Fadia Sara Ceccarelli *, Luz Abril Garduño-Villaseñor, Luis Carlos Hernández-Salgado
Centro de Investigación Científica y de Educación Superior de Ensenada-Cátedras Conacyt, Departamento de Biología de la Conservación, Carr. Tijuana-Ensenada 3918, Zona Playitas, 22860 Ensenada, Baja California, Mexico
*Corresponding author: ceccarelli@cicese.mx (F.S. Ceccarelli)
Received: 6 June 2022; accepted: 13 September 2022
Abstract
The northwestern Mexican Baja California state is an ecologically unique area being the only part of the country with a Mediterranean ecosystem and high species diversity and endemism for many groups. Studies on the biodiversity of the region are scarce, especially regarding ecologically important groups such as terrestrial arthropods. Even scarcer are studies on another important aspect of diversity, namely genetic diversity. Due to the paucity of information, the genetic diversity and population histories of the 2 most abundant species of Phidippus (Araneae: Salticidae) of the region are presented, based on nuclear and mitochondrial DNA data. Phidippus johnsoni, the more widespread of the 2, belongs to 1 large, panmictic population in Baja California, while P. phoenix was divided into 2 populations. Both species have high genetic diversity and estimated large population sizes, which increased during the last 150,000 years for P. johnsoni, and the last 30,000 years, after the Last Glacial Maximum for P. phoenix. This study, therefore, reinforces the importance of this area, not only for its species diversity but also for the genetic diversity found within the populations, which reflects ecological stability during the Pleistocene, allowing for the accumulation of genetic diversity through time.
Keywords: Demography; Pleistocene; Baja California; Phidippus johnsoni; Phidippus phoenix
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Diversidad genética e historias poblacionales de dos especies de Phidippus (Araneae: Salticidae) del noroeste de México
Resumen
Baja California es un área importante ecológicamente siendo la única zona del país con un ambiente mediterráneo y cuenta con una alta diversidad de especies y endemismos. Los estudios de biodiversidad y diversidad genética en esta región de México son escasos, sobre todo para grupos ecológicamente importantes como los artrópodos terrestres. Debido a la escasez de información, se utilizaron datos moleculares para obtener la diversidad genética y las historias poblacionales de las 2 especies de Phidippus (Araneae:Salticidae) más abundantes de la región. La especie más ampliamente distribuida es Phidippus johnsoni y se encontró que pertenece a una gran población panmíctica en Baja California, mientras que P. phoenix se divide en 2 poblaciones. Ambas especies presentaron una alta diversidad genética y grandes tamaños poblacionales, los cuales aumentaron durante los últimos 150,000 años para P. johnsoni y los últimos 30,000 años, después del último Máximo Glacial, para P. phoneix. Este estudio refuerza la importancia de esta región, no solo por su diversidad de especies, sino también por la diversidad genética que se encuentra dentro de las poblaciones, la cual se ha acumulado desde el Pleistoceno, gracias a la relativa estabilidad ecológica de la zona durante ese periodo.
Palabras clave: Demografía; Pleistoceno; Baja California; Phidippus johnsoni; Phidippus phoenix
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Despite its status as a megadiverse country (Myers et al., 2000; Sarukhan, 2008), there is a paucity of studies for many taxa in large areas of Mexico. One such region is the northwestern state of Baja California, which hosts ecologically interesting habitats such as the Sonoran Desert and the California Floristic Province, the latter of which spans a large area of California in the USA, and a smaller area of Baja California (Burge et al., 2016; González-Abraham et al., 2010). For Mexico, this is a unique area, being the only part of the country with a Mediterranean climate and several endemic species of flora and fauna (Riddle et al., 2000; Riemann & Exequiel, 2007). In fact, Morrone and Márquez (2008) marked “California Norte” in the state of Baja California as one of 15 panbiogeographical nodes in Mexico, representing areas of high diversity that should receive priority for conservation. While the flora of the region has been well documented (Riemann & Exequiel, 2007; Wiggins, 1980), only a tiny fraction of terrestrial arthropods containing ecologically crucial taxa have been thoroughly studied (Clark & Sankey, 1999; Due & Polis, 1986). For spiders, to date, there are about 414 described species for the whole Baja California peninsula (Jiménez et al., 2018), a number certainly due to increase with further studies (Hernández-Salgado et al., 2022). Furthermore, a track-analysis found that the Baja California peninsula hosts 3 of 9 generalized tracks for Mexico based on agelenid spiders (Maya-Morales et al., 2018).
The spider family Salticidae (also known as jumping spiders) has more than 6,000 described species worldwide (World Spider Catalog, 2022). In addition to being species-rich and diverse, salticids have been model organisms for studies on phylogenetics and evolution (Bodner & Maddison, 2012; Hill & Richman, 2009; Maddison et al., 2017; Penney, 2010), ecology (Maldonado-Carrizales & Ponce-Saavedra, 2017; Rubio et al., 2019) and behavior (Ceccarelli, 2008; Jackson & Pollard, 1996; Lim & Li, 2006), given their varied and at times highly specialized life strategies. A North American genus of jumping spiders, Phidippus has attracted particular attention due to the relatively large size of the species, conspicuous colors, and diversity, counting to date 61 described and valid species for North America, of which 42 are found in Mexico (Edwards, 2004, 2020; Hernández-Salgado et al., 2022).
Based on recent studies of the genus Phidippus diversity in Baja California, at least 11 species are found in the peninsula, of which 4 are new records and at least one undescribed species (Hernández-Salgado et al., 2022; Guerrero-Fuentes, pers. comm.). Most of the peninsula’s diversity regarding Phidippus is concentrated in the northern state of Baja California. Considering the whole geographic range of Phidippus, from Alaska to Costa Rica, it is remarkable that an approximate 17% of species can be found in an area the size of Baja California. In addition to species richness, an important dimension of diversity is genetic diversity since it gives an idea of the uniqueness and viability of a species throughout its distributional range (Vellend & Geber, 2005). In population genetics, genetic diversity is assessed through metrics such as haplotype diversity and population structure, while studying demographic processes provides an overview of past events such as historical gene flow and population size changes (Hamilton, 2021).

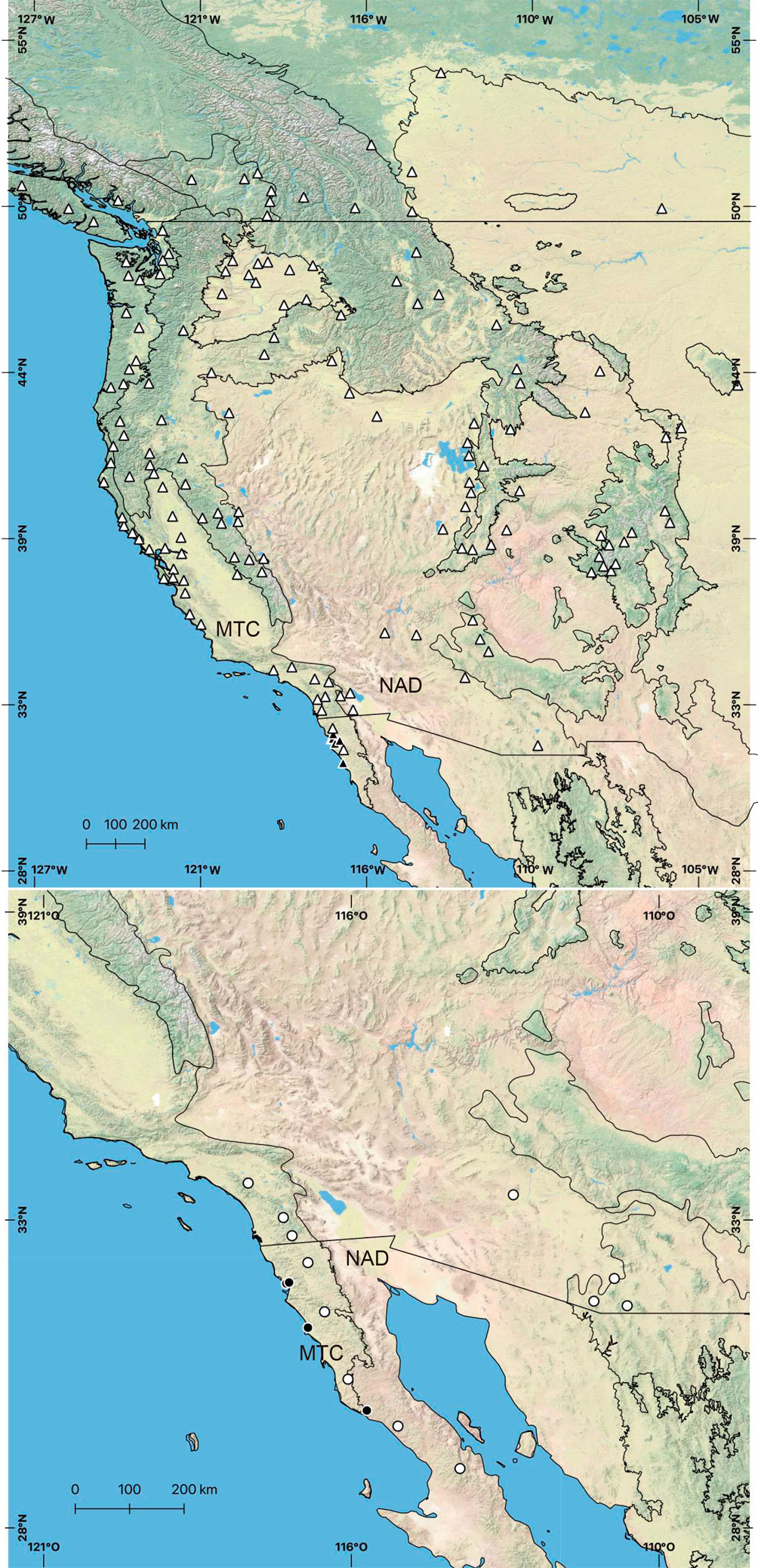
Hernández-Salgado et al. (2022) found that 2 of the most abundant Phidippus species from northwestern Mexico were Phidippus johnsoni (G. W. Peckham & E. G. Peckham, 1883) and Phidippus phoenix Edwards, 2004 (Fig. 1). The first species, P. johnsoni, has been a model organism for a range of studies, such as its life history (Jackson, 1978), nest structure (Jackson, 1979), the structural origin of their cheliceral iridescence (Ingram et al., 2011), and a range of behavioral studies on predatory and reproductive behavior and strategies (Jackson, 1977a, b, c, 1980, 1981, 1986). Phidippus johnsoni is also described as being the species of its genus found in the most varieties of habitats, including beaches, alpine meadows, coniferous forest, chaparral, and coastal sage (Edwards, 2004). Less is known about the biology and evolution of P. phoenix, described as a “typical desert species”, found only in this habitat (Edwards, 2004). These 2 species are of a similar size, but, given their habitat preferences, they differ in the extent of their distributions (Edwards, 2004). While P. johnsoni is more widely distributed, ranging from western Canada throughout western U.S.A., with its southernmost distributional limit being in the Mediterranean region of northwestern Mexico, P. phoenix ranges from southwestern U.S.A. to the middle of the Baja California peninsula, which means that the Mediterranean region of Mexico is central to its distribution (Fig. 2).
Considering the biological and ecological differences between P. johnsoni and P. phoenix, in this study, the population structure and demographic histories of the 2 species’ populations from northwestern Mexico are compared using mitochondrial and nuclear sequence data. Thus, in addition to knowing the species richness and diversity of Phidippus in the region, a further dimension is added, namely the genetic diversity of 2 of the species of Phidippus in time and space.
Materials and methods
Phidippus johnsoni and P. phoenix individuals were collected during the day by hand between 2018 and 2020 in a variety of habitats, such as chaparral, desert, agro-ecosystems, and urban environments in the Mexican state of Baja California (Fig. 2; Supplementary material, Table S1). The individuals were stored in vials with ethanol 96% at -20 °C until they were used for DNA extraction. All individuals used in this study had been identified in a previous study by morphological examination of the male and female genitalia and genetic analysis based on a fragment of the mitochondrial Cytochrome Oxidase CI (COI) gene and deposited in the arachnid collection of the Museo de Artrópodos de Baja California (for further information, please see Hernández-Salgado et al., 2022).
Whole genomic DNA was extracted from the legs and prosoma using Qiagen’s DNeasy Blood & Tissue Kit, following the manufacturers protocol. Subsequently, Polymerase Chain Reactions were carried out in a mix containing 5 ul 5x GoTaq Flexi Buffer, 1 mM MgCl2, 1.25 units of GoTaq DNA Polymerase (Promega), 0.2 mM of each dNTP, 0.5 uM of each primer, 3ul DNA and nuclease-free water to a final volume of 25 ul. The primers used to amplify a 268 base-pair fragment of the nuclear gene fragment Histone 3a (H3a) were H3a_F (3’-ATG GCT CGT ACC AAG CAG ACV GC-5’) and H3a_R (3’-ATA TCC TTR GGC ATR ATR GTG AC-5’) (Colgan et al., 1998). The thermal cycling protocol consisted of an initial denaturing step of 95 °C for 5 minutes, followed by 35 cycles of 95 °C for 30 seconds, 51 °C for 45 seconds, and 72 °C for 1 min, with a final extension step of 72 °C for 10 minutes. PCR products were checked in a 1.5% agarose gel by electrophoresis and sent for purification and bi-directional sanger sequencing to Macrogen Inc., Korea. Additionally, for the same individuals of P. johnsoni and P. phoenix, nucleotide sequences of a 658 base-pair fragment of the initial portion of the COI mitochondrial gene were obtained from a previous study (Hernández-Salgado et al., 2022).
Table 1
Mean LnP(K), (SD), ∆K calculated through Structure and output by Structure Harvester using K = 1-6. The results shown are from markers analyzed independently and combined. The numbers in bold typeface show the value of K with the highest likelihood.
| K | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Phidippus phoenix | H3 | LnP(K) | -488.23 | -361.3 | -293.6 | -298.5 | -288 | -293.23 |
| Stdev LnP(K) | 0.35 | 0.17 | 11.62 | 24.25 | 1.73 | 3.6 | ||
| DeltaK | – | 341.98 | 6.24 | 0.63 | 9.06 | – | ||
| COI | LnP(K) | -174.63 | -87.46 | -97.3 | -107.3 | -108.86 | -115.8 | |
| Stdev LnP(K) | 0.057 | 0.47 | 1.249 | 3.13 | 2.96 | 6.27 | ||
| DeltaK | – | 205.25 | 0.13 | 2.69 | 1.8 | – | ||
| Both | LnP(K) | -663.16 | -530.33 | -468.53 | -449.5 | -480.26 | -520.23 | |
| Stdev LnP(K) | 0.7 | 1.6 | 2.3 | 9.18 | 22.05 | 21.99 | ||
| DeltaK | – | 44.28 | 18.57 | 5.42 | 0.41 | – | ||
| Phidippus johnsoni | H3 | LnP(K) | -1614.16 | -1665.03 | -1627.9 | -1806.1 | -1667.8 | -1657.66 |
| Stdev LnP(K) | 0.3 | 49.75 | 22.68 | 209.97 | 91.1 | 52.96 | ||
| DeltaK | – | 1.76 | 9.49 | 1.5 | 1.4 | – | ||
| COI | LnP(K) | -1995.13 | -1208.966 | -1107.26 | -1036.73 | -1029.1 | -1112.3 | |
| Stdev LnP(K) | 0.15 | 17.12 | 6.67 | 53.48 | 176.42 | 356.89 | ||
| DeltaK | 39.96 | 4.67 | 1.17 | 0.51 | – | |||
| Both | LnP(K) | -1995.23 | -1897.9 | -2040.43 | -2027.53 | -2094.5 | -3191.16 | |
| Stdev LnP(K) | 0.92 | 0.9 | 42.88 | 115.43 | 212.83 | 1922.64 | ||
| DeltaK | – | 266.51 | 3.62 | 0.69 | 4.83 | – |
Table 2
Characteristics of the mitochondrial (COI) and nuclear (H3a) gene fragments for Baja California’s Phidippus johnsoni and Phidippus phoenix, including the 2 inferred populations for the latter
| Data matrix | N taxa | N hap | H | N sites | π | θ |
| P. johnsoni COI | 46 | 19 | 0.877 | 658 | 0.00872 | 0.01198 |
| P. phoenix COI | 34 | 14 | 0.859 | 658 | 0.00599 | 0.00849 |
| P. phoenix north COI | 12 | 5 | 0.576 | 658 | 0.00351 | 0.00512 |
| P. phoenix south COI | 22 | 11 | 0.844 | 658 | 0.00321 | 0.00755 |
| P. johnsoni H3a | 39 | 72 | 0.986 | 268 | 0.03435 | 0.01466 |
| P. phoenix H3a | 32 | 8 | 0.784 | 268 | 0.01758 | 0.01184 |
| P. phoenix north H3a | 13 | 7 | 0.840 | 268 | 0.02522 | 0.01467 |
| P. phoenix south H3a | 19 | 5 | 0.706 | 268 | 0.00982 | 0.00622 |
N taxa = Number of individual sequences, N hap = number of haplotypes, H = haplotype diversity, N sites = number of nucleotides in the sequence, π = nucleotide diversity, θ = Watterson’s parameter.
The nuclear H3a sequences were edited in Sequencher v. 4.1.4., examining the chromatograms carefully by eye to detect double-peaks and assigning base calls based on the IUPAC ambiguity code. Alignments were created for each species and for each gene fragment separately (658 base-pairs for COI and 268 base-pairs for H3a), using the MAFFT online server with default settings (Katoh et al., 2019).
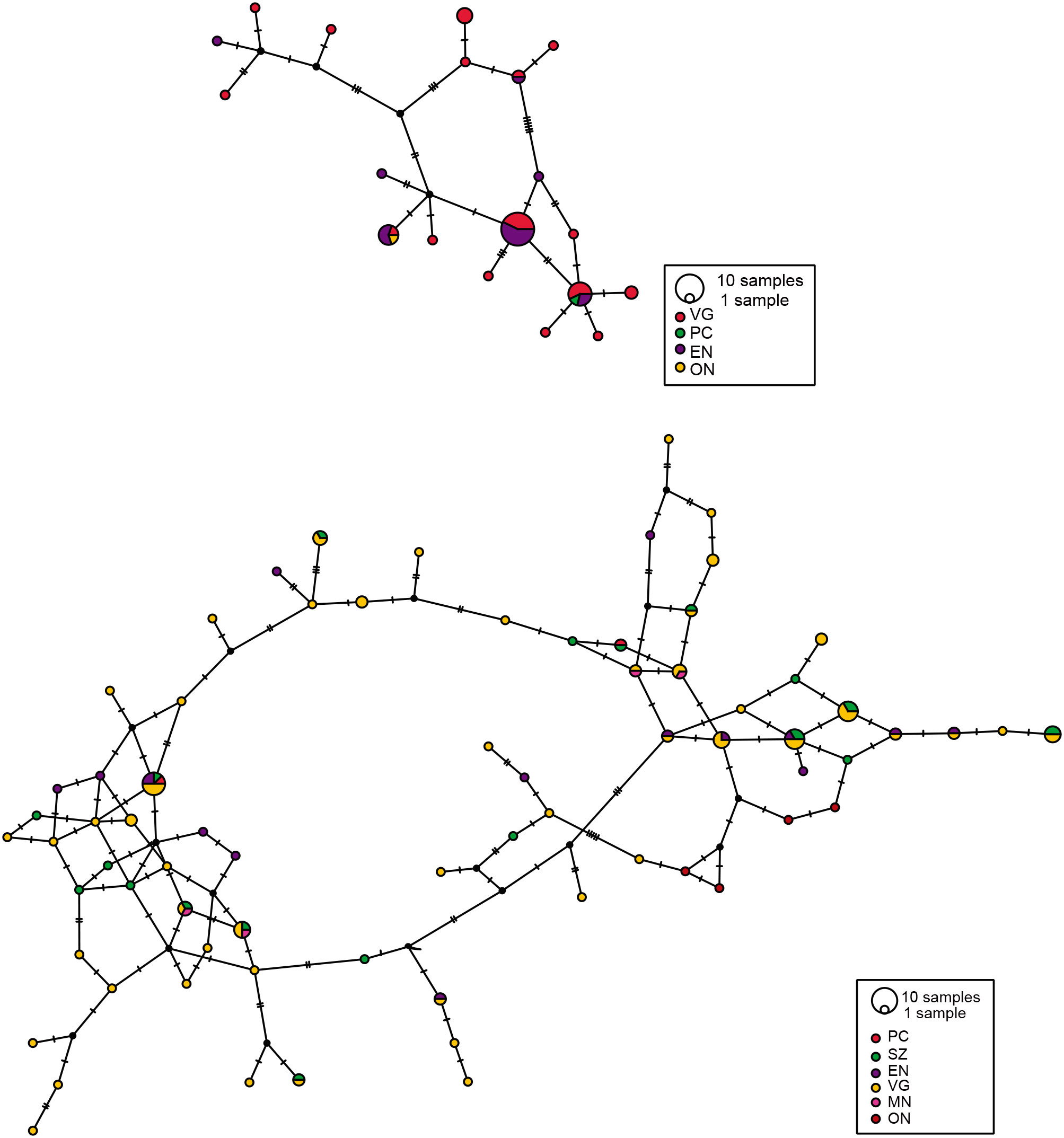
The H3a alignments with ambiguous base calls were loaded into DnaSP v. 6.12.03 (Rozas et al., 2017), and the PHASE algorithm was applied with 10,000 iterations and assuming recombination to obtain the most likely sequences (haplotypes) for all the alleles present in the samples for each species, P. johnsoni, and P. phoenix (Stephens & Donnelly, 2003). Haplotype networks were then obtained in the program PopART (Leigh & Bryant, 2015) under the TCS algorithm for each species and marker (COI and H3a) separately (Clement et al., 2002), to visualize the geographical distribution of the haplotypes and evaluate whether there was overall genetic structuring between the sampled localities. Since none of the third codon positions in the matrices were highly variable, they were included in the analyses. Furthermore, being aware that possible recombination events in nuclear markers will skew analyses such as haplotype networks, in this study the H3a marker was still included, to evaluate the difference between mitochondrial and nuclear data sources.

Population structure and demographic histories
The number of P. johnsoni and P. phoenix populations was estimated using the COI and H3a haplotype matrices. The nuclear and mitochondrial markers were analyzed separately and combined through Structure (Pritchard et al., 2000), each with 3 runs of 50,000 generations of Markov Chain Monte Carlo (mcmc) simulations with a burn-in of 5,000. The admixture and the correlated population allele frequencies models were utilized. The input format for combined analysis was phased diploid data, while the second allele for haploid data was marked as missing data. For the approximation K (number of populations), the method by Evanno et al. (2005) was used to calculate ∆K through Structure Harvester (Earl & vonHoldt, 2012).
To get an overview of the diversity present both for P. johnsoni and P. phoenix, the COI and H3a haplotype files for the inferred populations of each species were analyzed in DnaSP, calculating the haplotype diversity (H), nucleotide diversity (π; Nei, 1987), and the Watterson parameter (θ; Watterson, 1975). Additionally, the Fst value (Hudson et al., 1992) for P. phoenix’s populations was calculated to evaluate gene flow between them and their structuring.
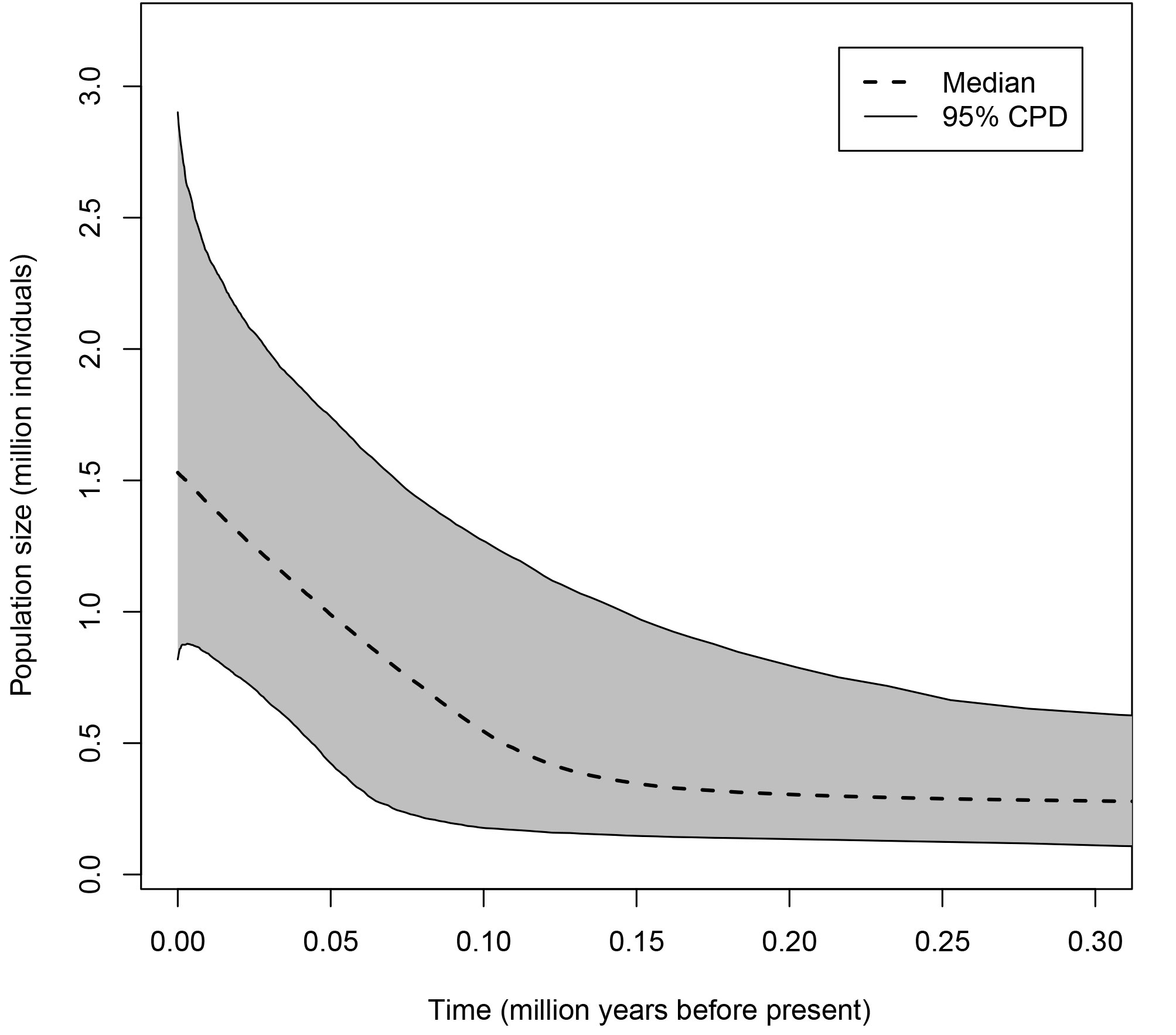
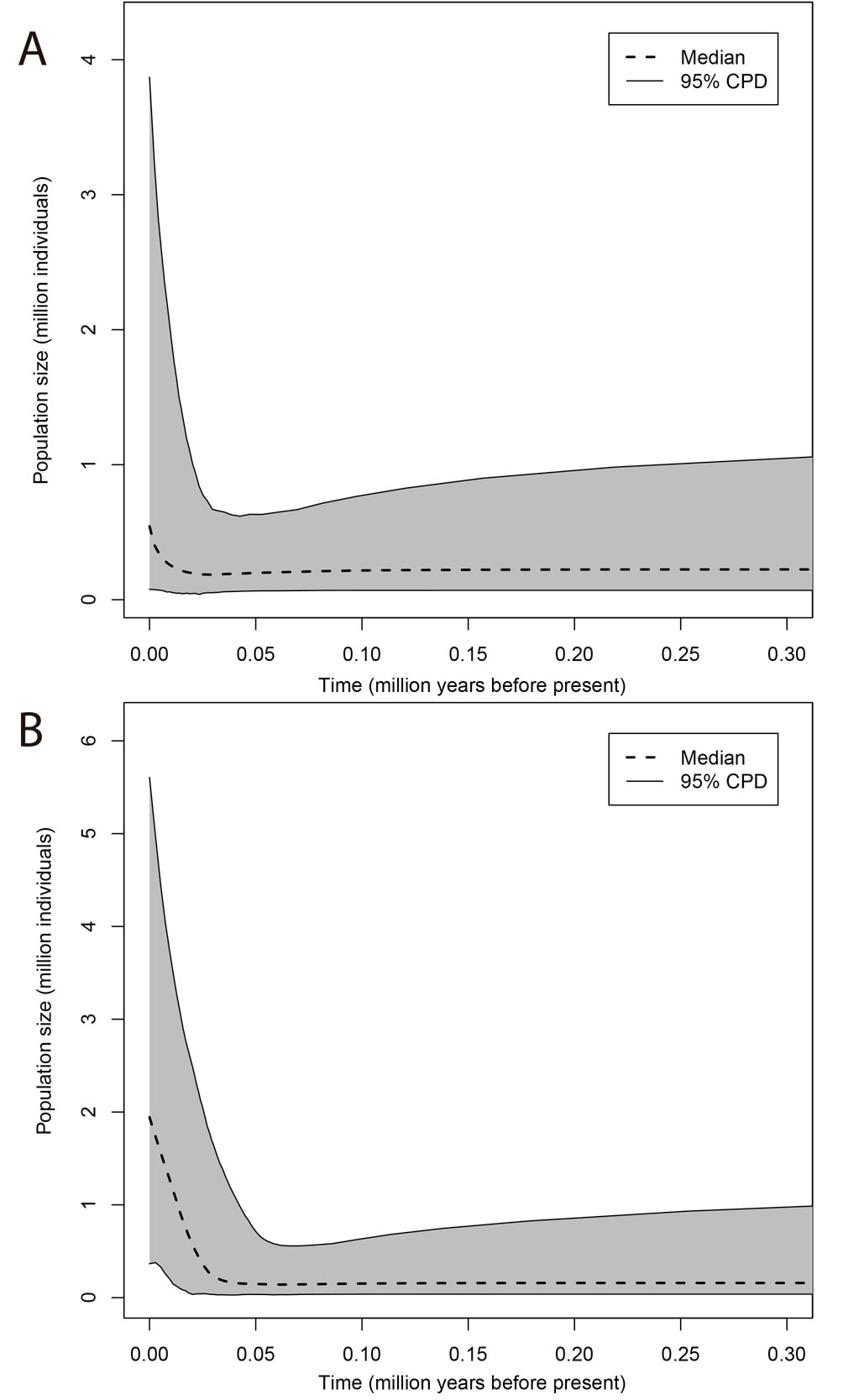
For each population, Extended Bayesian Skyline Plots (EBSPs; Heled & Drummond, 2008) were produced in BEAST 2.6 (Bouckaert et al., 2014). EBSP provide historical information on population size changes and estimate the current population sizes. For the analyses, linear EBSP coalescent tree priors and uncorrelated relaxed lognormal clock priors were selected, setting a clock rate of 0.0225 substitutions/site/million years for the COI fragment and HKY substitution models for both COI and H3a fragments. The mcmc simulations were run for 50 million generations, sampling every
5,000th generation. The runs were then checked in Tracer v. 1.7.1 (Rambaut et al., 2018), and EBSP plots were drawn using the plotEBSP.R script (available online at: https://www.beast2.org/tutorials/) in R version 4.2.0 (R core team, 2022).
Most algorithms used for inferring demographic histories of individuals and populations do not consider recombination in nuclear sequences. To check for recombination in the nuclear fragment used here, the recombination parameter R was calculated in DnaSP for each H3a haplotype matrix to estimate the minimum number of recombination events (Hudson, 1987). Additionally, to infer demographic histories that take nuclear recombination into account, Ancestral Recombination Graphs (ARGs) were built using the Kwarg package (Ignatieva et al., 2021), which implements a parsimony-based approach to estimate the number of recombination events that explain the history of a given data set. Kwarg was run on the P. johnsoni and P. phoenix H3a haplotype matrices, using the default parameters. Since KWARG does not estimate the number of generations between the trees’ nodes, ARGweaver (Hubisz et al., 2020) was used, which implements an mcmc algorithm. The mcmc simulations for each species were run for 2,000 iterations, sampling every 10th generation. The priors for population size and mutation rate were based on the outcomes of the EBSP analyses, set as 500,000 and 6.20e-08 substitutions/site for P. johnsoni and 500,000 and 1.04e-02 substitutions/site for P. phoenix, respectively.
Results
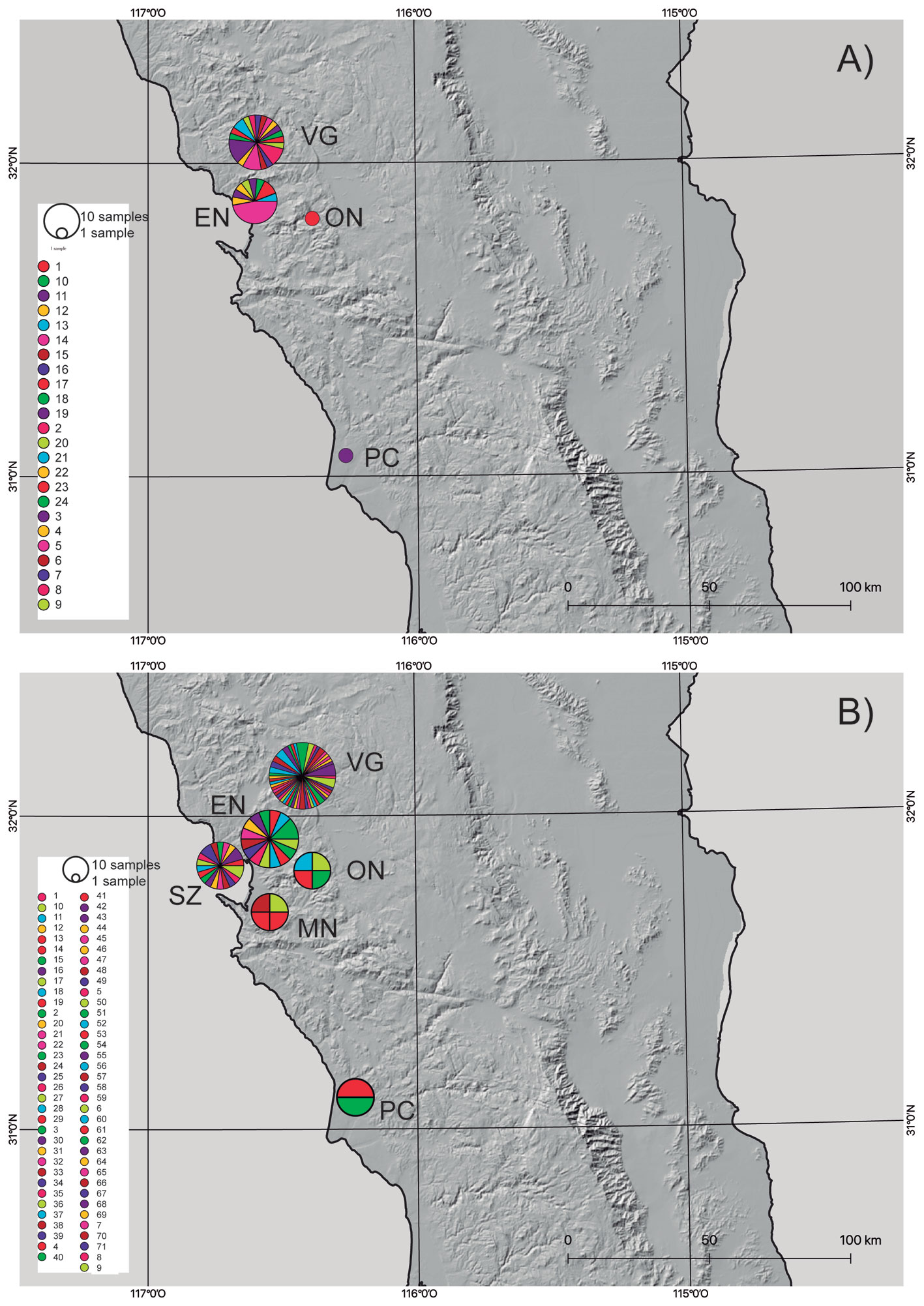
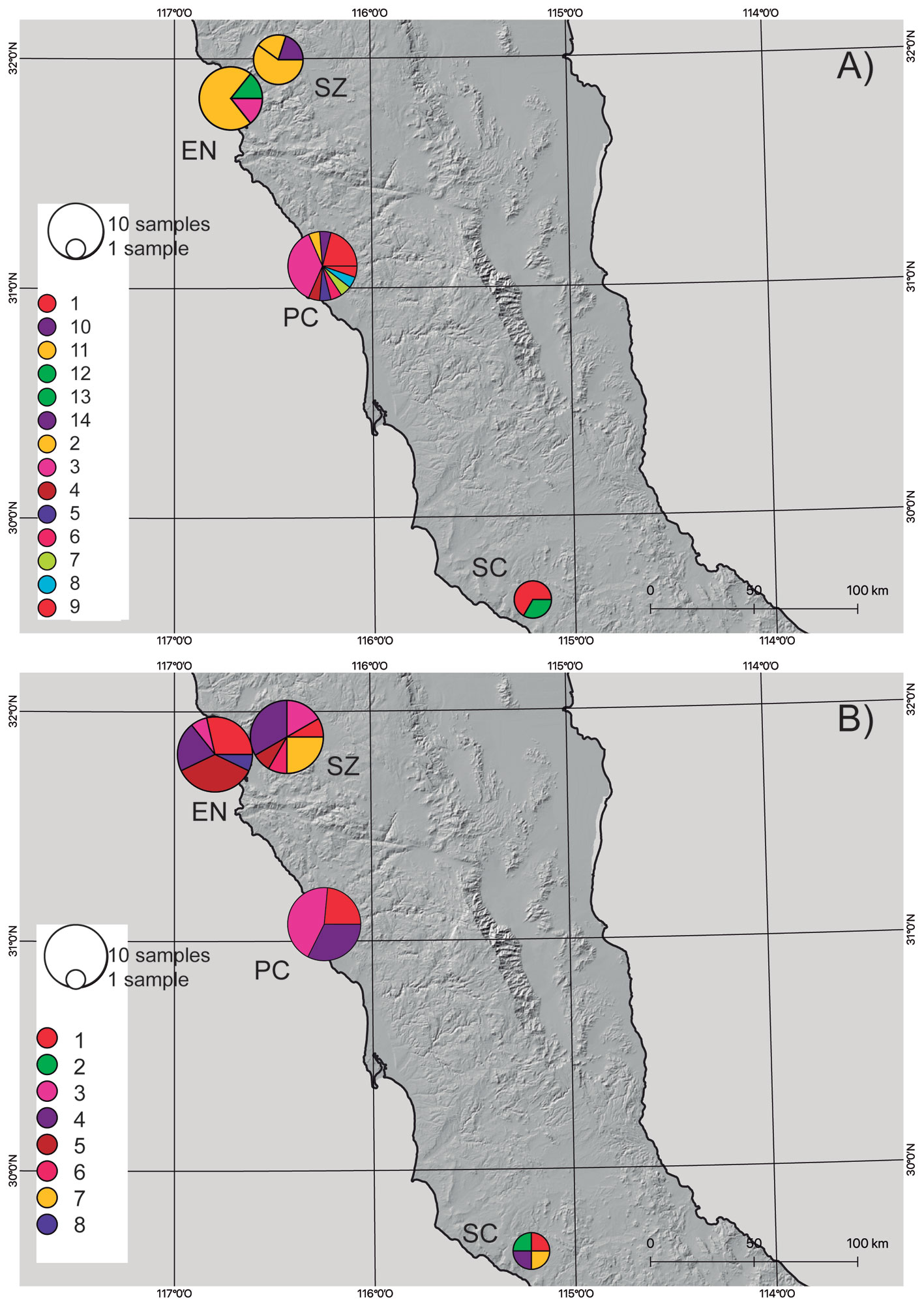
Based on the results from the mcmc runs in structure combining the mitochondrial and nuclear markers, P. johnsoni and P. phoenix comprise 2 populations each (Table 1; Supplementary material: Figs. S1, S2). The haplotype networks of P. johnsoni (Fig. 3) lack structure and generally show between 1 and 3 mutations from one haplotype to the other. For P. phoenix, more mutations are found between the general Ensenada/El Sauzal population than the rest, but only visible in the mitochondrial haplotype network (Fig. 4). Comparing the outcomes of the structure runs to the haplotype networks and the geographical congruence (Figs. 5, 6), the 2 hypothetical populations of P. johnsoni will be considered as one (note: structure does not allow for one single population as an output), and P. phoenix as 2 populations: a “northern” population which includes the localities in and around Ensenada and a “southern” population with individuals from Punta Colonet and Santa Catarina.
The general characteristics of the DNA sequence data used in this study, considering the inferred populations, can be found in table 2, where the highest haplotype and nucleotide diversity was found in the H3a marker of P. johnsoni. Comparatively, the diversity values for COI were also higher in P. johnsoni than for P. phoenix. For P. phoenix, COI haplotype diversity was higher in the southern population, whereas COI nucleotide —H3a haplotype— and H3a nucleotide diversity were higher in the northern populations. For the northern versus southern populations of P. phoenix, the Fst values were 0.149 and 0.571, based on the H3a and the COI gene fragments, respectively.
Based on the EBSP plots of the single P. johnsoni population and the 2 P. phoenix populations from this study (Figs. 7, 8), the estimated current population sizes (assuming a generation time of 1 year) are 1.5 million for P. johnsoni, ca. 500,000 for P. phoenix’ northern- and 2 million for P. phoenix’ southern population. All 3 populations are shown to have increased in size, with the population size increase starting around 150,000 years ago (kya) for P. johnsoni, 20 kya for P. phoenix’ northern and 30 kya for P. phoenix’ southern population.
The recombination parameter (R) calculated for the H3a fragment of P. johnsoni was 13.7, with an estimated Theta (recombination frequency) value of 9.21 and the minimum number of recombination events (Rm; Hudson & Kaplan, 1985) being 11. For P. phoenix, R = 0.001, Theta = 4.71 and Rm = 1. On the other hand, based on the KWARG analysis of the H3a matrices, there were 135 recurrent mutations and 27 recombination events for the P. johnsoni dataset and 2 recurrent mutations and 4 recombination events for P. phoenix. Considering these possible recombination events, the ancestral recombination graphs obtained from ARGweaver (Supplementary material, Figs. S3, S4) show again that there is no clear structuring by locality based on this nuclear marker for either species. The analyses estimated the average population size of P. johnsoni to be between 328,000 and 342,000, with an approximate time to the most recent common ancestor (tmrca) of 134,000 years, while for P. phoenix, the average size of both populations combined was between 249,000 and 267,000 and the tmrca ca. 171,000 years.
Discussion
Northwestern Mexico harbors unique and high biological diversity for several taxa, an example being the jumping spider genus Phidippus, for which at least 10 of its 61 species can be found in the state of Baja California. Additionally, from this study, it is clear that the area’s 2 most abundant species, P. johnsoni and P. phoenix, also have high genetic diversity. Although studies on genetic diversity of spiders from this region are scarce, another spider species, Pardosa sierra Banks, 1898 was found to have higher diversity in Baja California compared to its southern distribution (González-Trujillo et al., 2016). The link between species diversity and genetic diversity in an area is often ignored, as the former is measured as part of community ecology studies, while the latter is a component of population genetics. However, in many studies, the 2 measures were found to go together, be it because an area’s geo-climatic characteristics can promote both high species and genetic diversity in groups of organisms or because there is a direct correlation between the 2 measures, with one influencing the other (Vellend & Geber, 2005). This study adds another example to support the link between high species and genetic diversity, using Mexico’s Baja California state as the study area and Phidippus as the study taxon.
Although both Phidippus species in this study had high genetic diversity, it was markedly higher for P. johnsoni than for P. phoenix. Additionally, differences were found between the population structures and demographic histories of the 2 species in Baja California. For example, all P. johnsoni individuals from Baja California belonged to a single, panmictic large population, while P. phoenix seems to have structured populations in this same area, but the structuring was mainly found for the mitochondrial COI marker. This pattern of nuclear versus mitochondrial discordance in structuring may be due to their relative signal strength based on coalescence times being shorter in mitochondria, or it may reflect differences in male versus female-driven gene flow, often seen in species with sedentary females and more mobile males which move relatively large distances looking for females. In salticids, this strategy is common, as in other spiders, such as Homalonychus theologus Chamberlin, 1924 (Crews & Hedin, 2006). For P. johnsoni, the high recombination rate found in the H3a nuclear marker may have compromised the outcomes of certain analyses such as the haplotype networks, but can also be indicative of a large, panmictic population with a long, relatively stable demographic history (Hey & Wakeley, 1997).
Both species were also estimated to have large population sizes with hundreds of thousands of individuals, although there were discrepancies between the population sizes estimated with EBSP and ARGweaver, possibly due to the latter only using nuclear data and the former not taking recombination into account. The time to the most recent common ancestors in this area for each species was estimated around the late Pleistocene, while the population size increases were estimated to have started earlier for the P. johnsoni population than either of the P. phoenix populations. More specifically, the P. johnsoni population has a longer history than P. phoenix, starting from the late Pleistocene, approximately 150 kya during a glacial period, while the P. phoenix populations increased in size starting from 30-20 kya, corresponding to the Last Glacial Maximum (LGM). As P. johnsoni are found at higher altitudes and latitudes than P. phoenix, the former species is presumably more tolerant to colder climates (Edwards, 2004). This adaptation to the cold could explain why the LGM did not seem to have affected population sizes of P. johnsoni, as by 30 kya, they might have already been widespread and adapted to different climatic conditions. Pleistocene stability has also been found in several scorpion species of Baja California, whose distributions did not seem to be significantly affected by the LGM either (Graham et al., 2014).
The population genetic differences for these 2 Phidippus species with overlapping distributions in Baja California can be explained by their habitat preferences and distributional ranges. Phidippus johnsoni, being adapted to a wide range of habitats and thus found distributed in a much larger area, is likely to migrate more easily, thus contributing to high gene flow between localities. The relative vagility and distributional range, among other traits, have been found to influence genetic diversity in a range of other species (Meyer et al., 2009; Leffler et al., 2012; Levy et al., 2016). Additionally, the sampled P. johnsoni individuals came from the southernmost portion of the species’ entire distribution, so their population genetic parameters are unlikely to represent other P. johnsoni populations (Goldberg & Lande, 2007). On the other hand, P. phoenix, sampled from the central part of the species’ range, has traits more commonly seen in populations with narrower ecological niches (Lozier et al., 2011; Roderick et al., 2012). This work contributes to adding information on another important aspect of diversity —namely genetic diversity— to a biologically unique area while considering the species’ most important biological and ecological traits, thus providing a more holistic approach to studying biodiversity. To achieve a better understanding of the population dynamics of P. johnsoni and P. phoenix, more molecular data would be needed (Meirmans, 2015), as well as samples from the complete ranges of the species’ distributions.
Acknowledgments
To all those who contributed to this study with fieldwork and specimens, especially Eulogio López, Khutzy Munguía, Heriberto Murillo, Dariana Guerrero, Mario Salazar, Andrés Aquino, Diego de Pedro, Le Roy Sankey, Bryan Meza, Marshal Hedin, Bill and Mary Clark, Sergio González Piñuelas, Jorge Quintana, Ramiro Santos, Christian Baiza, Ana Alfaro and D. Parra. Special thanks also to Lita Castañeda Betancur for her help in the molecular laboratory and to Alejandro Valdez Mondragón and an anonymous reviewer for comments which greatly improved the manuscript. This work was funded by Fondo Sectorial de Investigación para la Educación SEP-Conacyt Investigación Científica Básica 2017–2018 grant A1-S-15134 to FSC; Jiji Foundation grant of the International Community Foundation awarded to FSC (award number 20180127) and Becas Nacionales Conacyt to LAGV and LCHS. Collections were carried out under the Semarnat permit SGPA/DGVS/11596/17.
References
Bodner, M. R., & Maddison, W. P. (2012). The biogeography and age of salticid spider radiations (Araneae: Salticidae). Molecular Phylogenetics and Evolution, 65, 213–240. https://doi.org/10.1016/j.ympev.2012.06.005
Bouckaert, R., Heled, J., Kühnert, D., Vaughan, T., Wu, C. H., Xie, D. et al. (2014). BEAST 2: a software platform for Bayesian evolutionary analysis. Plos Computational Biology, 10, e1003537. https://doi.org/10.1371/journal.pcbi.1003537
Burge, D. O., Thorne, J. H., Harrison, S. P., O’Brien, B. C., Rebman, J. P., Shevock, J. R. et al. (2016). Plant diversity and endemism in the California Floristic Province. Madroño, 63, 3-206. https://doi.org/10.3120/madr-63-02-3-206.1
Ceccarelli, F. S. (2008). Behavioral mimicry in Myrmarachne species (Araneae, Salticidae) from North Queensland, Australia. The Journal of Arachnology, 36, 344–351. https://doi.org/10.1636/CSt07-114.1
Clark, W. H., & Sankey, J. T. (1999). Late Holocene Sonoran Desert arthropod remains from a packrat midden, Catavina, Baja California Norte, Mexico. Pan Pacific Entomologist, 75, 183–199.
Clement, M., Snell, Q., Walker, P., Posada, D., & Crandall, K. (2002). TCS: Estimating gene genealogies. Parallel and Distributed Processing Symposium, International Proceedings, 2, 184.
Colgan, D. J., McLauchlan, A., Wilson, G. D. F., Livingston, S. P., Edgecombe, G. D., Macaranas, J. et al. (1998). Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Australian Journal of Zoology, 46, 419–437. https://doi.org/10.1071/ZO98048
Crews, S. C., & Hedin, M. (2006). Studies of morphological and molecular phylogenetic divergence in spiders (Araneae: Homalonychus) from the American southwest, including divergence along the Baja California Peninsula. Molecular Phylogenetics and Evolution, 38, 470–487. https://doi.org/10.1016/j.ympev.2005.11.010
Due, A. D., & Polis, G. A. (1986). Trends in scorpion diversity along the Baja California peninsula. The American Naturalist, 128, 460–468.
Earl, D. A., & VonHoldt, B. M. (2012). Structure Harvester: a website and program for visualizing Structure output and implementing the Evanno method. Conservation Genetics Resources, 4, 359–361. https://doi.org/10.1007/s12686-011-9548-7
Edwards, G. B. (2004). Revision of the jumping spiders of the genus Phidippus. Occasional Papers of the Florida State Collection of Arthropods, 11. Gainesville, Florida: The Center for Systematic Entomology.
Edwards, G. B. (2020). Description of Phidippus pacosauritus sp. nov. (Salticidae: Salticinae: Dendryphantini: Dendryphantina) with a reanalysis of related species in the Mystaceus Group. Peckhamia, 221.1, 1–18.
Evanno, G., Regnaut, S., & Goudet, J. (2005). Detecting the number of clusters of individuals using the software Structure: a simulation study. Molecular Ecology, 14, 2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Goldberg, E. E., & Lande, R. (2007). Species’ borders and dispersal barriers. The American Naturalist, 170, 297–304. https://doi.org/10.1086/518946
González-Abraham, C. E., Garcillán, P. P., Ezcurra, E., & Trabajo de Ecorregiones, G. de T. de E. (2010). Ecorregiones de la península de Baja California: una síntesis. Botanical Sciences, 87, 69. https://doi.org/10.17129/botsci.302
González-Trujillo, R., Correa-Ramírez, M. M., Ruiz-Sanchez, E., Salinas, E. M., Jiménez, M. L., & García-De León, F. J. (2016). Pleistocene refugia and their effects on the phylogeography and genetic structure of the wolf spider Pardosa sierra (Araneae: Lycosidae) on the Baja California Peninsula. Journal of Arachnology, 44, 367–379. https://doi.org/10.1636/R15-84.1
Graham, M. R., Bryson, Jr., R. W., & Riddle, B. R. (2014). Late Pleistocene to Holocene distributional stasis in scorpions along the Baja California peninsula. Biological Journal of the Linnean Society, 111, 450–461. https://doi.org/10.1111/bij.12216
Hamilton, M. B. (2021). Population Genetics. Hoboken, New Jersey: John Wiley & Sons.
Heled, J. & Drummond, A. J. (2008). Bayesian inference of population size history from multiple loci. BMC Evolutionary Biology, 8, 1–15. https://doi.org/10.1186/1471-2148-8-289
Hernández-Salgado, L. C., Guerrero-Fuentes, D. R., Garduño-Villaseñor, L. A., Castañeda-Betancur, L., López-Reyes, E., & Ceccarelli, F. S. (2022). New distributional records of Phidippus (Araneae: Salticidae) for Baja California and Mexico: an integrative approach. Diversity, 14, 159–172. https://doi.org/10.3390/d14030159
Hey, J., & Wakeley, J. (1997). A coalescent estimator of the population recombination rate. Genetics, 145, 833-46. https://doi.org/10.1093/genetics/145.3.833
Hill, D. E., & Richman, D. B. (2009). The evolution of jumping spiders (Araneae: Salticidae): a review. Peckhamia, 75, 1–7.
Hubisz, M., & Siepel, A. (2020). Inference of ancestral recombination graphs using ARGweaver. In J. Y. Dutheil (Ed), Statistical population genomics (pp. 231–266). New York: Humana. https://doi.org/10.1007/978-1-0716-0199-0_10
Hudson, R. R. (1987). Estimating the recombination parameter of a finite population model without selection. Genetics Research, 50, 245–250. https://doi.org/10.1017/S0016672300023776
Hudson, R. R., & Kaplan, N. L. (1985). Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics, 111, 147–164. https://doi.org/10.1093/genetics/111.1.147
Hudson, R. R., Slatkin, M., & Maddison, W. P. (1992). Estimation of levels of gene flow from DNA sequence data. Genetics, 132, 583–589. https://doi.org/10.1093/genetics/132.2.583
Ignatieva, A., Lyngsø, R. B., Jenkins, P. A., & Hein, J. (2021). KwARG: Parsimonious reconstruction of ancestral recombination graphs with recurrent mutation. Bioinformatics, 37, 3277–3284. https://doi.org/10.1093/bioinformatics/btab351
Ingram, A. L., Deparis, O., Boulenguez, J., Kennaway, G., Berthier, S., & Parker, A. R. (2011). Structural origin of the green iridescence on the chelicerae of the red-backed jumping spider, Phidippus johnsoni (Salticidae: Araneae). Arthropod Structure & Development, 40, 21–25. https://doi.org/10.1016/j.asd.2010.07.006
Jackson, R. R. (1977a). Prey of the jumping spider Phidippus johnsoni (Araneae: Salticidae). Journal of Arachnology, 5, 145–149.
Jackson, R. R. (1977b). Courtship versatility in the jumping spider, Phidippus johnsoni (Araneae: Salticidae). Animal Behaviour, 25, 953–957.
Jackson, R. R. (1977c). An analysis of alternative mating tactics of the jumping spider Phidippus johnsoni (Araneae, Salticidae). Journal of Arachnology, 5, 185–230.
Jackson, R. R. (1978). Life history of Phidippus johnsoni (Araneae, Salticidae). Journal of Arachnology, 6, 1–29.
Jackson, R. R. (1979). Nests of Phidippus johnsoni (Araneae, Salticidae): characteristics, pattern of occupation, and function. Journal of Arachnology, 7, 47–58.
Jackson, R. R. (1980). The mating strategy of Phidippus johnsoni (Araneae, Salticidae): II. Sperm competition and the function of copulation. Journal of Arachnology, 8, 217–240.
Jackson, R. R. (1981). Relationship between reproductive security and intersexual selection in a jumping spider Phidippus johnsoni (Araneae: Salticidae). Evolution, 35, 601–604.
Jackson, R. R. (1986). Use of pheromones by males of Phidippus johnsoni (Araneae, Salticidae) to detect subadult females that are about to molt. The Journal of Arachnology, 14, 137–139.
Jackson, R. R., & Pollard, S. D. (1996). Predatory behavior of jumping spiders. Annual Review of Entomology, 41, 287–308. https://doi.org/10.1146/annurev.en.41.010196.001443
Jiménez, M. L., Palacios-Cardiel, C., Maya-Morales, J., Berrian, J. E., & Ibarra-Núñez, G. (2018). Nuevos registros de arañas (Arachnida: Araneae) para la Región Del Cabo, península de Baja California, México. Acta Zoológica Mexicana, 34, 1–13. https://doi.org/10.21829/azm.2018.3412159
Katoh, K., Rozewicki, J., & Yamada, K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. https://doi.org/10.1093/bib/bbx108
Leffler, E. M., Bullaughey, K., Matute, D. R., Meyer, W. K., Segurel, L. & Venkat, A. (2012). Revisiting an old riddle: what determines genetic diversity levels within species? Plos Biology, 10, e1001388. https://doi.org/10.1371/journal.pbio.1001388
Leigh, J. W., & Bryant, D. (2015). PopART: Full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6, 1110–1116. https://doi.org/10.1111/2041-210X.12410
Levy, E., Byrne, M., Coates, D. J., Macdonald, B. M., McArthur, S., & van Leeuwen, S. (2016). Contrasting influences of geographic range and distribution of populations on patterns of genetic diversity in two sympatric Pilbara Acacias. Plos One, 11, e0163995. https://doi.org/10.1371/journal.pone.0163995
Lim, M. L., & Li, D. (2006). Behavioural evidence of UV sensitivity in jumping spiders (Araneae: Salticidae). Journal of Comparative Physiology A, 192, 871-878. https://doi.org/10.1007/s00359-006-0126-5
Lozier, J. D., Strange, J. P., Stewart, I. J., & Cameron, S. A. (2011). Patterns of range-wide genetic variation in six North American bumble bee (Apidae: Bombus) species. Molecular Ecology, 20, 4870–4888 https://doi.org/10.1111/j.1365-294X.2011.05314.x
Maddison, W. P., Evans, S. C., Hamilton, C. A., Bond, J. E., Lemmon, A. R., & Lemmon, E. M. (2017). A genome-wide phylogeny of jumping spiders (Araneae, Salticidae), using anchored hybrid enrichment. Zookeys, 695, 89. https://doi.org/10.3897/zookeys.695.13852
Maldonado-Carrizales, J., & Ponce-Saavedra, J. (2017). Arañas Saltarinas (Araneae: Salticidae) en dos sitios contrastantes en grado de antropización en Morelia Michoacán, México. Entomología Mexicana, 4, 597–603.
Maya-Morales, J., Jiménez, M. L., & Morrone, J. J. (2018). Track analysis of the funnel-web spiders (Araneae: Agelenidae) of Mexico. Revista Mexicana de Biodiversidad, 89, 530–540. https://doi.org/10.22201/ib.20078706e.2018.2.2367
Meirmans, P. G. (2015). Seven common mistakes in population genetics and how to avoid them. Molecular Ecology, 24, 3223–3231. https://doi.org/10.1111/mec.13243
Meyer, C. F., Kalko, E. K., & Kerth, G. (2009). Small-scale fragmentation effects on local genetic diversity in two Phyllostomid bats with different dispersal abilities in Panama. Biotropica, 41, 95–102. https://doi.org/10.1111/j.1744-7429.2008.00443.x
Morrone, J. J., & Márquez, J. (2008). Biodiversity of Mexican terrestrial arthropods (Arachnida and Hexapoda): a biogeographical puzzle. Acta Zoológica Mexicana, 24, 15–41. https://doi.org/10.21829/azm.2008.241613
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853-858. https://doi.org/10.1038/35002501
Nei, M. (1987). Molecular evolutionary genetics. New York: Columbia University Press.
Penney, D. (2010). The evolution of jumping spiders (Araneae: Salticidae): the palaeontological evidence. Peckhamia, 81, 1–3.
Pritchard, J., K., Stephens, M., Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155, 945–959. https://doi.org/10.1093/genetics/155.2.945
R core team (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032
Riddle, B. R., Hafner, D. J., Alexander, L. F., & Jaeger, J. R. (2000). Cryptic vicariance in the historical assembly of a Baja California Peninsular desert biota. Proceedings of the National Academy of Sciences, 97, 14438–14443. https://doi.org/10.1073/pnas.250413397
Riemann, H., & Exequiel, E. (2007). Endemic regions of the vascular flora of the peninsula of Baja California, Mexico. Journal of Vegetation Science, 18, 327–336. https://doi.org/10.1111/j.1654-1103.2007.tb02544.x
Roderick, G. K., Croucher, P. J., Vandergast, A. G., & Gillespie, R. G. (2012). Species differentiation on a dynamic landscape: shifts in metapopulation genetic structure using the chronology of the Hawaiian archipelago. Evolutionary Biology, 39, 192–206. https://doi.org/10.1007/s11692-012-9184-5
Rozas, J., Ferrer-Mata, A., Sánchez-del Barrio, J. C., Guirao-Rico, S., Librado, P., Ramos-Onsins, S. E. et al. (2017). DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution, 34, 3299–3302. https://doi.org/10.1093/molbev/msx248
Rubio, G. D., Stolar, C. E., Ohashi, D. V., & Baigorria, J. E. (2019). Jumping spiders (Araneae: Salticidae) in agroecosystems: a case study to know how friendly some crops can be for native fauna. Studies on Neotropical Fauna and Environment, 54, 133–148. https://doi.org/10.1080/01650521.2019.1629174
Sarukhan, J. (Ed.). (2008). Capital natural de México. Ciudad de México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio).
Stephens, M., & Donnelly, P. A. (2003). A comparison of Bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics, 73, 1162–1169. https://doi.org/10.1086/379378
Vellend, M., & Geber, M. A. (2005). Connections between species diversity and genetic diversity. Ecology Letters, 8, 767–781. https://doi.org/10.1111/j.1461-0248.2005.00775.x
Watterson, G. A. (1975). On the number of segregating sites in genetical models without recombination. Theoretical Population Biology, 7, 256–276. https://doi.org/10.1016/0040-5809(75)90020-9
Wiggins, I. L. (1980). Flora of Baja California. Redwood, CA: Stanford University Press.
Wiken, E., Jiménez-Nava, F., & Griffith, G. (2011). North American Terrestrial Ecoregions —Level III. Montreal, Canada: Commission for Environmental Cooperation.
World Spider Catalog (2022). World Spider Catalog. Version 23.0. Natural History Museum Bern. Retrieved on June 01, 2022 from: http://wsc.nmbe.ch




