference of our results with those studies is that, except for C. multilineata, we did not find any of them as dominant in the VRSNP. The Isla Verde reef was the only one where C. personatus was dominant (Fig. 3), and this species ranked as the 6th most abundant in average for the whole VRSNP (Fig. 9). Instead, we found Chromis multilineata, Ocyurus chrysurus, Abudefduf saxatilis, Elacatinus jarocho, and Stegastes leucostictus as dominant. The difference in the reef fish species dominance order could be ascribed to differences in the zone and depth range of the reef sites where surveys were made. Their fish assemblage data were collected at zones exposed to heavy surge, the windward reef slopes in the VRSNP, and in a non-emergent patch-type reef in the Tuxtlas Reef System. Also, they surveyed at different range depths, 3-5 and 10-15 m (Pérez-España et al., 2015), 3-17 m (Olán et al., 2020), and 11-22 m (Gónzalez-Gándara, 2020b). So, the main difference is that we surveyed the leeward or protected side of the reef, and they surveyed on the windward or exposed side. In summary, in the SWGM’s reefs the following reef fishes are dominant: pomacentrids (Abudefduf, Chromis, Stegastes, Microspathodon, Neopomacentrus), haemulids (Haemulon), labrids (Bodianus, Halichoeres, Thalassoma), gobiids (Coryphopterus, Elacatinus), lutjanids (Ocyurus), scarids (Scarus), and tetraodontids (Canthigaster). Another important assumption is that the assemblage structure of reef fishes is different at distinct geomorphological reef zones.
The Gulf of Mexico is a large tropical and subtropical marine area, where coral reefs have developed at suitable places in the 4 geographical sectors: NW, NE, SW, and SE (Gil-Agudelo et al., 2020; Jaap et al., 2008; Schmahl et al., 2008; Tunnell et al., 2007;). That division in 4 sectors, was also used for the delimitation of the distribution ranges of fishes of the Gulf of Mexico by McEachran (2009). We compared the reef fish assemblage structure of the VRSNP (SW), with previous reports from other reef systems in the Gulf of Mexico, like the Flower Garden Banks (NW), the Florida Keys (NE), and Campeche Bank (SE), to know how different or similar is the reef fish assemblage structure of the VRSNP (Fig. 9). The Flower Garden Banks (FGB) comprise several submerged reefs, some 330 km to the west of Port Aransas, Texas, that also is influenced by the Mississippi River (Buckel et al., 2014). The Florida Keys (FKS) is composed by hundreds of shallow reefs, located south to the Florida Peninsula (Bohnsack et al., 1999). The Campeche Bank (CBR) also is composed by many emergent and non-emergent reefs (Chávez & Beaver, 2007). In the eastern half of the Gulf of Mexico, the FKS and the CBR lie on shallow carbonate shelves, which are not influenced by mainland drainage. On the other hand, in the western half of the Gulf of Mexico, the VRSNP and the FGB are on shelves with siliciclastic sediments sourced from the continent by rivers (Mendelssohn et al., 2017; Tunnell et al., 2007). As expected, due to the different climatological, oceanographical and ecological conditions of each sector of the gulf, the reef fish assemblages have some differences in the order of species abundance. The most dominant species for each reef system are C. multilineata in the VRSNP and the CBR, P. furcifer in the FGB, and T. bifasciatum in the FKS. Fifteen different species are among the top 5 most abundant at any locality, including Pomacentrids (Abudefduf, Chromis, Stegastes), Labrids (Clepticus, Thalassoma), Haemulids (Haemulon), Gobiids (Elacatinus), Lutjanids (Ocyurus), Scarids (Scarus), Grammatids (Gramma) and Serranids (Paranthias). Hence, as the 4 reef systems share most of these species, it can be concluded that the reef fish assemblage structure found in the VRSNP, in general is similar to those found at other coral reefs in the Gulf of Mexico, especially with the FGB.
Although, the reef fish assemblage structure of the VRSNP has been previously studied, only general results for the whole reef system were reported (Olán-Gutiérrez et al., 2020; Pérez-España et al., 2015; Rangel-Ávalos et al., 2008). Hence, this study focused on the numerical abundance of reef fish species, regardless of their trophic structure or their functional diversity, as this kind of data are useful as a baseline for ecological comparative purposes with other coral reefs. Also, as the reef system is composed by several reefs, we also searched for a spatial distribution pattern of reef fish assemblages. From our results, the most important findings were: 1) 116 reef species were recorded; 2) the reefs with high fish densities also had high species numbers; 3) there were no differences in fish density between the northern and southern reef groups, and the dry and rainy climate seasons; 4) average total reef fish density in the VRSNP is similar to the records for the Caribbean before the 2000 year, which suggests that VRSNP could be considered as a resilient ecosystem; 5) we found evidence of a spatial distribution pattern with 3 well-defined groups of reefs, which more or less resemble the classification based on the cover of the photosynthetic benthic communities; 6) higher fish densities are associated to both high hermatypic coral and low crustose coralline algae covers, which is additional evidence in support that their abundance might be influenced by the same environmental drivers; 7) the assemblage structure of reef fishes is different at distinct reef zones (windward and leeward slopes); 8) as expected, with some differences in the species abundance order, the assemblage structure of reef fishes is similar at all coral reefs in the Gulf of Mexico. Finally, coral reefs have been declining worldwide due to several local environmental stressors. In addition, global warming and ocean acidification have driven a generalized coral bleaching and death, a reduction in coral calcification rates, and a flattening of the reef bottom. Therefore, reef fish species richness and abundance will tend to decrease. So, ecological descriptive studies, like this, are an important tool to determine the impact of environmental threats on coral reefs in the future.
Acknowledgements
Thanks to the authorities of the Veracruz Reef System National Park (Conanp), who allowed us to conduct this study, and for the use of their boats. We are grateful to Manuel Victoria Muguira (Dorado Buceo) and Joseph Loustalot Laclette (Scubaver), who provided us with the scuba dive gear. We would like to thank an anonymous reviewer, his/her comments helped us to improve our manuscript. This study was financed by a grant from Conabio (GM005: Sistema Arrecifal Veracruzano, condición actual y programa permanente de monitoreo) to G. Horta-Puga.
References
Álvarez-Filip, L., Carricart-Ganivet, J. P., Horta-Puga, G., & Iglesias-Prieto, R. (2013). Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Scientific Reports, 3, 3486. https://doi.org/10.1038/srep03486
Álvarez-Filip, L., Dulvy, N. K., Côté, I. M., Watkinson, A. R., & Gill, J. A. (2011). Coral identity underpins architectural complexity on Caribbean reefs. Ecological Applications, 21, 2223–2231. https://doi.org/10.2307/41416650
Álvarez-Filip, L., Dulvy, N. K., Gill, J. A., Côté, I. M., & Watkinson, A. R. (2009). Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proceedings of the Royal Society of London B, 276, 3019–3025. https://doi.org/10.1098/rspb.2009.0339
Álvarez-Filip, L., Paddack, M. J., Collen, B., Robertson, D. R., & Côté, & I. M. (2015). Simplification of Caribbean Reef-Fish Assemblages over Decades of Coral Reef Degradation. Plos One, 10, e0126004. https://doi.org/10.1371/journal.pone.0126004
Amezcua-Linares, F., Amezcua, F., & Gil-Manrique, B. (2014). Effects of the Ixtoc I oil spill on fish assemblages in the southern Gulf of Mexico. In J. B. Alford, M. S. Peterson, & C. C. Green (Eds.), Impacts of oil spill disasters on marine habitats and fisheries in North America (pp. 201–229). Boca Raton: CRC Press.
Arenas-Fuentes, V., & Jiménez-Badillo, L. (2004). La pesca en el Golfo de México. Hacia mayores biomasas de explotación. In M. Caso, I. Pisanty, & E. Ezcurra (Eds.), Diagnóstico ambiental del golfo de México, Vol. 2 (pp. 755–769). Ciudad de México: INE-Semarnat.
Atta, C. J., Coker, D. J., Sinclair-Taylor, T. H., DiBattista, J. D., Kattan, A., Monroe, A. A., & Berumen, M. L. (2019). Conspicuous and cryptic reef fishes from a unique and economically important region in the northern Red Sea. Plos One, 14, e0223365. https://doi.org/10.1371/journal.pone.0223365
Avendaño-Álvarez, O., Salas-Monreal, D., Marín-Hernández, M., Salas de León, D. A., & Monreal-Gómez, M. A. (2017). Annual hydrological variation and hypoxic zone in a tropical reef system. Regional Studies in Marine Science, 9, 145–155. https://doi.org/10.1016/j.rsma.2016.12.007
Ayala-Rodríguez, G. A., Ordóñez-López, U., Meiners, C., & Marín-Hernández, M. (2016). Listado taxonómico, aspectos ecológicos y biogeográficos de las larvas de peces del Sistema Arrecifal Veracruzano, Suroeste del Golfo de México (junio 2011-junio 2013). Revista de Biología Marina y Oceanografía, 51, 255-264. http://dx.doi.org/10.4067/S0718-19572016000200004
Basso, D. (2012). Carbonate production by calcareous red algae and global change Geodiversitas, 34, 13-33. https://doi.org/10.5252/g2012n1a2
Beaver, C. R., & Chávez, E. A. (2007). Reef fisheries. In J. W. Tunnell, E. A. Chávez, & K. Whithers (Eds.), Coral reefs of the southern Gulf of Mexico (pp. 112–118). College Station, TX: Texas A&M University Press.
Beger, M., & Possingham, H. P. (2008) Environmental factors that influence the distribution of coral reef fishes: modeling occurrence data for broad-scale conservation and management. Marine Ecology Progress Series, 361, 1-13. https://doi.org/10.3354/meps07481
Bryant, W. R., Lugo, J., Cordova, C., & Salvador, A. (1991). Physiography and bathymetry. In A. Salvador (Ed.), The Gulf of Mexico basin, Geology of North America, Vol. J. (pp. 13–30). Boulder, CO: Geological Society of America.
Bohnsack, J. A., & Bannerot, S. P. (1986). A stationary visual census technique for quantitatively assessing community structure of coral reef fishes. NOAA Technical Report NMFS41.
Bohnsack, J. A., McClellan, D. B., Harper, D. E., Davenport, G. S., Konoval, G. J., Eklund, A. M. et al. (1999). Baseline data for evaluating reef fish populations in the Florida Keys, 1979-1998. NOAA Technical Memorandum NMFS-SEFSC-427.
Buckel, C. A., Muñoz, R. C., Whitfield, P. E., & Degan, B. P. (2014). Fish communities of the coral reef. In R. Clark, J. C. Taylor, C. A. Buckel, & L. M. Kracker (Eds.), Fish and benthic communities of the Flower Garden Banks National Marine Sanctuary: Science to Support Sanctuary Management (pp. 74–199). NOAA Technical Memorandum NOS NCCOS 179.
Caldwell, Z. R., Zglinczynski, B. J., Williams, G. J., & Sandin, S. A. (2016). Reef fish survey techniques: assessing the potential of standardizing methodologies. Plos One, 11, e0153066. https://doi.org/10.1371/journal.pone.0153066
Carricart-Ganivet, J. P., Beltrán-Torres, A. U., & Horta-Puga, G. (2011). Distribution and prevalence of coral diseases in the Veracruz Reef System, Southern Gulf of Mexico. Diseases of Aquatic Organisms, 95, 181-187. https://doi.org/10.3354/dao0239
Carricart-Ganivet, J. P., & Horta-Puga, G. (1993). Arrecifes de coral en México. In S. Salazár-Vallejo, & N. E. González NE (Eds.), Biodiversidad marina y costera de México (pp. 80–90). Ciudad de México: Conabio/ CIQRO.
Carrillo, L., Horta-Puga, G., & Carricart-Ganivet, J. P. (2007). Climate and oceanography. In J. W. Tunnell, E. A. Chávez, & K. Whithers (Eds.), Coral reefs of the southern Gulf of Mexico (pp. 34–40). College Station, TX: Texas A&M University Press.
Carriquiry, J. D., & Horta-Puga, G. (2010). The Ba/Ca record of corals from the southern Gulf of Mexico: contributions from land-use changes, fluvial discharge and oil-drilling muds. Marine Pollution Bulletin, 60, 1625–1630. https://doi.org/10.1016/j.marpo lbul.2010.06.007
Chávez, E. A., & Beaver, C. R. (2007). Reef fish. In J. W. Tunnell, E. A. Chávez, & K. Whithers (Eds.), Coral reefs of the southern Gulf of Mexico (pp. 102–111). College Station, TX: Texas A&M University Press.
Chávez, E. A., Tunnell, J. W., & Whiters, K. (2007). Reef zonation and ecology: Veracruz shelf and Campeche Bank. In J. W. Tunnell, E. A. Chávez, & K. Whithers (Eds.), Coral reefs of the southern Gulf of Mexico (pp. 41–67). College Station, TX. Texas A&M University Press.
Claro, R. (1994). Ecología de los peces marinos de Cuba. Chetumal, Q. Roo: Instituto de Oceanología y de la Academia de Ciencias de Cuba/ Centro de Investigaciones de Quintana Roo.
Claro, R., Cantelar, K., Pina-Amargós, F., & García-Arteaga, J. P. (2007). Cambios en las comunidades de peces de los arrecifes coralinos del Archipiélago Sabana-Camagüey, Cuba. Revista de Biología Tropical, 55, 537-547.
Coker, D. J., Wilson, S. K., & Pratchett, M. S. (2014). Importance of live coral habitat for reef fishes. Reviews in Fish Biology and Fisheries, 24, 89-126. https://doi.org/10.1007/s11160-013-9319-5
CNP (Carta Nacional Pesquera). (2018). Acuerdo por el que se da a conocer la Carta nacional Pesquera. Diario Oficial de la Federación, México, 11/06/2018.v Available at:
https://www.gob.mx/inapesca/documentos/carta-nacional-
pesquera-2017
Díaz-de-León, A., Fernández, J. I., Álvarez-Torres, P., Ramírez-Flores, O., & López-Lemus, L. G. (2004). La sustentabilidad de las pesquerías del Golfo de México. In M. Caso, I. Pisanty, & E. Ezcurra (Eds.), Diagnóstico ambiental del golfo de México, Volumen 2 (pp. 725–753). Ciudad de México: INE-Semarnat.
Del Mónaco, C., Narciso, S., Alfonso, F., Giménez, E., & Bustillos, F. (2010). Evaluación de las comunidades de corales y peces de algunos arrecifes de la Isla Tortuga y cayos adyacentes, Venezuela. Boletín del Centro Investigaciones Biológicas, 44, 355–378.
Del Moral-Flores, L. F., Tello-Musi, J. L., Reyes-Bonilla, H., Pérez-España, H., Martínez-Pérez, J. A., Horta-Puga, G. et al. (2013). Lista sistemática y afinidades zoogeográficas de la ictiofauna del Sistema Arrecifal Veracruzano. México. Revista Mexicana de Biodiversidad, 84, 825–846. https://doi.org/10.7550/rmb.34912
Emery, K. O. (1963). Regional studies, coral reefs off Veracruz, Mexico. Geofísica Internacional, 3, 11– 17
Flores-Coto, C., Sanvicente-Añorve, L., Zavala-García, F., Zavala-Hidalgo, J., & Funes-Rodríguez, R. (2014). Environmental factors affecting structure and spatial patterns of larval fish assemblages in the southern Gulf of Mexico. Revista de Biología Marina y Oceanografía, 49, 307–321. http://dx.doi.org/10.4067/S0718-19572014000200010
Friedlander, A. M., & DeMartini, E. E. (2002). Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian Islands: the effects of fishing down apex predators. Marine Ecology Progress Series, 230, 253–264. https://doi.org/10.3354/meps230253
Gil-Agudelo, D. L., Cintra-Buenrostro, C. E., Brenner, J., González-Díaz, P., Kiene, W., Lustic, C. et al. (2020). Coral reefs in the Gulf of Mexico large marine ecosystem: conservation status, challenges, and opportunities. Frontiers in Marine Science, 6, 807. https://doi.org/10.3389/fmars.2019.00807
Graham, N. A. J., & Nash, K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral Reefs, 32, 315–326. https://doi.org/10.1007/s00338-012-0984-y
González-Gándara, C. (2020a). Composition, distribution and relative abundance of fishes linked to fringing reefs of southern Veracruz, Gulf of Mexico. GSC Biological and Pharmaceutical Sciences, 12, 217–228. https://doi.org/10.30574/gscbps.2020.12.2.0268
González-Gándara, C. (2020b). The fish community of Palo Seco reef, Veracruz, Gulf of Mexico and its relationship with the morpho functional groups. Ciencia UAT, 14, 34–50. https://doi.org/10.29059/cienciauat.v14i2.1347
González-Gándara, C., & Arias-González, J. E. (2001a). Nuevos registros de peces en el Arrecife Alacrán, Yucatán, México. Revista de Biolología Tropical, 42, 765–772.
González-Gándara, C., & Arias-González, J. E. (2001b). Lista actualizada de peces del arrecife Alacranes, Yucatán, México. Anales del Instituto de Biología, UNAM, Serie Zoología, 72, 245–258.
González-Gándara, C., & Chávez, E. A. (2020). Fish and fisheries of the eastern coast of Mexico, with emphasis con coral reef species. In L. Hufnagel (Ed.), Natural history and ecology of Mexico and Central America (pp. 1–19) London: IntechOpen. https://www.intechopen.com/online-first/74513
González-Gándara, C., Lozano-Vilano, M. L., Vivencio-de la Cruz, F., & Domínguez-Barradas, C. (2013). Peces del Sistema Arrecifal Lobos-Tuxpan, Veracruz, México. Universidad y Ciencia, 29, 191–208. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0186-29792013000200008
Gutiérrez-de-Velasco, G., & Winant, C. D. (1996). Seasonal patterns of wind stress and wind stress curl over the Gulf of Mexico. Journal of Geophysical Research, 101, 127–140. https://doi.org/10.1029/96JC01442
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). Past: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 9.
Horta-Puga, G. (2003). Condition of selected reef sites in the Veracruz Reef System (stony corals and algae). Atoll Research Bulletin, 496, 360–369
Horta-Puga, G. (2007). Environmental impacts. In J. W. Tunnell, E. A. Chávez, & K. Whithers (Eds.), Coral reefs of the southern Gulf of Mexico (pp. 126–142). College Station, TX: Texas A&M University Press.
Horta-Puga, G. (2017). Lead geochemical partitioning in biogenic carbonate sediments at a coral reef depositional environment. Marine Pollution Bulletin, 116, 71–79. https://doi.org/10.1016/j.marpolbul.2016.12.049
Horta-Puga, G., Álvarez-Filip, L., Cabral-Tena, R. A., López-Pérez, A., Ortiz-Lozano, L., Pérez-España, H.et al. (2019). Coastal coral reefs in Mexico. In A. V. Botello, S. Villanueva, & J. Gutiérrez (Eds.), Costas y mares mexicanos: contaminación, impactos, vulnerabilidad y cambio climático (pp. 329–366). Campeche, México: UNAM/ UAC.
Horta-Puga, G., Tello-Musi, J. L., Beltrán-Torres, A. U., Carricart-Ganivet, J. P., Carriquiry, J. D., & Villaescusa-Celaya, J. (2015). Veracruz Reef System: a hermatypic coral community thriving in a sedimentary terrigenous environment. In A. Granados-Barba, L. Ortiz-Lozano, D. Salas-Monreal, & C. González-Gándara (Eds.), Aportes al conocimiento del Sistema Arrecifal Veracruzano: hacia el corredor arrecifal del suroeste del golfo de México (pp. 181–208). Campeche, México: UAC.
Horta-Puga, G., Tello-Musi, J. L., Córdova, A., Gutiérrez-Carrillo, A., Gutiérrez-Martínez, J., & Morales-Aranda, A. A. (2020). Spatio-temporal variability of benthic macroalgae in a coral reef system highly influenced by fluvial discharge: Veracruz, Gulf of Mexico. Marine Ecology, 41, e12596. https://doi.org/10.1111/maec.12596
Humann, P., & DeLoach, N. (2014). Reef fish identification, Florida, Caribbean, Bahamas. 4th Ed. Jacksonville, FLA: New World Publications.
Jaap, W. C., Szmant, A., Jaap, K., Dupont, J., Clarke, R., Somerfield, P. et al. (2008). A perspective on the biology of Florida Keys coral reefs. In B. M. Riegl, & R. E. Dodge (Eds.), Coral reefs of the USA (pp. 75–125). Switzerland: Springer.
Johnston, M. A., Nuttall, M. F., Eckert, R. J., Embesi, J. A., Sterne, T. K., Hickerson, E. L. et al. (2016). Persistence of coral assemblages in Flower Garden Banks National Marine Sanctuary, Gulf of Mexico. Coral Reefs, 35, 821–826. https://doi.org/10.1007/s00338-016-1452-x
Jones, G. P., & Syms, C. (1998). Disturbance, habitat structure and the ecology of fishes on coral reefs. Australian Journal of Ecology, 23, 287–297. https://doi.org/10.1111/j.1442-9993.1998.tb00733.x
Jordán-Garza, G., Gónzalez-Gándara, C., Salas-Pérez, J. J., & Morales-Barragán, A. M. (2017). Coral assemblages are structured along a turbidity gradient on the Southwestern Gulf of Mexico, Veracruz. Continental Shelf Research, 138, 32–40. http://dx.doi.org/10.1016/j.csr.2017.03.002
Lidell, W. D. (2007). Origin and geology. In J. W. Tunnell, E. A. Chávez, & K. Whithers (Eds.), Coral reefs of the southern Gulf of Mexico (pp. 23–33). College Station, TX: Texas A&M University Press.
López-Ordaz, A., & Rodríguez-Quintal, J. G. (2010). Ictiofauna asociada a un arrecife somero en el Parque Nacional Morrocoy, Venezuela. Revista de Biología Tropical, 58, 163–174.
Mallela, J., Roberts, C., Harrod, C., & Goldspink, C. R. (2007) Distributional patterns and community structure of Caribbean coral reef fishes within a river-impacted bay. Journal of Fish Biology, 70, 523–537. https://doi.org/10.1111/j.1095-8649.2007.01323.x
Mayorga-Martínez, M., Bello-Pineda, J., Perales-Valdivia, H., Pérez-España, H., & Heyman, W. (2021). Characterizing geomorphology of mesophotic coral reef ecosystems in the southwestern Gulf of Mexico: implications for conservation and management. Frontiers in Marine Science, 8, 639359. https://doi.org/10.3389/fmars.2021.639359
McEachran, J. D. (2009). Fishes (Vertebrata: Pisces) of the Gulf of Mexico. In D. L. Felder, & D. K. Camp (Eds.), Gulf of Mexico origin, waters and biota, Volume 1 Biodiversity (pp. 1223–1316). College Station, TX: Texas A & M University.
Mejía, L. S., & Garzón-Ferreira, J. (2000). Estructura de comunidades de peces arrecifales en cuatro atolones del Archipiélago de San Andrés y Providencia (Caribe sur occidental). Revista de Biología Tropical, 48, 883–896.
Mendelssohn, I. A., Byrnes, M. R., Kneib, R. T., & Vittor, B. A. (2017). Coastal habitats of the Gulf of Mexico. In C. H. Ward (Ed.), Habitats and biota of the Gulf of Mexico: before the Deepwater Horizon oil spill, Voume 1 (pp. 359–640). New York: Springer. https://doi.org/10.1007/978-1-4939-3447-8
Menza, C. W., Ault, J. S., Beets, J., Bohnsack, J. A., Caldow, C., Christensen, J. et al. (2006). A guite to monitoring reef fish in the National Park Service’s South Florida/Caribbeab network. NOAA Technical Memorandum NOS NCCOS 39.
Morelock, J., & Koenig, K. J. (1967). Terrigenous sedimentation in a shallow water coral reef environment. Journal of
Sedimentary Petrology, 37, 1001–1005. https://doi.org/10.
1306/74D71811-2B21-11D7-8648000102C1865D
Murawski, S. A., Peebles, E. B., Gracia, A., Tunnell, J. W., & Armenteros, M. (2018). Comparative abundance, species composition, and demographics of continental shelf fish assemblages throughout the Gulf of Mexico. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science, 10, 325–346. https://doi.org/10.1002/mcf2.10033
Nelson, J. S., Grande, T. C., & Wilson, M. V. H. (2016). Fishes of the World. 5th Ed. New Jersey: John Wiley & Sons
Núñez-Lara, E., González-Salas, C., Ruiz-Zárate, M. A., Hernández-Landa, R., & Arias-González, J. E. (2003). Condition of coral reef ecosystems in central-southern Quintana Roo (part 2. Reef fish communities). Atoll Research Bulletin, 496, 338–358
Olán-González, M., Reyes-Bonilla, H., Álvarez-Filip, L., Pérez-España, H., & Olivier, D. (2020). Fish diversity divergence between tropical eastern pacific and tropical western Atlantic coral reefs. Environmental Biology of Fishes, 103, 1323–1341. https://doi.org/10.1007/s10641-020-01026-y
Ortiz-Lozano, L. (2012). Identification of priority conservation actions in marine protected areas: using a causal networks approach. Ocean and Coastal Management, 55, 74–83. https://doi.org/10.1016/j.ocecoaman.2011.10.013
Ortiz-Lozano, L., Colmenares-Campos, C., & Gutiérrez-Velázquez, A. (2018). Submerged coral reefs in the Veracruz Reef System, Mexico and its implications for marine protected area management. Ocean and Coastal Management, 158, 11–23. https://doi.org/10.1016/j.ocecoaman.2018.03.012
Ortiz-Lozano, L., Pérez-España, H., Granados-Barba, A., González-Gándara, C., Gutiérrrez-Velázquez, A., & Martos, J. (2013). The reef corridor of the southwest Gulf of Mexico: challenges for its management and conservation. Ocean and Coastal Management, 86, 22–32. http://dx.doi.org/10.1016/j.ocecoaman.2013.10.006
Paddack, M. J., Reynolds, J. D., Aguilar, C., Appeldoorn, R. S., Beets, J., Burkett, E. W. et al. (2009). Recent region-wide declines in Caribbean reef fish abundance. Current Biology, 19, 590–595. https://doi.org/10.1016/j.cub.2009.02.041
PD. (2021). Pescando datos. Sector pesquero en números. Updated may/2020. https://pescandodatos.org/sector-pesquero-en-numeros.html. Accessed 25/Jun/2021.
Pérez-España, H., Ávila-Gutiérrez, P. S., Melo-Merino, S. M., Berumen-Solórzano, P., & Flores-Arévalo, R. R. (2015). Patrones interanuales e interarrecifales de las comunidades de peces, corales y equinodermos en el Sistema Arrecifal Veracruzano. In A. Granados-Barba, L. Ortiz-Lozano, D. Salas-Monreal, & C. González-Gándara (Eds.), Aportes al conocimiento del Sistema Arrecifal Veracruzano: hacia el corredor arrecifal del suroeste del golfo de México (pp. 159–178). Campeche, México: UAC.
Pittman, S. J., Hile, S. D., Jeffrey, C. F. G., Clark, R., Woody, K., Herlach, B. D. et al. (2010). Coral reef ecosystems of Reserva Natural de La Parguera (Puerto Rico): spatial and temporal patterns in fish and benthic communities (2001-2007). NOAA Technical Memorandum NOS NCCOS 17.
Rangel-Ávalos, M. A., Jordan, L. K. B., Walker, B. K., Gilliam, D. S., Carvajal-Hinojosa, E., & Spieler, R. E. (2008). Fish and coral reef communities of the Parque Nacional Sistema Arrecifal Veracruzano (Veracruz Coral Reef System National Park) Veracruz, Mexico: preliminary results. Proceedings of the 60th Gulf and Caribbean Fisheries Institute, 60,
427–435.
Robertson, D. R., & Cramer, K. L. (2014). Defining and dividing the Greater Caribbean: insights from the biogeography of shorefishes. Plos One, 9, e102918. https://doi.org/10.1371/journal.pone.0102918
Robertson, D. R., Pérez-España, H., Domínguez-Domínguez, O., Estapé, C.J., & Estapé, A.M. (2019). An update to the inventory of shore-fishes from the Parque Nacional Sistema Arrecifal Veracruzano, Veracruz, México. Zookeys, 882, 127–157. https://doi.org/10.3897/zookeys.882.38449
Robertson, D. R., Pérez-España, H., Núñez-Lara, E., Puc-Itza, F., & Simões, N. (2016). The fishes of Cayo Arcas (Campeche Bank, Gulf of Mexico): an updated checklist. Zookeys, 640, 139–155. https://doi.org/10.3897/zookeys.640.10862
Salmerón-García, O., Zavala-Hidalgo, J., Mateos-Jasso, A., & Romero-Centeno, R. (2010). Regionalization of the Gulf of Mexico from space-time chlorophyll-a concentration variability. Ocean Dynamics, 61, 439–448. https://doi.org/10.1007/s10236-010-0368-1
Samoilys, M. A., & Carlos, G. (2000). Determining methods of underwater visual census for estimating the abundance of coral reef fishes. Environmental Biology of Fishes, 57, 289–304. https://doi.org/10.1023/A:1007679109359
Sandin, S. A., Smith, J. E., DeMartini, E. E., Dinsdale, E. A., Donner, S. D., Freidlander, A. M. et al. (2008). Baselines and degradation of coral reefs in the northern Line Islands. Plos One, 3, e1548. https://doi.org/10.1371/journal.pone.0001548
Sanvicente-Añorve, L., Flores-Coto, C., & Sánchez-Velasco, L. (1998). Spatial and seasonal patterns of larval fish assemblages in the southern Gulf of Mexico. Bulletin of Marine Science, 62, 17–30.
Schmahl, G. P., Hickerson, E. L., & Precht, W. F. (2008). Biology and ecology of coral reefs and coral communities in the Flower Garden Banks region, northwestern Gulf of Mexico. In B. M. Riegl, & R. E. Dodge (Eds.), Coral reefs of the USA (pp. 221–261). Switzerland: Springer.
Smith, J. E., Brainard, R., Carter, A., Grillo, S., Edwards, C., Harris, J. et al. (2016). Re-evaluating the health of coral reef communities: baselines and evidence for human impacts across the central Pacific. Proceedings of the Royal Society B, 283, 10151985. https://doi.org/10.1098/rspb.2015.1985
Stallings, C. D. (2009). Fishery-independent data reveal negative effect of human population density on Caribbean predatory fish communities. Plos One, 4, e5333. https://doi.org/10.1371/journal.pone.0005333
Teichert, S., Steinbauer, M., & Kiessling, W. A. (2020). Possible link between coral reef success, crustose coralline algae and the evolution of herbivory. Scientific Reports, 10, 17748. https://doi.org/10.1038/s41598-020-73900-9
Tello-Musi, J. L., Chávez-Arteaga, M., Cruz-López, F. J., & Martínez-Pérez, J. A. (2018) Adenda a la lista sistemática y afinidades zoogeográficas de la ictiofauna del Sistema Arrecifal Veracruzano, México. Revista de Zoología (FESI, UNAM), 29, 81–83. https://www.redalyc.org/articulo.oa?id=49855322007
Toller, W., Debrot, A. O., Vermeij, M. J. A., & Hoetjes, P. C. (2010). Reef fishes of Saba Bank, Netherland Antilles: assemblage structure across a gradient of habitat types. Plos One, 5, e9207. https://doi.org/10.1371/journal.pone.0009207
Tunnell, J. W. (1988). Regional comparison of southwestern Gulf of Mexico to Caribbean Sea coral reefs. Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 3, 303–308.
Tunnell, J. W. (2007) Reef distribution. In J. W. Tunnell, E. A. Chávez, & K. Whithers (Eds.), Coral reefs of the southern Gulf of Mexico (pp. 14–22). College Station, TX: Texas A&M University Press.
Tunnell, J. W., Chávez, E. A., & Whithers, K. (2007). Coral reefs of the southern Gulf of Mexico. College Station, TX: Texas A&M University Press.
Vargas-Hernández, J. M., Badillo, M. L. J., & Fuentes, V. A. (2002). El Sistema Arrecifal Veracruzano y las pesquerías asociadas. In P. Guzmán-Amaya, C. Quiroga-Brahms, C. Díaz-Luna, D. Fuentes-Ceballos, C. M. Contreras, & G. Silva-López (Eds.), La pesca en Veracruz y sus perspectivas de desarrollo (pp. 13–16). Xalapa, Ver: Sagarpa/
INP/ UV.
Vargas-Hernández, J. M., Nava-Martínez, G., & Román-Vives, M. A. (2002). Peces del Sistema Arrecifal Veracruzano. In P. Guzmán-Amaya, C. Quiroga-Brahms, C. Díaz-Luna, D. Fuentes-Ceballos, C. M. Contreras, & G. Silva-López (Eds.), La pesca en Veracruz y sus perspectivas de desarrollo (pp. 17–29). Xalapa, Ver: Sagarpa/ INP/ UV.
Wetmore, L. S., Dance, M. A., Hill, R. L., & Rooker, J. R. (2020). Community dynamics of fish assemblages on mid-shelf and outer-shelf coral reefs in the Northwestern Gulf of Mexico. Frontiers in Marine Science, 7, 152. https://doi.org/10.3389/fmars.2020.00152
Weiss, A., & Martindale, R. C. (2017). Crustose coralline algae increased framework and diversity on ancient coral reefs. Plos One, 12, e0181637. https://doi.org/10.1371/journal.pone.0181637
Williams, I. D., Baum, J. K., Heenan, A., Hanson, K. M., Nadon, M. O., & Brainard, R. E. (2015) Human, oceanographic and habitat drivers of Central and Western Pacific Coral Reef Fish Assemblages. Plos One, 10, e0120516. https://doi.org/10.1371/journal.pone.0120516