Early establishment of endangered and valuable tree species in cloud forest restoration plantings
Siunelly Landero-Lozada a, Tarin Toledo-Aceves a, *, Fabiola López-Barrera a, Vinicio J. Sosa a, Neptalí Ramírez-Marcial b
a Red de Ecología Funcional, Instituto de Ecología, A.C., Carretera Antigua a Coatepec 351, El Haya, 91070 Xalapa, Veracruz, Mexico
b Departamento de Conservación de la Biodiversidad, El Colegio de la Frontera Sur, Carretera Panamericana y Periférico Sur s/n, 29290 San Cristóbal de Las Casas, Chiapas, Mexico
*Corresponding author: tarin.toledo@inecol.mx (T. Toledo-Aceves)
Abstract
Tropical montane cloud forests (TMCF) host high biodiversity and endemicity and are severely threatened by illegal selective logging, deforestation, fragmentation and climate change. The fragments of TMCF present high heterogeneity over short spatial intervals and a regional forest restoration approach must therefore incorporate seedling performance throughout the TMCF elevation range. We examined early seedling establishment in 12 endangered and valuable TMCF species in the understory of unplanned selectively logged TMCF and assessed the influence of elevation and canopy cover on seedling performance. Tree seedlings (10-18 mo-old) were transplanted into restoration plantings in 6 forest sites along an elevation range (1,250 to 1,995 m) in Veracruz, Mexico. In each forest, 30 seedlings per species were planted and their survival and relative growth rates in height (RGRh) and basal stem diameter (RGRd) recorded, along with canopy cover. Survival was high for all species after one year (81.1 to 99.4%) and was unaffected by elevation. Canopy cover positively affected the survival probability of 3 species. Both RGRh and RGRd decreased at lower elevations in 4 species; however, overall growth rates were positive. Our results indicate a positive early seedling establishment response in the evaluated tree species under the TMCF canopy (66-97% of canopy cover).
Keywords:
Elevation; Enrichment planting; Forest restoration; Growth; Seedling survival
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Establecimiento temprano de especies de árboles amenazadas y valiosas en plantaciones de restauración en bosque mesófilo de montaña
Resumen
Los bosques mesófilos de montaña (BMM) albergan alta biodiversidad y endemismos y están severamente amenazados por la tala selectiva ilegal, la deforestación, la fragmentación y el cambio climático. Los fragmentos de BMM son altamente heterogéneos en intervalos espaciales cortos, por lo que su restauración, desde una aproximación regional, debe incorporar su rango de elevación. Evaluamos el establecimiento temprano de 12 especies de BMM amenazadas y valiosas, y la influencia de la elevación y la cobertura del dosel sobre su desempeño. Las plántulas (10 a 18 meses de edad) se plantaron en 6 sitios de BMM en un gradiente de elevación (1,250 a 1,995 m) en Veracruz, México. En cada bosque se plantaron 30 plántulas por especie y se registró la sobrevivencia y crecimiento en altura (RGRh), el diámetro basal (RGRd) y la cobertura del dosel. La sobrevivencia fue alta para todas las especies después de un año (81.1 to 99.4%) y no fue afectada por la elevación. La cobertura del dosel afectó positivamente la probabilidad de sobrevivencia de 3 especies. En general, las tasas de crecimiento fueron positivas y en 4 especies disminuyeron a menor elevación. Nuestros resultados indican una respuesta positiva del establecimiento temprano de las plántulas en las especies estudiadas bajo el dosel del BMM (66-97% de cobertura).
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Elevación; Plantación de enriquecimiento; Restauración forestal; Crecimiento; Sobrevivencia de plántulas
Introduction
Tropical Montane Cloud Forest (TMCF) is one of the ecosystems most threatened by deforestation, fragmentation and global climate change (Bruijnzeel et al., 2011; Feeley et al., 2013). It naturally occupies a very restricted area and has been estimated to account for between 2.5 and 14.2% of the total area of tropical forests worldwide (Bubb et al., 2004; Mulligan, 2011). Typically, TMCF is located in zones in frequent contact with clouds at vegetation level (Hamilton et al., 1995). As a result of global climate change, changes in the patterns of precipitation, cloud distribution and increased temperatures could be among the most important threats to the high biodiversity of this ecosystem (Feeley et al., 2013; Lutz et al., 2013; Pounds et al., 1999; Solomon, 2007). The TMCF is also under severe pressure because of deforestation and fragmentation. It was estimated that, by 2002, only 28% of the original TMCF cover remained in Mexico, of which 52.4% corresponded to degraded or secondary forests (Challenger et al., 2009). Added to TMCF loss, chronic selective logging threatens the regeneration of certain species (González-Espinosa et al., 2011; Ortiz-Colín et al., 2017; Ramírez-Marcial et al., 2001). As a result of these factors, 60% of TMCF tree species in Mexico have been reported to be under some category of threat (González-Espinosa et al., 2011).
Threatened tree species are subjected to strong population bottlenecks and therefore are at a high risk of local extinction because of demographic and genetic stochasticity (Millet et al., 2013). The introduction of seedlings and saplings of key tree species under the forest canopy might be a viable strategy for the recovery of their populations. Furthermore, the likelihood of forest conversion to more profitable land uses could be reduced if possibilities for subsequent timber harvests are increased (Grauel & Putz, 2004). Given that traditional selective logging is a common practice in TMCF (Bruijnzeel et al., 2011; Ortiz-Colín et al., 2017; Toledo-Aceves et al., 2011) enrichment plantings with endangered and valuable plant species for the local communities could favour the long-term maintenance of forest biodiversity (Millet et al., 2013). Under the canopy, assemblages of late-successional and animal dispersed tree species can be introduced; these may not otherwise arrive because of limited seed sources or dispersal agents (Martínez-Garza & Howe, 2003). However, insufficient information regarding microenvironmental requirements for the early establishment of native TMCF tree species limits their use for restoration purposes. Moreover, lack of knowledge about the species-specific requirements for establishment increases the risk of failure through the inappropriate selection of species and sites (Lamb et al., 2005; van Breugel et al., 2011).
Several abiotic and biotic factors, operating at different temporal and spatial scales, may influence the success of enrichment plantings (Paquette et al., 2006; Rappaport & Montagnini, 2014). Light availability is a determining factor in the establishment of tree plantings in humid regions; intermediate levels of canopy cover have been found to increase the success of assisting regeneration by enrichment planting in degraded tropical forests in Asia and the Americas (Millet et al., 2013; Paquette et al., 2006). Nevertheless, the influence of other factors on early seedling establishment in enrichment plantings requires further investigation (van Breugel et al., 2011). TMCF exhibit a remarkable turnover of tree species assemblages due to the presence of elevation gradients and environmental heterogeneity (e.g., soil type, slope) and their interaction (Williams-Linera et al., 2013; Williams-Linera & Vizcaíno-Bravo, 2016). Tree species distribution is therefore concentrated, but not clearly limited in certain elevation zones (Toledo-Garibaldi & Williams-Linera, 2014; Vázquez & Givnish, 1998). Restoration of TMCF structure and function requires a regional approach (Williams-Linera, 2002). In order to achieve these goals, it is necessary to evaluate multi-specific native species responses along the elevation range of TMCF distribution. Restoration plantings are most vulnerable during their first year of establishment, yet seedling response to environmental factors varies across species and spatial scales (e.g., Bertacchi et al., 2015). In this study, we examine early tree seedling establishment of 12 valuable TMCF species in the understory of unplanned selectively logged TMCF forests and assess the influence of elevation and canopy cover on the variability in seedling performance. To our knowledge, this is the first study to evaluate early seedling performance in species-rich plantings along an elevation gradient. This information will provide a base line with which to select adequate species assemblages for TMCF restoration considering environmental variation at different spatial scales in order to avoid premature failure of forest restoration practices at regional level.
Materials and methods
The experiment was conducted along an elevation gradient from 1,250 to 1,995 m asl, within the TMCF distribution range in the Pixquiac River micro-watershed, part of the La Antigua basin, in Veracruz, Mexico. This is a region classified as being of high priority for TMCF conservation in Mexico (Toledo-Aceves et al., 2011). Along this gradient, mean annual temperature ranges from 12 to 20 °C and mean annual precipitation from 1,800 to 2,000 mm (Vidriales-Chan et al., 2012). Six forest fragments were selected based on shared attributes (fragment area > 1 ha, absence of cattle grazing inside the forest, ≤ 40° slope) (Table 1). For at least the last 80 years, there has been unplanned selective logging in all the forest fragments; this activity tends to target species with high wood density, mainly for use in the production of charcoal or in rural construction, and thus causes floristic impoverishment (Ortíz-Colín et al., 2017).
In each forest, one 50 × 55 m plot was delimited in order to establish one enrichment planting plot. To assess forest structure and composition, one 50 × 10 m transect was delimited along the middle of the plot and the diameter at breast height (DBH; at 1.3 m height) of all trees ≥ 5 cm DBH recorded. To estimate natural regeneration, all the seedlings and saplings (≥ 0.5 m height and ≤ 5 cm DBH) were recorded in 3 subplots (4 × 5 m) at either end and in the middle of the transect. The forest characteristics for each plot are shown in Table 1. Plot 1 was the only plot in which the exotic plants Eriobotrya japonica (loquat) and Syzygium jambos (pomarrosa) were recorded, and this site was previously used as a shaded coffee plantation.
A sensor was placed in the canopy of a tree (to reduce the risk of theft) at the centre of the plot in order to record air temperature (iButton Thermochron) at 10 minutes intervals from October 2015 to February 2016 and during April 2016. Volumetric soil water content was quantified with a soil moisture probe (10 HS soil moisture sensor) and a data logger (HOBO, Onset Computer Corporation, USA) buried 20-30 cm beneath the soil at the centre of each plot. Soil water content measurements were recorded at one sampling point every 2 hours from February to June 2016; these measurements were taken simultaneously in all plots. To characterize the upper layer of soil at each plot, at the beginning of the experiment (rainy season), 6 soil samples were collected from depth 0-10 cm and combined into one compound sample for laboratory analysis. The superficial litter was removed, and samples were taken only from the topsoil, corresponding to the A1 sub-horizon. Soil samples were collected within one week and stored at 4 ºC for less than a week prior to the laboratory analysis of pH, soil density, total soil Carbon (tot-C), total soil Nitrogen (tot-N), and total soil Phosphorus (tot-P) (additional data are presented in appendix 1). Due to the high spatial heterogeneity of soil nutrients, controlled in part by plant root and plant residue patterns (Stoyan et al., 2000), the effects of tot-C, tot-N and tot-P on seedling performance were not analysed.
Table 1
Characteristics of 6 forest plots along an elevation gradient, Veracruz, Mexico. For diameter at breast height (DBH), juvenile density, and canopy cover the mean ± 1 standard error is presented. NA = not available.
|
Plot |
Elevation (m asl) |
DBH (cm) |
Juvenile density (stem/ m2) |
Canopy cover (%) |
Mean temperature (°C) |
Soil water content (%) |
|
1 |
1,250 |
21.2 ± 3.6 |
0.38 ± 0.07 |
93.3 ± 0.4 |
18.7 |
25 |
|
2 |
1,526 |
24.7 ± 2.6 |
0.15 ± 0.08 |
93.2 ± 0.6 |
17.4 |
36 |
|
3 |
1,573 |
19.3 ± 1.8 |
0.10 ± 0.05 |
84.2 ± 1.8 |
18.2 |
37 |
|
4 |
1,680 |
24.8 ± 2.3 |
0.35 ± 0.10 |
92.1 ± 0.6 |
16.5 |
28 |
|
5 |
1,853 |
28.3 ± 3.2 |
0.30 ± 0.08 |
91.9 ± 1.1 |
16.1 |
35 |
|
6 |
1,995 |
23.9 ± 2.2 |
0.33 ± 0.19 |
92.3 ± 0.7 |
15.1 |
NA |
Twelve TMCF tree species were selected based on seed availability, conservation status and local value (Table 2). Nine of the studied species are included on the Red List of Mexican Cloud Forest Trees under some category of threat (González-Espinosa et al., 2011; see status in Table 2) and all are associated with advanced successional phases. Pioneer or light demanding, and shade tolerant species represent 2 contrasting strategies, but a continuous gradient of shade tolerance exists among tree species (Wright et al., 2003). Six species are reported as shade tolerant and 6 as intermediate tolerant; however, some shade tolerance classifications were proposed based on empirical observations in a specific area and contradictions have arisen among categories assigned to a given species (Toledo-Aceves et al., 2017). For example, Ulmus mexicana has been reported as a shade tolerant species but a preliminary study suggests an intermediate shade tolerance strategy (Toledo-Aceves et al., 2017). Four species are endemic to Mexico: Juglans pyriformis, Ocotea disjuncta, Quercus germana and Q. sartorii. Given their desirable wood properties, some of the studied species have been overharvested by local communities and some of their populations have disappeared locally in the study region; e.g., Juglans pyriformis, Ocotea disjuncta, Oreomunnea mexicana (Paré & Gerez, 2012), Meliosma alba and Sideroxylon contrerasii (T. Toledo-Aceves, pers. obs). Natural regeneration of some of these species is also absent or very low in the logged forest of the region (Ortíz-Colín et al., 2017).
The reported tree species elevation ranges are based on their presence, not on frequency of occurrence (Table 2). The latter parameter would be more precise for determining the suitable range of habitats provided for each species in different elevation zones, but this information was unavailable for most species. Considering this, and in order to gain more information for the management of native tree diversity, we used several tree species distributed along the elevation gradient.
Seeds of at least 3 individuals per species were collected in 2013 and 2014 from TMCF fragments in the region. Seeds were collected directly from the trees instead of the forest floor in order to reduce the risk of contamination by soil pathogens. Voucher specimens of all species were deposited in the herbarium of the Instituto de Ecología A.C. (IE-XAL). Immediately after collection, the seeds were cleaned and sown in seedbeds with forest soil from the forest adjacent to the nursery and, approximately 2 months after germination, the seedlings were transplanted into polythene bags (30 × 16 cm) filled with forest soil. The plants were kept in a rustic nursery covered with 30% shade mesh, and no chemicals were applied. Seedling age varied from 10 to 18 months at the time of planting because of differences in phenology and germination among species (initial seedling height at transplanting time is presented in appendix 2).
Table 2
Tree species studied for the restoration of degraded tropical montane cloud forest in Veracruz, Mexico. CR = Critically endangered, EN = endangered, VU= vulnerable, NT = near threatened and LC = least concern (González-Espinosa et al., 2011). Shade tolerance: I = intermediate, T = shade tolerant.
|
Acronym |
Species |
Family |
Conservation status |
Elevation range (m asl) |
Shade tolerance |
|
CM |
Clethra macrophylla M. Martens & Galeotti |
Clethraceae |
LC |
750-1,5001 |
I |
|
CT |
Carpinus tropicalis (Donn. Sm.) Lundell |
Betulaceae |
NT |
1,200-2,5002 |
T |
|
FU |
Fraxinus uhdei (Wenz.) Lingelsh. |
Oleaceae |
LC |
1,300-2,2401 |
I |
|
JP |
Juglans pyriformis Liebm. |
Juglandaceae |
EN |
1,200-1,4001 |
T |
|
MA |
Meliosma alba (Schltdl.) Walp. |
Sabiaceae |
EN |
700-1,9001 |
I |
|
OD |
Ocotea disjuncta Lorea-Hern. |
Lauraceae |
EN |
1,700-2,5001 |
T |
|
OM |
Oreomunnea mexicana (Standl.) J.F. Leroy |
Juglandaceae |
EN |
1,100-2,0001 |
T |
|
PR |
Prunus rhamnoides Koehne |
Rosaceae |
VU |
1,500-2,400 |
T |
|
QG |
Quercus germana Schltdl. & Cham. |
Fagaceae |
CR |
800-1,8001 |
I |
|
QS |
Quercus sartorii Liebm. |
Fagaceae |
EN |
1,300-2,0001 |
I |
|
SC |
Sideroxylon contrerasii (Lundell) T.D.Penn. |
Sapotaceae |
VU |
800-1,8001 |
T |
|
UM |
Ulmus mexicana (Liebm.) Planch. |
Ulmaceae |
EN |
150-2,1501 |
I |
1González-Espinosa et al. (2011)
2Furlow (1987)
In each of the 6 plots, 30 seedlings per species were transplanted at the beginning of the rainy season (May-June 2015). A map was generated for species distribution at random in each plot and the seedlings were transplanted ~2.6 m apart. A hole of approximately 30 cm in depth and 20 cm in width was created with a spade for each seedling. Seedlings were transplanted with all the soil contained in the original nursery bag. Since weeding is a common practice in enrichment plantings to reduce aggressive non-woody competitors (ITTO, 2002), a ~1 m radius around each seedling was cleared of weeds with a machete at the time of transplantation and again after 3 and 6 months. Seedling survival was recorded for all the plants after one year. From each plot, 10 seedlings per species were selected at random and their height and stem diameter measured immediately after transplanting and again after 12 months. Relative growth rates (RGR) in height and diameter were calculated as follows: RGR = (ln H2– ln H1) / t2-t1, where H is seedling height; t is time, and the initial and final measurements are denoted by subscripts 1 and 2 respectively (Hunt, 1982). Canopy cover (as a surrogate for solar radiation reaching the seedling) was measured using hemispherical photographs above each of the seedlings selected for growth measurements in September 2015 (3 months post-transplantation). A camera (Canon Eos rebel XSi) with a fish eye lens was set up 1.3 m above the ground on a tripod, levelled and orientated towards north. The photographs were analysed using Gap Light Analyzer (Version 2.0.).
In order to compare the forest structure variables among plots, an ANOVA was performed for adult tree DBH and for density of natural tree regeneration. Prior to analysis the BoxCox transformation was applied to tree seedling and sapling density (no. plants /m2) values in order to comply with the normality of the residuals. Since recording of soil nutrients was conducted in only one sampling event per plot, no measurements of the variation were calculated.
Replicated regression analyses were used considering seedlings as the experimental units (Cottingham et al., 2005). For each species, a logistic regression with logit link function was used to evaluate the effect of elevation and canopy cover on seedling survival after one year. Since elevation and temperature were significantly correlated, temperature was excluded from the model to reduce collinearity. Elevation was included because, in addition to changes in temperature, changes in atmospheric pressure, solar radiation and ultraviolet light occur with changes in elevation (Körner, 2007). Elevation and canopy cover were included as covariables. To evaluate the combined effect of elevation and canopy cover on RGRh and RGRd, a general linear model (GLM) was used for each species. All statistical analyses were run in Minitab 16.
Results
A summary of forest plot mean DBH, canopy cover, natural regeneration density and microclimate are shown in table 1. Tree mean DBH and density of natural regeneration were similar among all plots (p = 0.24 and p = 0.99, respectively). Canopy cover and tree regeneration density were not correlated with elevation (r = -0.049, r = -0.561 and r = 0.057, respectively, p > 0.05). As expected, mean temperature was highly correlated with elevation; temperature decreased as elevation increased (r = -0.955, p = 0.003; Table 1). Volumetric soil water content at 20-30 cm depth was relatively constant within each plot, ranging from 25 to 37% among plots (Table 1), and was not correlated with elevation (p > 0.05). Soil bulk density ranged from 0.32 to 0.90 g /cm2 and was not correlated with elevation (r = -0.809, p = 0.51). Soil tot-C increased with elevation (r = 0.90, p = 0.014), while soil tot-N, and tot-P were not correlated with elevation (r = -0.65, p = 0.16, and r = -0.15, p = 0.76 respectively).
Over the course of one year (2015/2016), seedling survival was very high in all the species; the lowest survival on average for the 6 plots was 81.1% in Meliosma alba and the highest was 99.4% in Sideroxylon contrerasii (Fig. 1). Elevation had no significant effect on seedling survival in any of the tree species (Table 3; Fig. 1). Mean canopy cover in the plot had a significant positive effect on the survival probability of Carpinus tropicalis, Juglans pyriformis and Prunus rhamnoides (p < 0.05; Table 3).
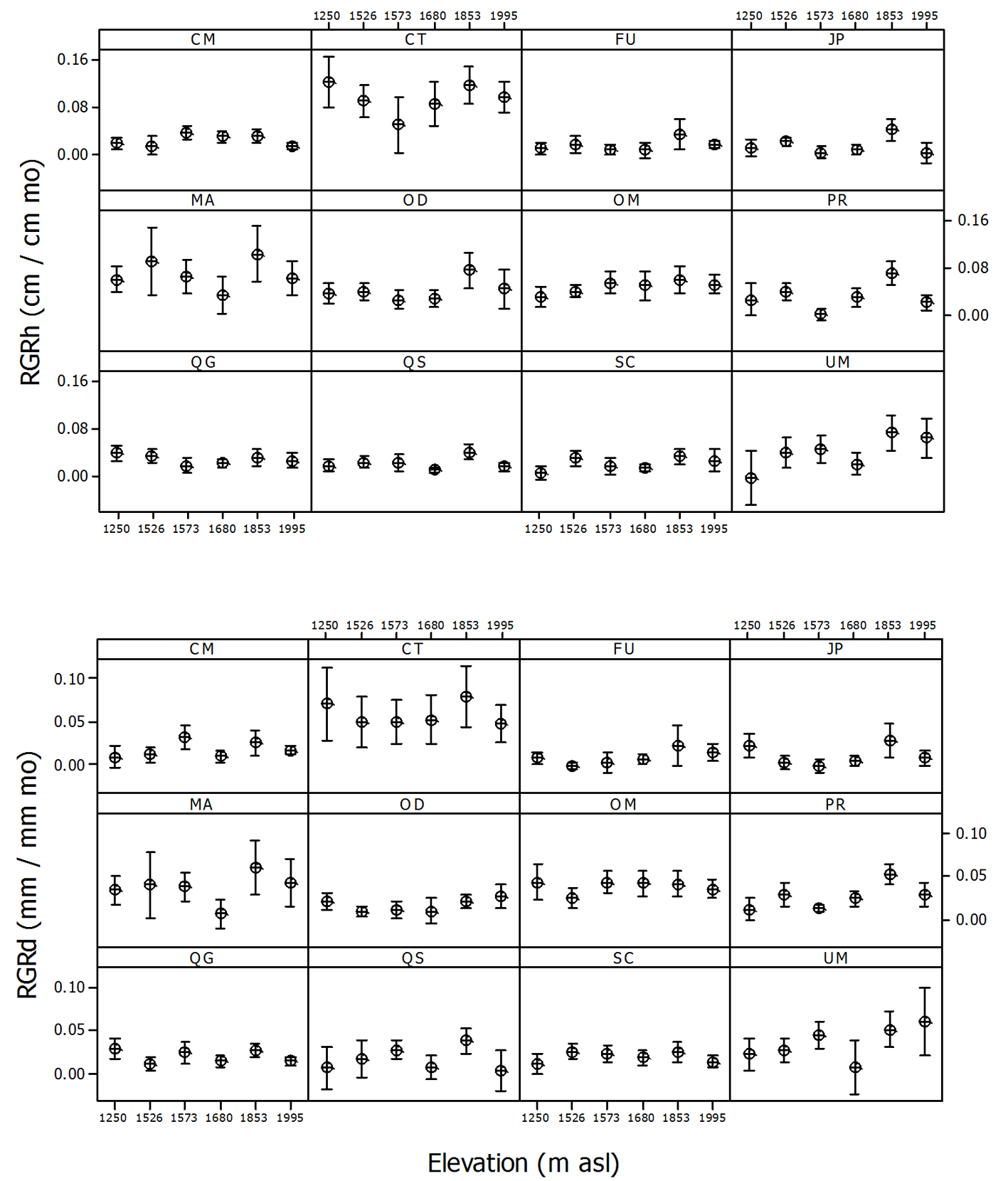
Mean relative growth rates were positive in all species. The highest values were recorded for C. tropicalis (RGRh = 0.095 ± 0.006 cm cm-1 mo-1, RGRd = 0.059 ± 0.005 mm mm-1 mo-1), followed by M. alba (RGRh = 0.069 ± 0.007 cm cm-1 mo-1, RGRd = 0.037 ± 0.004 mm mm-1 mo-1), while the lowest rates were for Fraxinus uhdei (RGRh = 0.013 ± 0.002 cm cm-1 mo-1, RGRd = 0.008 ± 0.002 mm mm-1 mo-1) and J. pyriformis (RGRh = 0.013 ± 0.002 cm cm-1 mo-1, RGRd = 0.011 ± 0.002 mm mm-1 mo-1) (Fig. 2). The effect of elevation on growth rates was only significant for 4 species (Table 4): in Oreomunnea mexicana, S. contrerasii and Ulmus mexicana, RGRh increased with elevation and in P. rhamnoides and U. mexicana, RGRd also increased with increasing elevation (p < 0.05). Canopy cover above the individual plant had a significant effect only on 2 species: Clethra macrophylla and Quercus sartorii displayed lower growth rates under increased canopy cover (Table 4).
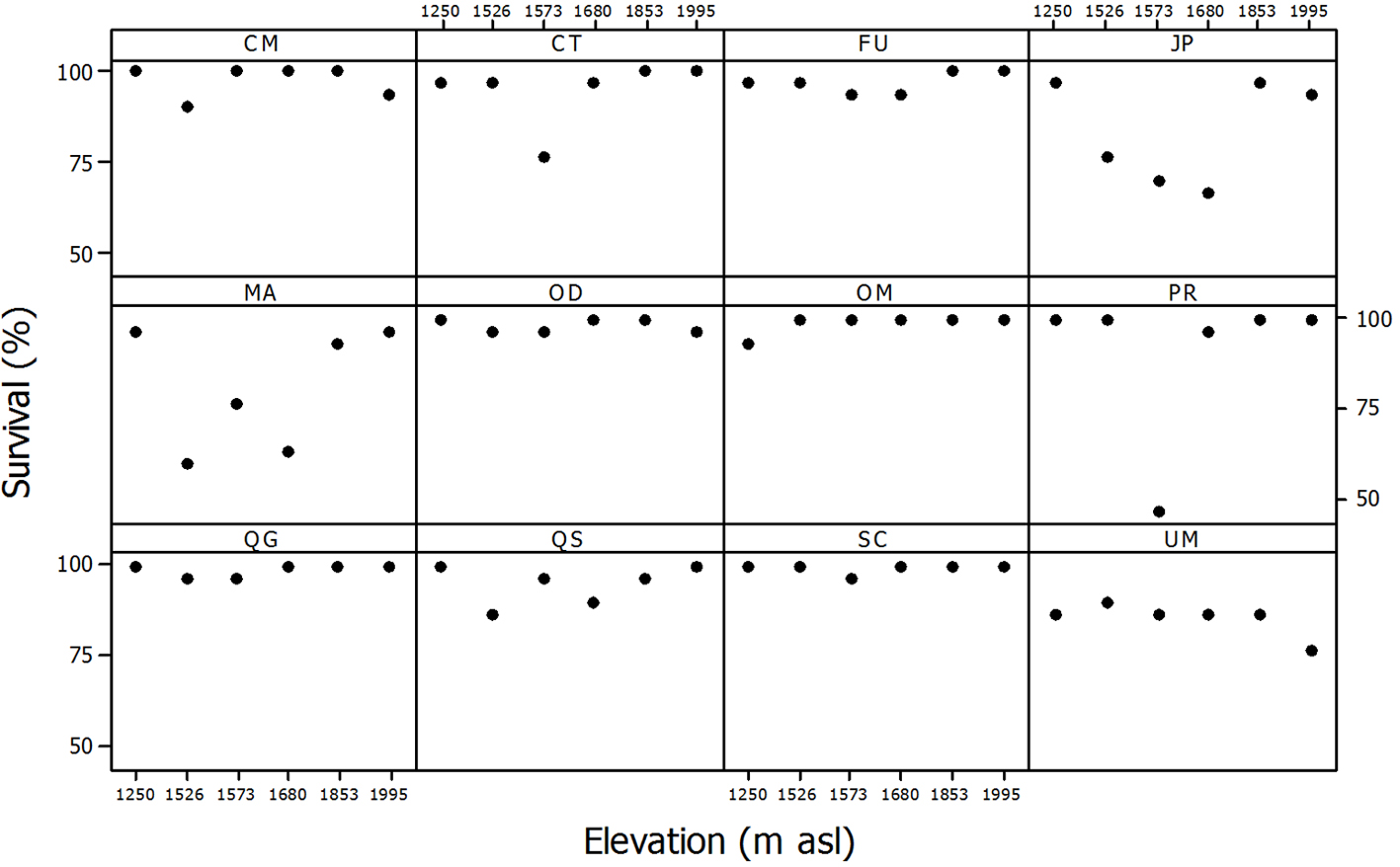
Table 3
Logistic regression results for the effect of elevation and canopy cover on seedling survival in 12 cloud forest species in enrichment plantings established along an elevation gradient. Each regression is for an individual species across all plots (N = 180). Coef = Coefficient, Z = Z-test statistic value. Significant values are in bold (p ≤ 0.05), DF = degrees of freedom. Results for which the model did not converge due to very low mortality were excluded (mortality: Clethra macrophylla = 2.78%, Oreomunnea mexicana = 1.11%, Sideroxylon contrerasii = 0.56%).
|
Species |
Intercept |
Elevation |
Canopy cover |
DF |
||||||
|
Coef |
Z |
p |
Coef |
Z |
p |
Coef |
Z |
p |
||
|
Carpinus tropicalis |
-30.69 |
-3.19 |
0.001 |
0.003 |
1.36 |
0.17 |
0.31 |
3.58 |
< 0.0001 |
2 |
|
Fraxinus uhdei |
-10.12 |
-0.97 |
0.330 |
0.002 |
1.06 |
0.29 |
0.11 |
1.07 |
0.28 |
2 |
|
Juglans pyriformis |
-9.47 |
-1.85 |
0.064 |
0.0004 |
0.50 |
0.616 |
0.113 |
2.08 |
0.037 |
2 |
|
Meliosma alba |
-2.81 |
-0.53 |
0.599 |
0.0007 |
0.97 |
0.33 |
0.032 |
0.58 |
0.562 |
2 |
|
Ocotea disjuncta |
-4.38 |
-0.32 |
0.752 |
-0.001 |
-0.45 |
0.651 |
0.117 |
0.73 |
0.468 |
2 |
|
Prunus rhamnoides |
-53.15 |
-4.63 |
0.00 |
0.0007 |
0.18 |
0.854 |
0.615 |
4.40 |
< 0.001 |
2 |
|
Quercus germana |
-14.98 |
-0.87 |
0.385 |
0.002 |
0.59 |
0.553 |
0.175 |
1.04 |
0.298 |
2 |
|
Quercus sartorii |
7.81 |
0.65 |
0.514 |
0.0004 |
0.32 |
0.748 |
-0.061 |
-0.49 |
0.626 |
2 |
|
Ulmus mexicana |
3.69 |
0.57 |
0.569 |
-0.000 |
-1.03 |
0.304 |
-0.003 |
-0.06 |
0.955 |
2 |
Discussion
The results support the notion that the tree species assemblage evaluated may be able to establish successfully under the canopy of TMCF throughout the elevation range. While our study reports only the first-year post-transplantation, previous studies have found that early establishment can be representative of the success of enrichment planting in the mid-term (Simoes-Macayo & Renison, 2015) and since enrichment plantings are more vulnerable during the first year, our results indicate the potential for establishment of the species (Bertacchi et al., 2015). The high survival values recorded are like previous reports for transplanted tree seedlings within secondary TMCF at elevations of between 1,340 and 1,580 m asl by Álvarez-Aquino et al. (2004), who reported tree survival after 14 months in Carpinus caroliniana (70%), Fagus grandifolia (78%), Quercus acutifolia (78%), and Symplocos coccinea (91%). In addition, Muñiz-Castro et al. (2015) reported more than 80% tree survival after one year in Fagus grandifolia, Quercus germana, and Q. xalapensis. In some forest restoration studies, the main causes of tree seedling mortality were not only related to the microenvironment associated with the canopy cover, but also to biotic factors such as herbivory (Muñiz-Castro et al., 2015; Pedraza & Williams-Linera, 2003). In this context, we observed high leaf insect herbivory of P. rhamnoides in site 3, which could have caused the high mortality of this species in this site.
The early success of enrichment planting is determined by 1) species-specific traits that allow seedlings to ameliorate relocation stresses, and 2) seedling responses to environmental and biotic factors operating at different spatial and temporal scales following transplantation (Bertacchi et al., 2015; van Breugel et al., 2011). The seedling performance recorded in this study indicates that species were moderately affected by ecological factors at the local or microhabitat (canopy cover) scale, and by changes in environmental factors associated with elevation (e.g., mean temperature). The high levels of canopy cover in the studied forests seem to favour early seedling survival in the studied species. Paquette et al. (2006) also found that seedling survival is improved under high levels of canopy cover (> 75%) in enrichment plantings in tropical forests. In this study, canopy cover had a significant positive effect on the survival probability of C. tropicalis, J. pyriformis, and P. rhamnoides. Higher survival in C. tropicalis has been reported under the canopy of secondary TMCF compared to that in open areas (Álvarez-Aquino et al., 2004). Previous transplantation experiments also report higher seedling survival in other native TMCF tree seedlings under the canopy of secondary TMCF compared to recently abandoned (< 1 year previously) pastures and agricultural land (Álvarez-Aquino et al., 2004; Muñiz-Castro et al., 2015). The canopy can reduce the proliferation of competitive vegetation, buffer extreme temperature variation and provide a more humid understory (Lamb et al., 2005; Montes-Hernández & López-Barrera, 2013; Muñiz-Castro et al., 2015). Thus, the high canopy cover (84-93%) in TMCF subjected to chronic selective logging can provide favourable habitat conditions for the early establishment of intermediate to shade tolerant tree seedlings by enrichment plantings. It should be noted that while some species may display high survival during the early years of establishment under canopy cover, their mortality can increase as these species become light demanders or the canopy cover changes as a result of natural succession and the creation of forest canopy gaps (Bertacchi et al., 2015). This emphasizes the requirement for long term monitoring of tree performance.
Table 4
General linear model results for the effects of elevation and canopy cover on relative growth rate in height (RGRh) and diameter (RGRd) in 12 cloud forest tree species in enrichment plantings along an elevation gradient. Coef = Coefficient, t-student (t) and probability of rejecting null hypothesis are given; significant values are presented in bold (p ≤ 0.05). The number of plants measured varied due to mortality.
|
Species |
Intercept |
Elevation |
Canopy cover |
|||||||
|
RGRh |
Coef |
t |
p |
Coef |
t |
p |
Coef |
t |
p |
Error DF |
|
Clethra macrophylla |
0.106 |
3.00 |
0.004 |
0.000002 |
0.24 |
0.810 |
-0.0009 |
-2.62 |
0.011 |
57 |
|
Carpinus tropicalis |
0.112 |
0.89 |
0.379 |
-0.00001 |
-0.51 |
0.614 |
0.00008 |
0.06 |
0.952 |
51 |
|
Fraxinus uhdei |
-0.046 |
-0.87 |
0.390 |
0.00001 |
1.52 |
0.135 |
0.0003 |
0.55 |
0.583 |
56 |
|
Juglans pyriformis |
-0.016 |
-0.26 |
0.796 |
0.000006 |
0.50 |
0.620 |
0.0002 |
0.33 |
0.743 |
50 |
|
Meliosma alba |
0.004 |
0.04 |
0.969 |
0.00001 |
0.44 |
0.665 |
0.0004 |
0.32 |
0.752 |
49 |
|
Ocotea disjuncta |
-0.045 |
-0.57 |
0.569 |
0.00003 |
1.91 |
0.061 |
0.0003 |
0.44 |
0.662 |
56 |
|
Oreomunnea mexicana |
0.051 |
0.70 |
0.487 |
0.00003 |
2.20 |
0.032 |
-0.0006 |
-0.78 |
0.436 |
56 |
|
Prunus rhamnoides |
0.034 |
0.22 |
0.825 |
0.00001 |
1.0 |
0.321 |
-0.0002 |
-0.18 |
0.854 |
50 |
|
Quercus germana |
0.032 |
0.97 |
0.338 |
-0.00001 |
-1.37 |
0.175 |
0.0002 |
0.64 |
0.522 |
57 |
|
Quercus sartorii |
0.062 |
1.76 |
0.085 |
0.00001 |
1.17 |
0.247 |
-0.0006 |
-1.68 |
0.099 |
53 |
|
Sideroxylon contrerasii |
-0.045 |
-1.09 |
0.281 |
0.00002 |
2.60 |
0.012 |
0.0002 |
0.57 |
0.568 |
57 |
|
Ulmus mexicana |
-0.005 |
-0.05 |
0.958 |
0.00009 |
4.14 |
< 0.001 |
-0.001 |
-1.14 |
0.259 |
54 |
|
RGRd |
||||||||||
|
Clethra macrophylla |
0.132 |
4.40 |
< 0.0001 |
0.00001 |
1.96 |
0.055 |
-0.001 |
-4.90 |
< 0.001 |
57 |
|
Carpinus tropicalis |
0.15 |
1.37 |
0.178 |
-0.000009 |
-0.39 |
0.698 |
-0.0008 |
-0.74 |
0.462 |
51 |
|
Fraxinus uhdei |
-0.066 |
-1.54 |
0.130 |
0.00001 |
1.79 |
0.078 |
0.0005 |
1.06 |
0.292 |
56 |
|
Juglans pyriformis |
-0.025 |
-0.48 |
0.634 |
-0.000002 |
-0.19 |
0.848 |
0.0004 |
0.79 |
0.434 |
50 |
|
Meliosma alba |
0.041 |
0.48 |
0.631 |
0.00002 |
1.10 |
0.276 |
-0.0004 |
-0.48 |
0.636 |
49 |
|
Ocotea disjuncta |
-0.020 |
-0.54 |
0.591 |
0.00001 |
1.34 |
0.187 |
0.0001 |
0.51 |
0.609 |
56 |
|
Oreomunnea mexicana |
0.065 |
1.13 |
0.262 |
-0.00003 |
-0.23 |
0.816 |
-0.0002 |
-0.42 |
0.678 |
56 |
|
Prunus rhamnoides |
0.008 |
0.09 |
0.929 |
0.00003 |
3.27 |
0.002 |
-0.0004 |
-0.41 |
0.681 |
50 |
|
Quercus germana |
0.056 |
2.19 |
0.033 |
-0.000009 |
-1.17 |
0.249 |
-0.0002 |
-0.93 |
0.355 |
57 |
|
Quercus sartorii |
0.130 |
2.31 |
0.025 |
0.00001 |
0.86 |
0.396 |
-0.001 |
-2.49 |
0.016 |
53 |
|
Sideroxylon contrerasii |
0.044 |
1.39 |
0.169 |
0.000006 |
0.72 |
0.474 |
-0.0003 |
-1.14 |
0.26 |
57 |
|
Ulmus mexicana |
0.105 |
1.21 |
0.232 |
0.00005 |
2.63 |
0.011 |
-0.0017 |
-1.85 |
0.069 |
54 |
While a positive effect of canopy cover on tree seedling survival was previously reported in comparison to open areas, growth rates have also been found to decrease under high forest canopy cover (Álvarez-Aquino et al., 2004; Avendaño-Yáñez et al., 2014; Muñiz-Castro et al., 2015). We found that the growth of only 2 species (Clethra macrophylla and Quercus sartorii) was negatively correlated to canopy cover, probably due to the dense canopy in some sites (> 90%) and the lower shade tolerance of these broad-leaved species. This suggests that these tree species are likely to be limited by the available light. Other Clethra species have been reported to display high growth rates in response to the opening of small clearings (Arriaga, 2000). Previous studies have reported higher growth rates in various Quercus species with increased light availability (Ramírez-Bamonde et al., 2005; Ramírez-Marcial, 2003; Muñiz-Castro et al., 2015) and high growth rates were also reported in Q. sartorii seedlings one year after transplantation into a recently abandoned pasture (Williams-Linera et al., 2015). Species such as Carpinus tropicalis, Meliosma alba and Ulmus mexicana, which displayed the highest growth rates and could be expected to be more influenced by light availability, were unaffected by changes in canopy cover, possibly because the range of canopy cover did not encompass the conditions to which these species respond; even microsites with the lowest canopy cover (66%) may be too shaded for these species to respond. In a shade-house experiment, the seedling growth rates of 10 TMCF tree species (including M. alba and U. mexicana) did not differ between 8 and 12 mol m-2 d-1 for daily cumulative PAR (Toledo-Aceves et al., 2017).
While seedling survival was unaffected by elevation, increased growth rates with elevation were found in 4 species (Oreomunnea mexicana, Prunus rhamnoides, Sideroxylon contrerasii, and Ulmus mexicana). The mean distribution of the elevation range of these species is no higher than in the rest of the species and the lower growth rates could indicate stress caused by higher temperatures at the lowest plots; a difference of about 3 °C in mean temperature was recorded along the elevation gradient. If this pattern persists, these species could eventually die off or be outcompeted by other species at the lowest elevation; however, since yearly variation in climate may cause important fluctuations in tree seedling survival, a longer observation period is necessary in order to obtain a clearer picture of the fate of these species. The lack of a stronger response to canopy cover and elevation in most species could also be explained by the high heterogeneity within and among plots as a result of past management history, location, topography and soil conditions (Williams-Linera et al., 2013). These variables could add to the variation found and produce a homogenizing effect at the regional scale and thus obscuring specific responses. Soil water content, for example, varied between 25 and 37% among plots and is also highly variable in temporal and spatial terms.
With the current reduction and degradation of TMCF cover, even floristically impoverished TMCF remnants have become essential to the conservation of a significant portion of biodiversity and play an important role in the promotion of restoration plans (Lutz et al., 2013; Toledo-Aceves et al., 2011). As a component of restoration for production purposes, the establishment of enrichment plantings for timber harvesting could be a viable practice given that: 1) approximately 52% of the remaining TMCF in Mexico is classified as secondary (Challenger et al., 2009), 2) several threatened tree species are shade tolerant and display higher survival under the canopy (Álvarez-Aquino et al., 2004; González-Espinosa et al., 2011; Ramírez-Marcial et al., 2010), 3) selective logging is a common practice among local communities and targets many shade tolerant species (Ortiz-Colín et al., 2017), and 4) competition with grasses or other herbaceous plants under the forest canopy is lower than that of open areas, thus reducing maintenance costs. Enrichment planting can also help reduce restoration costs because of lower seedling mortality under shade conditions (Bertacchi et al., 2015). However, as part of restoration and management, future costs, such as those incurred by monitoring and intervention actions, should be considered in the mid and long term. For example, reduction of canopy cover as part of planned timber harvesting could promote higher growth rates. Previous studies in Costa Rica report positive effects of selective logging on tree growth and seed production in TMCF (Guariguata & Saenz, 2002; Saenz & Guariguata, 2001). Our results could serve to encourage efforts on the part of forest managers to use native TMCF trees, including threatened species, in order to foster higher levels of biodiversity in otherwise impoverished forests. While the starting height of the transplants varied greatly among and within species, the use of one-year-old seedlings seems suitable where competition control is applied at the beginning of the enrichment practice. There are few screening trials that include high numbers of TMCF species under a forest restoration context (Ramírez-Marcial et al., 2006); however, this type of study represents an essential initial step towards the appropriate selection of ecologically viable and socially acceptable species (van Breugel et al., 2011; Millet et al., 2013). Overall, our results support the potential of enrichment planting to facilitate the recovery of endangered and valuable TMCF species at the regional level, but long-term monitoring of reintroduced populations is necessary given potential fluctuations in plant survival and growth over time.
Acknowledgements
This work was funded by the Consejo Nacional de Ciencia y Tecnología (Conacyt) (CB2014-01/238831), Rufford Small Grants (14476-1) to TTA, and SENDAS A.C. Conacyt also provided a scholarship to Siunelly Landero-Lozada to conduct her MSc thesis (338206). Our gratitude to Rogelio Macías, Yareni Perroni, Claudia Gallardo, Ricardo Hernández, Antonio SanGabriel and Francisco SanGabriel, for granting us permission to work on their respective properties. We thank INECOL, A.C., Carlos Iglesias and the staff of the “Francisco Javier Clavijero Botanical Garden” for the use of their facilities. We thank Claudia Gallardo, Constanza Pinto, Magdaleno Mendoza, Maricela Bautista, Martín SanGabriel and Víctor Vásquez for their valuable help establishing and monitoring the enrichment plantings. Claudia Gallardo identified tree species and Keith Macmillan revised the English text.
Appendix 1. Soil characteristics of 6 cloud forest plots along an elevation gradient, Veracruz, Mexico. Total soil Carbon (tot-C), total soil Nitrogen (tot-N), and total soil Phosphorus (tot-P).
|
Plot |
Elevation (m asl) |
pH |
Soil density (g /cm2) |
Tot-C (%) |
Tot-N (%) |
Tot-P (%) |
|
1 |
1,250 |
4.5 |
0.90 |
5.8 |
0.66 |
0.03 |
|
2 |
1,520 |
4.7 |
0.82 |
9.81 |
0.95 |
0.05 |
|
3 |
1,600 |
4.2 |
0.43 |
16.90 |
1.33 |
0.07 |
|
4 |
1,680 |
5.4 |
0.47 |
13.24 |
1.17 |
0.07 |
|
5 |
1,845 |
4.4 |
0.60 |
16.34 |
1.14 |
0.03 |
|
6 |
1,984 |
4.2 |
0.32 |
18.63 |
1.10 |
0.02 |
Appendix 2. Initial seedling height of 12 cloud forest tree species at transplanting time (pooled across elevations, N = 90, mean ± 1SE).
|
Acronym |
Species |
Height (cm) |
|
CM |
Clethra macrophylla M. Martens & Galeotti |
58.0 ± 1.6 |
|
CT |
Carpinus tropicalis (Donn. Sm.) Lundell |
15.5 ± 0.5 |
|
FU |
Fraxinus uhdei (Wenz.) Lingelsh. |
28.9 ± 1.4 |
|
JP |
Juglans pyriformis Liebm. |
49.2 ± 1.7 |
|
MA |
Meliosma alba (Schltdl.) Walp. |
9.8 ± 0.5 |
|
OD |
Ocotea disjuncta Lorea-Hern. |
18.4 ± 0.9 |
|
OM |
Oreomunnea mexicana (Standl.) J.F. Leroy |
28.4 ± 1.4 |
|
PR |
Prunus rhamnoides Koehne |
28.7 ± 1.4 |
|
QG |
Quercus germana Schltdl. & Cham. |
63.5 ± 2.4 |
|
QS |
Quercus sartorii Liebm. |
60.6 ± 2.4 |
|
SC |
Sideroxylon contrerasii (Lundell) T.D.Penn. |
35.6 ± 2.1 |
|
UM |
Ulmus mexicana (Liebm.) Planch. |
62.1 ± 4.3 |
References
Álvarez-Aquino, C., Williams-Linera, G., & Newton, A. C. (2004). Experimental native tree seedling establishment for the restoration of a Mexican Cloud Forest. Restoration Ecology, 12, 412–418.
Arriaga, L. (2000). Gap building phase regeneration in a tropical montane cloud forest of north-eastern México. Journal of Tropical Ecology, 16, 535–562.
Avendaño-Yáñez, M. L., Sánchez-Velasquez L. R., Meave, J. A., & Pineda-López, M. R. (2014). Is facilitation a promising alternative for cloud forest restoration? Forest Ecology and Management, 329, 328–333.
Bertacchi, M. I. F., Amazonas, N. T., Brancalion, P. H. S., Brondani, G. E., de Oliveira, A. C. S., de Pascoa, M. A. R. et al. (2015). Establishment of tree seedlings in the understory of restoration plantations: natural regeneration and enrichment plantings. Restoration Ecology, 24, 100–108.
Bruijnzeel, L. A., Scatena, F. N., & Hamilton, L. S. (2011). Tropical montane cloud forests: science for conservation and management. Cambridge: Cambridge University Press.
Bubb, P., May, I., Miles, L., & Sayer, J. (2004). Cloud forest agenda. Cambridge: UNEP-WCMC.
Challenger, A., Dirzo, R., López, J. C., Mendoza, E., Lira-Noriega, A., & Cruz, I. (2009). Factores de cambio y estado de la biodiversidad. In Conabio (Eds.), Capital natural de México, vol. II. Estado de conservación y tendencias de cambio (pp. 37–73). México City: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Cottingham, K. L., Lennon, J. T., & Brown, B. L. (2005). Knowing when to draw the line: designing more informative ecological experiments. Frontiers in Ecology and the Environment, 3, 145–152.
Feeley, K. J., Hurtado, J., Saatchi, S., Silman, M. R., & Clark, D. B. (2013). Compositional shifts in Costa Rican forests due to climate-driven species migrations. Global Change Biology, 19, 3472–3480.
Furlow, J. J. (1987). The Carpinus caroliniana complex in North America. II. Systematics Source. Systematic Botany, 12, 416–434.
González-Espinosa, M., Meave, J. A., Lorea-Hernández, F. G., Ibarra-Manríquez, G., & Newton, A. (2011). The Red List of Mexican cloud forest trees. Cambridge: Fauna and Flora International.
Grauel, W., & Putz, F. (2004). Effects of lianas on growth and regeneration of Prioria copaifera in Darien, Panama. Forest Ecology and Management, 190, 99–108.
Guariguata, M. R., & Saenz, G. P. (2002). Post-logging acorn production and oak regeneration in a tropical montane forest, Costa Rica. Forest Ecology and Management, 67, 285–293.
Hamilton, L. S., Juvik, J. O., & Scatena, F. N. (1995). Tropical montane cloud forest, ecological studies. New York: Springer.
Hunt, R. (1982). Plant growth curves: the functional approach to plant growth analysis. London: Edward Arnold.
ITTO (International Tropical Timber Organization). (2002). ITTO Guidelines for the restoration, management and rehabilitation of degraded and secondary tropical forests. Yokohama, Japan: ITTO Policy Development Series No. 13.
Körner, C. (2007). The use of ‘altitude’ in ecological research. Trends in Ecology and Evolution, 22, 569–574.
Lamb, D., Erskine, P. D., & Parrotta, J. A. (2005). Restoration of degraded tropical forest landscapes. Science, 310, 1628–1632.
Lutz, D. A., Powell, R. L., & Silman, M. R. (2013). Four decades of Andean timberline migration and implications for biodiversity loss with climate change. Plos One, 8, e74496.
Martínez-Garza, C., & Howe, H. F. (2003). Restoring tropical diversity: beating the time tax on species loss. Journal of Applied Ecology, 40, 423–429.
Millet, J., Tran, N., Vien-Ngoc, N., Tran-Thi, T., & Prat, D. (2013). Enrichment planting of native species for biodiversity conservation in a logged tree plantation in Vietnam. New Forest, 44, 369–383.
Montes-Hernández, B., & López-Barrera, F. (2013). Seedling establishment of Quercus insignis: a critically endangered oak tree species in southern Mexico. Forest Ecology and Management, 310, 927–934.
Mulligan, M. (2011). Modelling the tropics-wide extent and distribution of cloud forest and cloud forest loss, with implications for conservation priority. In L. A. Bruijnzeel, F. N. Scatena, & Hamilton, L. S. (Eds.), Tropical montane cloud forests: science for conservation and management (pp. 14–38). Cambridge: Cambridge University Press.
Muñiz-Castro, M., Williams-Linera, G., & Benítez-Malvido, J. (2015). Restoring montane cloud forest: establishment of three Fagaceae species in the old fields of central Veracruz, Mexico. Restoration Ecology, 23, 26–33.
Ortiz-Colín, P., Toledo-Aceves, T., López-Barrera, F., & Gerez-Fernández, P. (2017). Can traditional selective logging secure tree regeneration in cloud forest? iForest, 10, 369–375.
Paquette, A., Bouchard, A., & Cogliastro, A. (2006). Survival and growth of under-planted trees: a meta-analysis across four biomes. Ecological Applications, 16, 1575–1589.
Paré, O. L., & Gerez, P. (2012) Al filo del agua: cogestión de la subcuenca del río Pixquiac, Veracruz. México D.F.: Juan Pablos Editores.
Pedraza, R. A., & Williams-Linera, G. 2003. Evaluation of native tree species for the rehabilitation of deforested areas in a Mexican cloud forest. New Forests, 26, 83–99.
Pounds, J. A., Fogden, M. P. L., & Campbell, J. H. (1999). Biological response to climate change on a tropical mountain. Nature, 398, 611–615.
Ramírez-Bamonde, E. S., Sánchez-Velásquez, L. R., & Andrade-Torres, A. (2005). Seedling survival and growth of three species of mountain cloud forest in Mexico, under different canopy treatments. New Forest, 30, 95–101.
Ramírez-Marcial, N. (2003). Survival and growth of tree seedlings in anthropogenically disturbed Mexican montane rain forest. Journal of Vegetation Science, 14, 881–890.
Ramírez-Marcial, N., Camacho-Cruz, A., González-Espinosa, M., & López-Barrera, F. (2006). Establishment, survival and growth of tree seedlings under successional montane oak forests in Chiapas, México. In M. Kappelle (Ed.), Ecology and conservation of Neotropical montane oak forests (pp. 177–189). Berlin: Springer-Verlag.
Ramírez-Marcial, N., González-Espinosa, M., Camacho-Cruz, A., & Ortiz-Aguilar, D. (2010). Forest restoration in Lagunas de Montebello National Park, Chiapas, México. Ecological Restoration, 28, 354–360.
Ramírez-Marcial, N., González-Espinosa, M., & Williams-Linera, G. (2001). Anthropogenic disturbance and tree diversity in montane rain forests in Chiapas, Mexico. Forest Ecology and Management, 154, 311–326.
Rappaport, D., & Montagnini, F. (2014). Tree species growth under a rubber (Hevea brasiliensis) plantation: native restoration via enrichment planting in southern Bahia, Brazil. New Forest, 45, 715–732.
Saenz, G. P., & Guariguata, M. R. (2001). Demographic response of tree juveniles to reduced-impact logging in a Costa Rican montane forest. Forest Ecology and Management, 140, 75–84.
Simoes-Macayo, N., & Renison, D. (2015) ¿Cuántos años monitorear el éxito de plantaciones con fines de restauración? Análisis en relación al micrositio y procedencia de las semillas. Bosque, 36, 315–322.
Solomon, S. (2007). Climate change 2007-the physical science basis: working group I contribution to the fourth assessment report of the IPCC, (Vol. 4). Cambridge: Cambridge University Press.
Stoyan, H., De-Polli, H., Böhm, S., Robertson, G. P., & Paul, E. A. (2000). Spatial heterogeneity of soil respiration and related properties at the plant scale. Plant and Soil, 222, 203–214.
Toledo-Aceves, T., López-Barrera, F., & Vásquez-Reyes, V. (2017). Preliminary analysis of functional traits in cloud forest tree seedlings. Trees, 31, 1253–1262.
Toledo-Aceves, T., Meave, J. A., González-Espinoza, M., & Ramírez-Marcial, N. (2011). Tropical montane cloud forests: current threats and opportunities for their conservation and sustainable management in Mexico. Journal of Environmental Management, 92, 974–981.
Toledo-Garibaldi, M., & Williams-Linera, G. (2014). Tree diversity patterns in successive vegetation types along an elevation gradient in the Mountains of Eastern Mexico. Ecological Restoration, 29, 1097–1104.
van Breugel, M., Hall, J. S., Craven, D. J., Gregoire, T. G., Park, A., Dent, D. H. et al. (2011). Early growth and survival of 49 tropical tree species across sites differing in soil fertility and rainfall in Panama. Forest Ecology and Management, 261, 1580–1589.
Vázquez, J. A., & Givnish, T. J. (1998). Altitudinal gradients in tropical forest composition, structure, and diversity in the Sierra de Manantlán. Journal of Ecology, 86, 999–1020.
Vidriales-Chan, G., García-Coll, I., Martínez, A., Gerez, P., & Muñiz-Castro, M. (2012). Características del medio natural. In L. Paré y P. Gerez (Eds.), Al filo del agua (pp. 75–134). México D.F.: Juan Pablos Editores.
Williams-Linera, G. (2002). Tree species richness complementary, disturbance and fragmentation in a Mexican tropical montane cloud forest. Biodiversity Conservation, 11, 1825–1843.
Williams-Linera, G., López-Barrera, F., & Bonilla-Moheno, M. (2015). Estableciendo la línea de base para la restauración del bosque de niebla en un paisaje periurbano. Madera y Bosques, 21, 89–101.
Williams-Linera, G., Toledo-Garibaldi, M., & Hernández, C. G. (2013). How heterogeneous are the cloud forest communities in the mountains of central Veracruz, Mexico? Plant Ecology, 214, 685–701.
Williams-Linera, G., & Vizcaíno-Bravo, Q. (2016). Cloud forests on rock outcrop and volcanic soil differ in indicator tree species in Veracruz, Mexico. Revista Mexicana de Biodiversidad, 87, 1265–1274.
Wright, S. J., Muller-Landau, H., Condit, R., & Hubbell, S. P. (2003). Gap-dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology, 84, 3174–3185.



