First reports of Zoophthora radicans (Entomophthoraceae) and Metarhizium rileyi (Clavicipitaceae) as pathogens of Plutella xylostella (Lepidoptera: Plutellidae) in Argentina
Romina G. Manfrino *, Alejandra C. Gutierrez, Claudia C. López-Lastra
Centro de Estudios Parasitológicos y de Vectores, Universidad Nacional de La Plata- Consejo Nacional de Investigaciones Cientificas, Boulevard 120, entre 61 y 62, La Plata, 1900 Buenos Aires, Argentina
* Corresponding author: manfrino@cepave.edu.ar (R.G. Manfrino)
Abstract
Plutella xylostella (L.), diamondback moth (DBM), is considered among the 20 most resistant arthropods in the world. Entomopathogenic fungi are important mortality factors of Lepidoptera in the field. However, in Argentina entomopathogenic fungi as mortality factors of P. xylostella have not been identified. The aim of this study was to identify and isolate fungal pathogens of Plutella xylostella in Santa Fe, Argentina. The prevalence and seasonality of fungal infections were also investigated. Zoophthora radicans (Bref.) A. Batko (Entomophthoromycota: Entomophthorales) and Metarhizium rileyi (Farl.) Kepler, Rehner & Humber (Ascomycota: Hypocreales) as fungal pathogens of Plutella xylostella L. are reported for the first time in Argentina. The pathogens were identified from larvae, pupae and adults of P. xylostella on cauliflower and on cabbage. Zoophthora radicans was the predominant fungal pathogen, while M. rileyi was recorded from only one infected larva.
Keywords:
Diamondback moth; Fungal pathogens; Prevalence; Seasonality
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Primeros registros de Zoophthora radicans (Entomophthoraceae) y Metarhizium rileyi (Clavicipitaceae) como patógenos de Plutella xylostella (Lepidoptera: Plutellidae) en Argentina
Resumen
Plutella xylostella (L.), la polilla de las coles, es considerada entre los 20 artrópodos más resistentes en el mundo. Los hongos entomopatógenos son importantes factores de mortalidad de lepidópteros en el campo. Sin embargo, en Argentina no han sido identificados hongos entomopatógenos como factores de mortalidad de P. xylostella. El objetivo de este estudio fue identificar y aislar hongos patógenos de Plutella xylostella en Santa Fe, Argentina. La prevalencia y la estacionalidad de las infecciones fúngicas fue también investigada. Zoophthora radicans (Bref.) A. Batko (Entomophthoromycota: Entomophthorales) y Metarhizium rileyi (Farl.) Kepler, Rehner & Humber (Ascomycota: Hypocreales) como hongos patógenos de Plutella xylostella L. son registrados por primera vez en Argentina. Los patógenos fueron aislados e identificados desde larvas, pupas y adultos de P. xylostella en repollo y coliflor. Zoophthora radicans fue el hongo patógeno predominante mientras que M. rileyi fue registrado infectando una única larva.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Polilla de las coles; Hongos patógenos; Prevalencia; Estacionalidad
Introduction
Plutella xylostella (L.), diamondback moth (DBM), is the most destructive pest of Brassicaceae crops (Talekar & Shelton, 1993) and is considered among the 20 most resistant arthropods in the world (Mota-Sánchez et al., 2002). The extraordinary ability of this insect to develop resistance to various active ingredients of chemicals used against it means that other approaches are essential for the long-term management of this destructive pest.
Microbial pest control agents are welcome additions to Integrated Pest Management programs by their specificity against target pests and minimal environmental impacts. Entomopathogenic fungi are important mortality factors of lepidopteran in the field. Several species of fungal pathogens have been isolated from DBM (Cherry et al., 2004; Kirk et al., 2004; Vandenberg et al., 1998), but few have been studied in detail.
Zoophthora radicans (Bref.) A. Batko is an entomophthoralean fungus that has been reported to cause natural epizootics in several different insect species, including P. xylostella (Galaini-Wraight et al., 1991; Vandenberg & Soper, 1987). Metarhizium rileyi (Farl.) Kepler, S.A Rehner & Humber (Ascomycota: Hypocreales) is able to cause epizootics and dramatically reduce pest populations, as has been recorded in the U.S., Brazil and Australia (Corrêa & Smith, 1975).
In Argentina Bertolaccini et al. (2010) have mentioned entomopathogenic fungi as mortality factors of P. xylostella but the pathogenic species have not been identified. Thus, the aim of the present study was to isolate and identify the pathogenic fungi of P. xylostella, as well as to determine the prevalence of fungal infections.
Materials and methods
Surveys were conducted in conventional crop production in Monte Vera ((31º32’58.21” S, 60º41’34.74” W), Santa Fe province, Argentina. Brassica oleracea L. var. botrytis (cauliflower) and B. oleracea L. var. capitata (cabbage) were surveyed during the time of crop production. On the cauliflower crop, the survey was made from April 08 to July 20, 2011, whereas on the cabbage crop, the survey was made from March 29 to May 27, 2011. Surveys were conducted from planting to harvest.
Plutella xylostella sampling: P. xylostella populations were sampled weekly. Twenty plants of each crop were randomly selected and checked to quantify for larvae, pupae and adults of P. xylostella. Dead P. xylostella with evidence of external fungal growth (showing sporulation) were collected. Dead P. xylostella without external signs of mycosis were also collected and placed in Petri dishes (60 mm in diameter) with a filter paper moistened with a few drops of distilled water (humid chambers) and maintained at 20 °C for 24-72 h to allow the development of overt mycoses.
To obtain pure cultures, P. xylostella infected with Z. radicans were placed on a moistened piece of sterile filter paper attached with double coated tape to the lid of a sterile 60 mm Petri dish, and then the lid was inverted over the bottom of a sterile Petri dish containing SEMA (80% Sabouraud dextrose agar and 20% of a mixture of egg yolk and skim milk) (Papierok & Hajek, 2012) plus 40,000 units/mL penicillin G (Merckâ, Germany) and 80,000 units/ml streptomycin (Parafarmâ, Argentina). This assembly was left for 12 h in darkness at 22 ± 1 ºC. The lid with the attached P. xylostella was replaced with a sterile lid after 12 h. Metarhizium rileyi was isolated on Sabouraud maltose agar + 1% yeast extract (SMAY). All isolates were incubated at 22 ± 1 ºC with a photoperiod of 16:8 (L: D). Isolates were deposited in the Mycological Collections at the Centro de Estudios Parasitológicos y de Vectores (CEP, La Plata, Argentina). Herbarium materials such as dried infected specimens and microscope slides were also deposited in the Mycological Collections at the CEP (La Plata, Argentina).
Infected P. xylostella were examined under a Zeiss DV4 dissecting stereomicroscope. Mycelia of M. rileyi were mounted in lactophenol/cotton blue (0.01% w/v), while fungal structures of Z. radicans were mounted in aceto-orcein (LPAO) (1:1) or stained with 1% aceto-orcein plus glycerine for semipermanent mounts. Fungal preparations were observed by phase contrast with an Olympus CH3 microscope and were photographed using a Nikon Optiphot microscope equipped with differential interference contrast (DIC) fitted with a Canon PowerShot A80 camera. Measurements of length and width of fungal structures (conidia, conidiogenous cells and mycelia) from fresh infected cadavers were made to enable specific identification. Metarhizium rileyi was identified according to taxonomic keys and monographs in Samson (1974), Samson et al. (1988) and Zare and Gams (2001) and Z. radicans according to taxonomic keys and monographs of Bałazy (1993), Humber (2012) and Keller (1991).
The numbers of healthy and infected P. xylostella in each development stage (larvae, pupae and adults) were compared by parametric Anova and Tukey’s (HSD) post hoc test with p = 0.05 after log transformation of data. The Mann-Whitney U-test was used to compare the density of healthy and infected P. xylostella between crops and between developmental stages. Analyses were performed using InfoStat statistical software (2004).
Results
Zoophthora radicans and M. rileyi were the pathogens identified as mortality factors of P. xylostella. Zoophthora radicans was the predominant fungal pathogen on both crops and was recorded infecting larvae and pupae. One adult of P. xylostella infected with Z. radicans was also recorded. Infected adult was found in a lettuce crop located adjacent to the cabbage crop. Metarhizium rileyi was recorded from only one infected larva.
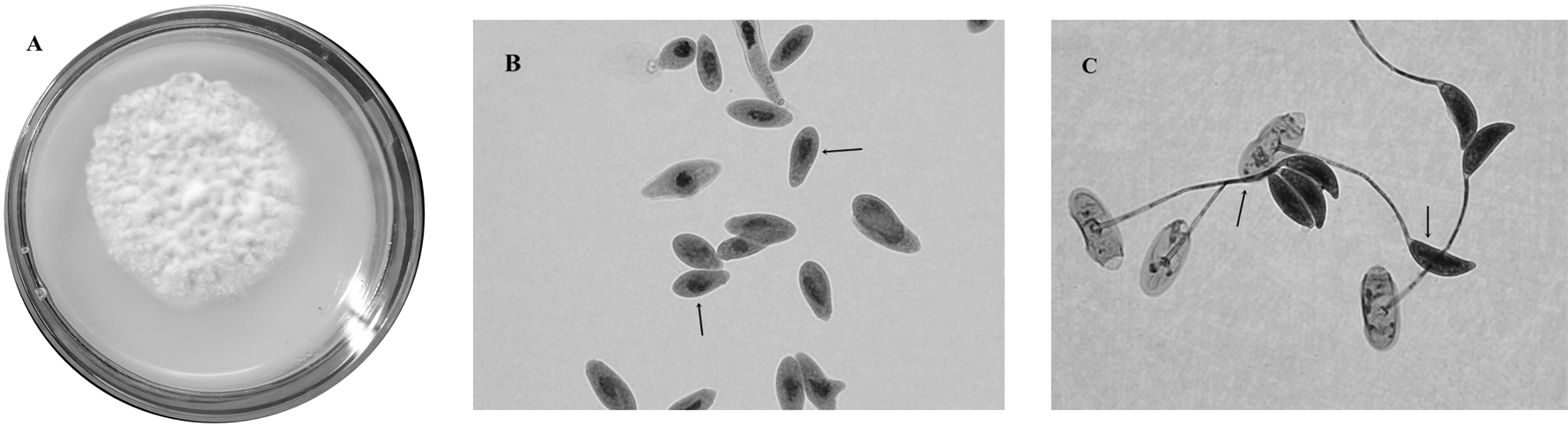
Zoophthora radicans (Bref.) A. Batko, Bull. Acad. Polon. Sci., Cl. II. sér. sci. biol. 12: 323 (1964) (Fig. 1).
=Synonymy:
Empusa radicans Bref. 1870
Classification: Zygomycota, Entomophthoromycotina, Entomophthorales, Entomophthoraceae.
Herbarium access number: CEP 50 (specimen number)
Description. Microscopic characters: conidia uninucleate, primary conidia elongate, papilla generally conical demarcated with a slight protuberance from the body of the conidia: 20.15 ± 2.32 (15.67–24.02) in length × 6.19 ± 0.83 (4.94-7.44) µm in width. Secondary conidia as capilliconidia: 20.04 ± 1.83 (18.89-24.4) × 7.09 ± 0.54 (6.39-7.93) × 46.95 ± 9.70 (35.81-60.31) µm. Thin rhizoids compounds with specialized adhesive disc. No resistance spores were observed. Macroscopic characters: colony diameters reached 20 mm in 10 d on SMYA at 24 ºC in darkness, rather compact, white, with clear yellow on reverse. Isolated but not maintained in pure cultures.
Metarhizium rileyi (Farl.) Kepler, Rehner & Humber, in Kepler, Humber, Bischoff & Rehner, Mycologia 106 (4): 824 (2014) (Fig. 2).
=Synonymy:
Botrytis rileyi Farl. 1883
Classification: Ascomycota, Pezizomycotina, Sordariomycetes, Hypocreomycetidae, Hypocreales, Cordycipitaceae.
Culture collection access number: CEP 367
Description. Microscopic characters: conidiogenous cells usually subglobose to short cylindrical or ampulliform with a globose base, 4.34 µm ± 0.69 (3.4-5.3 µm) in length x 1.8 µm ± 0.23 (1.4-2.2 µm) in width with necks short, smooth-walled. Conidia strongly ellipsoidal, smooth-walled, hyaline 4.2 µm ± 0.47 (3.6-5.3 µm) in length x 2.1 µm ± 0.21 (1.7-2.4 µm) in width (average). Hyphae septate, smooth-walled, hyaline 1.8 µm ± 0.33 (1.0-2.2 µm) wide occasionally branched. Macroscopic characters: colonies growing up to 33.6 mm in diameter after 10 d at 25 ºC. Circular colonies, with regular borders, reverse white to slightly yellowish. Spore colonies malachite green in according to the color chart Rayner (1970). Isolated and maintained in pure cultures.

Population variations: the averages of healthy and infected P. xylostella on each crop were calculated per sample date and are shown in Table 1.
On cabbage, the peak in population density of P. xylostella was observed on April 8, 2011 (X¯ = 1.70 insects/plant), and the first infected insects were observed on May 13, 2011. Significant differences were not recorded in the number of infected-P. xylostella among sampling dates (p > 0.05) (Table 1). The highest prevalence of fungal infection (40%, n = 5) was observed on May 19, 2011 and occurred with 18.1 ºC temperature and 87% relative humidity (Fig. 3). After this date, there was a sharp decline in the number of healthy and infected insects.
On cauliflower, the peak in population density of P. xylostella was observed on May 13, 2011 (X¯ = 1.25 insects/plant) (Table 1). There were significant differences between study weeks on the number of infected-P. xylostella (p < 0.05) (Table 1). The first infected insects were observed on May 5, 2011, and the highest prevalence of fungal infection (90%, n = 10) was observed on May 27, 2011 (Table 1). Also, important prevalences were recorded until the end of the crop cycle. The highest percentage of infection occurred with 12.8 ºC temperature and 82% relative humidity (Fig. 4).
The number of infected insects was significantly different among both crops (p < 0.05; Mann Whitney U = 4,612.0). On cabbage, there were significant differences in the abundance of infected-P. xylostella between developmental stages (larvae and pupae) (p < 0.05; Mann Whitney U = 756.00). On cauliflower, the abundance of infected insects among larvae and pupae were not significantly different (p > 0.05; Mann Whitney U = 1,515.00).
Discussion
In Argentina there are reports of Z. radicans and M. rileyi as fungal pathogens of lepidopteran moths. López-Lastra and Scorsetti (2006) have recorded Z. radicans as a pathogen of Epinotia aporema (Walsingham) (Lepidoptera: Tortricidae) and Tuta absoluta Meyrick (Lepidoptera: Gelechiidae), while M. rileyi has been identified from Anticarsia gemmatalis (Hübner) on soybean crops (Toledo et al., 2004). Zoophthora radicans and M. rileyi as pathogens of P. xylostella are first records for Argentina.
In this study, we recorded infection levels of Z. radicans of 40 to 90% on P. xylostella. Aragón et al. (1997) cited prevalences of 40 to 80% of Pandora gammae (Entomophthoraceae) on Rachiplusia nu (Guen) on soybean crops.
Entomopathogenic fungi possess distinct advantages over other microbial control agents because they are able to attack all the developmental stages of insects, including pupal stages (Ferron, 1978). Zoophthora radicans infects insects of several orders and is a widespread pathogen of diamondback moths, usually attacking larvae but also sometimes pupae (Kanervo, 1946). In the present study, we found infections of Z. radicans on larvae and pupae of P. xylostella. The prevalence was higher in larvae than in pupae probably because the thick and sclerotized cuticle of the pupa is a major barrier to fungal infection (De La Rosa et al., 2002; Hajek & St. Leger, 1994).
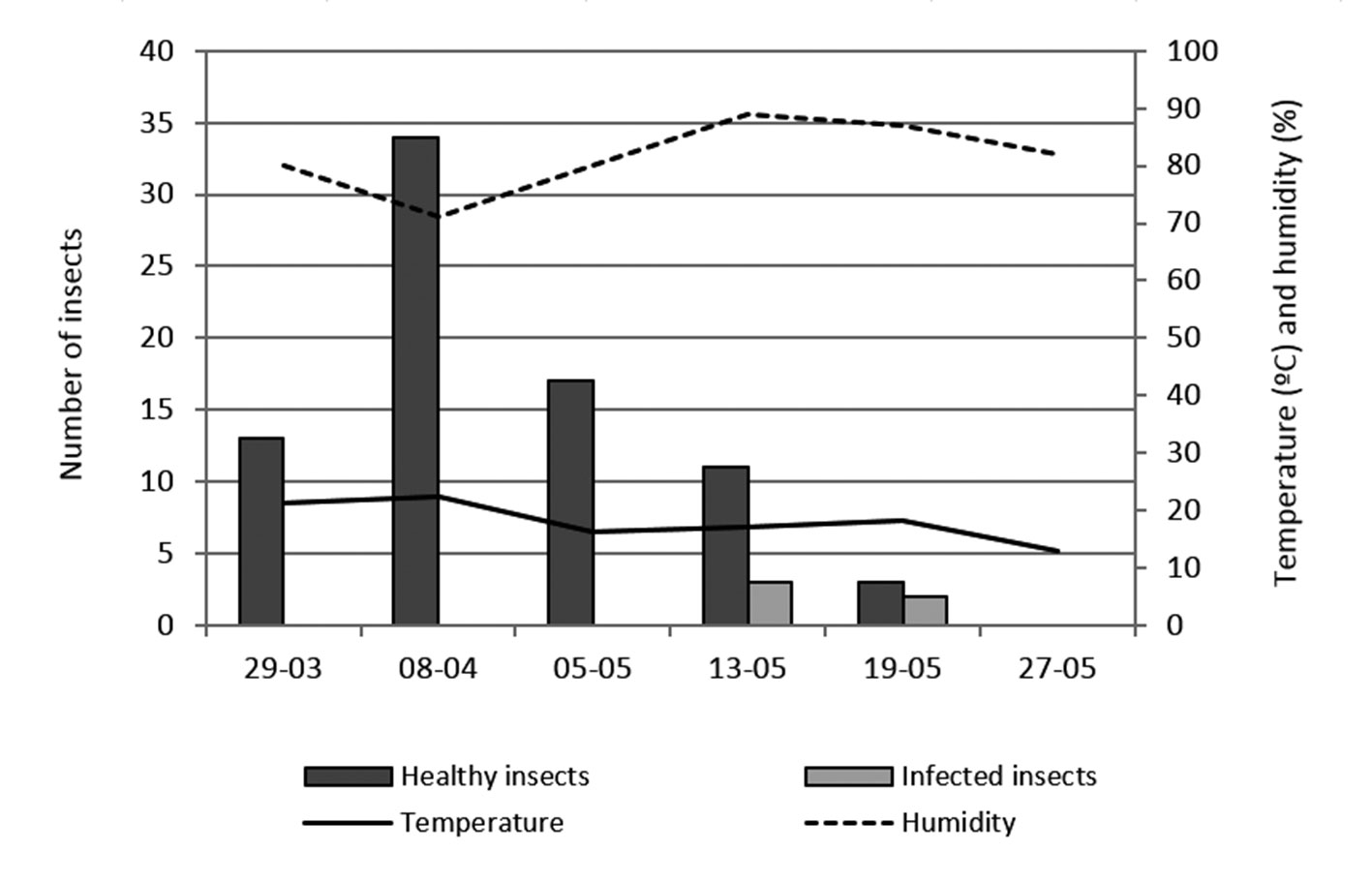
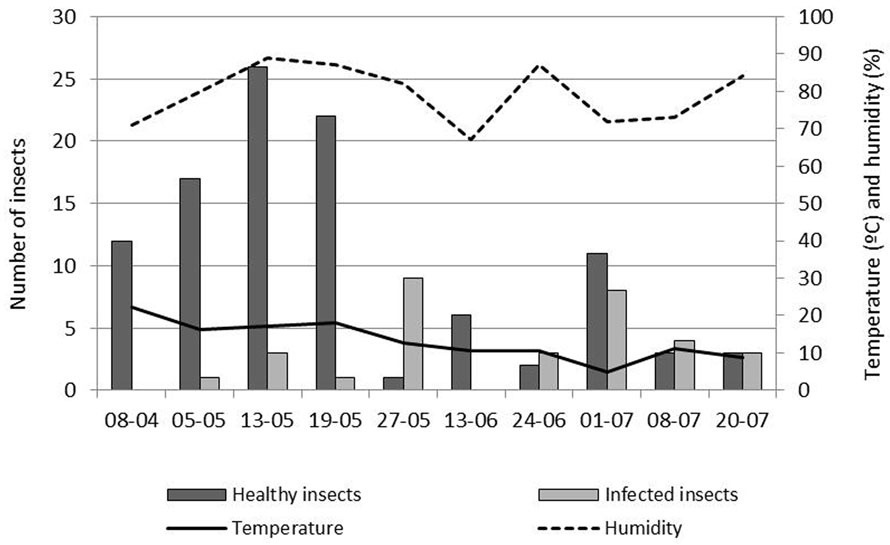
Table 1
Mean number of healthy and infected P. xylostella and percentage of infection by sampling date on each cropa.
|
Crops |
N |
Sample date |
Healthy P. xylostella |
Infected P. xylostella |
Percentage of infection |
|
Brassica oleracea var. capitata (cabbage) |
13 |
29.03.2011 |
0.65 (0.74) a |
0.00 (0.00) a |
0 |
|
34 |
08.04.2011 |
1.70 (1.86) b |
0.00 (0.00) a |
0 |
|
|
17 |
05.05.2011 |
0.80 (1.64) a |
0.00 (0.00) a |
0 |
|
|
14 |
13.05.2011 |
0.55 (1.05) a |
0.10 (0.30) a |
21.4 |
|
|
5 |
19.05.2011 |
0.15 (0.36) a |
0.10 (0.30) a |
40 |
|
|
Brassica oleracea var. botrytis (cauliflower) |
12 |
08.04.2011 |
0.65 (0.93) abd |
0.00 (0.0) a |
0 |
|
18 |
05.05.2011 |
0.85 (1.18) ade |
0.05 (0.22) a |
5.6 |
|
|
29 |
13.05.2011 |
1.25 (1.20) e |
0.15 (0.67) ab |
10.3 |
|
|
23 |
19.05.2011 |
1.10 (1.41) de |
0.05 (0.22) a |
4.3 |
|
|
10 |
27.05.2011 |
0.05 (0,22) c |
0.45 (0.60) b |
90 |
|
|
6 |
13.06.2011 |
0.30 (0.57) abc |
0.00 (0.0) a |
0 |
|
|
5 |
24.06.2011 |
0.10 (0.30) bc |
0.15 (0.36) ab |
60 |
|
|
19 |
01.07.2011 |
0.60 (0.68) abcd |
0.40 (0.68) b |
42.1 |
|
|
7 |
08.07.2011 |
0.10 (0.30) bc |
0.20 (0.41) ab |
57.1 |
|
|
6 |
20.07.2011 |
0.15 (0.48) bc |
0.15 (0.36) ab |
50 |
a Entries show the mean number (±SE) of healthy and infected P. xylostella. Means sharing the same lower-case letter are not statistically different (p >0.05).
In the present study, the highest prevalences of fungal infection on both crops were recorded during May, with high humidity percentages (over 80%). In accordance with Steinkraus (2006), relative humidity is the single most critical factor in transmission of fungal pathogens. Fungal pathogens generally require high relative humidity for survival, germination and sporulation. In Argentina, previous studies on the phenology of entomophthoroid fungi in populations of insects have reported that fungal infections are more common in the autumn-winter season (in the southern hemisphere, from March to August) (López-Lastra et al., 2003; Toledo et al., 2008). In Uruguay, Alzugaray et al. (2010) recorded P. neoaphidis as one of the main mortality agents of aphids but emphasized that its action is restricted to autumn and winter. The low temperatures and humid conditions prevalent during the winter growing season tend to be favorable for the occurrence of entomophthoroid mycoses (Poprawski & Wraight, 1998; Wraight et al., 1993).
Our results showed that Z. radicans is an important factor in the mortality of P. xylostella under natural conditions. This study is the first record of Z. radicans and M. rileyi from P. xylostella in Argentina.
References
Alzugaray, R., Ribeiro, A., Silva, H., Stewart, S., Castiglioni, E., Bartaburu, S. et al. (2010). Prospección de agentes de mortalidad natural de áfidos en leguminosas forrajeras en Uruguay. Agrociencia, 14, 27–35.
Aragón, J. R., Molinari, A., & Lorenzatti-De Diez, S. (1997). Manejo integrado de plagas. In L. M. Giorda, & H. E. Baigorri (Eds.) El cultivo de la soja en la Argentina. Córdoba, Argentina: Instituto Nacional de Tecnología Agropecuaria.
Bałazy, S. (1993). Flora of Poland. Cracow: Polish Academy of Sciences.
Bertolaccini, I., Sánchez, D., & Arregui, C. (2010). Incidencia de algunos factores naturales de mortalidad de Plutella xylostella (L.) (Lepidoptera: Plutellidae) en el área centro-este de Santa Fe, Argentina. Horticultura Argentina, 29, 20–24.
Cherry, A. J., Mercadier, G., Meikle, W., Castelo-Blanco, M., & Schroer, S. (2004). The role of entomopathogens in DBM biological control. In A. Kirk, & D. Bordat (Eds.), Improving biocontrol of Plutella xylostella, Proceedings of the International Symposium. Montpellier, France: CIRAD, USDA-ARS.
Corrêa, B. S., & Smith, J. G. (1975). Nomuraea rileyi attacking the velvetbean caterpillar, Anticarsia gemmatalis Hubner, in Parana. Florida Entomologist, 58, 280.
De La Rosa, W., López, F. L., & Liedo, P. (2002). Beauveria bassiana as a pathogen of the Mexican fruit fly (Diptera: Tephritidae) under laboratory conditions. Journal of Economic Entomology, 95, 36–43.
Ferron, P. (1978). Biological control of insect pests by entomopathogenic fungi. Annual Review of Entomology, 23, 409–442.
Galaini-Wraight, S., Wraight, S. P., Carruthers, R. I., Magalhaes, B. P., & Roberts, D. W. (1991). Description of a Zoophthora radicans (Zygomycetes: Entomophthoraceae) epizootics in a population of Empoasca kraemeri (Homoptera: Cicadellidae) on beans in central Brazil. Journal of Invertebrate Pathology, 58, 311–326.
Hajek, A. E., & Leger, R. J. (1994). Interactions between fungal pathogens and insect hosts. Annual Review of Entomology, 39, 293–322.
Humber, R. A. (2012). Entomophthoromycota: a new phylum and reclassification of entomophthoroid fungi. Mycotaxon, 120, 477–492.
InfoStat. (2004). InfoStat version 2004. Grupo InfoStat, Facultad Ciencias Agrarias, Universidad Nacional de Córdoba, Argentina.
Kanervo, V. (1946). Sporadic observations concerning diseases in certain species of insects 3. Diseases attacking Plutella maculipennis Curt. Annual Review of Entomology, 12, 143–153.
Keller, S. (1991). Arthropod-pathogenic Entomophthorales of Switzerland II. Erynia, Eryniopsis, Neozygites, Zoophthora, and Tarichium. Sydowia, 43, 39–122.
Kirk, A. A., Mercadier, G., Bordat, D., Delvare, G., Pichon, A., Arvanitakis, L. et al. (2004). Variability in Plutella and its natural enemies: implications for biological control. In N. M. Endersby, & P. M. Ridland (Eds.), The management of diamondback moth and other crucifer pests. Proceedings of the Fourth International Workshop. 26-29 November 2001. Melbourne: Department of Natural Resources and Environment.
López-Lastra, C. C., & Scorsetti, A. C. (2006). Hongos patógenos de insectos en Argentina (Zygomycetes: Entomopthorales). Revista de Biologia Tropical, 54, 311–315.
López-Lastra, C. C., Mazzucchelli, M. G., & Dikgolz, V. (2003). Temporal changes in the prevalence of three species of Trichomycetes (Zygomycota: Zygomycotina) in Dipteran aquatic larvae from Argentina. Fungal Diversity, 14, 85–93.
Mota-Sánchez, D., Bills, P. S., & Whalon, M., E. (2002). Arthropod resistance to pesticides: status and overview. In W. B. Wheeler (Eds.), Pesticides in Agriculture and the Environment (pp. 241–272) New York: Marcel Dekker Inc.
Papierok, B., & Hajek, A. E. (2012). Fungi: Entomophthorales. In L. A. Lacey (Eds.), Manual of techniques in invertebrate pathology. Second Ed (pp. 151–187). Amsterdam: Academic Press.
Poprawski, T. J., & Wraight, S. P. (1998). Fungal pathogens of Russian wheat aphid (Homoptera: Aphididae). In S. Quisenberry, & F. Peairs (Eds.), Response model for an introduced pest-the russian wheat aphid (pp. 209–233). Proceedings, Entomological Society of America. Lanham, Maryland: Thomas Say Publications, Entomology.
Rayner, R. W. A. (1970). Mycological colour chart. Kew, Surrey, England. Commonwealth Mycological Institute. Available in: http://trove.nla.gov.au/version/25379954.
Samson, R. A. (1974). Paecilomyces and some allied Hyphomycetes. Studies in Mycology 6. The Netherlands: Baarn.
Samson, R. A., Evans, H. C., & Latgé, J. P. (1988). Atlas of entomopathogenic fungi. Berlin: Springer-Verlag.
Steinkraus, D. C. (2006). Factors affecting transmission of fungal pathogens of aphids. Journal of Invertebrate Pathology, 92, 125–131.
Talekar, N. S., & Shelton, A. M. (1993). Biology, ecology, and management of the diamondback moth. Annual Review of Entomology, 38, 275–301.
Toledo, A. V., Scorsetti, A. C., Dikgolz, V. E., & López-Lastra, C. C. (2004). Paecilomyces fumosoroseus y Nomuraea rileyi (Deuteromycotina: Hyphomycetes), hongos patógenos de insectos plaga de la agricultura en la Argentina. Boletín de la Sociedad Argentina de Botánica, 39, 21–26.
Toledo, A. V., Giambelluca, L., Marino de Remes-Lenicov, A. M., & López-Lastra, C. C. (2008). Pathogenic fungi of planthoppers associated with rice crops in Argentina. International Journal of Pest Management, 54, 363–368.
Vandenberg, J. D., Shelton, A. M., Wilsey, W. T., & Ramos, M. (1998). Assessment of Beauveria bassiana sprays for control of diamondback moth (Lepidoptera: Plutellidae) on crucifers. Journal of Economic Entomology, 91, 624–630.
Vandenberg, J. D., & Soper, R. S. (1987). Prevalence of Entomophthorales mycosis in populations of spruce budworm, Choristoneura fumiferana. Environmental Entomology, 7, 847–853.
Wraight, S. P., Poprawski, T. J., Meyer, W. L., & Peairs, F. B. (1993). Natural enemies of Russian wheat aphid (Homoptera: Aphididae) and associated cereal aphid species in spring-planted wheat and barley in Colorado. Environmental Entomology, 22, 1383–1391.
Zare, R., & Gams, W. (2001). A revision of Verticillium sect. Prostata IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia, 73, 1–50.



