Carlos Luis Leopardi-Verde a, *, Salvador Guzmán-González a, German Carnevali b, c, Rodrigo Duno de Stefano b, José Luis Tapia-Muñoz b
a Universidad de Colima, Facultad de Ciencias Biológicas y Agropecuarias, Km. 40 Autopista Colima-Manzanillo, Crucero de Tecomán, 28930 Tecomán, Colima, Mexico
b Centro de Investigación Científica de Yucatán, A.C., Herbarium CICY, Calle 43. Núm. 130 x 32 x 34, Col. Chuburná de Hidalgo, 97205 Mérida, Yucatán, Mexico
c Harvard University, Harvard University Herbaria, Orchid Herbarium of Oakes Ames, 22 Divinity Avenue, Cambridge, Massachusetts 02138, USA
*Corresponding author: cleopardi@ucol.mx (C.L. Leopardi-Verde)
Received: 3 July 2020; accepted: 8 January 2021
Abstract
Weeds are plants adapted to habitats modified by people and that often interfere with different human activities. These plants constitute an economically and ecologically relevant group because of their implications for agriculture. Because the agrestal weeds of the state of Colima, Mexico have been poorly documented, we surveyed these plants in commercial agricultural fields and plantations of the state, from February 2015 through May 2019. We surveyed 25 sites, each of about 1 ha, dedicated to cash-crops (blueberry, blackberry, coffee, maize, onion, jalapeño pepper, papaya, Mexican lime, and sugarcane). We found 222 weedy species (43 eudicots, 6 monocots, 2 magnoliids, 1 fern, and 1 liverwort), belonging to 53 families. The most species-rich families were Poaceae (29 species) and Asteraceae (25). A high percent of the weed flora was native (84.2%) and 15.8% alien. The most common species were Euphorbia hirta and Heliotropium procumbens. We found 21 endemic species and Manihot chlorosticta in the near threatened (NT) risk category of the IUCN. Also, we found that each crop tends to have a distinctive weed community, which in general appears to be determined by bioclimatic factors, principally temperature and precipitation.
Keywords: Agroecosystem; Bioclimatic variables; Floristics; Ruderals; Western Mexico
Malezas de cultivos comerciales en Colima, México
Resumen
Las malezas son plantas adaptadas a hábitats modificados por el ser humano y que interfieren con sus actividades. Éstas constituyen un grupo ecológica y económicamente importante por sus implicaciones para la agricultura. Debido a que las malezas arvenses del estado de Colima, México, han sido poco documentadas, se presenta una lista al respecto con base en el muestreo de campos agrícolas dedicados a cultivos comerciales en el estado. El trabajo de campo se desarrolló desde febrero del 2015 hasta mayo del 2019. Se muestrearon 25 campos agrícolas, cada uno de alrededor de 1 ha, dedicados a cultivos comerciales (mora azul, zarzamoras, café, maíz, cebolla, chile jalapeño, papaya, limón mexicano y caña de azúcar). Se encontraron 222 especies de malezas, que representan 53 familias (43 eudicotiledóneas, 6 monocotiledóneas, 2 magnólidos, 1 helecho y 1 hepática). Las familias más ricas fueron Poaceae (29 especies) y Asteraceae (25). Un alto porcentaje de la flora de malezas es nativa (84.2%) y el 15.8% exótica. Las especies más comunes fueron Euphorbia hirta y Heliotropium procumbens. Se encontró que 21 especies son endémicas y que Manihot chlorosticta se encuentra en la categoría de casi amenazada (NT) de la UICN. Además, se encontró que cada cultivo tiende a tener una comunidad distintiva de malezas asociada, que en general parece estar determinada por factores bioclimáticos, principalmente la temperatura y la precipitación.
Palabras clave: Agroecosistema; Variables bioclimáticas; Florística; Ruderales; Occidente de México
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Weeds are plants adapted to habitats modified by humans and that may interfere with their activities (Holzner, 1978). Their habitats range from home gardens to agricultural fields, and they establish self-sustained populations (Baker, 1974; Hanan et al., 2015). They are also evolutionarily interesting because of their adaptations to novel selective pressures associated with human-managed environments. As the concept of weeds is broad, Holzner (1982) divided them into several classes, of which the 2 most commonly used have been agrestal and ruderal weeds. Agrestals have been defined as weeds that grow associated with crops grown in tilled, arable land cropping. Although this concept usually refers to weeds of cereals or other annual crops, the definition can be broadened to include all weeds of cultivated lands, such as, for example, coffee plantations (Holzner, 1982). Ruderals, instead, are weeds that grow in highly disturbed, human-modified habitats, such as trash dumps, home gardens, city parks, roofs, roadsides, etc. For the purposes of this article, we will focus on agrestal weeds.
In general, the composition of a weed community is regulated as in natural ecosystems; it is also influenced by the regional species pool, distance from propagule sources, climatic constraints, and management practices (such as the use of herbicides, fertilizers, etc.), as predicted by the community assembly theory (Booth & Swanton, 2002; Colorado-Zuluaga, 2015; Götzenberger et al., 2011; Holzner, 1982).
Weeds have variable ecological requirements; some of them are specialists that may behave like the crops they grow with, and others are non-specialist that may have requirements similar to those of wild plants (Holzner, 1978). However, it is usually agreed that they are capable of high ecophysiological plasticity, fast-growth, and the capability of completing their life cycles within a short period of time (Baker, 1974; Holzner, 1982). Furthermore, they generally produce large amounts of seeds (the so-called r strategy) (Baker, 1974; Holzner, 1982; Storkey et al., 2010). For these reasons, weeds can compete with (and often, out-compete) crops for resources (light, space, water, nutrients), generating economic losses, which can be as high as 80% of the production (Espinosa-García & Sarukhán, 1997; Tanver et al., 2013). They can mechanically obstruct the harvest (Campiglia et al., 2018), or be hosts to pests or diseases (Datar, 2012; Nebreda et al., 2004; Santos-Martins et al., 2016).
However, despite the burden they may represent, weeds can also provide benefits. Weeds are usually pioneers in ecological succession and they can provide pollination services by maintaining populations of suitable pollinators or attracting them into the managed area; they can improve soil quality by preventing erosion. Also, they may contribute to maintaining a favorable microclimate for edaphic fauna; some species can even help to incorporate nitrogen into the soil (e.g., many Fabaceae) and aid in pest control, among other benefits (Blanco & Leyva, 2007; Campiglia et al., 2018; Holzner, 1982). From a socio-economic point of view, several weed species have potential as ornamental, medicinal, forage, and food plants (Blanckaert et al., 2007; Espinosa-García & Sarukhán, 1997; González-Amaro et al., 2009; Vieyra-Odilon & Vibrans, 2001).
About 12.3% of the Mexican flora is composed of species with potential weedy behavior (Espinosa-García et al., 2004a; 2009). If the vascular plants are represented in Mexico by 23,314 (Villaseñor, 2016) to 24,360 taxa (Sosa et al., 2018), then possibly as many as 2,800 species may display weedy behavior given the right circumstances (Espinosa-García et al., 2009). However, despite the importance that weeds may have for Mexico’s agri-food industry, our knowledge of this group is, as a rule, relatively poor for many areas of the country (Espinosa-García & Villaseñor, 2017). The state of Colima is no exception to this assumption. In general, there is scanty information about its flora, although several authors have noted its great floristic diversity (Moreno-Gómez et al., 2016; Padilla-Velarde et al., 2006; Ruiz-Villarreal, 2016; Velarde et al., 2008; Villaseñor, 2016). Published knowledge of Colima’s weeds is also poor and consists of only 3 publications (Orozco-Santos, 2001; Orozco-Santos & Farías-Larios, 2014; Villaseñor & Espinosa, 1998).
Here, we present the results of a survey of the agrestal weeds in agricultural areas of Colima devoted to cash-crops. We analyze the proportion of native and alien weed species. Then, we try to elucidate some of the biogeographical patterns of the weed communities with a non-metric multidimensional scaling analysis to identify weed communities and then, we identify the most influential bioclimatic variables determining the composition of the weed communities with the Maxent algorithm.
Materials and methods
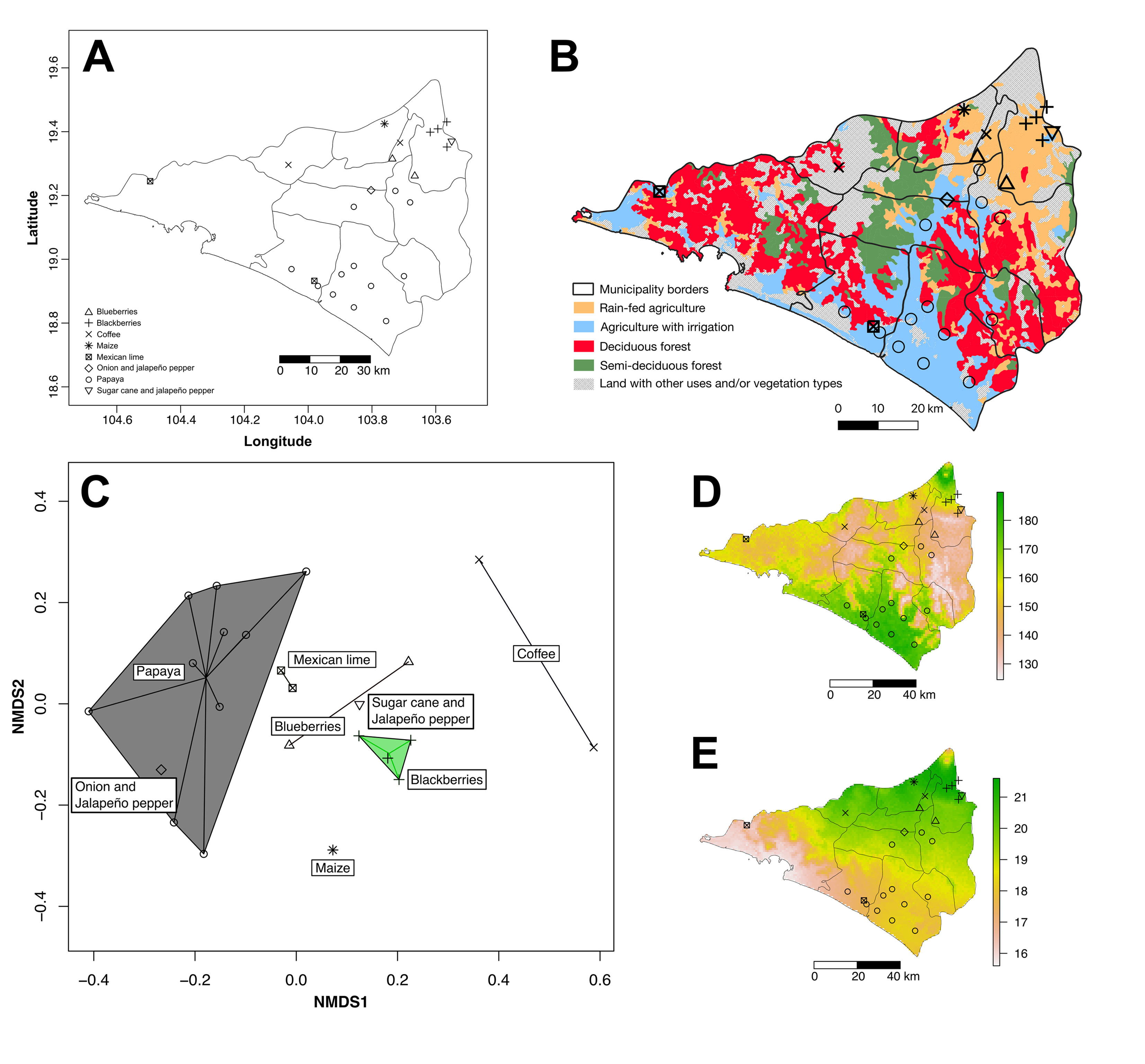
The state of Colima is located in western Mexico, along the central Pacific coast (19°31’-18°41’ N, 103°29’-104°41’ W). It borders on the Pacific Ocean to the west and southwest, the states of Jalisco (north and northwest) and Michoacán (to the south); its elevation ranges from sea level to 3,820 m and it covers an area of approximately 5,543 km2 (Fig. 1). The main type of climate in the state is warm subhumid (Aw), followed by semi-dry warm (BSh), semi-warm subhumid (ACw), subhumid temperate (Cw), and a small area of subhumid semi-cold climate (Cew) (García, 2004). The average annual rainfall is 1,000 mm and it is strongly seasonal, with rainfall mostly concentrated from June through October. The average annual temperature is 26 °C (Conabio, 2016; Conafor, 2014). The main type of vegetation is dry forest (deciduous and sub-deciduous), although it is also possible to find small areas of other types of vegetation such as Quercus forests, coniferous forests, thorn scrub, gallery forests, mountain mesophilic forest, mangrove, and savanna-like vegetation (Martínez-Cruz & Ibarra-Manríquez, 2012; Padilla-Velarde et al., 2006).
According to SIAP (2018), the state has approximately 760,000 inhabitants, 90% of which live in urban areas, and 10% in rural areas. The primary sector employs 11.5% of the population. Almost 90% of the people in the primary sector work in agriculture, which in 2017 contributed ca. 4.6% of the Colima gross domestic product (GDP). In 2017, the state yielded about 3.7 million tons of food, of which 97% corresponded to agricultural products. Due to the diversity of climates in Colima, it is possible to find a wide range of crops, from bananas in the tropical lowlands to others that require more temperate conditions, such as blackberry. The most important crops (in terms of surface area and contribution to GDP) are papaya and Mexican lime. In Colima, 45% of the planted area is irrigated, and 55% is rain-fed. Perennial crops grow on 85% of the planted area and contribute 91% of the harvested volume. The municipality with the highest production value is Tecomán, followed by Manzanillo and Armería.
Between February 2015 and May 2019, we surveyed 25 cultivated sites devoted to cash-crops in the state of Colima (municipality of Armería [3 plots], Colima [2 plots], Comala [1 plot], Coquimatlán [2 plots], Cuauhtémoc [6 plots], Ixtlahuacán [1 plot], Manzanillo [1 plot], Minatitlán [1 plot], Tecomán [7 plots], and Villa de Álvarez [2 plots]; Fig. 1, Appendix 1). Each site was surveyed once, but at a different time of the year or in different years; thus, we sampled across all seasons, although some surveyed crops (coffee, maize) were sampled only in one season. Safety and ethical considerations limited the survey to companies or individuals willing to participate. Due to the importance of papayas to the Colima agri-food industry, our sample was biased toward this crop; but we also sampled other relevant crops, such as Mexican lime and some currently expanding crops like blackberry and blueberry (see Appendix 1 for a complete list).
Most of the surveyed crops grew on units of several hectares, and they were divided into blocks. We selected one block (usually of ca. 1 ha) and searched the interior and the edges (within the managed area) of the field until we could find no additional weed species. Exceptions to this procedure were coffee and sugarcane plantations. In the first case, because there is no division, we worked within the plantation without a previously determined direction, continuously documenting the species until we were unable to find additional ones. In sugarcane fields, we only searched at the edge because the plant density of the crop made it difficult to work inside. Our sample was heterogeneous because it included perennial and annual crops. Some crops were grown inside greenhouses or used occasional protection such as covers, like plastic tunnels; also, most of the sampled agricultural fields and plantations had irrigation systems and intensive or semi-intensive management programs (Appendix 1).
Each weed morphospecies was photographed. Then, vouchers were collected and processed according to standard herbarium techniques. For specimens with few, small, or delicate flowers, all or part of the material was preserved in a solution of 96% ethanol and glycerol (3:1) for further study; this material was used for identification and later integrated into a formal spirits collection of Colima weeds. The specimens were identified using general and regional floristic treatments (Cullen, 2006; Espinosa-García & Sarukhán, 1997; Levin & Gillespie, 2016; McVaugh & Anderson, 1984; Pruski & Robinson, 2018; Vibrans, 2012) or sent to specialists in particular plant groups for determinations (see acknowledgments for a list of specialists). The first set of the collections (including the spirit collection) was housed at the herbarium of the Universidad de Colima (UCOL), and duplicates were sent to the herbarium of the Centro de Investigación Científica de Yucatán (CICY), and the herbarium of the Universidad de Guadalajara, Centro Universitario de la Costa Sur (ZEA); acronyms according to Thiers (2020, continuously updated). The nomenclature was updated using the Taxonomic Resolution Service (Boyle et al., 2013), but we cross-checked to verify the status of the names and synonymy following Villaseñor (2016) and international databases such as IPNI (2020), The Plant List (2013), and Tropicos (2020). In cases of nomenclatural conflicts, we preferred the names used by the most recent or authoritative source (Levin & Gillespie, 2016; Pruski & Robinson, 2018).
The status of the species as native or alien was assessed based on Villaseñor and Espinosa-García (2004), Villaseñor (2016), Espinosa-García and Villaseñor (2017), and Sosa et al. (2018). We assigned native species to one of 3 levels of geographic restriction, according to the distribution data available in Villaseñor (2016): i) native species that grow naturally in Mexico, but their distribution is not restricted to the country, ii) the endemics of Mexico (their distribution is naturally restricted to the country, but are widely distributed across it), and iii) the endemics to the Mexican Pacific Coast. The third level is comprised of 2 biogeographical provinces: the Pacific Coast province, which is in the Neotropical Domain and the Sierra Madre del Sur province, which is in the Mexican Transition Zone. Both provinces are included because according to Morrone (2019) regionalization system, Colima is encompassed within both; the lowlands correspond to the Pacific Coast province and the highlands are in the Sierra Madre del Sur province.
To analyze the distinctiveness of the weed floras of the surveyed crops and fields or plantations, a non-metric multidimensional scaling (NMDS) was carried out in R with the vegan package (Oksanen et al., 2019; R Core Team, 2019). We used data from 25 surveyed fields and plantations in the analysis; because we only had presence/absence data, we used the Jaccard index as a distance measure. The NMDS is a non-parametric multivariate ordering technique that is based on the rankings of distances between points (Chahouki, 2012). In addition, we calculated the proportion of the samples that each weed species represented and examined whether it is exclusive to any crop or bioclimatic zone of the state. The NMDS clustered the crops according to the similarities of their weed flora. Then, we selected the locations to assess the bioclimatic variables most relevant in determining the composition of the weed floras. These bioclimatic variables were used at the finest grain size available (ca. 1 km2) from the WorldClim database (Fick & Hijmans, 2017). Data were analyzed with Maxent within R using the dismo package (Hijmans et al., 2017; Phillips et al., 2017). The R script used is available in the data resources section (see below). For illustrative purposes, we include a map with information on the main vegetation types and irrigation regimes, using land-use data layers series V (INEGI, 2013) and assembled in QGIS 3.12.
The underlying data of the analysis reported in this paper (including Matrices and R scripts used in NMDS and bioclimatic analyses) are available in https://doi.org/10.7910/DVN/JIU7LP
Results
We recorded 222 weed species for Colima, from 53 families and 163 genera (Table 1, Appendices 2, 3). Ferns and liverworts were represented by a single family and genus each; 2 families and 3 genera were Magnoliids, 6 families and 28 genera were Monocotyledons, and 43 families and 129 genera were Eudicotyledons. The most speciose plant families were Poaceae (29 species), Asteraceae (25), and Fabaceae (18). The genera with the most species were Euphorbia L. (7 species), Solanum L. (6), Physalis L., and Ipomoea L. (5 species each; Appendix 2). Also, 84.2% of the species were native and 15.8% were alien; 21 (9.4%) of 222 species included on our list were endemic to Mexico, 5 restricted to the Mexican Pacific, the remaining 16 with broader distributions (Table 1, Appendix 2).
Forty-six percent (46.85%, 104/222) of the species were found in 4% (1/25) of the surveyed sites and 20.72% (46/222) of the species were in 8% (2/25) of the sites; together this represents 67.57% of the species. In contrast, only 4 species occurred in more than 40% of the sites and were thus found in most of the sampled crops: Euphorbia hirta (60%, 15/25 sites), Heliotropium procumbens (48%, 12/25 sites), Richardia scabra (44%, 11/25 sites), and Euphorbia heterophylla (40%, 10/25 sites). We also found a few, much more crop-specific species, such as Piper hispidum, which grows only in coffee plantations, whereas Marchantia polymorpha or Lemna aequinoctialis were found only in blueberry plantations with intensive management. Papaya plantations were commonly invaded by Amaranthus palmeri, along with Anoda cristata, Acalypha aristata, and Boerhavia erecta. In Mexican lime plantations, a distinctive weed species was Struthanthus interruptus, a hemiparasite, whereas in maize Castilleja arvensis was commonly found (Appendix 2).
In a more detailed comparison of the surveyed crops, we found that the crop with the most associated weed species was papaya (106 species), followed by blackberry (73), and Mexican lime (54) (Table 1). The crop associated with the largest number of endemic species was coffee (6 species, 16.7%), whereas not a single endemic species behaving as a weed was found in Mexican lime plantations, although these plantations, along with those of blackberries, sugarcane, and papaya, had the highest proportion of alien taxa: 20.4%, 19.2%, 15.8%, and 14.2%, respectively (Table 1, Appendix 2).
In comparing crops and all species using NMDS, we found that coffee and maize had very characteristic weed floras. Papaya, onions, and Mexican lime fields were more similar, whereas blueberry, blackberry, and sugarcane also had weed components in common (Fig. 1C). This pattern reflects the 2 biogeographic areas in the state: the onion field and all our papaya and Mexican lime plantations were within the Pacific Coast province. In contrast, the sugarcane, blackberry, maize fields, and one of the coffee plantations were located within the Sierra Madre del Sur province. One coffee orchard was in a highly conserved area near the Sierra de Manantlán protected area, whereas the blueberry fields were found in areas that can be considered as transitional between both biogeographic areas.
To further explore the pattern of weed composition across the surveyed sites, we reviewed our data to identify unique species for each group of crops or area and found that taxa such as Acalypha setosa, Cissus verticillata, Boerhavia erecta, Corynandra viscosa, Momordica charantia, and Priva lappulacea, among others (Appendix 2), were common or unique in papaya and Mexican lime plantations. Also, species like Bidens alba, Glandularia bipinnatifida, Lepidium virginicum, Pseudognaphalium viscosum, and Verbena carolina, among others, were common or restricted to blueberry, blackberry, and sugarcane fields (Appendix 2).
To identify the potentially most influential bioclimatic variables in each area, we pooled localities of papaya, onion, and Mexican lime plantations and found that the main bioclimatic variables identified by Maxent (potentially with more than 40% of weight each) were “temperature seasonality” (bio4, Fig. 1D), and “mean temperature of wettest quarter” (bio8). This implies that the weed species that grow in the Pacific Coast province of Colima are adapted to a high monthly average variability in temperature across the year (ca. 17 ºC). At the same time, they appear to prefer warmer temperatures in the most humid period (ca. 28 ºC). In contrast, the species occurring within the Sierra Madre del Sur province or near its limits, were favored by the combination of temperature with a lower monthly variation (ca. 15 ºC), and lower mean temperature (ca. 23 ºC) in the most humid quartile.
The same analyses for localities with blueberry, blackberry, and sugarcane plantations found that the main bioclimatic variables (potentially with more than 40% of weight each one) were “temperature annual range” (bio7, Fig. 1 E) and “precipitation of driest quarter” (bio17). This means that weed species that grow in or near the Sierra Madre del Sur province tend to withstand more extreme temperatures, with an annual variation of up to 20 ºC, when compared with the species that grow in the Pacific Coast province zone of Colima (up to 18 ºC). Similarly, there was variation in the amount of precipitation during the driest quartile of the year, although the surveyed areas tended to behave similarly, on average 8.92 vs. 8.37 mm of rainfall in the crops sampled in the Pacific Coast and Sierra Madre del Sur provinces, respectively.
Table 1. Families and genera by sampled crop, as well as their number and percentage of native, endemic, and exotic species.
|
Crop |
Families |
Genera |
Species |
Native (%) |
Endemics (%) |
Aliens (%) |
|
Blueberries |
24 |
39 |
43 |
33 (76.7) |
4 (9.3) |
6 (14) |
|
Blackberries |
26 |
58 |
73 |
56 (76.7) |
3 (4.1) |
14 (19.2) |
|
Coffee |
18 |
32 |
36 |
28 (77.8) |
6 (16.7) |
2 (5.6) |
|
Maize |
10 |
17 |
21 |
18 (85.7) |
1 (4.8) |
2 (9.5) |
|
Mexican lime |
25 |
47 |
54 |
43 (79.6) |
0 |
11 (20.4) |
|
Onion and jalapeño pepper |
9 |
9 |
12 |
11 (91.7) |
1 (8.3) |
0 |
|
Papaya |
26 |
84 |
106 |
83 (78.3) |
8 (7.5) |
15 (14.2) |
|
Sugarcane and jalapeño pepper |
19 |
32 |
38 |
29 (76.3) |
3 (7.9) |
6 (15.8) |
|
Total |
53 |
163 |
222 |
166 (74.8) |
21 (9.4) |
35 (15.8) |
Discussion
The first compilation of weeds of Colima was published by Villaseñor and Espinosa (1998), who conceptualized weeds as plants that were reported as unwanted in human-controlled habitats. Their list included agrestal, ruderal, grassland, aquatic, forest, and environmental weeds, and comprised 510 species. The large difference between lists (510 vs. 222 species) can be explained by the differences in scope, but also by different methods based on either bibliography or fieldwork. Both lists share 121 species (54% of the 222 species listed in Appendix 2), including such common taxa as Anoda cristata, Euphorbia hirta, Amaranthus palmeri, and Argemone mexicana, among others. However, we also report 101 additional species for the state; Evolvulus alsinoides, Chloris barbata, and Urochloa meziana are some of the more common in surveyed sites.
The other published weed lists for Colima were restricted to tamarind plantations, and Mexican lime plantations (Orozco-Santos, 2001; Orozco-Santos & Farías-Larios, 2014). In the first study, 21 species of weeds (8 monocots and 13 eudicots) were reported, but only 3 species were not shared with our list, namely Digitaria sanguinalis, Euphorbia hypericifolia, and Commelina erecta (Appendix 2).
Orozco-Santos and Farias Larios (2014) reported 44 species (15 monocots and 29 eudicots), but only 10 (22.7%) of their species were not found on our list (Digitaria sanguinalis, Chloris virgata, Brachiaria fasciculata, Rottboellia cochinchinensis, Acalypha alopecuroides, Euphorbia hypercifolia, Commelina erecta, Cuscuta americana, Senna occidentalis, and Crotalaria incana. Of the remaining 34 species, 18 were shared by both lists whereas 16 species were found by us associated with other crops, but not with Mexican lime; our list also includes another 34 species associated with Mexican lime that were not reported by Orozco-Santos and Farias Larios (2014) (Appendix 2).
The most speciose families on our list, Poaceae and Asteraceae (Appendix 2), are consistent with most weed studies in Mexico (León-de la Luz et al., 2009; Martínez-De La Cruz et al., 2015; Orozco-Santos, 2001; Orozco-Santos & Farías-Larios, 2014; Vibrans, 1998; Villaseñor & Espinosa, 1998). This reflects the high number of taxa that these families have in Mexico and elsewhere (Baker, 1974; Simpson, 2019; Villaseñor, 2016).
Other important families were Fabaceae and Euphorbiaceae, which are usually well-represented in the dry forests that are prevalent in Colima (Ceballos et al., 2010; Conafor, 2014; Martínez-Cruz & Ibarra-Manríquez, 2012; Padilla-Velarde et al., 2006). Some of the most widely distributed weedy plants in the state are taxa such as Euphorbia heterophylla (Aarestrup et al., 2008). One interesting pattern was that the most frequently collected weeds were several taxa of Euphorbia subgenus Chamaesyce, particularly Euphorbia hirta. This trend most likely reflects their wide climatic and edaphic tolerance, the fact that some of them feature C4 photosynthesis, the numerous seeds produced by each individual, toxic latex that deters herbivores, and their resistance to pesticides (Ferreira et al., 2017; Tanver et al., 2013).
Our results show that 15.8% of our list is composed of alien species; but the proportion of alien weeds ranges from 0-20.4% (Table 1), depending on the crop. These general numbers are consistent with the national estimates of 12.3% of the total flora being weedy (Espinosa-García et al., 2004a, 2009) and is below the range of 25.2-39.2% estimated for the alien weed flora of Colima (Espinosa-García et al., 2004a; Villaseñor & Espinosa-García,
2004).
The relatively small proportion of alien species in some of the surveyed crops, particularly in maize, coffee, and onions, may be related to their resistance to the establishment of alien species because of the history of agriculture in the region (Espinosa-García & Villaseñor, 2017). The somewhat higher levels in other types of crops (such as papaya, blueberry, blackberry, and Mexican lime) may be related to the movement of plantlets, seedlings, or other propagules for crop establishment, along with soil or other substrates. Good examples of introduced species that are currently weeds in crops are several grass species used for forage, including Echinochloa colona, Eleusine indica, Melinis repens, and Megathyrsus maximus, among others. These species can disperse with contaminated equipment or material, by adherence to vehicles or animals (Sánchez-Ken et al., 2012; Vibrans, 2012), or even by local birds that use them as food (Petit et al., 2013; Viana et al., 2016).
Most crops had their own weed assemblage, except for a field of onions and jalapeño pepper, which was embedded in the papaya group in the analysis. This pattern has been found in other crop systems such as wheat, watermelon, banana, and others (Gomaa, 2012; Holzner, 1982; Quintero-Pertúz et al., 2018; Suárez et al., 2001). In our data, the most distinctive communities were maize and coffee. Probably the key to understand this are the crop management practices: coffee is managed with traditional techniques, and also has a permanent tree cover; these characteristics tend to be friendly to native, shade-loving flora, and also explain the high weed endemicity, compared to other crops, although we report only 36 species, a far smaller figure than the 58 species found by Sanginés et al. (2014) in coffee plantations at Comapa (Veracruz). The differences may be associated with the study region —the flora of Veracruz is different from that of Colima; it may also be related to our survey techniques. We only sampled in the dry season and only once in each site.
Maize also had a distinctive community, with traditional milpa-like management (we found squash, but not beans plants inside the maize field), which is also friendly to native, sun-loving species. We found only 21 species associated with this crop, but we do not have much comparative data.
The other crops, which clustered in 2 larger groups (Fig. 1C), can probably be explained by several filtering factors (apart from management). They were related to the biogeographic provinces (Pacific Coast and Sierra Madre del Sur), and their different climates and species pools (Appendix 2), as was found elsewhere (Booth & Swanton, 2002; Nagy et al., 2017; Poggio, 2012).
Several temperature variables (bio4, bio7 and bio8; Fig. 1D, E) influenced the composition of the local weed flora. Group one (lowland, Pacific Coast province) was associated with the hottest temperatures (average at wettest quarter 28 ºC) with less extreme values (range up to +/-17 ºC), and group 2 (highland, Sierra Madre del Sur province) with the coldest temperatures (average at wettest quarter 23 ºC) and more extreme values (range up to +/-20 ºC). According to Espinosa-García et al. (2004b), in Mexico, low temperature is one of the limiting factors for the distribution of vegetation. The same was only partially true for Colima perhaps because no survey sites were located in areas with winter frosts, also the Maxent analysis did not identify that the minimum temperature of the coldest month (bio6) as a relevant variable, as suggested by Espinosa-García et al. (2004b).
Noteworthy is the high number of species collected in one or a few sites (ca. 70%) and the proportion of endemics (ca. 9%; Table 1; Appendix 2). Both observations are probably related to the heterogeneity of the landscape. The 9.4% endemic species found is within the expected value according to the information available for maize (5.7-13.7%) (Vibrans, 1998) and for ruderal floras such as Malinalco in the state of Mexico (8.7%) (Martínez-De La Cruz et al., 2015) and Coronango in Puebla (6.3%) (Flores-Huitzil et al., 2020).
The crops do not have the same proportion of endemics. There were no endemics in the Mexican lime plantations, whereas in coffee plantations they reached 16.7% of the total flora. Perhaps these variations are related to the fact that the first are irrigated and located in a highly modified landscape matrix. In mature plantations weeds are controlled by brush cutters and herbicides. On the other hand, coffee plantations are in a landscape matrix that tends to be more conserved and managed less intensively.
From our species list, only Manihot chlorosticta (which is widely distributed in Mexico) is included in the IUCN red list (as near threatened, NT) (Vera-Sánchez & Nassar, 2019). But we found another 15 endemic species of wide distribution in the country and 5 restricted only to the Pacific Coast: Tridax dubia and Melampodium tepicense were found in blackberries, Phyllanthus hexadactylus and Hilaria annua in papaya, and Piper abalienatum in coffee. They are currently under no legal protections
(Appendix 2).
Finally, 222 species were found, of these 166 (74.8%) are native. Furthermore, 21 of the listed species are endemic to Mexico, whether they are widely distributed (16 species) or restricted to the Pacific Coast (5 species). In general, each crop has its own distinctive community of weeds; however, they are grouped into 2 large clusters: those that correspond to lowland crops in the Pacific Coast province and that tend to have warmer and more stable temperatures throughout the year. The other group is made up of highland crops found within the Sierra Madre del Sur province, which tends to be subject to lower temperatures and greater annual variation. Of course, some crops are in transitional areas and have a flora that reflects this, whereas others such as maize and coffee feature very distinctive floras, due to different management requirements and particular characteristics.
The predictive power of our results is constrained because they are focused on commercial crops, most of them perennial and with a relatively high degree of technification; they also have a significant bias towards papaya. Although the sampling is multi-year and covers all seasons of the year, each site was visited just once. Therefore, it cannot be ruled out that by including more annual crops as well as other crops managed in a more traditional manner (low technification), it would be possible to add more species to this list, in accordance with the high richness of native species in the state (ca. 4,300 species sensu Villaseñor, 2016). Also, ruderal vegetation and aquatic weeds were not included. We expect to document the weed flora of urban areas of Colima and increase the sampling in crops such as maize as well as include other crops such as banana, coconut, melon, and cucumber, and with different management requirements (i.e., traditional, technified or organic).
Acknowledgments
To Manuel Bermúdez Guzmán (INIFAP), Noé Rosales Bonilla, Marco Tulio Buenrostro Nava, Javier Farias Larios, and Jesús Cuevas Anguiano (University of Colima) for their field support. To the Consejo Estatal de Productores de Papaya del Estado de Colima, A.C., in particular Nazario Rodríguez Guerra, for facilitating access to papaya producers and entrepreneurs. We are particularly grateful to the enterprises Red Starr, Frutioro Mexico, Mia Produce, Finis TRR, Harvest H52, Café La Huerta, and all other persons and corporations who gave us access to some of their farms to collect samples. We also are indebted to Gerardo Salazar, José Luis Villaseñor (both at Instituto de Biología, UNAM) and Aarón Rodríguez (CUCBA, Universidad de Guadalajara) for helping with the taxonomic determination of some samples. CLLV thanks the University of Colima for supporting this project. Last but not least, we thank the Secretaría de Educación Pública-Mexico (SEP) for the postdoctoral grants (PRODEP program: agreements DSA/103.5/14/11781 and DSA/103.5/16/526), and also for the support through the NPTC grant (511-6/18-9401), all awarded to CLLV. We also wish to thank 2 anonymous reviewers and Guillermo Ibarra-Manríquez (Associate Editor), whose comments and suggestions deeply improved this manuscript.
Appendix 1. Sampled localities and crops in the different municipalities of Colima.
|
Municipality |
Crop |
Crop typea |
Managementb |
GPS (decimal system) |
Elevation (m) |
|
|
1 |
Armería |
Papaya |
P |
S, OS, I |
18.96916667 N – 104.0522167 W |
18 |
|
2 |
Armería |
Mexican lime |
P |
S & OS, T |
18.93233333 N – 103.9807 W |
23 |
|
3 |
Armería |
Papaya |
P |
S & OS, I |
18.91636667 N –103.9694833 W |
17 |
|
4 |
Colima |
Papaya |
P |
S & OS, I |
19.17834722 N – 103.6803667 W |
489 |
|
5 |
Colima |
Papaya |
P |
S & OS, I |
19.21434444 N – 103.7265667 W |
438 |
|
6 |
Comala |
Maize |
A |
S & OS, I |
19.42466667N – 103.7606 W |
1,107 |
|
7 |
Coquimatlán |
Papaya |
P |
S, OS, I |
19.16433333 N – 103.8569333 W |
262 |
|
8 |
Coquimatlán |
Onion and jalapeño pepper |
A |
S, OS, I |
19.21640833 N – 103.8027944 W |
369 |
|
9 |
Cuauhtémoc |
Sugar cane and blackberries |
P |
S, OS, I |
19.35193333 N – 103.56485 W |
1,093 |
|
10 |
Cuauhtémoc |
Sugar cane and jalapeno pepper |
A/P |
S, OS, I |
19.37028333 N – 103.5502167 W |
1,060 |
|
11 |
Cuauhtémoc |
Blackberries |
P |
S, OS, I |
19.39843333 N – 103.61725 W |
1,430 |
|
12 |
Cuauhtémoc |
Blackberries |
P |
S, OPR, I |
19.4088 N – 103.59355 W |
1,387 |
|
13 |
Cuauhtémoc |
Blackberries |
P |
S, OPR, I |
19.43063333 N – 103.5654833 W |
1,477 |
|
14 |
Cuauhtémoc |
Blueberries |
P |
X, PR, I |
19.26116667 N – 103.6658167 W |
612 |
|
15 |
Ixtlahuacán |
Papaya |
P |
S, OS. I |
18.94711667 N – 103.6996 W |
126 |
|
16 |
Manzanillo |
Mexican Lime |
P |
S, OS, T |
19.24465 N – 104.4954 W |
30 |
|
17 |
Minatitlán |
Coffee |
P |
S, OS, T |
19.29596667 N – 104.06195 W |
798 |
|
18 |
Tecomán |
Papaya |
P |
S, OS, I |
18.80633333 N – 103.75565 W |
25 |
|
19 |
Tecomán |
Papaya |
P |
S, PR, I |
18.84922 N – 103.8567 W |
16 |
|
20 |
Tecomán |
Papaya |
P |
S, OS, I |
18.91636667 N – 103.8028167 W |
157 |
|
21 |
Tecomán |
Papaya |
P |
S, OS, I |
18.8746 N – 103.9447 W |
13 |
|
22 |
Tecomán |
Papaya |
P |
S, OS, I |
18.88961667 N – 103.9224 W |
23 |
|
23 |
Tecomán |
Papaya |
P |
S, OS, I |
18.97908333 N – 103.8567667 W |
68 |
|
24 |
Tecomán |
Mostly papayas, some other crops |
P |
S, OS, I |
18.95288333 N – 103.8954667 W |
56 |
|
25 |
Villa de Álvarez |
Blueberries |
P |
S, OS, I |
19.31445 N – 103.7360167 W |
675 |
|
26 |
Villa de Álvarez |
Coffee |
P |
S, OS, T |
19.36546667 N – 103.7118167 W |
991 |
a P = Perennial; A = annual. b S = Planted on soil; X = planted in containers; OS = open air; PR = protected (green house); OPR = occasionally protected (infrastructure to install plastic tunnels for protection of the crop when necessary); I = intensive management; T = traditional management.
Appendix 2. List of weeds by surveyed crops. S = Status (N = native, N*= endemic to Mexico, NW = restricted to Mexican Pacific Coast; I = introduced); B = blueberries; BB = blackberries; C = coffee; M= maize; ML = Mexican lime; OJ = onions and jalapeño pepper; P = papaya; SC = sugar cane and jalapeño pepper; % = percent of sites in which we found the species.
|
Family |
Species |
S |
Crops |
||||||||
|
B |
BB |
SC |
M |
C |
OJ |
P |
ML |
% |
|||
|
Marchantiophyta |
|||||||||||
|
Marchantiaceae |
Marchantia polymorpha L. |
N |
1/2 |
4.0 |
|||||||
|
Ferns (Polypodiopsida) |
|||||||||||
|
Pteridaceae |
Adiantum amplum C. Presl |
N |
1/2 |
4.0 |
|||||||
|
Adiantum trapeziforme L. |
N |
1/2 |
4.0 |
||||||||
|
Magnoliids |
|||||||||||
|
Aristolochiaceae |
Aristolochia taliscana Hook. & Arn. |
N* |
1/11 |
4.0 |
|||||||
|
Piperaceae |
Peperomia glabella (Sw.) A.Dietr. |
N |
1/2 |
4.0 |
|||||||
|
Piper abalienatum Trel. |
NW |
2/2 |
8.0 |
||||||||
|
Piper hispidum Kunth |
N |
2/2 |
8.0 |
||||||||
|
Monocots |
|||||||||||
|
Araceae |
Lemna aequinoctialis Welw. |
N |
1/2 |
4.0 |
|||||||
|
Commelinaceae |
Commelina diffusa Burm. f. |
N |
1/4 |
4.0 |
|||||||
|
Commelina rufipes Seub. |
N |
1/2 |
4.0 |
||||||||
|
Tradescantia zebrina hort. ex Bosse |
N |
1/2 |
4.0 |
||||||||
|
Cyperaceae |
Cyperus amabilis Vahl |
N |
1/1 |
4.0 |
|||||||
|
Cyperus hermaphroditus (Jacq.) Standl. |
N |
1/2 |
2/4 |
1/1 |
1/11 |
20.0 |
|||||
|
Cyperus rotundus L. |
I |
1/11 |
4.0 |
||||||||
|
Cyperus tenerrimus J. Presl & C. Presl |
N |
1/1 |
4.0 |
||||||||
|
Kyllinga odorata Vahl |
N |
1/2 |
4.0 |
||||||||
|
Orchidaceae |
Beloglottis costaricensis Schltr. |
N |
1/2 |
4.0 |
|||||||
|
Poaceae |
Anthephora hermaphrodita Kuntze |
N |
1/1 |
4.0 |
|||||||
|
Axonopus scoparius (Humb. & Bonpl. ex Flüggé) Kuhlm. |
I |
1/11 |
4.0 |
||||||||
|
Bouteloua dimorpha Columbus |
N |
1/11 |
4.0 |
||||||||
|
Cenchrus ciliaris L. |
I |
1/11 |
4.0 |
||||||||
|
Cenchrus echinatus L. |
N |
1/2 |
2/4 |
1/1 |
16.0 |
||||||
|
Chloris barbata Sw. |
I |
1/2 |
2/2 |
12.0 |
|||||||
|
Cynodon dactylon (L.) Pers. |
I |
1/2 |
1/4 |
2/2 |
16.0 |
||||||
|
Dactyloctenium aegyptium (L.) Willd. |
I |
2/2 |
8.0 |
||||||||
|
Digitaria abyssinica (Hochst. ex A. Rich.) Stapf |
I |
2/4 |
8.0 |
||||||||
|
Digitaria bicornis (Lam.) Roem. & Schult. |
I |
1/1 |
1/11 |
2/2 |
16.0 |
||||||
|
Echinochloa colona (L.) Link |
I |
2/2 |
1/11 |
1/2 |
16.0 |
||||||
|
Eleusine indica (L.) Gaertn. |
I |
2/2 |
3/4 |
1/1 |
2/11 |
1/2 |
32.0 |
||||
|
Eragrostis atrovirens (Desf.) Trin. ex Steud. |
I |
1/4 |
4.0 |
||||||||
|
Eragrostis ciliaris (L.) R. Br. |
I |
1/4 |
4.0 |
||||||||
|
Eragrostis mexicana (Hornem.) Link |
N |
1/2 |
1/4 |
1/1 |
12.0 |
||||||
|
Hilaria annua Reeder & C. Reeder |
NW |
1/1 |
4.0 |
||||||||
|
Hilaria belangeri (Steud.) Nash |
N |
1/2 |
4.0 |
||||||||
|
Ixophorus unisetus (J. Presl) Schltdl. |
N |
1/11 |
4.0 |
||||||||
|
Leptochloa mucronata (Michx.) Kunth |
N |
1/2 |
4.0 |
||||||||
|
Leptochloa panicoides (J. Presl) Hitchc. |
N |
1/2 |
1/11 |
8.0 |
|||||||
|
Megathyrsus maximus (Jacq.) B.K. Simon & S.W.L. Jacobs |
I |
1/2 |
4.0 |
||||||||
|
Melinis repens (Willd.) Zizka |
I |
1/2 |
2/4 |
12.0 |
|||||||
|
Paspalum notatum Flüggé |
N |
1/2 |
4.0 |
||||||||
|
Paspalum paniculatum L. |
N |
1/4 |
1/2 |
8.0 |
|||||||
|
Paspalum virgatum L. |
N |
1/11 |
4.0 |
||||||||
|
Setaria parviflora (Poir.) Kerguélen |
N |
1/4 |
4.0 |
||||||||
|
Sorghum halepense (L.) Pers. |
I |
3/11 |
12.0 |
||||||||
|
Sporobolus indicus (L.) R.Br. |
I |
1/4 |
4.0 |
||||||||
|
Urochloa meziana (Hitchc.) Morrone & Zuloaga |
N* |
1/11 |
4.0 |
||||||||
|
Zingiberaceae |
Costus pictus D. Don |
N |
1/2 |
4.0 |
|||||||
|
Curcuma longa L. |
I |
1/2 |
4.0 |
||||||||
|
Eudicots |
|||||||||||
|
Acanthaceae |
Dicliptera nervata Greenm. |
N* |
1/2 |
4.0 |
|||||||
|
Elytraria imbricata (Vahl) Pers. |
N |
2/4 |
8.0 |
||||||||
|
Henrya insularis Nees |
N |
2/2 |
8.0 |
||||||||
|
Justicia caudata A. Gray |
N |
1/2 |
1/2 |
8.0 |
|||||||
|
Justicia salviiflora Kunth |
N* |
1/2 |
4.0 |
||||||||
|
Ruellia blechum L. |
N |
1/2 |
1/11 |
1/2 |
12.0 |
||||||
|
Ruellia nudiflora (Engelm. & A. Gray) Urb. |
N |
1/11 |
4.0 |
||||||||
|
Tetramerium nervosum Nees |
N |
1/4 |
1/11 |
8.0 |
|||||||
|
Aizoaceae |
Trianthema portulacastrum L. |
N |
1/1 |
4.0 |
|||||||
|
Amaranthaceae |
Achyranthes aspera L. |
I |
1/2 |
4.0 |
|||||||
|
Amaranthus hybridus L. |
N |
1/4 |
1/1 |
1/11 |
12.0 |
||||||
|
Amaranthus palmeri S.Watson |
N |
1/2 |
4/11 |
2/2 |
28.0 |
||||||
|
Amaranthus spinosus L. |
N |
1/4 |
1/1 |
1/11 |
1/2 |
16.0 |
|||||
|
Chenopodium album L. |
I |
1/1 |
4.0 |
||||||||
|
Apiaceae |
Eryngium nasturtiifolium Juss. ex F. Delaroche |
N |
1/2 |
1/4 |
8.0 |
||||||
|
Spananthe paniculata Jacq. |
N |
1/4 |
4.0 |
||||||||
|
Apocynaceae |
Asclepias curassavica L. |
N |
1/2 |
2/11 |
12.0 |
||||||
|
Asclepias glaucescens Kunth |
N |
1/2 |
4.0 |
||||||||
|
Asteraceae |
Ageratina malacolepis (B.L. Rob.) R.M. King & H. Rob. |
N* |
1/2 |
4.0 |
|||||||
|
Aldama dentata La Llave |
N |
1/11 |
4.0 |
||||||||
|
Bidens alba (L.) DC. |
N |
4/4 |
1/1 |
1/2 |
24.0 |
||||||
|
Conyza canadensis (L.) Cronquist |
N |
1/2 |
1/4 |
1/1 |
12.0 |
||||||
|
Eclipta prostrata (L.) L. |
N |
1/2 |
4.0 |
||||||||
|
Emilia fosbergii Nicolson |
I |
1/4 |
1/1 |
8.0 |
|||||||
|
Emilia sonchifolia (L.) DC. |
I |
1/4 |
4.0 |
||||||||
|
Galinsoga parviflora Cav. |
N |
1/4 |
4.0 |
||||||||
|
Lagascea mollis Cav. |
N |
4/11 |
16.0 |
||||||||
|
Melampodium divaricatum DC. |
N |
1/2 |
1/4 |
1/11 |
1/2 |
16.0 |
|||||
|
Melampodium perfoliatum Kunth |
N |
1/4 |
4.0 |
||||||||
|
Melampodium tepicense B.L. Rob. |
NW |
1/4 |
4.0 |
||||||||
|
Parthenium hysterophorus L. |
N |
1/2 |
2/11 |
12.0 |
|||||||
|
Perityle microglossa Benth. |
N |
4/4 |
1/1 |
1/2 |
1/11 |
28.0 |
|||||
|
Pseudelephantopus spicatus (Juss. ex Aubl.) C.F.Baker |
N |
1/4 |
2/2 |
12.0 |
|||||||
|
Pseudognaphalium viscosum (Kunth) Anderb. |
N |
3/4 |
12.0 |
||||||||
|
Sclerocarpus uniserialis (Hook.) Benth. & Hook. f. ex Hemsl. |
N |
2/11 |
8.0 |
||||||||
|
Sonchus oleraceus L. |
I |
1/4 |
1/1 |
8.0 |
|||||||
|
Spilanthes urens Jacq. |
N |
2/4 |
1/1 |
12.0 |
|||||||
|
Synedrella nodiflora (L.) Gaertn. |
N |
1/2 |
4.0 |
||||||||
|
Tithonia rotundifolia S.F. Blake |
N |
1/2 |
3/11 |
16.0 |
|||||||
|
Tridax dubia Rose |
NW |
2/4 |
1/1 |
12.0 |
|||||||
|
Tridax mexicana A.M. Powell |
N* |
2/11 |
8.0 |
||||||||
|
Tridax procumbens L. |
N |
1/4 |
1/1 |
1/11 |
1/2 |
16.0 |
|||||
|
Zinnia maritima Kunth |
N* |
1/1 |
1/2 |
2/11 |
16.0 |
||||||
|
Boraginaceae |
Cordia alba (Jacq.) Roem. & Schult. |
N |
1/11 |
4.0 |
|||||||
|
Heliotropium angiospermum Murray |
N |
4/11 |
1/2 |
20.0 |
|||||||
|
Heliotropium indicum L. |
N |
1/2 |
4.0 |
||||||||
|
Heliotropium procumbens Mill. |
N |
1/2 |
1/4 |
1/1 |
1/1 |
6/11 |
2/2 |
48.0 |
|||
|
Heliotropium rufipilum (Benth.) I.M. Johnst. |
N |
1/2 |
4.0 |
||||||||
|
Tournefortia mutabilis Vent. |
N |
1/2 |
1/11 |
8.0 |
|||||||
|
Brassicaceae |
Lepidium didymum L. |
I |
1/4 |
4.0 |
|||||||
|
Lepidium virginicum L. |
N |
4/4 |
1/1 |
20.0 |
|||||||
|
Campanulaceae |
Lobelia fenestralis Cav. |
N |
1/4 |
4.0 |
|||||||
|
Cleomaceae |
Corynandra viscosa (L.) Cochrane & Iltis |
N |
4/11 |
1/2 |
20.0 |
||||||
|
Tarenaya spinosa (Jacq.) Raf. |
N |
1/11 |
4.0 |
||||||||
|
Convolvulaceae |
Cuscuta umbellata Kunth |
N |
1/11 |
4.0 |
|||||||
|
Dichondra sericea Sw. |
N |
1/4 |
4.0 |
||||||||
|
Evolvulus alsinoides (L.) L. |
N |
1/2 |
4/4 |
1/1 |
1/11 |
28.0 |
|||||
|
Ipomoea hederifolia L. |
N |
1/4 |
1/1 |
8.0 |
|||||||
|
Ipomoea minutiflora (M. Martens & Galeotti) House |
N |
1/1 |
4.0 |
||||||||
|
Ipomoea quamoclit L. |
I |
1/11 |
4.0 |
||||||||
|
Ipomoea ternifolia Cav. |
N |
2/11 |
8.0 |
||||||||
|
Ipomoea trifida (Kunth) G. Don |
N |
1/4 |
1/11 |
8.0 |
|||||||
|
Merremia quinquefolia (L.) Hallier f. |
N |
3/11 |
1/2 |
16.0 |
|||||||
|
Operculina pinnatifida (Kunth) O’Donell |
N |
2/11 |
8.0 |
||||||||
|
Cucurbitaceae |
Citrullus lanatus (Thunb.) Matsum. & Nakai |
I |
1/2 |
4.0 |
|||||||
|
Cucumis melo L. |
I |
3/11 |
12.0 |
||||||||
|
Luffa cylindrica (L.) M.Roem. |
I |
1/11 |
4.0 |
||||||||
|
Momordica charantia L. |
I |
5/11 |
2/2 |
28.0 |
|||||||
|
Sicyos microphyllus Kunth |
N* |
1/2 |
1/1 |
8.0 |
|||||||
|
Euphorbiaceae |
Acalypha aristata Kunth |
N |
1/2 |
1/1 |
3/11 |
1/2 |
24.0 |
||||
|
Acalypha setosa A. Rich. |
N |
2/11 |
1/2 |
12.0 |
|||||||
|
Euphorbia adenoptera Bertol. |
N* |
1/2 |
1/4 |
8.0 |
|||||||
|
Euphorbia graminea Jacq. |
N |
1/2 |
4.0 |
||||||||
|
Euphorbia heterophylla L. |
N |
2/4 |
1/1 |
1/1 |
4/11 |
2/2 |
40.0 |
||||
|
Euphorbia hirta L. |
N |
1/2 |
4/4 |
1/1 |
1/1 |
1/1 |
5/11 |
2/2 |
60.0 |
||
|
Euphorbia hyssopifolia L. |
N |
2/2 |
2/4 |
1/1 |
1/11 |
2/2 |
32.0 |
||||
|
Euphorbia nutans Lag. |
N |
1/1 |
1/1 |
2/11 |
16.0 |
||||||
|
Euphorbia ophthalmica Pers. |
N |
1/2 |
4/4 |
20.0 |
|||||||
|
Manihot chlorosticta Standl. & Goldman |
N* |
2/11 |
8.0 |
||||||||
|
Ricinus communis L. |
I |
1/2 |
1/4 |
1/1 |
1/2 |
16.0 |
|||||
|
Fabaceae |
Aeschynomene americana L. |
N |
1/4 |
4.0 |
|||||||
|
Chamaecrista rotundifolia (Pers.) Greene |
N |
1/4 |
1/1 |
8.0 |
|||||||
|
Coursetia caribaea (Jacq.) Lavin |
N |
1/11 |
4.0 |
||||||||
|
Crotalaria cajanifolia Kunth |
N |
1/2 |
1/4 |
1/1 |
1/2 |
16.0 |
|||||
|
Crotalaria pumila Ortega |
N |
1/11 |
4.0 |
||||||||
|
Desmodium procumbens (Mill.) Hitchc. |
N |
1/11 |
4.0 |
||||||||
|
Desmodium scorpiurus (Sw.) Poir. |
N |
1/4 |
2/2 |
12.0 |
|||||||
|
Indigofera miniata Ortega |
N |
1/11 |
4.0 |
||||||||
|
Macroptilium atropurpureum (DC.) Urb. |
N |
2/11 |
8.0 |
||||||||
|
Mimosa rosei B.L. Rob. |
N* |
1/11 |
4.0 |
||||||||
|
Phaseolus lunatus L. |
N |
1/2 |
1/2 |
8.0 |
|||||||
|
Rhynchosia minima (L.) DC. |
N |
1/1 |
4/11 |
1/2 |
24.0 |
||||||
|
Rhynchosia precatoria (Humb. & Bonpl. ex Willd.) DC. |
N |
1/2 |
4.0 |
||||||||
|
Senna obtusifolia (L.) H.S. Irwin & Barneby |
N |
1/1 |
1/11 |
8.0 |
|||||||
|
Senna uniflora (Mill.) H.S. Irwin & Barneby |
N |
3/11 |
12.0 |
||||||||
|
Vachellia pennatula (Schltdl. & Cham.) Seigler & Ebinger |
N |
1/11 |
4.0 |
||||||||
|
Vigna luteola (Jacq.) Benth. |
N |
1/11 |
4.0 |
||||||||
|
Zornia reticulata Sm. |
N |
1/1 |
4.0 |
||||||||
|
Lamiaceae |
Mesosphaerum suaveolens (L.) Kuntz |
N |
1/4 |
1/2 |
8.0 |
||||||
|
Salvia misella Kunth |
N |
3/4 |
12.0 |
||||||||
|
Stachys coccinea Ortega |
N |
1/4 |
1/2 |
8.0 |
|||||||
|
Loasaceae |
Gronovia scandens L. |
N |
1/11 |
8.0 |
|||||||
|
Mentzelia aspera L. |
N |
1/2 |
4.0 |
||||||||
|
Loranthaceae |
Struthanthus interruptus (Kunth) G. Don |
N |
1/2 |
4.0 |
|||||||
|
Lythraceae |
Cuphea leptopoda Hemsl. |
N |
1/11 |
4.0 |
|||||||
|
Malvaceae |
Abutilon trisulcatum (Jacq.) Urb. |
N |
2/11 |
8.0 |
|||||||
|
Anoda cristata (L.) Schltdl. |
N |
1/2 |
5/11 |
1/2 |
28.0 |
||||||
|
Corchorus aestuans L. |
N |
2/11 |
8.0 |
||||||||
|
Corchorus trilocularis L. |
I |
1/11 |
4.0 |
||||||||
|
Herissantia crispa (L.) Brizicky |
N |
4/11 |
1/2 |
20.0 |
|||||||
|
Kosteletzkya depressa (L.) O.J. Blanch., Fryxell & D.M. Bates |
N |
1/11 |
4.0 |
||||||||
|
Malachra alceifolia Jacq. |
N |
2/11 |
8.0 |
||||||||
|
Malvastrum coromandelianum (L.) Garcke |
N |
1/2 |
2/11 |
12.0 |
|||||||
|
Melochia pyramidata L. |
N |
1/4 |
1/1 |
4/11 |
24.0 |
||||||
|
Pseudabutilon umbellatum (L.) Fryxell |
N |
2/11 |
8.0 |
||||||||
|
Sida abutilifolia Mill. |
N |
1/2 |
4.0 |
||||||||
|
Sida rhombifolia L. |
N |
3/11 |
1/2 |
16.0 |
|||||||
|
Triumfetta semitriloba Jacq. |
N |
2/2 |
8.0 |
||||||||
|
Waltheria indica L. |
N |
2/11 |
8.0 |
||||||||
|
Martyniaceae |
Martynia annua L. |
N |
1/1 |
4.0 |
|||||||
|
Molluginaceae |
Mollugo verticillata L. |
N |
1/2 |
1/4 |
1/1 |
1/2 |
16.0 |
||||
|
Namaceae |
Nama jamaicensis L. |
N |
1/4 |
1/2 |
8.0 |
||||||
|
Nyctaginaceae |
Boerhavia coccinea Mill. |
N |
1/4 |
1/11 |
8.0 |
||||||
|
Boerhavia erecta L. |
N |
1/2 |
6/11 |
1/2 |
32.0 |
||||||
|
Salpianthus purpurascens (Cav. ex Lag.) Hook. & Arn. |
N |
1/2 |
4.0 |
||||||||
|
Onagraceae |
Ludwigia octovalvis (Jacq.) P.H. Raven |
N |
1/1 |
2/11 |
12.0 |
||||||
|
Orobanchaceae |
Castilleja arvensis Cham. & Schltdl. |
N |
1/1 |
4.0 |
|||||||
|
Oxalidaceae |
Oxalis corniculata L. |
N |
3/4 |
1/1 |
16.0 |
||||||
|
Papaveraceae |
Argemone mexicana L. |
N |
2/2 |
1/4 |
1/1 |
1/2 |
20.0 |
||||
|
Passifloraceae |
Passiflora foetida L. |
N |
1/1 |
4.0 |
|||||||
|
Pedaliaceae |
Sesamum indicum L. |
I |
1/11 |
4.0 |
|||||||
|
Petiveriaceae |
Petiveria alliacea L. |
N |
1/2 |
4.0 |
|||||||
|
Rivina humilis L. |
N |
1/11 |
4.0 |
||||||||
|
Phyllanthaceae |
Phyllanthus amarus Schumach. & Thonn. |
N |
1/1 |
2/11 |
12.0 |
||||||
|
Phyllanthus hexadactylus McVaugh |
NW |
1/11 |
4.0 |
||||||||
|
Plantaginaceae |
Mecardonia procumbens (Mill.) Small |
N |
2/4 |
1/1 |
1/2 |
16.0 |
|||||
|
Scoparia dulcis L. |
N |
1/4 |
1/1 |
8.0 |
|||||||
|
Stemodia verticillata (Mill.) Hassl. |
N |
1/2 |
4.0 |
||||||||
|
Plumbaginaceae |
Plumbago pulchella Boiss. |
N* |
1/2 |
4.0 |
|||||||
|
Polemoniaceae |
Loeselia amplectens (Hook. & Arn.) Benth. ex DC. |
N* |
1/2 |
4.0 |
|||||||
|
Loeselia ciliata L. |
N |
1/1 |
4.0 |
||||||||
|
Polygonaceae |
Rumex pulcher L. |
I |
1/1 |
4.0 |
|||||||
|
Portulacaceae |
Portulaca oleracea L. |
N |
1/2 |
1/4 |
1/1 |
1/1 |
1/11 |
1/2 |
24.0 |
||
|
Talinum paniculatum (Jacq.) Gaertn. |
N |
1/11 |
4.0 |
||||||||
|
Primulaceae |
Anagallis arvensis L. |
I |
2/4 |
8.0 |
|||||||
|
Rubiaceae |
Oldenlandia corymbosa L. |
N |
2/11 |
8.0 |
|||||||
|
Richardia scabra L. |
N |
1/2 |
4/4 |
1/1 |
1/1 |
3/11 |
1/2 |
44.0 |
|||
|
Spermacoce remota Lam. |
I |
4/4 |
1/1 |
1/11 |
1/2 |
28.0 |
|||||
|
Solanaceae |
Capsicum annuum L. |
N |
1/2 |
4.0 |
|||||||
|
Datura discolor Bernh. |
N |
1/2 |
4.0 |
||||||||
|
Physalis aggregata Waterf. |
N |
1/4 |
4.0 |
||||||||
|
Physalis cordata Mill. |
N |
1/11 |
4.0 |
||||||||
|
Physalis lassa Standl. & Steyerm. |
N |
1/1 |
4.0 |
||||||||
|
Physalis philadelphica Lam. |
N |
1/2 |
1/11 |
1/2 |
12.0 |
||||||
|
Physalis pubescens L. |
N |
1/4 |
1/1 |
8.0 |
|||||||
|
Solanum adscendens Sendtn. |
N |
1/11 |
4.0 |
||||||||
|
Solanum americanum Mill. |
N |
1/2 |
3/4 |
1/1 |
2/11 |
2/2 |
36.0 |
||||
|
Solanum angustifolium Mill. |
N |
1/1 |
1/11 |
8.0 |
|||||||
|
Solanum grayi Rose |
N* |
1/1 |
4.0 |
||||||||
|
Solanum houstonii Martyn |
N* |
1/11 |
4.0 |
||||||||
|
Solanum lycopersicum L. |
N |
1/2 |
2/4 |
1/1 |
1/11 |
20.0 |
|||||
|
Urticaceae |
Pilea microphylla (L.) Liebm. |
N |
1/2 |
4.0 |
|||||||
|
Verbenaceae |
Glandularia bipinnatifida (Nutt.) Nutt. |
N |
2/4 |
8.0 |
|||||||
|
Lantana camara L. |
N |
1/11 |
1/2 |
8.0 |
|||||||
|
Phyla nodiflora (L.) Greene |
N |
1/11 |
4.0 |
||||||||
|
Priva lappulacea (L.) Pers. |
N |
5/11 |
2/2 |
28.0 |
|||||||
|
Verbena carolina L. |
N |
3/4 |
12.0 |
||||||||
|
Violaceae |
Hybanthus attenuatus (Humb. & Bonpl. ex Willd.) Schulze-Menz |
N |
1/2 |
1/2 |
8.0 |
||||||
|
Vitaceae |
Cissus verticillata (L.) Nicolson & C.E. Jarvis |
N |
1/11 |
1/2 |
8.0 |
||||||
|
Zygophyllaceae |
Kallstroemia grandiflora Torr. ex A. Gray |
N |
3/11 |
1/2 |
16.0 |
||||||
|
Kallstroemia maxima (L.) Hook. & Arn. |
N |
1/1 |
3/11 |
16.0 |
|||||||
|
Kallstroemia parviflora Norton |
N |
1/11 |
4.0 |
||||||||
|
Kallstroemia rosei Rydb. |
N* |
1/2 |
4.0 |
||||||||
|
Tribulus cistoides L. |
I |
1/11 |
4.0 |
Appendix 3. Some weeds in Colima.
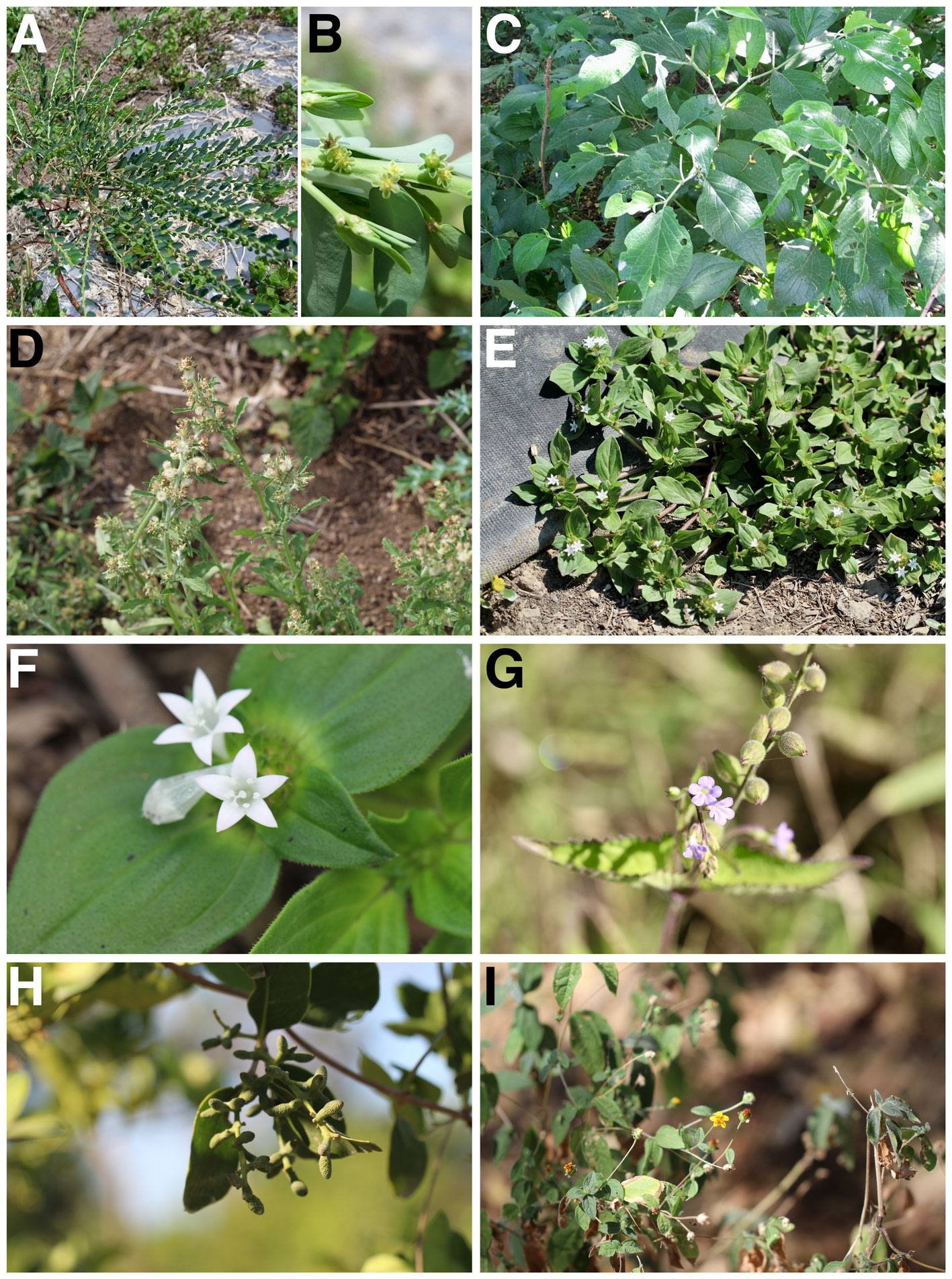
Part 1. A) Acalypha aristata, general view. B-D) Amaranthus palmeri. B) General view. C) Detail of a female inflorescence. D) Detail of a male inflorescence. E) Anoda cristata, general view and flower. F) Castilleja arvensis, general view and inflorescence. G) Boerhavia erecta, detail of an inflorescence. H) Corynandra viscosa, general view and inflorescence.
Appendix 3. Continued.
Part 2. A) Euphorbia heterophylla, detail of an inflorescence. B) E. hirta, general view. C) Evolvulus alsinoides, general view. D) Glandularia bipinnatifida, general view. E) Heliotropium procumbens, general view. F) Lemna aequinoctialis, general view. G) Marchantia polymorpha, general view. H) Melampodium tepicense, general view and flowers.
Appendix 3. Continued.
Part 3. A-B) Phyllanthus hexadactylus. A) General view. B) Detail of the flowers. C) Piper hispidum, general view and inflorescence. D) Pseudognaphalium viscosum, general view and inflorescence. E-F) Richardia scabra. E) General view of the plant. F) Detail of flowers. G) Priva lappulacea, general view and inflorescence. H) Struthanthus interruptus, general view and fruits. I) Tridax dubia, general view.
References
Aarestrup, J., Karam, D., Corrêa, E., & Fernandes, G. (2008). Análise da viabilidade de sementes de Euphorbia heterophylla. Planta Daninha, 26, 515–519. https://doi.org/10.1590/s0100-83582008000300006
Baker, H. G. (1974). The evolution of weeds. Annual Review of Ecology and Systematics, 5, 1–24.
Blanckaert, I., Vancraeynest, K., Swennen, R. L., Espinosa-García, F. J., Piñero, D., & Lira-Saade, R. (2007). Non-crop resources and the role of indigenous knowledge in semi-arid production of Mexico. Agriculture, Ecosystems and Environment, 119, 39–48. https://doi.org/10.1016/j.agee.2006.06.015
Blanco, Y., & Leyva, Á. (2007). Las arvenses en el agroecosistema y sus beneficios agroecológicos como hospederas de enemigos naturales. Cultivos Tropicales, 28, 21–28.
Booth, B. D., & Swanton, C. J. (2002). Assembly theory applied to weed communities. Weed Science, 50, 2–13. https://doi.org/10.1614/0043-1745(2002)050[0002:aiatat]2.0.co;2
Boyle, B., Hopkins, N., Lu, Z., Raygoza, G. J. A., Mozzherin, D., Rees, T. et al. (2013). The taxonomic name resolution service: an online tool for automated standardization of plant names. BMC Bioinformatic, 14, 16. https://doi.org/10.1186/1471-2105-14-16
Campiglia, E., Radicetti, E., & Mancinelli, R. (2018). Floristic composition and species diversity of weed community after 10 years of different cropping systems and soil tillage in a Mediterranean environment. Weed Research, 58, 273–283. https://doi.org/10.1111/wre.12301
Ceballos, G., Martínez, L., García, A., Espinoza, E., Bezaury-Creel, J., & Dirzo, R. (2010). Diversidad, amenazas y áreas prioritarias para la conservación de las selvas secas del pacífico de México. Ciudad de México: Fondo de Cultura Económica/ Conabio.
Chahouki, M. A. Z. (2012). Classification and ordination methods as a tool for analyzing of plant communities. In L. Freitas, & A. P. Barbosa (Eds.), Multivariate analysis in management, engineering and the sciences (pp. 221–254). London: IntechOpen.
Colorado-Zuluaga, G. J. (2015). How ecological communities are structured: a review on ecological assembly rules. Revista EIA, 12, 27–53.
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2016). La biodiversidad en Colima, estudio de Estado. Ciudad de México: Conabio.
Conafor (Comisión Nacional Forestal). (2014). Inventario estatal forestal y de suelos Colima 2013. Ciudad de México: Semarnat – Conafor.
Cullen, J. (2006). Practical plant identification. Including a key to native and cultivated flowering plants in north temperate regions. Cambridge, UK: Cambridge University Press.
Datar, V. V. (2012). Integrated management of Papaya ring spot virus in Papaya (Carica papaya). Indian Phytopathology, 65, 12–17.
Espinosa-García, F. J., & Sarukhán, J. (1997). Manual de malezas del Valle de México. Ciudad de México: Universidad Nacional Autónoma de México/ Fondo de Cultura Económica.
Espinosa-García, F. J., & Villaseñor, J. L. (2017). Biodiversity, distribution, ecology and management of non-native weeds in Mexico: a review. Revista Mexicana de Biodiversidad, 88, 76–96. https://doi.org/10.1016/j.rmb.2017.10.010
Espinosa-García, F. J., Villaseñor, J. L., & Vibrans, H. (2004a). The rich generally get richer, but there are exceptions: Correlations between species richness of native plant species and alien weeds in Mexico. Diversity and Distributions, 10, 399–407. https://doi.org/10.1111/j.1366-9516.2004.00099.x
Espinosa-García, F. J., Villaseñor, J. L., & Vibrans, H. (2004b). Geographical patterns in native and exotic weeds of Mexico. Weed Technology, 18, 1552–1558. https://dx.doi.org/10.1016/j.rmb.2017.10.010
Espinosa-García, F. J., Villaseñor, J. L., & Vibrans, H. (2009). Mexico: Biodiversity, distribution, and possible economic impact of exotic weeds. In T. R. Van Denver, F. J. García-Espinosa, & B. L. Harper-Lore (Eds.), Invasive plants on the move: controlling them in North America (pp. 43–52). Tucson: Arizona-Sonora Desert Museum.
Ferreira, D. T., Da Silva, I. C., Da Silva, V. M., Endres, L., De Souza, R. C., & Ferreira, V. M. (2017). Análise de crescimento de espécies daninhas do gênero Euphorbia. Revista Agro@mbiente On-Line, 11, 145. https://doi.org/10.18227/1982-8470ragro.v11i2.3851
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. The International Journal of Climatology, 37, 4302–4315. https://doi.org/10.1002/joc.5086
Flores-Huitzil, E. C., Coombes, A. J., & Villaseñor, J. L. (2020). Las angiospermas ruderales del municipio Coronango, Puebla, México. Acta Botanica Mexicana, 127, e1601. https://doi.org/10.21829/abm127.2020.1601
García, E. (2004). Modificaciones al sistema de clasificación climática de Köppen. Ciudad de México: Instituto de Geografía-UNAM.
González-Amaro, R. M., Martínez-Bernal, A., Basurto-Peña, F., & Vibrans, H. (2009). Crop and non-cop productivity in a traditional maize agroecosystem of the highland of Mexico. Journal of Ethnobiology and Ethnomedicine, 5, 38. https://doi.org/10.1186/1746-4269-5-38
Gomaa, N. H. (2012). Composition and diversity of weed communities in Al-Jouf province, northern Saudi Arabia. Saudi Journal of Biological Sciences, 19, 369–376. http://dx.doi.org/10.1016/j.sjbs.2012.05.002
Götzenberger, L., de Bello, F., Bråthen, K. A., Davison, J., Dubuis, A., Guisan, A. et al. (2011). Ecological assembly rules in plant communities-approaches, patterns and prospects. Biological Reviews, 87, 111–127. https://doi.org/10.1111/j.1469-185x.2011.00187.x
Hanan, A. A. M., Vibrans, H., Cacho, N. I., Villaseñor, J. L., Ortíz, E., & Gómez-G, V. A. (2015). Use of herbarium data to evaluate weediness in five congeners. AoB Plants, 8, plv144. https://doi.org/10.1093/aobpla/plv144
Hijmans, R. J., Phillips, S., Leathwick, J., & Elith, J. (2017). dismo: Species distribution modeling. Accessed 03 October 2020: https://CRAN.R-project.org/package=dismo
Holzner, W. (1978). Weed species and weed communities. Vegetatio, 38, 13–20. https://doi.org/10.1007/BF00141295
Holzner, W. (1982). Concepts, categories and characteristics of weeds. In W. Holzner, & N. Numada (Eds.), Biology and ecology of weeds (pp. 3–20). Dordrecht: Springer.
INEGI (Instituto Nacional de Estadística y Geografía). (2013). Uso de suelo y vegetación. Accessed 01 March 2020: https://www.inegi.org.mx/temas/usosuelo/
IPNI (International Plant Names Index). (2020). The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. Accessed 20 Septembre, 2020: http://www.ipni.org
León-de la Luz, J. L., Domínguez-León, M., & Van Denver, T. R. (2009). Baja California Sur: Native, exotic, and invasive weeds. In T. R. Van Denver, F. J. García-Espinosa, & B. L. Harper-Lore (Eds.), Invasive plants on the move: controlling them in North America (pp. 125–136). Tucson: Arizona-Sonora Desert Museum.
Levin, G. A., & Gillespie, L. J. (2016). Euphorbiaceae Jussieu. In Flora of North America Editorial Committee (Ed.), Flora of North America North of Mexico. New York: Oxford University Press.
Martínez-Cruz, J., & Ibarra-Manríquez, G. 2012. Áreas prioritarias de conservación para la flora leñosa del estado de Colima, México. Acta Botanica Mexicana, 99, 31–53. https://doi.org/10.21829/abm99.2012.18
Martínez-De La Cruz, I., Vibrans, H., Lozada-Pérez, L., Romero-Manzanares, A., Aguilera-Gómez, L. I., & Rivas-Manzano, I. V. (2015). Plantas ruderales del área urbana de Malinalco, Estado de México, México. Botanical Sciences, 93, 907–919. https://doi.org/10.17129/botsci.213
McVaugh, R., & Anderson, W. R. (1984). Flora Novo-Galiciana: a descriptive account of the vascular plants of Western Mexico, Volume 12 Compositae. Ann Arbor: University of Michigan Press.
Moreno-Gómez, S., Cuevas-Guzmán, R., Núñez-López, N. M., & Solís-Magallanes, J. A. (2016). Guía de árboles de la selva baja caducifolia de la microcuenca La Salada, Colima. Autlán de Navarro, Jalisco: Universidad de Guadalajara.
Morrone, J. J. (2019). Regionalización biogeográfica y evolución biótica de México: Encrucijada de la biodiversidad del nuevo mundo. Revista Mexicana de Biodiversidad, 90, e902980. https://doi.org/10.22201/ib.20078706e.2019.90.2980
Nagy, K., Lengyel, A., Kovács, A., Türey, D., Csergö, A. M., & Pinke, G. (2017). Weed species composition of small-scale farmlands bears a strong crop-related and environmental signature. Weed Research, 58, 46–56. https://doi.org/10.1111/wre.12281
Nebreda, M., Moreno, A., Pérez, N., Palacios, I., Seco-Fernández, V., & Fereres, A. (2004). Activity of aphids associated with lettuce and broccoli in Spain and their efficiency as vectors of Lettuce mosaic virus. Virus Research, 100, 83–88. https://doi.org/10.1016/j.virusres.2003.12.016
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D. et al. (2019). vegan: Community ecology package. Assesed 25 September 2020: https://CRAN.R-project.org/package=vegan
Orozco-Santos, M. (2001). El cultivo de tamarindo (Tamarindus indica L.) en el trópico seco de México. Tecomán, Colima: Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación.
Orozco-Santos, M., & Farías-Larios, J. (2014). El limón mexicano (Citrus aurantifolia). Manejo integrado de malezas. Tecomán, Colima: Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias.
Padilla-Velarde, E., Cuevas-Guzmán, R., Ibarra-Manríquez, G., & Moreno-Gómez, S. (2006). Riqueza y biogeografía de la flora arbórea del estado de Colima, México. Revista Mexicana de Biodiversidad, 77, 271–295. http://dx.doi.org/10.22201/ib.20078706e.2006.002.337
Petit, S., Alignier, A., Colbach, N., Joannon, A., Le, Cœur, D., & Thenial, C. (2013). Weed dispersal by farming at various spatial scales. A review. Agronomy for Sustainable Development, 33, 205–217. https://doi.org/10.1007/s13593-012-0095-8
Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E., & Blair, M. E. (2017). Opening the black box: an open-source release of Maxent. Ecography, 40, 887–893. https://doi.org/10.1111/ecog.03049
Poggio, S. L. (2012). Cambios florísticos en comunidades de malezas: un marco conceptual basado en reglas de ensamblaje. Ecología Austral, 22, 150–158.
Pruski, J. F., & Robinson, H. E. (2018). Asteraceae. In G. Davidse, M. Sousa, S. Knapp, & F. Chiang (Eds.), Flora Mesoamericana 5(2) (pp. 1–608). St. Louis, USA: Universidad Nacional Autónoma de México/ Missouri Botanical Garden/ The Natural History Museum (London).
Quintero-Pertúz, I., Carbonó-Delahoz, E., & Jarma-Orozco, A. (2018). Weeds associated with banana crops in Magdalena Deparment, Colombia. Planta Daninha, 38, e020217466. https://doi.org/10.1590/S0100-83582020380100015
R Core Team. (2019). R: a language and environment for statistical computing. Accessed 10 November 2019: https://www.r-project.org/
Ruiz-Villarreal, E. (2016). Árboles de Minatitlán, Colima. Guía de usos tradicionales. Colima: PACMYC/ Gobierno del estado de Colima.
Sánchez-Ken, J. G., Zita, G. A., & Mendoza, M. (2012). Catálogo de las gramíneas malezas nativas e introducidas de México. Ciudad de México: Conacofi-Sagarpa.
Sanginés, L., Dávila, P., Solano, L., & Pérez-Gil, R. F. (2014). Arvenses de cafetal: identificación, evaluación química y comportamiento etológico de ovinos en pastoreo. Ecosistemas y Recursos Agropecuarios, 1, 249–260.
Santos-Martins, D., Ventura, J. A., Paula, R. C. A. L., Fornazier, M. J., Rezende, J. A. M., Culik, M. P. et al. (2016). Aphid vectors of Papaya ringspot virus and their weed hosts in orchards in the major papaya producing and exporting region of Brazil. Crop Protection, 90, 191–196. https://doi.org/10.1016/j.cropro.2016.08.030
SIAP (Servicio de Información Agroalimentaria y Pesquera). (2018). Colima infografía agroalimentaria 2018. Ciudad de México: SIAP-Sagarpa.
Simpson, M. G. (2019). Plant systematics. San Diego: Academic Press-Elsevier.
Sosa, V., De-Nova, J. A., & Vásquez-Cruz, M. (2018). Evolutionary history of the flora of Mexico: Dry forests cradles and museums of endemism. Journal of Systematic and Evolution, 56, 523–536. https://doi.org/10.1111/jse.12416
Storkey, J., Moss, S. R., & Cussans, J. W. (2010). Using assembly theory to explain changes in a weed flora in response to agricultural intensification. Weed Science, 58, 39–46. https://doi.org/10.1614/WS-09-096.1
Suárez, S. A., de la Fuente, E. B., Ghersa, C. M., & León, R. J. C. (2001). Weed community as an indicator of summer crop yield and site quality. Agronomy Journal, 93, 524–530.
Tanver, A., Khaliq, A., Javaid, M., Chaudhry, M., & Awan, I. (2013). Implications of weeds of the genus Euphorbia for crop production: A review. Planta Daninha, 31, 723–731. https://doi.org/10.1590/s0100-83582013000300024
The Plant List (2013). Version 1.1. published on the internet. Accessed 20 September 2020: http://www.theplantlist.org
Thiers, B. M. (2020, continously updated). Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Accessed 09 March 2020: http://sweetgum.nybg.org/ih/
Tropicos (2020). Tropicos.org. Missouri Botanical Garden. Accessed 20 Septembre, 2020: http://www.tropicos.org
Velarde, E. P., Guzmán, R. C., & Koch, S. D. (2008). Plantas vasculares y vegetación de la parte alta del arroyo Agua Fría, municipio de Minatitlán, Colima, México. Acta Botanica Mexicana, 84, 25–72. https://doi.org/10.21829/abm84.2008.1066
Vera-Sánchez, K. S., & Nassar, N. (2019). Manihot chlorosticta. The IUCN Red List of Threatened Species 2019: e.T20752627A20755986. Accessed 02 April 2020: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T20752627A20755986.en
Viana, D. S., Santamaría, L., & Figuerola, J. (2016). Migratory birds as global dispersal vectors. Trends in Ecology & Evolution, 31, 763–775. http://dx.doi.org/10.1016/j.tree.2016.07.005
Vibrans, H. (1998). Native maize field weed communities in south-central Mexico. Weed Research, 38, 153–166. http://dx.doi.org/10.1046/j.1365-3180.1998.00082.x
Vibrans, H. (2012). Malezas de México. Accessed 17 January 2019: http://www.conabio.gob.mx/malezasdemexico/2inicio/home-malezas-mexico.htm
Vieyra-Odilon, L., & Vibrans, H. (2001). Weeds as crops: the value of maize field weed in the valley of Toluca, Mexico. Economic Botany, 53, 426–443. https://doi.org/10.1007/BF02866564
Villaseñor, J. L. (2016). Checklist of the native vascular plants of Mexico. Revista Mexicana de Biodiversidad, 87, 559–902. https://doi.org/10.1016/j.rmb.2016.06.017
Villaseñor, J. L., & Espinosa-García, F. J. (1998). Catálogo de malezas de México. Ciudad de México: Universidad Nacional Autónoma de México/ Fondo de Cultura Económica.
Villaseñor, J. L., & Espinosa-García, F. J. (2004). The alien flowering plants of Mexico. Diversity and Distributions, 10, 113–123. https://doi.org/10.1111/j.1366-9516.2004.00059.x