Reproductive characteristics of Tozeuma carolinense (Decapoda: Caridea: Hippolytidae) in the Gulf of Mexico
José Luis Bortolini a, *, María del Pilar Alonso b, Irma Lorraine Mercado a
a Departamento de Biología Comparada, Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Copilco El Bajo, 04510 Ciudad de México, Mexico
b Departamento de Matemáticas, Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Copilco El Bajo, 04510 Ciudad de México, Mexico
*Corresponding author: jlbr@ciencias.unam.mx (J.L. Bortolini)
Abstract
We studied reproductive characteristics of the phantom shrimp Tozeuma carolinense inhabiting sea-grass beds to test hypotheses about seasonality of reproduction, sex ratio and sexual system, and fecundity values in 3 Mexican collecting sites in the Gulf of Mexico. Four hundred and eighty collected individuals were measured and separated to 3 groups. Based on discriminant analysis, carapace length (CL) was selected as morphometric feature to define 10 size-class intervals. The relationship between sexes were analyzed. Embryos were counted, measured and classified into 4 stages of development. Regression analyses were performed to determine the correlation between the number and size of embryos vs. CL. Sex ratio was 1:1, however, for each location, the sex ratio was different. Males were smaller than females; ovigerous females were larger than non-ovigerous females and males. Fecundity analysis have a significant positive relationship, both in the number and size of embryos vs. female CL. Ovigerous females were found at all dates, and confirmed in El Cayo populations with monthly samples in a year, so that we conclude that T. carolinense belongs to the species category with a seasonal-continuous reproduction.
Keywords: Fecundity; Phantom shrimp; Population analysis; Tozeuma carolinense
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Características reproductivas de Tozeuma carolinense (Decapoda: Caridea: Hippolytidae) del golfo de México
Resumen
Estudiamos las características reproductivas de Tozeuma carolinense de la zona de pastos marinos para probar la hipótesis sobre estacionalidad de reproducción, proporción sexual, sistema sexual y fecundidad en 3 sitios del golfo de México. Cuatrocientos ochenta individuos fueron medidos y separados en 3 grupos. Un análisis discriminante determinó el largo del caparazón (CL) para establecer 10 intervalos de clase. La relación entre sexos fue analizada. Los embriones fueron contados, medidos y clasificados en 4 estadios de desarrollo. Se establecieron análisis de regresión para determinar la correlación entre el número y la talla de los embriones vs. el CL. La proporción sexual fue de 1:1, sin embargo, ésta difiere en cada sitio. Los machos fueron más pequeños que las hembras; las hembras ovígeras fueron más grandes que las hembras no ovígeras y que los machos. La fecundidad tiene una relación significativamente positiva para el número y el tamaño de los embriones vs. CL de la hembra. Se observaron hembras ovígeras en todas las fechas de recolecta, particularmente en la población de El Cayo, en donde se realizaron muestreos mensuales durante el año, por lo que concluimos que T. carolinense pertenece a la categoría de especie con reproducción estacional continua.
Palabras clave: Análisis poblacional; Camarón fantasma; Fecundidad; Tozeuma carolinense
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
The genus Tozeuma, of the family Hippolytidae, is found worldwide in temperate and tropical marine and estuarine waters with a distribution that includes North Carolina, both coasts of Florida, Bermuda, the Bahamas, Texas, Yucatán, Puerto Rico, and St. Thomas, Virgin Islands (Ewald, 1969). In Florida, it has been observed in Soldier Key, Biscayne Bay by Voss and Voss (1955) and from north-western Florida by Wass (1955), and Voss (1956). Tozeuma carolinense, commonly known as the phantom or grass shrimp, was described in 1878 by J. K. Kingsley and has been reported from Vineyard Sound, on the north-eastern coast of USA to Coração, Venezuela, inhabiting sea grass meadows (Bauer, 2004). According with Corey and Reid (1991), T. carolinense is a subtropical species; for Ewald (1969), it is a tropical caridean shrimp inhabiting temperate waters into the Gulf of Mexico linked to sea-grass beds of Zostera spp., Diplanthera spp., Cymodocea spp., but especially to turtle grass Thalassia spp. This species is found in depths of 150 m and is easily recognized by its elongate form and very long, slender, dagger-shaped rostrum which is longer than the carapace, thick and rounded with denticles or spines above, with many minute sharp serrulations beneath (Ewald, 1969). The shrimp has short pereiopods for gripping slender objects (Bauer, 2004). Sexual dimorphism is evident in adult stages. Females are considerably larger than males and have a more intense coloration. Even though male’s pleopods have an appendix interna, the second pair is reduced in size with few hooks and bearing an appendix masculina with series of prominent sharp spines (Abele & Kim, 1986; Ewald, 1969).
There are 2 hypotheses proposed to explain the reproductive behavior of individuals that seem to have a continuous reproduction through year. One is that tropical species should have continuous reproduction because there are relatively stable environmental conditions. Elevated water temperatures of tropical habitats would allow year-round breeding (Orton, 1920). The other hypothesis states that the selective pressure acting on timing of reproduction in marine invertebrates with planktotrophic larvae might be a temporal variation of larval supply, that is, the seasonal pattern of primary and secondary productivity (Thorson, 1950). Pinheiro and Fransozo (2002) proposed 2 categories of continuous reproduction: the first, a continuous reproduction in which the monthly percentages of ovigerous females are similar; and second, a seasonal continuous reproduction, when ovigerous females occur in all months, but in different percentages, and it is possible to recognize some peaks in some seasons.
In caridean shrimps associated with sea-grass meadows, many species with continuous breeding have been reported (Bauer, 1989, 2004; Bauer & Abdalla, 2000). The sex ratio, generally found to be close to 1:1 (female: male) in nature (Fisher, 1958), favors any sex in any season in an annual period (Kim et al., 2008; Zare et al., 2011). A sex ratio biased towards males seems to be caused by sexual dimorphism, which causes a differential mortality due to predation, larger individuals are more frequently preyed upon (Berglund, 1981); however, a predominance of females larger in size has been reported in T. carolinense (Ewald, 1969), which might indicate the reverse, given that there is no bias in sampling.
Reproduction is the most important aspect in biology of species; in crustaceans is frequently expressed in terms of fecundity (Zare et al., 2011). Fecundity is broadly defined as the gamete number produced by an organism in a specific period or reproductive event, and not the rate of gametes produced per year or lifetime. Authors such Stearns (1976) and Calow (1979) termed this definition as “apparent fecundity”. Anger and Moreira (1998) and Corey (1991) distinguished 3 fecundity types: potential fecundity (the number of oocytes in the ovary); realized fecundity (number of eggs attached under the abdomen), and actual fecundity (number of larvae hatched under the abdomen). According to Litulo (2004), internal factors such as biological characteristics of organisms and physical-chemical factors like salinity, temperature, moon cycle and photoperiod may determine reproductive phase periodicity, regulating the reproductive potential and the possible size of the resulting population (Mantelatto & Franzoso, 1997). Preliminary studies on the fecundity of crustaceans showed a positive relationship between fecundity and size of the parental female (Bertini & Baeza, 2014; Hynes, 1954; Ivanova & Vassilenko, 1987; Peixoto et al., 2004). To estimate fecundity in a proper way and not underestimate this value due to loss during incubation, the number of embryos in development stage I are counted or estimated (Martínez-Mayén & Román-Contreras, 2013, 2014). Strong lineal relationships exist between volume of the egg mass and carapace volume (or volume of female) for different species within a family or order of Crustacea (Corey & Reid, 1991). Bauer (1989, 1991) showed for several seagrass species, egg production in coastal areas of the tropics throughout the year is continuous after reaching maturity, with females producing successive broods of embryos at short intervals.
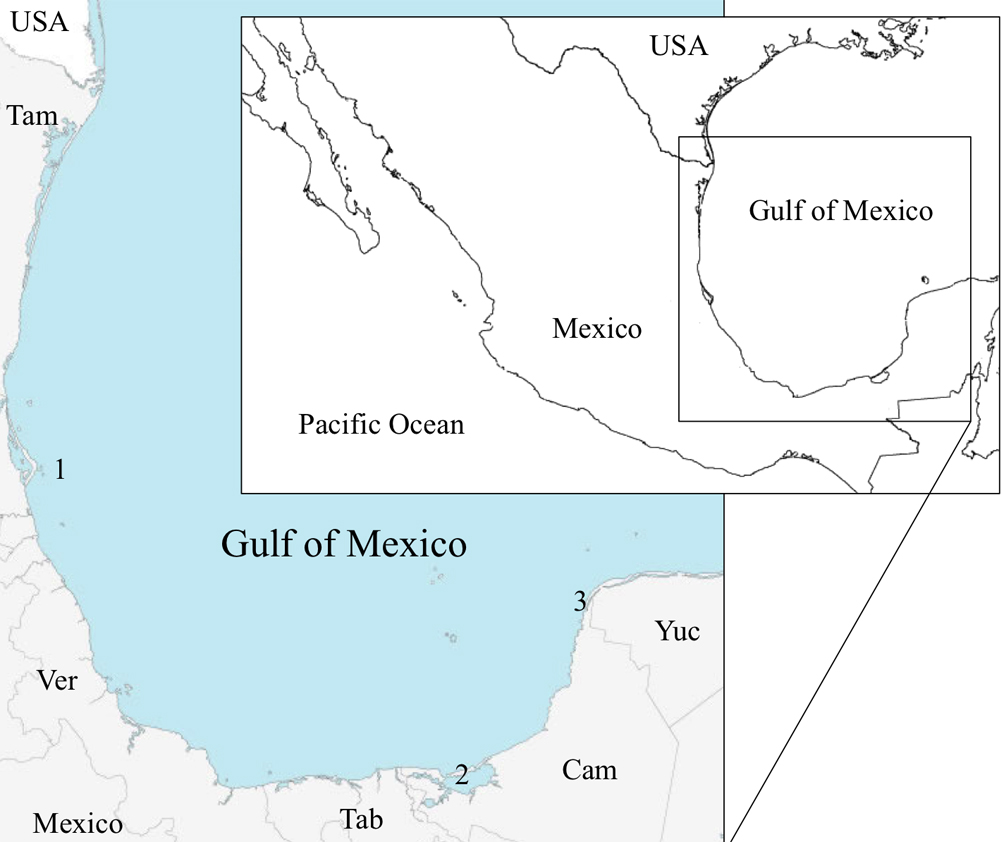
In this work, we describe reproductive characteristics of T. carolinense inhabiting sea-grass beds to test hypotheses about seasonality of reproduction, sex ratio and sexual system, and fecundity values in 3 Mexican collecting sites in the Gulf of Mexico (Fig. 1).
Materials and methods
Individuals of Tozeuma carolinense were obtained at 3 locations into the Gulf of Mexico, Isla Lobos at northern of Veracruz State (21º28’38.62” N, 97º13’26.46” W) in the WSW region of the gulf; El Cayo in Laguna de Términos (18º38’13” N, 91º41’18” W), and Celestún, at the north of Campeche State (20º46’20.77” N, 90º25’37.92” W), both in the SSW region of the gulf. Samples were collected on different dates between 2004 and 2015; all samples were taken in beds of Thalassia testudinum. Individuals were collected with hand nets (1,600 µm mesh size) and/or a beam-trawl net Colman-Seagrove type (mouth 80 cm width, 40 cm high and 125 cm length, external net 500 µm mesh size and internal net 1 cm mesh size). Samples were fixed with 10% seawater formalin in 1 l polyethylene terephthalate bottles. In the laboratory, samples were washed with tap water for 60 min and stored in EtOH 70%. For all individuals, the following information was obtained: sex, total length (TL) (rostrum edge to tip of telson); carapace length (CL) (postorbital edge to posterior margin of the carapace); body length (BL) (postorbital edge to tip of telson). The individuals were counted and classified into 3 groups: males (individuals with appendix masculina on second pleopods), non-ovigerous females (NOv) (individuals without appendix masculina and without embryos on the pleopods) and ovigerous females (Ov) (individuals with embryos on the pleopods). Juveniles (sexually undifferentiated individuals) were not considered. A multivariate analysis was carried out to determine which of 3 different morphometries, TL, BL, and CL provided less variance. We selected CL as the multivariate analysis indicated it as a stable highly significant morphometric feature, that is, a structure that does not change in its proportional dimensions (Bauer, 1989, 1991; Bauer & Lin, 1994; Cabrera et al., 2001; Somerton & Meyers, 1983). Ten class intervals with 0.98 mm in amplitude were established based in CL data which were obtained by determining the lower and upper limits of each of them (Table 1). In ovigerous females, embryos were removed from pleopods, counted and classified by the system suggested by Allen (1966) and modified by Bauer (1986): stage 1, early embryos with no visible blastoderm; stage 2, blastoderm distinct, no eye development; stage 3, embryos with eyes, abdomen not free from cephalothorax, and stage 4, embryos near to hatching, little or no yolk, large eyes, abdomen free from the cephalothorax. Following Anger and Moreira (1998), and Corey (1991), the realized fecundity was also evaluated. Correlation analyses were carried out to determine the relationship between both the number and the size of the embryos in development stage1 with the CL of all females collected at the 3 collecting sites. Thus, we investigated the allometric relationship between embryos number (#E) and CL using the quadratic equation Y = aX2, (#E = a*CL2), and the relationship between embryo’s size (ES) vs. CL using the lineal equation Y = aX, (ES = a*CL). In both models, assumptions of the regression analysis were verified (Pearson, 1931). To observe the female proportion and the tendency of egg production by date, female population was analyzed during an annual cycle, only for El Cayo which was monthly sampled from October 2012 to November 2013. To analyze a possible loss of embryos we compared the fecundity at early and later embryo development using a Student t-test for 2 samples (Pearson, 1931).
Results
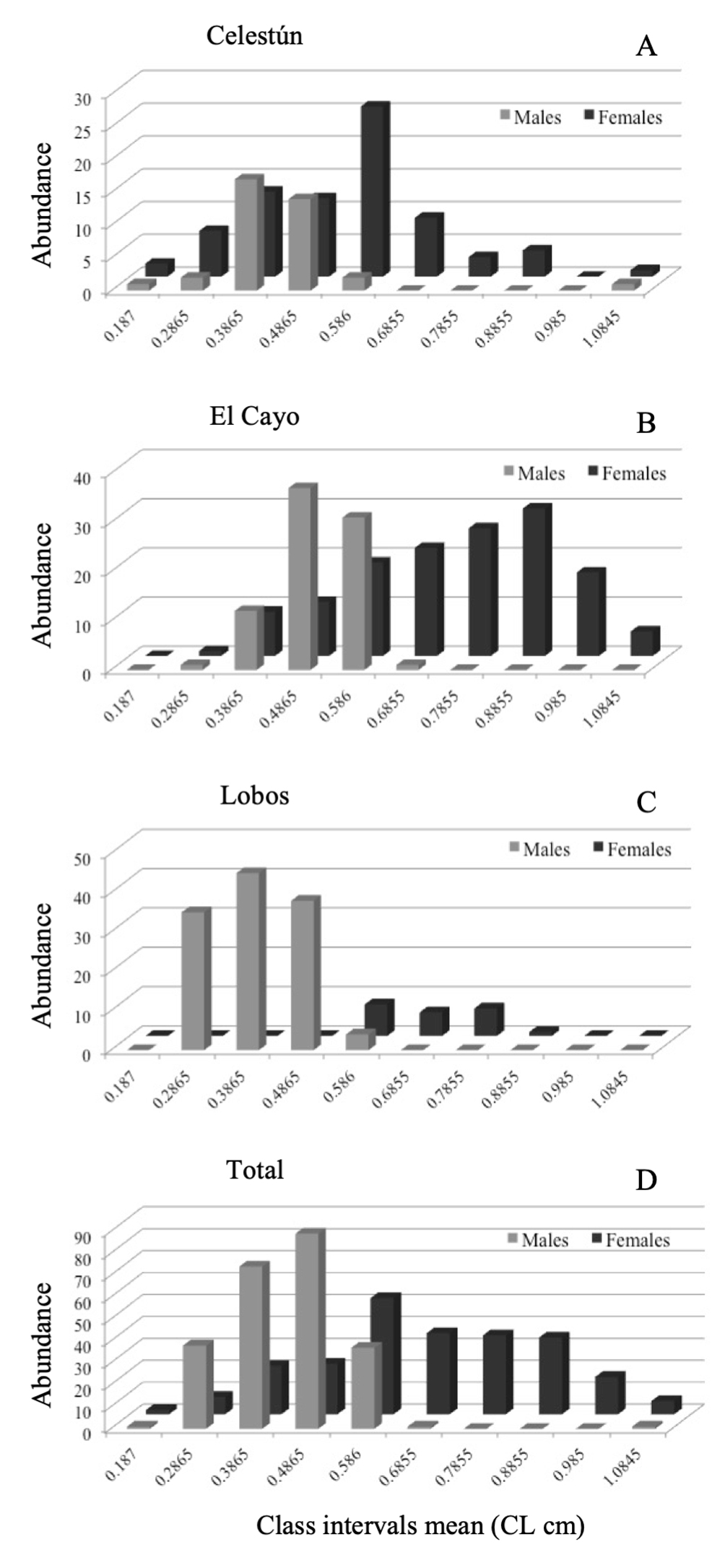
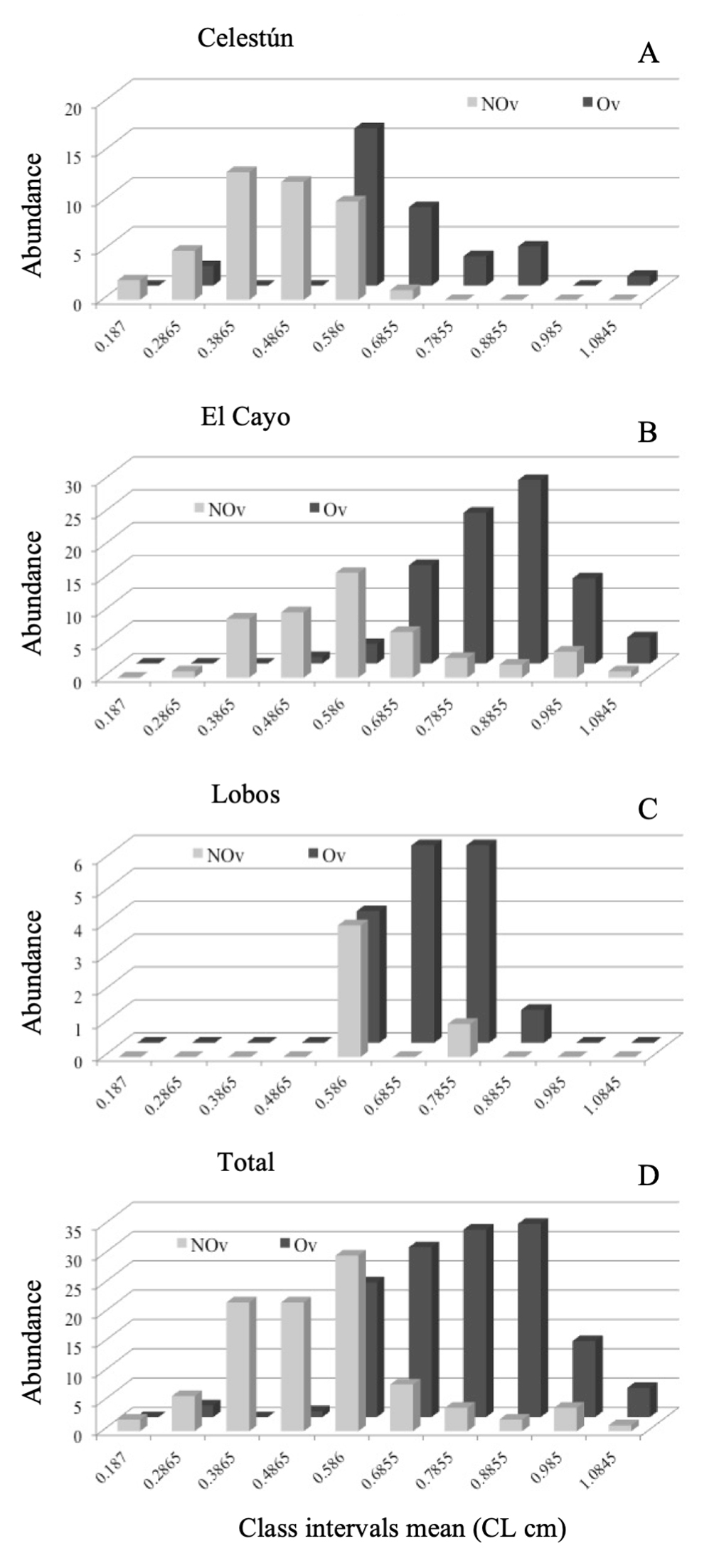
Four-hundred and eighty individuals were analyzed: 114 from Celestún, 222 from El Cayo, and 144 from Isla Lobos (Fig. 2D). Males and females from the 3 collecting sites, with sizes between 0.13 and 1.13 cm in CL are presented in table 1. Males were found in the initial intervals while the females were in the last ones (Fig. 2). Regarding the NOv and Ov females, the latter were observed in the last class intervals, showing a trend being the largest size in the population (Fig. 3). In all collecting sites, this pattern was maintained for all groups (males, NOv and Ov females) (Figs. 2, 3).
In Celestún, males were distributed in greater number with 0.23 to 0.43 cm of CL, while female distribution was throughout all size groups, however, the distribution of larger females in last intervals was evident, especially in sizes 0.53 to 0.63 cm of CL (Fig. 2A). In El Cayo, males grouped between 0.33 and 0.63 cm of CL intervals while females were distributed in all size intervals, even though there was an increased presence of them from 0.53 cm of CL onwards (Fig. 2B). In Isla Lobos, the presence of higher number of males vs. number of females was clear; males were better represented in size intervals of 0.23 to 0.53 cm of CL, while the few females were grouped in size intervals of 0.53 to 0.83 cm of CL (Fig. 2C).
In Celestún, Ov females were grouped after the 0.52 cm of CL, while NOv females were grouped from 0.13 to 0.63 cm of CL (Fig. 3A). In El Cayo, we observed few NOv and Ov females; NOv females were distributed from 0.33 to 1.08 cm of CL, and Ov females from 0.53 to 0.93 cm of CL (Fig. 3B). Finally, in Isla Lobos, we observed Ov females from 0.53 to 0.88 cm LC, while the NOv females were observed only in 2 class intervals, from 0.53 to 0.63 cm LC and 0.73 to 0.83 cm LC. Both subpopulations were the narrowest in terms of class intervals compared to the other 2 locations (Fig. 3C). Total abundance of non-ovigerous and ovigerous females of Tozeuma carolinense are grouped in figure 3D. The smallest individual in this work was from Celestún and the largest from El Cayo, the highest male average size was observed in El Cayo and the smallest male average size in Isla Lobos. In the case of females average size was (NOv and Ov), the highest were observed in El Cayo, and the smallest in Celestún.
Sex ratio in the total sampled population was 50.21% for males and 49.79% for females (1:1), however, for each location the sex ratio was different. In the Celestún and El Cayo populations, sex ratio was biased towards females, 0.25:1 and 0.35:1, respectively. Isla Lobos population had a sex ratio of 1:0.03, 84.70% males and 15.28% females. The relationship of Ov and NOv females in the entire sampled population was 57.74% and 42.26% respectively.
Table 1
Values of interval class, local and total abundance, and number of individuals by interval and type of individuals of Tozeuma carolinense.
|
Size intervals (cm CL) |
Celestún |
El Cayo |
Lobos |
Total |
|||||||||||||||||||
|
# |
Min. |
Max. |
Mean |
N |
♂ |
♀ |
♀Nov |
♀Ov |
N |
♂ |
♀ |
♀Nov |
♀Ov |
N |
♂ |
♀ |
♀Nov |
♀Ov |
N |
♂ |
♀ |
♀Nov |
♀Ov |
|
1 |
0.138 |
0.236 |
0.187 |
3 |
1 |
2 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
1 |
2 |
2 |
0 |
|
2 |
0.237 |
0.336 |
0.287 |
9 |
2 |
7 |
5 |
2 |
2 |
1 |
1 |
1 |
0 |
35 |
35 |
0 |
0 |
0 |
46 |
38 |
8 |
6 |
2 |
|
3 |
0.337 |
0.436 |
0.386 |
30 |
17 |
13 |
13 |
0 |
21 |
12 |
9 |
9 |
0 |
45 |
45 |
0 |
0 |
0 |
96 |
74 |
22 |
22 |
0 |
|
4 |
0.437 |
0.536 |
0.486 |
26 |
14 |
12 |
12 |
0 |
48 |
37 |
11 |
10 |
1 |
38 |
38 |
0 |
0 |
0 |
112 |
89 |
23 |
22 |
1 |
|
5 |
0.537 |
0.635 |
0.586 |
28 |
2 |
26 |
10 |
16 |
50 |
31 |
19 |
16 |
3 |
12 |
4 |
8 |
4 |
4 |
90 |
37 |
53 |
30 |
23 |
|
6 |
0.636 |
0.735 |
0.686 |
9 |
0 |
9 |
1 |
8 |
23 |
1 |
22 |
7 |
15 |
6 |
0 |
6 |
0 |
6 |
38 |
1 |
37 |
8 |
29 |
|
7 |
0.736 |
0.835 |
0.785 |
3 |
0 |
3 |
0 |
3 |
26 |
0 |
26 |
3 |
23 |
7 |
0 |
7 |
1 |
6 |
36 |
0 |
36 |
3 |
33 |
|
8 |
0.836 |
0.935 |
0.885 |
4 |
0 |
4 |
0 |
4 |
30 |
0 |
30 |
2 |
28 |
1 |
0 |
1 |
0 |
1 |
35 |
0 |
35 |
2 |
33 |
|
9 |
0.936 |
0.04 |
0.985 |
0 |
0 |
0 |
0 |
0 |
17 |
0 |
17 |
4 |
13 |
0 |
0 |
0 |
0 |
0 |
17 |
0 |
17 |
4 |
13 |
|
10 |
0.035 |
1.134 |
1.085 |
2 |
1 |
1 |
0 |
1 |
5 |
0 |
5 |
1 |
4 |
0 |
0 |
0 |
0 |
0 |
7 |
1 |
5 |
1 |
5 |
|
Total |
114 |
37 |
77 |
43 |
34 |
222 |
82 |
140 |
53 |
87 |
144 |
122 |
22 |
5 |
17 |
480 |
241 |
239 |
100 |
139 |
In Celestún, 34 Ov females were collected (44.15%) and 43 NOv females (55.80%); in El Cayo, there were 87 Ov females (62.14%) and 53 NOv females (37.85%). In Isla Lobos the proportions were different, 17 Ov females (77.27%) and 5 NOv females (22.72%).
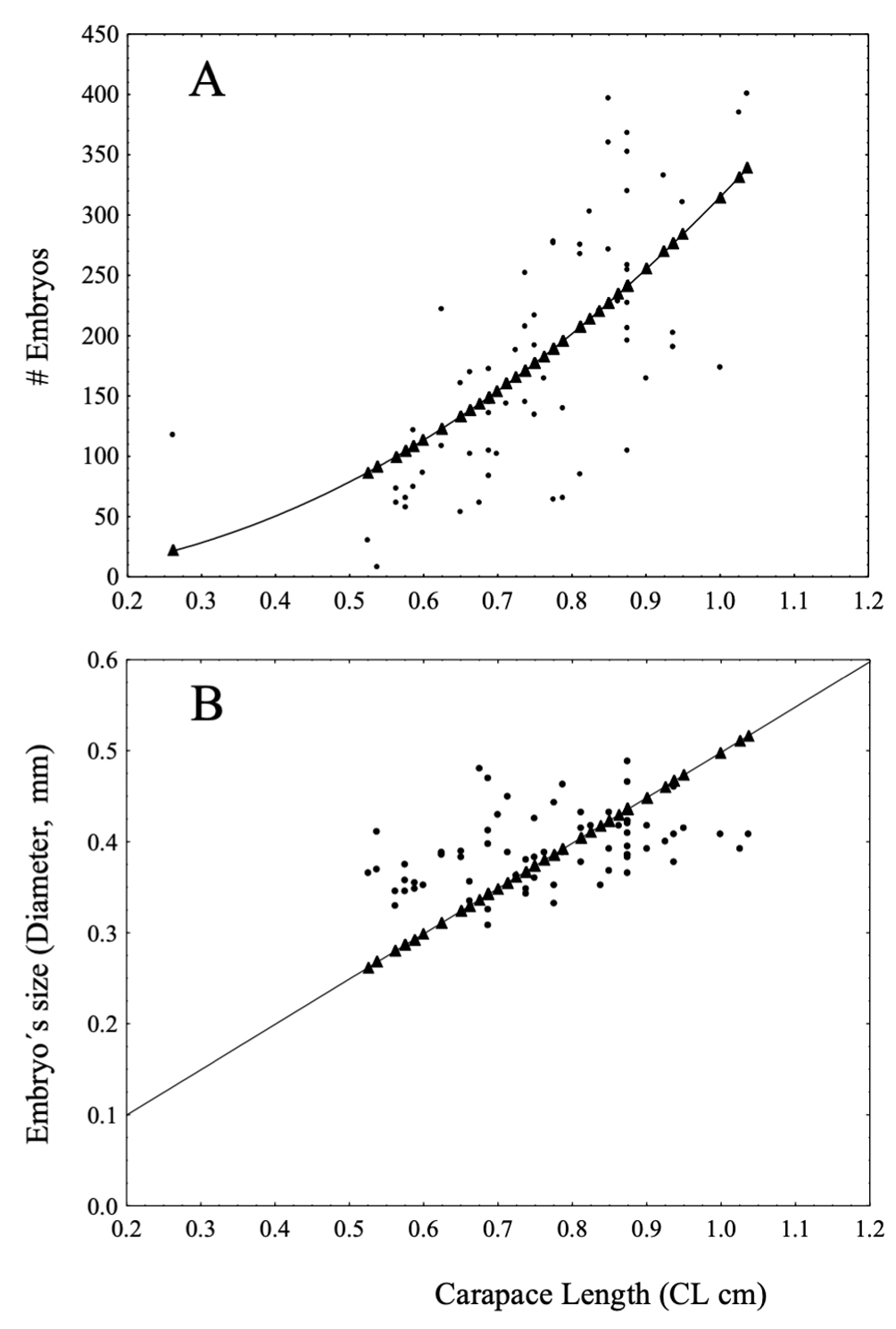
Fecundity analysis was performed with embryos in development stage 1. The mean number of embryos incubated by females was 159 with a diameter average of 0.39 mm: Celestún, 131 with 0.38 mm; El Cayo, 174 with 0.41 mm and Isla Lobos 170 with 0.34 mm. The relationship between #E and CL (adjusted to a quadratic model) was positive and significantly correlated, R2 = 0.89 (Fig. 4A) with an increment in #E = 314.97 * CL2. For each collecting site this relationship had similar values: Celestún, R2 = 0.87 (#E = 273.45 * CL2); El Cayo, R2 = 0.88 (#E = 393.95 * CL2) and Isla Lobos, R2 = 0.95 (#E = 263.64 * CL2). The relationship between ES vs. CL was positive in the entire sampled population R2 = 0.96 (ES = 0.0498 * CL) (Fig. 4B); Celestún had a R2 = 0.95 (ES = 0.0027 * CL); El Cayo, R2 = 0.98 (ES = 0.049 * CL) and Isla Lobos R2 = 0.99 (ES = 0.046 * CL).
We observed ovigerous females with embryos in stages 1, 2 and 3; stage 4 was not recorded. Differences in #E between different development were obtained. In stages 1 and 2, was recorded a T = 4.48 and p-value = 0.00002, which means that the stages are different. A significant loss was observed since the means were #E = 206.23 and #E = 81.64, respectively. For stages 2 and 3, we obtained a T = 0.1 and p-value = 0.92, the means are equal, i.e., no significant differences in embryo loss between these stages. El Cayo, except for March and June, was the collecting site which noted a regular sampling effort during the 2013 annual cycle. During this period, we observed Ov females during all months with an abundance peak between December 2012 and February 2013 (Table 2).
Table 2
Abundance of males and females of Tozeuma carolinense during annual cycle (2012-2013) in El Cayo. ♀NOv and ♀Ov, and minimum and maximum embryos/ Ov female values by collecting date (% is based in total population).
|
Date |
N |
♂ (%) |
♀ (%) |
♀ Nov (%) |
♀ Ov (%) |
# Egg min. |
# Egg max. |
|
|
2012 |
Oct |
5 |
1 (20) |
4 (80) |
3 (60) |
1 (20) |
229 |
229 |
|
Dec |
20 |
7 (35) |
13 (65) |
2 (10) |
11 (55) |
2 |
567 |
|
|
2013 |
Jan |
32 |
15 (46.8) |
17 (53.1) |
6 (18.7) |
11 (34.3) |
46 |
608 |
|
Feb |
55 |
23 (41.8) |
32 (58.1) |
10 (18.1) |
22 (40) |
2 |
490 |
|
|
Apr |
11 |
2 (18.1) |
9 (81.8) |
2 (18.1) |
7 (63.6) |
8 |
397 |
|
|
May |
14 |
10 (71.4) |
4 (28.5) |
2 (14.2) |
2 (14.2) |
222 |
459 |
|
|
Jul |
8 |
3 (37.5) |
5 (62.5) |
2 (40) |
3 (60) |
144 |
506 |
|
|
Aug |
1 |
0 (0) |
1 (100) |
0 (0) |
1 (100) |
50 |
50 |
|
|
Sep |
9 |
0 (0) |
9 (100) |
8 (88.8) |
1 (11.1) |
202 |
202 |
|
|
Oct |
2 |
0 (0) |
2 (100) |
0 (0) |
2 (100) |
2 |
2 |
|
|
Nov |
12 |
0 (0) |
12 (92.3) |
12 (92.3) |
0 (0) |
0 |
0 |
|
|
Total |
169 |
61 (35.6) |
108 (63.1) |
47 (27.4) |
61 (35.6) |
Discussion
In this work, we observed a sexual ratio of 1:1 in the total sampled population. According to Correa and Thiel (2003), equal sexual proportions in a population is characteristic of gonochoric species. We observed differences in sex ratio locally during all collecting dates. According to Kim et al. (2008) in Caridina fossarum, and Zare et al. (2011) in C. fossarum, these species showed more females than males during the reproductive season. A bias of this kind makes sense when we can analyze the sizes of both sexes, where males are small and therefore more agile, and the females are large, which makes them slower; then, probabilities to collect (to predate) a female, are higher than the odds of collecting a male. However, under this argument, in Isla Lobos, where we observed a highest male concentration, a greater male agility to escape from predators makes no sense because there is a large percentage of males compared to females. According to Berglund (1981), it may not be adaptive to diminish female body size beyond a certain limit, since egg number in each brood is positively related to cephalothorax size. A possible cause of the differences between sex ratio in collecting sites/dates is referred by Martínez-Mayén and Romero-Rodríguez (2018) to a competitive exclusion, due to a competition for substrate or food. Kim (2005) refers to different habitat preferences between sexes, differential primary sex ratio, differential mortality and/or different behavioral characteristics such as migration. Sforza et al. (2010) discussed spatial distribution of Callinectes danae and the partial sexual segregation due to the reproductive cycle. However, one should always consider that a bias in sampling (e.g., net mesh size, low sample size) might account for differences in sex ration. Upon the sex ratio and sexual dimorphism in size, we suggest that T. carolinense of the Gulf of Mexico has a gonochoric sexual system (separated sexes).
In relation to differences in CL between males and females, in this work males were found in lowest size intervals whilst females were in the highest intervals. Ewald (1969) mentioned that females were considerably larger than males. This sexual dimorphism coincides with others caridean species like Hippolyte obliquimanus (Terossi & Mantelatto, 2010). Smaller males can reduce the energetic waste during the development (Berglund, 1981). Correa and Thiel (2003) described 4 mating systems in Caridea, among them is the “pure searching” system, which is the common strategy within free living caridean shrimp, where successful males are those who possess small sizes because their size allows them to move with agility and find females. Presence of males with small size, also can be taken as an indication of the protandry phenomenon. This would require a study in great detail in T. carolinense sexual system to test this hypothesis. One of the features of protandry is a differentiate distribution in sizes in class intervals (Bauer, 2004).
Ov females were larger than NOv in the total sample as well as in each collecting sites. This data coincides with Ewald (1969). T. carolinense females seem to use a great quantity of energy in body growth. Once a female has certain size, energy is investing on reproduction and gametes production. Benefits that the Ov females are largest at the time of sexual maturity are clear; individual age is related with individual size and therefore with the number of embryos produced (Jensen, 1958). A great quantity of caridean species opt to postpone reproduction until they reach large sizes (Berglund, 1981). On the other hand, a female with embryos attached to its pleopods may show reduced swimming capacity and has less opportunities to escape if attacked by a predator.
Fecundity: we found that high proportion of females carried embryos at all dates and collecting sites throughout the year. Ov females were observed with largest sizes in CL. Although extraordinary, 3 females with embryos in pleopods than smaller to 0.53 cm of CL and located in the smallest class intervals. Relationship between #E produced and CL was positive for females. According with Berglund and Rosenqvist (1986), this is a pattern observed in several crustaceans; in carideans Hippolyte zostericola (Negreiros-Fransozo et al., 1996), Synalpheus filidigitus (Duffy & Macdonald, 1999), possess this reproductive characteristic. The best fit of our data on the variation of #E vs. CL for T. carolinense females was the quadratic equation. Emmerson (1985) suggested a logarithmic model to describe fecundity of Palaemon pacificus, and the same happened with Macrobrachium spp. described by Mejía-Ortíz et al. (2001), or Atya margaritacea fecundity analyzed by Sánchez et al. (2008), which indicates that the relationship between embryo number and carapace length is geometric, varying from species to species. Berglund and Rosenqvist (1986) established for Palaemon adspesus that there are 2 cost types associated with reproduction: reduction in the size of the individual and the increase in vulnerability to predators. For ES vs. CL, we applied a lineal relationship which is positive and a better data estimation. This means that larger size in the female, the greater the size of spawned ova. According to Guerao et al. (1994), this is an expected pattern if we consider that larger size in females, higher embryos number can be spawned. These similarity in the values of coefficients can be explained by the proximity of the latitudinal values between the analyzed localities. Finally, we observed significant differences in embryos loss between development stages 1 and 2.
Continuous reproduction: according to Bauer (1989, 1992), females at high latitudes have a well-established seasonality in the reproduction, e.g., seagrass caridean species from Tomioka Bay, Japan 32° N and caridean shrimp populations off the northeast coasts of England 55° N. In the studied populations, which are located in tropical latitudes (18-21°N) in the Gulf of Mexico, we found high proportions of T. carolinense females carrying embryos at all dates and confirmed in the El Cayo population with monthly samples in a year. Thus, in agreement with Pinheiro and Fransozo (2002), we conclude that T. carolinense in the Gulf of Mexico belongs to the species category with a seasonal-continuous reproduction, but with annual peaks in the late fall and early winter.
Acknowledgements
Ilse Rivera and Ana Cristina Martínez for support in laboratory work; Jorge Luis and Luis Díaz, fishermen in Tamiahua lagoon; Mario A. Gómez and team for field work at Términos lagoon. To Raymond T. Bauer for the revision of two versions of the manuscript, his constructive comments, corrections and suggestions are greatly appreciated, and Ofelia Delgado for the English edition.
References
Abele, L. G., & Kim, W. (1986). An illustrated guide to the marine decapod crustaceans of Florida. Florida: Florida State University.
Allen, J. A. (1966). The dynamics and relationships of mixed populations of caridea found off the northeast coast of England. In H. Barnes (Ed.), Some contemporary studies in marine science (pp. 45–65). London: Allen and Unwin.
Anger, K., & Moreira, G. S. (1998). Morphometric and reproductive traits of tropical caridean shrimps. Journal of Crustacean Biology, 18, 823–838. https://doi.org/10.1163/193724098X00674
Bauer, R. T. (1986). Sex change and life history pattern in the shrimp Thor manningi (Decapoda: Caridea): a novel case of partial protandric hermaphroditism. Biological Bulletin, 170, 11–31. https://doi.org/10.2307/1541377
Bauer, R. T. (1989). Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow. Journal of Experimental Marine Biology and Ecology, 127, 175–187. https://doi.org/10.1016/0022-0981(89)90183-4
Bauer, R. T. (1991). Analysis of embryo production in a caridean shrimp guild from a tropical seagrass meadow. In A. Wenner, & A. Kuris. (Eds.), Crustacean egg production, crustacean issues (181–191). Rotterdam, The Netherlands: Balkema Press.
Bauer, R. T. (1992). Testing generalizations about latitudinal variation in reproductive and recruitment patterns with caridean and sicyoniid shrimps. Invertebrate Reproduction & Development, 22, 193–202. https://doi.org/10.1080/07924259.1992.9672272
Bauer, R. T. (2004). Remarkable shrimps. Adaptations and natural history of the carideans. Oklahoma City: University of Oklahoma Press.
Bauer, R. T., & Abdalla, J. H. (2000). Patterns of brood production in the grass shrimp Palemonetes pugio (Decapoda: Caridea). Invertebrate Reproduction and Development, 38, 107–113. https://doi.org/10.1080/07924259.2000.9652445
Bauer, R. T., & Lin, J. (1994). Temporal patterns of reproduction and recruitment in populations of the penaeid shrimps Trochypenaeus similis (Smith) and T. constrictus (Stimpson) (Crustacea: Decapoda) from the Northcentral Gulf of Mexico. Journal of Experimental Marine Biology and Ecology, 182, 205–222. https://doi.org/10.1016/0022-0981(94)90052-3
Berglund, A. (1981). Sex dimorphism and skewed sex ratios in the prawn species Palaemon adspersus and P. squilla. Oikos, 36, 158–162. https://www.jstor.org/stable/3544440
Berglund, A., & Rosenqvist, G. (1986). Reproductive costs in the prawn Palaemon adspersus: effects on growth and predator vulnerability. Oikos, 46, 349–354. https://www.jstor.org/stable/3565833
Bertini, G., & Baeza, J. A. (2014). Fecundity and fertility in a freshwater population of the neotropical amphidromous shrimp Macrobrachium acanturus from the southeastern Atlantic. Invertebrate Reproduction & Development, 58, 207–217. https://doi.org/10.1080/07924259.2014.894948
Cabrera, P. J., Protti, M., Urriola, M., Sáenz O., & Alfaro, R. (2001). Tallas de fecundidad de Juxtafabia muliniarum (Brachyura: Pinnoteheridae) asociado con Saccostrea palmula (Bivalvia: Ostreidae), Costa Rica. Revista de Biología Tropical, 49, 889–894.
Calow, P. (1979). The cost of reproduction: a physiological approach. Biological review, 54, 23–40.
Corey, S. (1991). Comparative potential reproduction and actual production in several species of North American crayfish. In A. Wenner, & A. Kuris (Eds.) Crustacean egg production (69–76). Rotterdam, The Netherlands: A. A. Balkema
Corey, S., & Reid, D. M. (1991). Comparative fecundity of decapod crustacean. I. The fecundity of thirty-three species of nine families of caridean shrimp. Crustaceana, 60, 271–293. https://doi.org/10.1163/156854091X00056
Correa, C., & Thiel, M. (2003). Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76, 187–203. http://dx.doi.org/10.4067/S0716-078X2003000200006
Duffy, J. E., & Macdonald, K. S. (1999). Colony structure of the social snapping shrimp Synalpheus filidigitus in Belize. Journal of Crustacean Biology, 19, 283–292. https://doi.org/10.2307/1549235
Emmerson, W. (1985). Fecundity, larval rearing and laboratory growth of Palaemon pacificus (Stimpson) (Decapoda, Palaemonidae). Crustaceana, 49, 277–289.
Ewald, J. J. (1969). Observations on the biology of Tozeuma carolinense (Decapoda, Hippolytidae) from Florida, with special reference to larval development. Bulletin of Marine Science, 19, 510–549.
Fisher, R. A. (1958). The genetical theory of natural selection. 2nd edition. Dover, New York: HardPress Publishing.
Guerao, G., Pérez-Baquera, J., & Ribera, C. (1994). Growth and reproductive biology of Palaemon xiphias Risso, 1816 (Decapoda: Caridea: Palaemonidae). Journal of Crustacean Biology, 14, 280–288.
Hynes, H. B. N. (1954). The ecology of Gammarus duebeni Lilljeborg and its occurrence in fresh water in western Britain. Journal of Animal Ecology, 23, 38–84.
Ivanova, M. B., & Vassilenko, S. V. (1987). Relationship between number of eggs, brood weight, and female body weight in crustacea. International Review of Hydrobiology, 72, 147–169. https://doi.org/10.1002/iroh.19870720203
Jensen, J. P. (1958). The relation between body size and number of eggs in marine malacostrakes. Meddelelser Danmarks Fiskeri- og Havundersøgelser, Ny Series, 2, 1–25.
Kim, S. (2005). Population structure, growth, mortality, and size at sexual maturity of Palaemon gravieri (Decapoda: Caridea: Palaemonidae), Journal of Crustacean Biology, 25, 226–232. https://doi.org/10.1651/C-2510
Kim, C. K., Ma, C. W., Oh, C. W., & Paik, S. G. (2008). Reproduction and growth of the fresh- water prawn, Palaemon paucidens (Decapoda, Palaemonidae) in a lake of Korea. Environmental Biology, 29, 163–168.
Litulo, C. (2004). Fecundity of the pan-tropical fiddler crab Uca annulipes (H. Milne Edwards, 1837) from Costa do Sol Mangrove, Maputo Bay, southern Mozambique. Western Indian Ocean Journal of Marine Science, 3, 87–91. https://doi.org/10.1023/B:HYDR.000
Mantelatto, F. L. M., & Fransozo, A. (1997). Fecundity of the crab Callinectes ornatus Ordway, 1863 (Decapoda, Brachyura, Portunidae) from the Ubatuba region, São Paulo, Brazil. Crustaceana, 70, 214–224. https://doi.org/10.1163/156854097X00852
Martínez-Mayén, M., & Román-Contreras, R. (2013). Data on reproduction and fecundity of Processa bermudensis (Rankin, 1900) (Caridea, Processidae) from the southern coast of Quintana Roo, Mexico. Crustacena, 86, 84–97. https://doi.org/10.1163/15685403-00003152
Martínez-Mayén, M., & Román-Contreras, R. (2014). Sexual maturity, fecundity, and embryo loss in the pontoniine shrimp Cuapetes americanus (Kingsley, 1878) (Decapoda: Palemonidae) in Bahía de la Ascención, Quintana Roo, Mexico. Journal of Crustacean Biology, 34, 342–348. https://doi.org/10.1163/1937240X-00002235
Martínez-Mayén, M., & Romero-Rodríguez, J. (2018). Population structure and reproduction of the longtail grass shrimp Urocaris longicaudata (Decapoda, Caridea, Pontoniinae) in Laguna de Términos, SW Gulf of Mexico. Invertebrate Reproduction and Development, 62, 10–18. https://doi.org/10.1080/07924259.2017.1362483
Mejía-Ortíz, L. M., Álvarez, F., Román R., & Viccon-Pale, J. A. (2001). Fecundity and distribution of freshwater prawns of the genus Macrobrachium in the Huitzilapan River, Veracruz, Mexico. Crustaceana, 74, 69–77. https://doi.org/10.1163/156854001505442
Negreiros-Fransozo, M. L., Barba, E., Sánchez, J., Fransozo, A., & Raz-Guzmán, A. (1996). The species of Hippolyte Leach (Crustacea, Caridea, Hippolytidae) from Términos lagoon, southwestern Gulf of Mexico. Revista Brasileira de Zoología, 13, 539–551. http://dx.doi.org/10.1590/S0101-81751996000300002
Orton, J. H. (1920). Sea temperatures, breeding and distribution of marine animals. Journal of the Marine Biological Association of the United Kingdom, 12, 339–366.
Pearson, E. S. (1931). The analysis of variance in case of non-normal variation. Biometrika, 23, 114–133.
Peixoto, S., Soares, R. Wasielesky, W., Cavalli, R. O., & Jensen, L. (2004). Morphometric relationship of weight and length of cultured Farfantepenaeus paulensis during nursery, grow out, and broodstock production phases. Aquaculture, 241, 291–299. https://doi.org/10.1016/j.aquaculture.2004.08.008
Pinheiro, M. A. A., & Fransozo, A. (2002). Reproduction of the speckled swimming crab Arenaeus cribrarius (Brachyura: Portunidae) on the Brazilian coast near 23° 30’S. Journal of Crustacean Biology, 22, 416–428. https://doi.org/10.1163/20021975-99990249
Sánchez, P. J., Beltrán, R., & Ramírez-Lozano, J. P. (2008). Crecimiento y reproducción del camarón Atya margaritacea (Decapoda: Atyidae) en el río Presidio, Sinaloa, México. Revista de Biología Tropical, 56, 513–522. https://doi.org/10.15517/rbt.v56i2.5604
Sforza, R., Nalesso, R. C., & Joyeux, J. C. (2010). Distribution and population structure of Callinectes danae (Decapoda: Portunidae) in a tropical brazilian estuary. Journal of Crustacean Biology, 30, 597–606. https://doi.org/10.1651/09-3223.1
Somerton, D. A., & Meyers, W. S. (1983). Fecundity differences between primiparous and multiparous female Alaskan tanner crab (Chionoecetes bairdi). Journal of Crustacean Biology, 3, 183–186. https://doi.org/10.2307/1548254
Stearns, S. C. (1976). Life-history tactics: a review of the ideas. Quarterly Review of Biology, 51, 3–47.
Terossi, M., & Mantelatto, F. L. (2010). Sexual ratio, reproductive period and seasonal variation of the gonochoric shrimp Hippolyte obliquimanus (Caridea: Hippolytidae). Marine Biology Research, 6, 213–219. https://doi.org/10.1080/17451000903078630
Thorson, G. (1950). Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews of the Cambridge Philosophical Society, 25, 1–45.
Voss, G. L. (1956). Protective coloration and habitat of the shrimp Tozeuma carolinensis Kingsley, (Caridea: Hippolytidae). Bulletin of Marine Science of the Gulf and Caribbean, 6, 359–363.
Voss, G. L., & Voss, N. A. (1955). An ecological survey of Soldier Key, Biscayne Bay, Florida. Bulletin of Marine Science of the Gulf and Caribbean, 5, 203–229.
Wass, M. L. (1955). The decapod crustaceans of Alligator Harbor and adjacent inshore areas of northwestern Florida. Quarterly Journal of the Florida Academy of Science, 18, 129–176.
Zare, P., Naderi, M., Eshghi, H., & Anastasiadou, C. (2011). Reproductive traits of the freshwater shrimp Caridina fossarum Heller, 1862 (Decapoda, Caridea, Atyidae) in the Ghomp-Atashkedeh spring (Iran). Limnologica, 41, 244–248. https://doi.org/10.1016/j.limno.2010.11.003



