David Uriel Hernández-Becerril a, *, Graciela Arce-Rocha b
a Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
b Universidad Nacional Autónoma de México, Facultad de Ciencias, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
*Corresponding author: dhernand@cmarl.unam.mx (D.U. Hernández-Becerril)
Received: 13 November 2020; accepted: 9 March 2021
Abstract
The genus Blepharocysta Ehrenberg currently comprises 5 species of truly marine planktonic thecate dinoflagellates, which are solitary, globose, subspherical and heterotrophic cells, lack typical depressed cingulum and sulcus, and are mainly distributed in tropical areas. The morphology of 2 species, Blepharocysta splendor-maris and B. paulsenii, found in net samples from the tropical Mexican Pacific, was studied by LM and SEM, especially plate arrangement and theca ornamentation. Blepharocysta splendor-maris showed more ovoid cells, developed sulcal lists, and theca with shallow poroids having a relative low density, whereas B. paulsenii had more spherical cells, shorter epitheca, reduced and slightly ornamented sulcal lists, and theca with deep poroids having a relatively higher density. The thecal tabulation in both species agrees with conventional tabulation, including the anterior intercalary plate in B. paulsenii. A comparison of both morphological and ecological characteristics among all species of the genus is done, outstanding shape of cells, relative size, development of sulcal lists, and especially the theca ornamentation as important morphological characters to distinguish species. Blepharocysta splendor-maris is the only species distributed from tropical to Antarctic waters, whereas the others are limited to tropical and subtropical waters.
Keywords: Blepharocysta; Planktonic thecate dinoflagellates; Podolampadaceae; Tropical Mexican Pacific
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Morfología de dos especies de dinoflagelados tecados del género Blepharocysta (Dinophyta) del Pacífico tropical mexicano
Resumen
El género Blepharocysta Ehrenberg comprende 5 especies de dinoflagelados tecados, planctónicos marinos. Incluye células solitarias, globulares, subesféricas y heterótrofas, que carecen de cingulum y sulcus típicos, de distribución principal en áreas tropicales. Se estudió la morfología de las especies Blepharocysta splendor-maris y B. paulsenii, encontradas en muestras de red del Pacífico mexicano tropical, usando ML y MEB, particularmente el arreglo de placas y ornamentación de la teca. Blepharocysta splendor-maris mostró una forma ovoide, aletas sulcales desarrolladas, teca con poroides poco profundos y mediana densidad, mientras que B. paulsenii tuvo forma más esférica, epiteca más corta, aletas sulcales reducidas, ligeramente ornamentadas y teca con poroides profundos, de mayor densidad. La tabulación de placas en ambas especies concuerda con la tabulación convencional, incluida la placa intercalar anterior en B. paulsenii. Se comparan las características morfológicas y ecológicas de las especies del género, destacando forma de células, tamaño relativo, desarrollo de las aletas sulcales y especialmente la ornamentación de la teca como caracteres morfológicos para distinguir especies. Blepharocysta splendor-maris es la única especie distribuida desde aguas tropicales a antárticas, mientras que las otras están limitadas a aguas tropicales y subtropicales.
Palabras clave: Blepharocysta; Dinoflagelados tecados planctónicos; Podolampadaceae; Pacífico tropical mexicano
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Dinoflagellates are a very important protist taxonomic group in the marine plankton. Dinoflagellates include thecate, athecate and “thin-walled” forms, depending on the type of cell covering. Most members of the family Podolampadaceae (Podolampaceae) Lindemann have the unique characteristic among other thecate dinoflagellates of lacking the typical depressed cingulum and sulcus, such as species of the genera Blepharocysta Ehrenberg, Lissodinium Matzenauer and Podolampas Stein (Abé, 1966; Balech, 1963, 1988; Carbonell-Moore, 1991, 1994a; Fensome et al., 1993; Gómez et al., 2010; Steidinger & Tangen, 1997), but there have been new additions including Gaarderiella Carbonell-Moore, Heterobractum Carbonell-Moore, and Mysticella Carbonell-Moore (Carbonell-Moore, 1994a, 2010; Gómez et al., 2011).
More recently, with the addition of the genus Roscoffia Balech to the family (Gómez, 2012), the podolampaceans might include 7 genera, but there are other positions, considering only 6 genera (with Roscoffia out of the family) (Hoppenrath, 2017). Roscoffia has the same tabulation and clades together with Blepharocysta sp. in molecular phylogenies, although it differs in many other aspects like the presence of a typical cingulum and the benthic habits (Gómez et al., 2010, 2011, 2019; Yamaguchi et al., 2018). The genus Lessardia Saldarriaga et Taylor was also considered to be part of that family (Saldarriaga et al., 2003), although this classification remains controversial, as Lessardia has been proposed to belong to the family Lessardiaceae Carbonell-Moore based on morphological differences (Carbonell-Moore, 2004). Recently, the marine benthic species Cabra matta Murray et Patterson also formed a clade together with Roscoffia and the podolampaceans in molecular phylogenies (Yamaguchi et al., 2018).
The genus Blepharocysta currently comprises 5 species (Guiry & Guiry, 2020), all of them marine and planktonic, of warm-water, tropical to subtropical affinity, and having a globose, spherical or subspherical shape, mostly apparently smooth, with no neck or spines but conspicuous sulcal lists usually present at the antapex of cells (Abé, 1966; Balech, 1963; Carbonell-Moore, 1994a; Guiry & Guiry, 2020; Steidinger & Tangen, 1997). Although chloroplasts have been mentioned to be present in Blepharocysta (Steidinger & Tangen, 1997), there is strong evidence that its species do not have chloroplasts and they have a feeding mechanism by extruding a pallium, as other heterotrophic thecate dinoflagellates (Carbonell-Moore, 2004; Jacobson, 1999).
Classic papers on dinoflagellates have included observations of certain species of the genus Blepharocysta, redescribing well-known species (Blepharocysta splendor-maris (Ehrenberg) Ehrenberg, the type species) or describing new species (B. paulsenii Schiller, B. denticulata Nie, B. okamurae Abé) and their tabulation, basically using Light Microscopy (LM) (e.g., Abé, 1966; Balech, 1963, 1988; Gaarder, 1954; Nie, 1939; Schiller, 1937). Later, another new species (Blepharocysta hermosillae Carbonell-Moore) was described and illustrated with light microscopy and Scanning Electron Microscopy (SEM) (Carbonell-Moore, 1992), and the species B. compressa Gaarder and B. schilleri (Gaarder) Ballantine in Parke et Dixon (= B. matzenaueri Gaarder) were found to be synonyms of the type species of the closely related genus Lissodinium, Lissodinium schilleri Matzenauer (Carbonell-Moore, 1991).
Other studies are the revisions on taxonomy and biogeography of the family Podolampadaceae (Carbonell-Moore, 1994a, b), including the illustrations of whole cells in lateral or ventral view from the 5 Blepharocysta species by SEM (Carbonell-Moore, 1994b, plate I, Figs. 1-5); additionally, 3 new genera of the family were proposed (Gaarderiella, Heterobractum and Mysticella), with taxonomic changes of species originally described as Blepharocysta, as B. striata Schütt transferred to Mysticella striata (Schütt) Carbonell-Moore (Carbonell-Moore, 1994a). The taxonomic revision of the family Podolampadaceae also included a new interpretation on the plate formula: APC, 3’, 1a, 5”, 3C, 4S, 4-5”’, 1””, considering 5 postcingular (5”’) plates instead of the 3 traditional ones, with no intercalary posterior plate, which adjusts to the Blepharocysta species (Carbonell-Moore, 1994a). Additionally, molecular phylogenies of Podolampadaceae members, including Blepharocysta and Podolampas (and Lessardia) species have been provided (Gómez et al., 2010; Saldarriaga et al., 2003). The species Blepharocysta splendor-maris was recently documented by SEM micrographs from China (Yang & Li, 2014). Finally, there has been a large debate on a nomenclatural and taxonomic issue derived from the name Peridinium splendor-maris Ehrenberg, on which the type species of the genus Blepharocysta is based (Carbonell-Moore, 2018; Elbrächter et al., 2018, 2019).
During an oceanographic cruise along the coasts of the central Mexican Pacific, net samples were taken off the coast of Acapulco, and the microscopical analysis yielded a number of tropical forms of dinoflagellates, including 2 Blepharocysta species. This study shows details of the plate arrangement and ornamentation of the theca of Blepharocysta splendor-maris and B. paulsenii, using LM and SEM, and discusses important morphological and ecological characters to distinguish the species of the genus, providing a comparative table.
Material and methods
This study is based on material collected during the oceanographic cruise “MareaR VI” carried out from 10-21 June, 2014, along coasts of the central Mexican Pacific. Net samples (mesh 64 µm) were obtained by vertical hauls, the hauling depths depending on the depth of the fixed stations; a set of samples were fixed with formalin (4%) and another set with ethanol (70%). One sample obtained from 40 m depth to surface from station 42 (off the coast of Acapulco, State of Guerrero, 16°49’ N, 99°53’ W), yielded many tropical forms of dinoflagellates, including the Blepharocysta species studied herein. The total depth of the station was 56 m, the surface temperature was 29.8 °C, and a chlorophyll a maximum was recorded at 20 m.
Specimens belonging to Blepharocysta were isolated by micropippeting either from raw or rinsed samples, and analyzed by LM (Olympus BX 40, attached camera Hitachi KP-D50 Color digital) for identification, measuring and making the theca transparent, using sodium hypochlorite, following recommendations by Taylor (1978) and Taylor et al. (2003), whereas other additionally isolated specimens were studied by SEM (JEOL JSM6360LV) for details of the fine morphology, after rinsing, air-drying and coating with gold. General terminology for dinoflagellates follows recommendations by Fensome et al. (1993) and Steidinger and Tangen (1997), and more specific for the tabulation of podolampadacean members by Balech (1963), Abé (1966) and Carbonell-Moore (1994a) (Figs. 3, 15). Poroids density was calculated from SEM micrographs considering poroids numbers in 10 µm2 at low magnifications, especially focused in the plates C1 and C3, and poroids numbers in 5 µm2 at high magnifications, only in postcingular plates.
Results
Blepharocysta splendor–maris (Ehrenberg) Ehrenberg (Figs. 1-12)
References: Schiller, 1937, p. 477, Figs. 550 a-c; Nie, 1939, p. 31, text-Figs. 1, 2, pl. 1, Figs. 1-19; Balech, 1963, p. 16, pl. III, Figs. 34-44; Wood, 1963, p. 51, Fig. 188; Abé, 1966, p. 141, Figs. 21-32; Pesantes, 1978, p. 6, pl. 2, Fig. 6; Dodge, 1982, p. 254, Fig. 33H; Balech, 1988, 125, pl. 52, Figs. 16-19; Carbonell-Moore, 1994b, pl. I, Fig. 1; Steidinger and Tangen, 1997, p. 533, pl. 7, Fig. 49; Konovalova, 1998, p. 168, Fig. 36 6a, 6b; McMinn and Scott, 2005, p. 231, Fig. 3.17c; Iwataki et al., 2012, p. 127; Yang and Li, 2014, p. 162, 163.
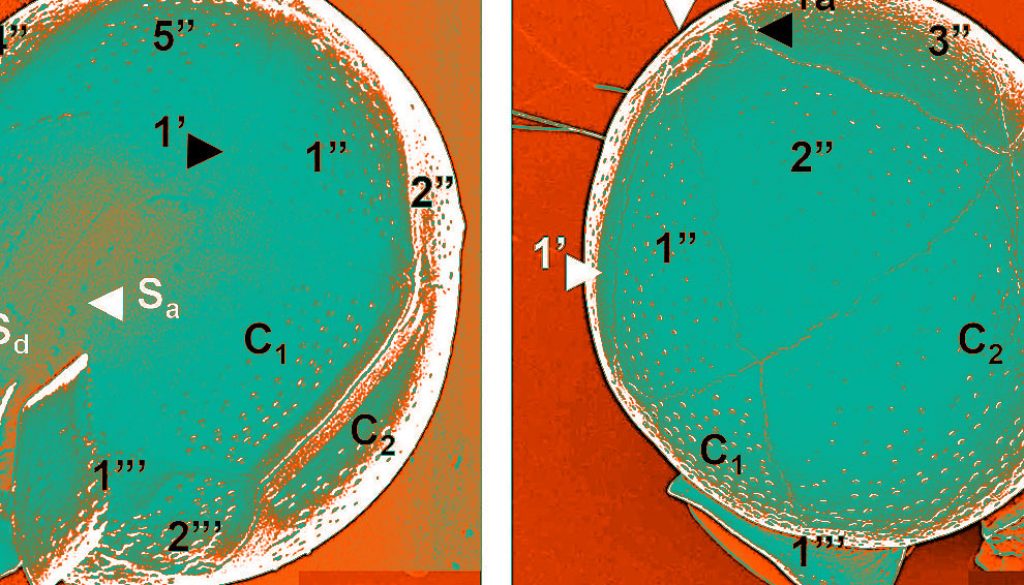
Only solitary cells of medium size were found, with a globose appearance, spherical, subspherical or ovoid in shape, some specimens slightly more elongate (larger than wide) and wider at the posterior hypotheca (Figs. 1-4). No conspicuous structures, apart from the sometimes salient sulcal list, which may be variable in length and development, and the apical pore, which may be completely unapparent or observed as a short and truncated knob (Figs. 1-4). Untreated cells showed a slightly eccentric and large nucleus, but no trace of chloroplast (Figs. 1, 2). Conversely, a specimen made transparent in ventral view showed the epitheca considerably larger than the hypotheca, the plates of the apical (1’), precingular (1” to 4”, except 3”), cingular (C1 to C3), and postcingular series (1”’ and 5”’, only), 2 sulcal platelets (Sa and Sd) and also the apical pore complex (APC) at the anterior apex and the sulcal lists at the posterior apex (Figs. 3, 4). The theca is completely covered by small poroids (Figs. 3, 4).
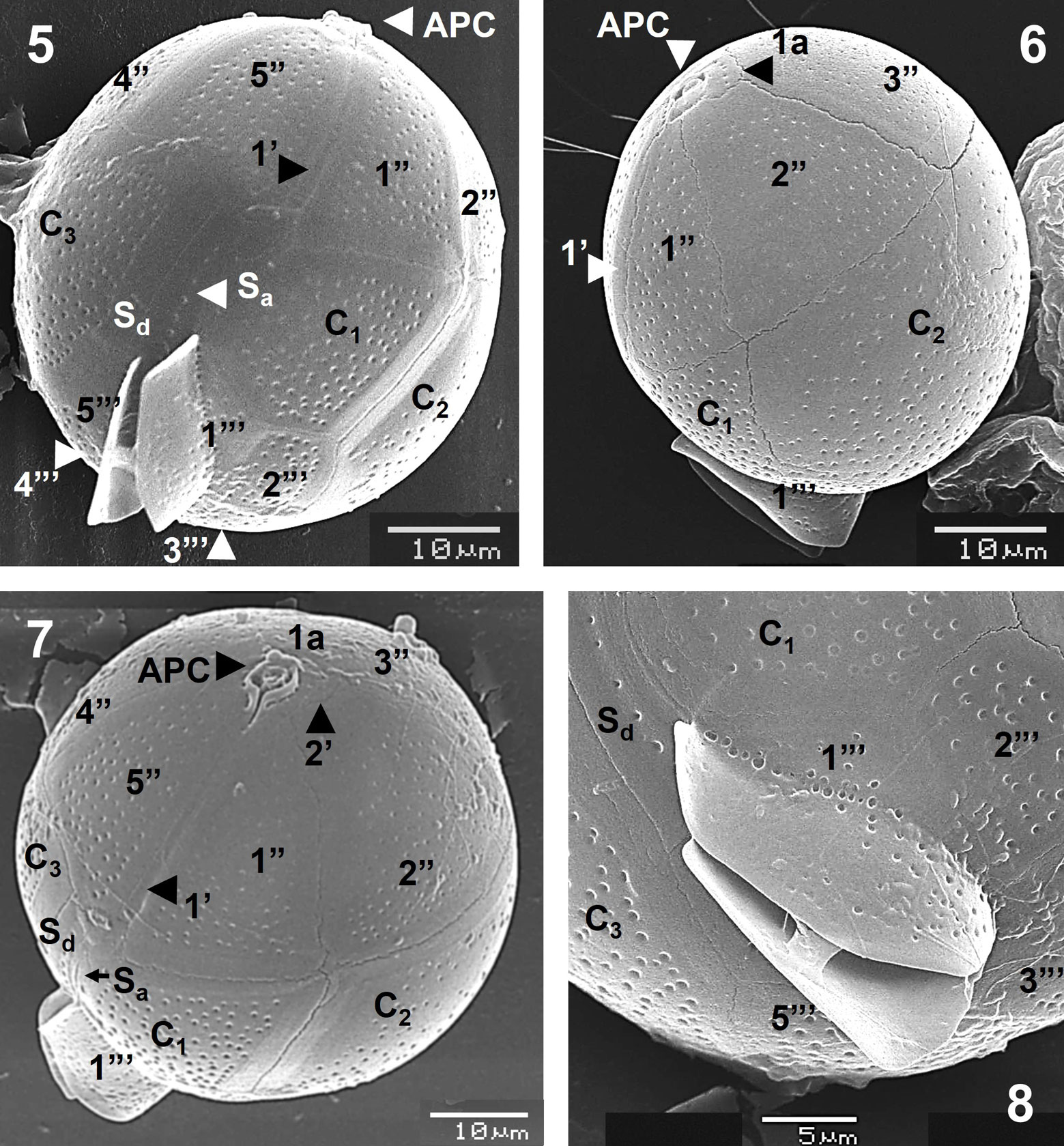
Observations by SEM confirmed the proportions of the epi- and hypotheca and plate arrangement of the species in ventral view, although postcingular plates (1”’ to 5”’) and the sulcal lists can be also visible (Fig. 5). The small postcingular plates constitute the hypotheca (Fig. 5), together with the antapical plate (1””, not shown). The plate 1’ is very narrow and long (Figs. 5-7, 9). Another specimen in lateral view made possible to observe the only intercalary plate (1a), which is rectangular and small (6.2 × 3.5 µm), close to the APC, the third precingular plate (3”) and the cingular plate C2, the largest of the species (Fig. 6).
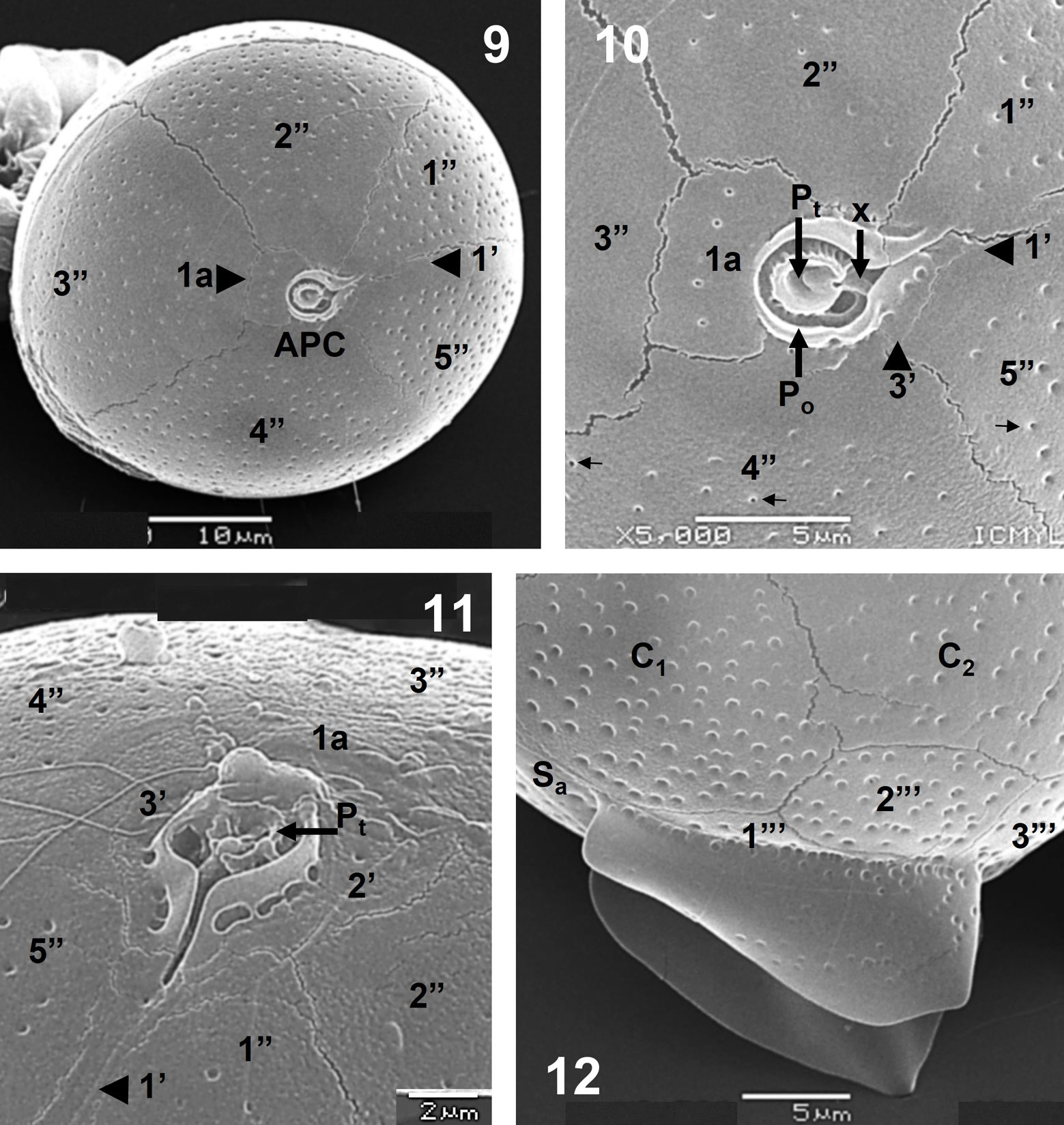
An apical view showed the APC, 1a and the second apical plate (2’), with the conspicuous sulcal plates (Sa and Sd) (Fig. 7), whereas a detailed view of the posterior part showed the sulcal list, the postcingular plates and the Sd platelet (Fig. 8). The APC, the first apical (1’), the intercalary (1a) and all precingular plates are seen in apical view (Fig. 9), whereas a detail of that view showed platelets of the APC: Po, Pt (cover platelet) and X (canal platelet) and the third apical plate (3’) (Fig. 10); the 3 apical plates (1’ to 3’), 2 being very small (2’ and 3’), and the APC are also shown (Fig. 11). The sulcal lists fairly developed and almost smooth, some plates (cingular and postcingular) and the anterior sulcal platelet (Sa) at the posterior zone, in lateral view, can be observed (Fig. 12).

The theca is covered by regularly distributed small and shallow poroids, which have a relative low density (39-43 pores in 10 µm2, in plates C1 and C3, and 12-16 pores in 5 µm2, in postcingular plates) (Figs. 5-12), and only few poroids have pores (perforations in the theca) (Fig. 10). The small, rectangular intercalary plate (1a) has usually a row of 5 pores (perforated poroids) (Figs. 6, 9, 10).
Measurements: 41-50 µm length, 39-46 µm width, 9-10 µm length of sulcal lists.
Blepharocysta paulsenii Schiller (Figs. 13-20)
References: Schiller, 1937, p. 478, Figs. 552 a-i; Gaarder, 1954, p. 7, Figs. 6-8; Hallegraeff, 1988, p. 96; Carbonell-Moore, 1991, Fig. 20; Delgado and Fortuño, 1991, p. 9, Fig. 5 X, pl. XXV c; Carbonell-Moore, 1994b, pl. I, Fig. 2; Carbonell-Moore, 2004, Fig. 3.
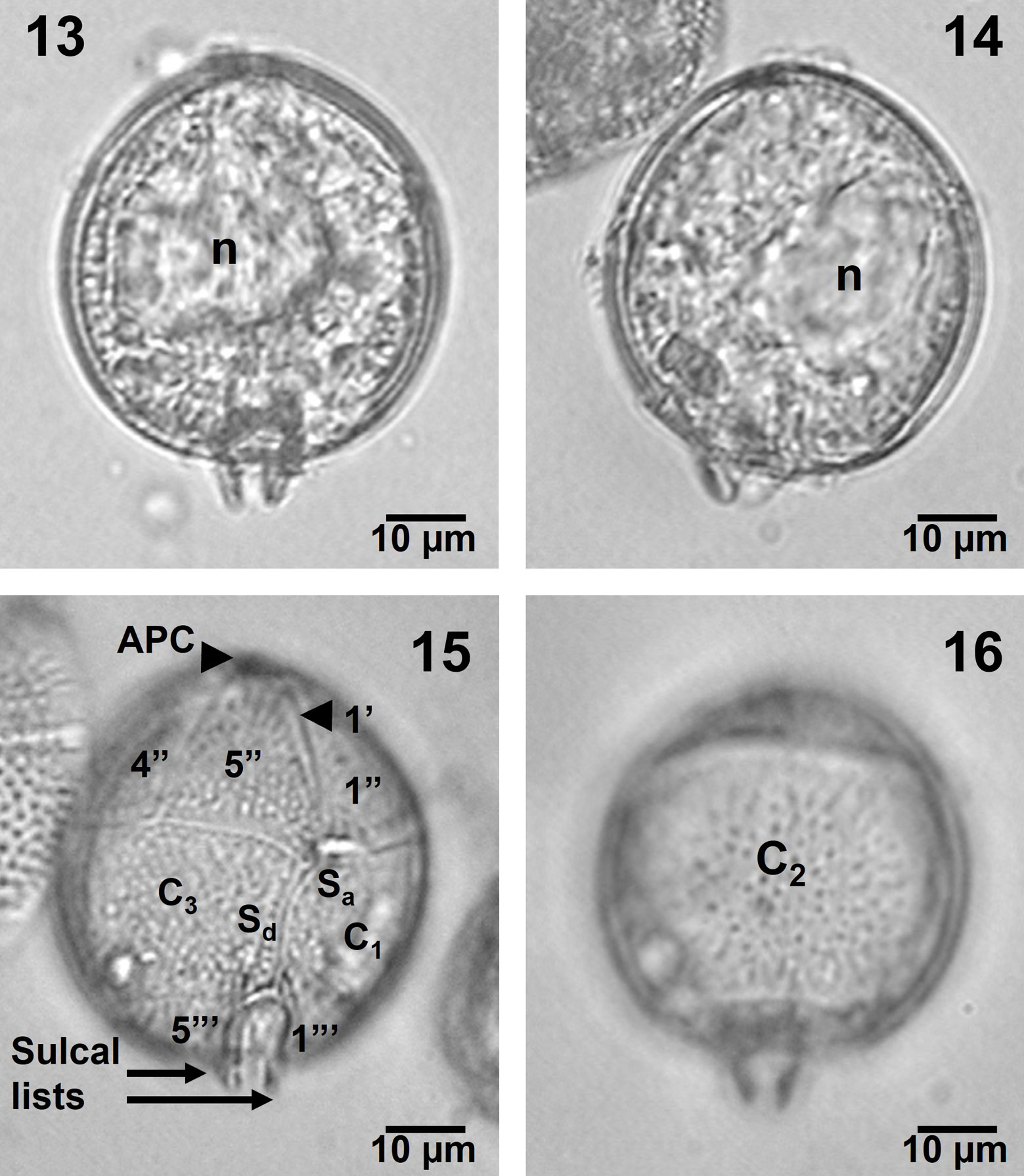
Solitary cells of medium size with a globose and spherical shape (Figs. 13-16). A very short apical protrusion indicating the APC and slight salient sulcal lists were apparent (Figs. 13-16). An untreated specimen showed a large, eccentrically located nucleus and no chloroplasts (Figs. 13, 14). Treated, transparent specimens in ventral and dorsal views showed the more reduced proportion of the epitheca in this species, and the plates arrangement (especially the precingular and cingular plates), very similar to the precedent species, but also showing the large cingular plate, C2 (Figs. 15, 16). The theca appeared covered by thick poroids and pores (Figs. 15, 16).
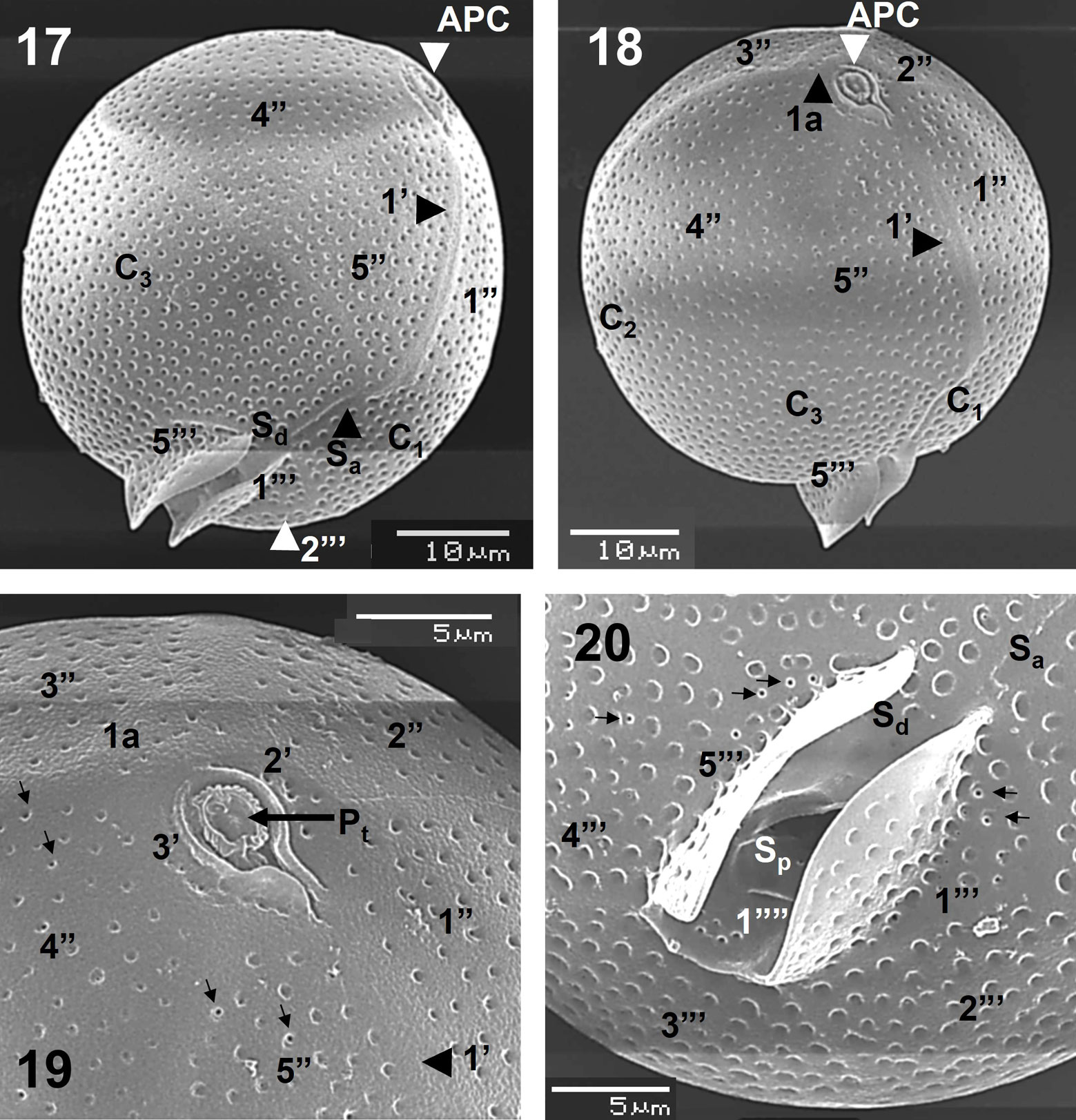
Cell shape and conspicuous plates of a specimen in ventral view are confirmed by SEM (Figs. 17, 18). The epitheca and the sulcal lists appeared more reduced than in Blepharocysta splendor–maris (Figs. 17, 18, 20). Shape and proportions of all plates seem similar to the precedent species, although the first apical (1’) is more evident in this species, and the sulcal platelets and the sutures of the plates are not as conspicuous as in Blepharocysta splendor–maris (Figs. 17-19). However, it is possible to observe the complete precingular plate series (Fig. 18).
The APC is not prominent (Figs. 17, 18) and the small platelets forming it were not completely seen, although the 3 apical plates (1’ to 3’) were observed (Fig. 19). The intercalary plate (1a) has, as in Blepharocysta splendor–maris, a row of 5-6 small pores (perforated poroids) (Fig. 19). An antapical view allowed to see the complete series of postcingular plates (1”’ to 5”’), the antapical plate (1””), and 3 sulcal platelets (Sa, Sd and Sp), plus the sulcal lists well ornamented with poroids (Fig. 20). The theca is covered by well-defined and deep poroids, which are fairly homogeneously distributed, with a relative high density (52-56 pores in 10 µm2, in plates C1 and C3, and 18-19 pores in 5 µm2, in postcingular plates), and some are perforated by small pores (Figs. 19, 20).
Measurements: 44-46 µm length, 42-45 µm width, 6-7 µm length of sulcal lists.
Discussion
Morphology, taxonomy and phylogeny
Species of the genus Blepharocysta are mainly distributed in warm, tropical and subtropical waters, but they have never been reported as frequent or abundant. The characteristic shape of all the species, globose forms with slightly prominent sulcal lists, make their recognition and posterior positive identification not always easy, for they may be easily ignored or confused with a range of different organisms (Balech, 1988; Carbonell-Moore, 1994a; Taylor, 1976). This fact partially explains the inaccurate knowledge of their diversity and distribution all over the world.
We confirmed previous descriptions and observations in the literature mainly using LM (e.g., Abé, 1966; Balech, 1963, 1988; Dodge, 1982; Gaarder, 1954; Nie, 1939; Schiller, 1937; Steidinger & Tangen, 1997) for both species, particularly the theca tabulation, which applies to members of the family Podolampadaceae: APC, 3’, 1a, 5”, 3C, 4S, 4-5”’, 1””. More recent observations by SEM were also comparable with those shown here (Carbonell-Moore, 1994b, 2004; Delgado & Fortuño, 1991; McMinn & Scott, 2005; Yang & Li, 2014).
In general terms, Blepharocysta splendor-maris appears subspherical to ovoid (larger than wide) than B. paulsenii, with large epitheca, fairly developed sulcal lists, and theca ornamented with shallow poroids (and pores) having a relative low density (39-43 poroids in 10 µm2 at cingular plates). On the other hand, Blepharocysta paulsenii has a more spherical (globose) shape, with a shorter epitheca, reduced and slightly ornamented sulcal lists, and the theca is covered regularly by deep and marked poroids (and especially pores) with a relative high density (52-56 poroids in 10 µm2 at cingular plates); additionally, the anterior intercalary plate, 1a, was also detected in this study (Figs 17, 18) in B. paulsenii (Carbonell-Moore, 1994a, pl. I, Fig. 1). Measurements of the 2 species were among the ranges given in the literature (Abé, 1966; Balech, 1963, 1988; Dodge, 1982; Gaarder, 1954; Schiller, 1937; Steidinger & Tangen, 1997) (Table 1).
There are different opinions on the recognition and validity of some species of Blepharocysta, especially B. paulsenii and B. okamurae, which are superficially similar to the best known species and type of the genus, B. splendor-maris. Nie (1939) considered that B. paulsenii is conspecific with B. splendor-maris, with the latter having priority, whereas Balech (1988) stated that B. okamurae should be considered as a synonym of B. splendor-maris. Taylor (1976) illustrated a specimen (pl. 28, Fig. 289) of Blepharocysta okamurae which is very similar to B. paulsenii as shown here; Hallegraeff (1988) showed a specimen of B. paulsenii almost identical to the current specimens in this work, and more recently, Iwataki et al. (2012) provided 2 LM illustrations with a specimen (p. 127) of B. okamurae, which also resembles those given here for B. paulsenii. This shows the difficulty to positively identify the species of Blepharocysta, only based on LM observations.
Most probably, due to its morphological variability, Gaarder (1954) proposed 2 forms of B. paulsenii, f. bullata Gaarder and f. depressa Gaarder, 2 rare variants of the species, and based on one report of B. paulsenii by Gaarder (1954) a new species, B. hermosillae, was described (Carbonell-Moore, 1992). Gárate-Lizárraga et al. (2007) reported Blepharocysta paulsenii in a coastal lagoon of the western coasts of Baja California, in the northern Mexican Pacific, following the plates’ arrangement (presumably the epitheca proportion), but judging from their illustration (pl. 5, Fig. 17) the specimen most possibly represents B. splendor-maris, as the shape of the cell is ovoid and the sulcal lists are very prominent and conspicuous.
Regarding the rest of recognized species, Blepharocysta denticulata may be distinguished by the position and structure of the short sulcus, and especially for its dentate plate sutures, lack of one postcingular plate and the sulcal lists extremely reduced (Balech, 1963; Carbonell-Moore, 1994b; Nie, 1939), Blepharocysta okamurae has a drop-shape, conspicuous apical pore, poroid theca, and short and poroid sulcal lists which are close to the cingulum (Abé, 1966, Carbonell-Moore, 1994a), and finally, Blepharocysta hermosillae which is the largest species, almost perfectly spherical, with wide apical plate 1’, and asymmetric sulcal lists, the left one being more developed (Carbonell-Moore, 1992, 1994b) (Table 1).
Table 1
Comparative morphological and distribution characters of the 5 recognized species of the genus Blepharocysta. 1: Nie (1939), 2: Balech (1963), 3: Schiller (1937), 4: This study, 5: Balech (1988).
|
Character |
B. denticulata |
B. hermosillae |
B. okamurae |
B. paulsenii |
B. splendor-maris |
|
Outline (Shape) |
Subspherical to ellipsoidal |
Spherical, globose |
Drop-shape, conspicuous APC |
Spherical, globose |
Subspherical to ovoid |
|
Size |
57-65 µm L, 51-55 µm W1; 38-47 µm L, 38-46 µm W2 |
56-79 µm L, 59-72 µm W Largest species |
56-63 µm L, 53-60 µm W |
50-65 µm W3; 44-46 µm L, 42-45 µm W4 (6-7 µm sulcal lists)4 |
40.5-71 µm L, 37-65 µm W5; 41-50 µm L, 39-46 µm W4 (9-10 µm sulcal lists)4 |
|
Proportion of epitheca or hypotheca |
Epitheca reduced |
Larger hypotheca: postcingular and antapical plates |
Epitheca large |
Shorter epitheca |
Epitheca large |
|
Tabulation and sutures |
Only 4””. 1a quadrangular. Sutures denticulate (serrate) |
APC small. 1’ wider than other species. 1a quadrangular |
Similar to the type species |
Similar to the type species. 1a present, rectangular |
Typical tabulation: Po Pt X 3’ 1a 5” 3C 4S 5”’ 1”” |
|
Sulcal lists |
Inconspicuous, extremely reduced |
Reduced and asymmetric, left one more developed |
Reduced, ornamented with poroids |
Reduced, smooth or fairly ornamented with poroids |
More developed, apparently smooth |
|
Theca ornamentation |
Poroids (?) and pores numerous |
Deep pores of different sizes. No poroids apparently |
Strong ornamentation: poroids (?) and pores marked and numerous |
Deep poroids and pores, high density, regularly distributed |
Shallow poroids and pores, low density. Variable character |
|
Global distribution |
Warm-water: subtropical Pacific, north Atlantic (Gulf of Mexico, Caribbean), Indian Ocean, |
Warm-water: equatorial Pacific, subtropical and north Atlantic (Gulf Stream, Mediterranean), Indian Ocean |
Warm-water: equatorial and tropical Pacific, north Atlantic |
Warm-water: Pacific, north Atlantic (Mediterranean), Indian Ocean |
Widely distributed, from tropical to Antarctic waters |
We gathered important morphological characters to distinguish all Blepharocysta species, and some distribution information is additionally included (Table 1), in order to compare these characters, facilitate the identification of the species, and emphasize their importance. From this information it is interesting to evaluate which morphological characters should be considered valuable, outstanding the “traditional” characters such as: 1) the shape of cells, 2) the relative size, 3) the development of sulcal lists, 4) position and structure of the sulcus, 5) the theca arrangement (including plates which may be lacking), and 6) type of plate sutures (linear or dentate).
In this work, we have also observed that the character of the theca ornamentation, type, distribution and density of poroids and pores should be an additional, important and useful morphological character. This character was already suggested by Steidinger and Tangen (1997, p. 533), who stated that “plate pore patterns may help in differentiating species”. Some specimens of Blepharocysta splendor-maris, the most spread and best known species of the genus, observed with SEM show different patterns of poroids and pores, and possibly the most remarkable example could be the illustrations provided by Carbonell-Moore (2018, Fig. 1 B-E), exhibiting a rather smooth theca with scarce pores (and no poroids) of different sizes, including some few and considerable large pores distributed in the hypotheca, particularly in the postcingular plates 2”’ and 4”’, and the antapical plate 1””, where they resemble the couple of rows of large pores at the postcingular plates in species of the closely related genus Podolampas (Andreis & Andreoli, 1975, Burns & Mitchell, 1982; Dodge, 1985; Hernández-Becerril, 1988; Yang & Li, 2014). Additionally, the intercalary plate 1a shows only 3 small pores, which are scattered but not in a row (as found in this study) (Carbonell-Moore, 2018, Fig. 1 C). Unfortunately, this character should be considered only for “mature” cells and it can be viewed only using SEM facilities.
Molecular phylogenies of members of the family Podolampadaceae, basically the genera Blepharocysta and Podolampas, have been provided (Gómez et al., 2010; Yamaguchi et al., 2018), with the species Cabra matta Murray et Patterson and Roscoffia capitata Balech, cladding together with Blepharocysta sp., but differing considerably in morphological (presence of a typical cingulum) and ecological characters (benthic habits) (Gómez et al., 2010, 2011; Yamaguchi et al., 2018).
There has been a large debate on a nomenclatural and taxonomic issue derived from the name Peridinium splendor-maris Ehrenberg, on which it is based the type species of the genus Blepharocysta (Carbonell-Moore, 2018; Elbrächter et al., 2018, 2019). We are still using the concept and names of all Blepharocysta species from the literature until a reliable conclusion may be achieved.
Distribution and ecology
Blepharocysta splendor-maris is the only species of the genus with a wide distribution from tropical to Antarctic waters (Balech, 1988; Carbonell-Moore 1994b; McMinn & Scott, 2005), whereas the others seem to be limited to tropical and subtropical waters (Table 1). All species appear in the Pacific Ocean, the Atlantic Ocean (except the southern Atlantic, where only B. splendor-maris is present), and the Indian Ocean (where only B. okamurae is absent) (Carbonell-Moore, 1994b) (Table 1).
Precisely, according to the habitat preferences of most, if not all, species of Blepharocysta, which is relatively deep in the water column (Carbonell-Moore, 1994b), where light should be of very low intensity and quality or rather absent, we can confirm that the 2 species studied here, Blepharocysta splendor-maris and B. paulsenii, do not contain chloroplasts, despite they were mainly found in subsurface or shallow sampling depths (42 m depth), and they should be regarded as heterotrophic forms (Carbonell-Moore, 2004; Jacobson, 1999). In the closely related species Podolampas bipes Stein, Schweikert and Elbrächter (2004) detected the presence of endocytobionts with chloroplasts. This fact may count for Steidinger and Tangen’s (1997) statements that Blepharocysta (and Lissodinium and Podolampas) have chloroplasts.
Acknowledgements
To M.C. Carbonell-Moore for her help in identifying the 2 species studied here. Additionally, Yolanda Hornelas Orozco (ex-technician SAMEB, ICML, UNAM) provided skilled technical support at the SEM. Partial support for this study was provided by PAPIIT, DGAPA, UNAM (projects IN226209-3 and IN296516). Coordinación de la Investigación Científica (CIC), Universidad Nacional Autónoma de México (UNAM) approved and supported the use of the R/V “El Puma” to carry out the oceanographic cruise “MareaR VI” from 10-21 June, 2014. Comments and recommendations by the editor-in-chief and an anonymous reviewer greatly helped to improve a previous version of this paper.
References
Abé, T. H. (1966). The armoured dinoflagellata: I. Podolampidae. Publications of the Seto Marine Biological Laboratory, 14, 129–154.
Andreis, C., & Andreoli, C. (1975). SEM survey on Mediterranean species of Podolampas. Giornale Botanico Italiano, 109, 387–397.
Balech, E. (1963). La familia Podolampacea (Dinoflagellata). Boletín del Instituto de Biología Marina, 2, 1–27.
Balech, E. (1988). Los dinoflagelados del Atlántico sudoccidental. Madrid: Publicaciones Especiales, Instituto Español de Oceanografía 1.
Burns, D. A., & Mitchell, J. S. (1982). Some coastal marine dinoflagellates from around New Zealand. New Zealand Journal of Marine and Freshwater Research, 16, 69–79. https://doi.org/10.1080/00288330.1982.9515947
Carbonell-Moore, M. C. (1991). Lissodinium Matzenauer emend., based upon the rediscovery of L. schilleri Matz., another member of the family Podolampadaceae Lindemann (Dinophyceae). Botanica Marina, 34, 327–340. https://doi.org/10.1515/9783112328101-039
Carbonell-Moore, M. C. (1992). Blepharocysta hermosillae, sp. nov. a new member of the family Podolampadaceae Lindemann (Dinophyceae). Botanica Marina, 35, 273–281. https://doi.org/10.1515/botm.1992.35.4.273
Carbonell-Moore, M. C. (1994a). On the taxonomy of the family Podolampadaceae Lindemann (Dinophyceae) with descriptions of three new genera. Review of Palaeobotany and Palynology, 84, 73–99. https://doi.org/10.1016/0034-6667(94)90042-6
Carbonell-Moore, M. C. (1994b). On the biogeography of the family Podolampadaceae Lindemann (Dinophyceae) vertical and latitudinal distribution. Review of Palaeobotany and Palynology, 84, 23–44. https://doi.org/10.1016/0034-6667(94)90039-6
Carbonell-Moore, M. C. (2004). On the taxonomical position of Lessardia Saldarriaga et Taylor within the family Podolampadaceae Lindemann (Dinophyceae). Phycological Research, 52, 340–345. https://doi.org/10.1111/j.1440-183.2004.00353.x
Carbonell-Moore, M. C. (2010). Gaarderiella, gen nov., a new name to replace Gaarderia Carbonell-Moore (Peridiniales, Dinophyceae, Podolampadaceae). Phycologia, 49, 402. https://doi.org/10.2216/10-10.1
Carbonell-Moore, M. C. (2018). Proposal to conserve the name Peridinium splendor-maris (Blepharocysta splendor-maris) (Dinophyceae) with a conserved type. Taxon, 67, 633–635. https://doi.org/10.12705/673.17
Delgado, M., & Fortuño, J. M. (1991). Atlas de fitoplancton del Mar Mediterráneo. Scientia Marina, 55 (Suppl. 1), 1–133.
Dodge, J. D. (1982). Marine Dinoflagellates of the British Isles. London: HMSO.
Dodge, J. D. (1985). Atlas of dinoflagellates: a scanning electron microscope survey. London: Farrand Press.
Elbrächter, M., Hoppenrath, M., Jahn, R., & Kusber, W. H. (2018). Stability of the generic names Alexandrium Halim and Gessnerium Halim at risk because of Peridinium splendor-maris Ehrenberg, the first documented bloom of Alexandrium (Dinophyceae). Notulae Algarum, 60, 1–6.
Elbrächter, M., Gottschling, M., Hoppenrath, M., Jahn, R., Montresor, M., Tillmann, U. et al. (2019). Proposal to conserve the name Alexandrium against Blepharocysta (Dinophyceae). Taxon, 68, 589–590. https://doi.org/10.1002/tax.12074
Fensome, R. A., Taylor, F. J. R., Norris, G., Sarjeant, W. A. S., Wharton, D. I., & Williams, G. L. (1993). A classification of living and fossil dinoflagellates. Micropaleontol, Special Pub. No. 7. Hanover: Sheridan Press.
Gaarder, K. R. (1954). Dinoflagellatae from the “Michael Sars” north Atlantic deep-sea expedition 1910. Report on the Scientific Results of the “Michael Sars” North Atlantic Deep-Sea Expedition 1910, University of Bergen, John Grieg, Bergen 2.
Gárate-Lizárraga, I., Band-Schmidt, C. J., Verdugo-Díaz, G., Muñetón-Gómez, M. S., & Félix-Pico, E. F. (2007). Dinoflagelados (Dinophyceae) del sistema lagunar Magdalena-Almejas. In R. Funes-Rodríguez, J. Gómez-Gutiérrez, & R. Palomares-García (Eds.), Estudios ecológicos en bahía Magdalena (pp. 145–174). México D.F.: FONMAR/ IPN/ CICIMAR.
Gómez F. (2012). A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata). CICIMAR Oceánides, 27, 65–140.
Gómez, F., Moreira, D., & López-García, P. (2010). Molecular phylogeny of the dinoflagellates Podolampas and Blepharocysta (Peridiniales, Dinophyceae). Phycologia, 49, 212–220. https://doi.org/10.2216/PH09-29.1
Gómez, F., Moreira, D., & López-García, P. (2011). Avances en el estudio de los dinoflagelados (Dinophyceae) con la filogenia molecular. Hidrobiológica, 21, 343–364.
Gómez, F., Nakamura, Y., & Artigas, L F. (2019). Molecular phylogeny of the sand-dwelling dinoflagellate Planodinium striatum and Chrysodinium gen. nov. for Plagiodinium ballux (Dinophyceae). Acta Protozoologica, 58, 115–124. https://doi.org/10.4467/16890027AP.19.012.11421
Guiry, M. D., & Guiry, G. M. (2020). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Retrieved on 02 October 2020 from: https://www.algaebase.org
Hallegraeff, G. M. (1988). Plankton. A microscopic world. Leiden: E.J. Brill.
Hernández-Becerril, D. U. (1988). Observaciones de algunos dinoflagelados (Dinophyceae) del Pacífico mexicano con microscopios fotónico y electrónico de barrido. Investigación Pesquera, 52, 515–529.
Hoppenrath, M. (2017). Dinoflagellate taxonomy – a review and proposal of a revised classification. Marine Biodiversity, 47, 381–403. https://doi.org/10.1007/s12526-016-0471-8
Iwataki, M., Takayama, H., Nguyen, N. V., Lim, P. T., & Fukuyo, Y. (2012). Dinoflagellates (Dinophyceae). In T. Omura, M. Iwataki, V. M. Borja, H. Takayama, & Y. Fukuyo (Eds.), Marine phytoplankton of the western Pacific (pp. 53–134). Tokyo: Kouseisha koisekaku, Ltd.
Jacobson, D. M. (1999). A brief history of dinoflagellate feeding research. Journal of Eukaryotic Microbiology, 46, 376–381. https://doi.org/10.1111/j.1550-7408.1999.tb04616.x
Konovalova, G. V. (1998). Dinoflagellatae (Dinophyta) of the Far Eastern seas of Russia and adjacent waters of the Pacific Ocean (In Russian). Dalnauka, Vladivostok.
McMinn, A., & Scott, F. J. (2005). Dinoflagellates. In F. J. Scott, & H. J. Marchant (Eds.), Antarctic marine protists (pp. 202–250). Canberra: Australia Biological Resources Study and Australian Antractic Division.
Nie, D. (1939). Dinoflagellata of the Hainan region. II. On the thecal morphology of Blepharocysta, with a description of a new species. Contributions from the Biological Laboratory of the Science Society of China, Zoological Series, 13, 23–39.
Pesantes, F. (1978). Dinoflagelados del fitoplancton del Golfo de Guayaquil. Publicación INOCAR, 2, 1–98.
Saldarriaga, J. F., Leander, B. S., Taylor, F. J. R., & Keeling, P. J. (2003). Lessardia elongata gen. et sp. nov. (Dinoflagellata, Peridiniales, Podolampaceae) and the taxonomic position of the genus Roscoffia. Journal of Phycology, 39, 368–378. https://doi.org/10.1046/j.1529-8817.2003.02113.x
Schiller, J. (1937). Dinoflagellatae (Peridineae). Teil II. Raben-
horst’s Kryptogamen-Flora. Leipzig: Akademie Verlag.
Schweikert, M., & Elbrächter, M. (2004). First ultrastructural investigations of the consortium between a phototrophic eukaryotic endocytobiont and Podolampas bipes (Dinophyceae). Phycologia, 43, 614–623. https://doi.org/10.2216/i0031-8884-43-5-614.1
Steidinger, K. A., & Tangen K. (1997). Dinoflagellates. In C.R. Tomas (Ed.), Identifying marine phytoplankton (pp. 387–584). San Diego: Academic Press.
Taylor, F. J. R. (1976). Dinoflagellates from the International Indian Ocean Expedition. A report on material collected by the R.V. “Anton Bruun” 1963-1964. Stuttgart: Bibliotheca Botanica 132.
Taylor, F. J. R. (1978). Dinoflagellates. In A. Sournia (Ed.), Phytoplankton manual (pp. 143–147). Paris: UNESCO.
Taylor, F. J. R., Fukuyo, Y., Larsen, J., & Hallegraeff, G. M. (2003). Taxonomy of harmful dinoflagellates. In G. M. Hallegraeff, D. M. Anderson, & A. D. Cembella (Ed.), Manual on harmful marine microalgae (pp. 389–432). Paris: UNESCO/ IOC.
Wood, E. J. F. (1963). Dinoflagellates of the Australian region. II. Recent collections. Division of Fisheries and Oceanography, Technical paper, 14. Melbourne: CSIRO.
Yamaguchi, A., Wakeman, K. C., Hoppenrath, M., Horiguchi, T., & Kawai, H. (2018). Molecular phylogeny of the benthic dinoflagellate Cabra matta (Dinophyceae) from Okinawa, Japan. Phycologia, 57, 630–640. https://doi.org/10.2216/18-7.1
Yang, S. M., & Li, R. X. (2014). Atlas of dinoflagellates in China’s seas. Beijing: Ocean Press.