Berenice Ramírez-López a, Ireri Suazo-Ortuño a, Luis H. Escalera-Vázquez b, Omar Domínguez-Domínguez b, Yurixhi Maldonado-López c, *
a Universidad Michoacana de San Nicolás de Hidalgo, Instituto de Investigaciones sobre los Recursos Naturales, Avenida Juanito Itzícuaro s/n, Nueva Esperanza, 58330 Morelia, Michoacán, Mexico
b Universidad Michoacana de San Nicolás de Hidalgo, Facultad de Biología, Calle Gral. Francisco J. Múgica s/n A-1, Felícitas del Río, 58030 Morelia, Michoacán, Mexico
c Universidad Michoacana de San Nicolás de Hidalgo, Cátedra Conacyt-Instituto de Investigaciones sobre los Recursos Naturales, Avenida Juanito Itzícuaro s/n, Nueva Esperanza, 58330 Morelia, Michoacán, Mexico
*Corresponding author: yurixhimaldonado@gmail.com (Y. Maldonado-López)
Received: 2 February 2022; accepted: 9 May 2023
Abstract
Ambystoma dumerilii, known as “achoque”, is a microendemic salamander from Lake Pátzcuaro, considered as a critically endangered species according to the IUCN (2020). The main threats are high levels of water contamination, high levels of eutrophication in addition to the fact that invasive species can be found within the “achoque” habitat. For these reasons, an important conservation effort has been the maintenance of “achoque” in captivity. However, captivity is known to be a stressor derived from non-optimal conditions that can have important physiological consequences that are reflected in body conditions. Therefore, our objective was to evaluate the condition of A. dumerilii individuals through a morphological analysis using different parameters such as morphological character sizes, geometric morphometrics, fluctuating asymmetry and allometry, in individuals from Lake Pátzcuaro and captivity. We found that almost all the traits have a negative allometric relationship with the body size in individuals from both conditions. Our results showed that individuals from the lake presented greater sizes, slimmer bodies and higher levels of fluctuating asymmetry than captive individuals, all results are consistent in the context of performance with greater potential adaptations to increase swimming performance than individuals from captivity.
Keywords: Ambystoma; Achoque; Allometric patterns; Fluctuating asymmetry; Geometric morphometrics; Habitat perturbation
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Diferenciación morfológica en poblaciones de Ambystoma dumerilii en condiciones de cautiverio y vida libre
Resumen
Ambystoma dumerilii, conocido como “achoque”, es una salamandra microendémica del lago de Pátzcuaro, considerada como en peligro crítico. Las principales amenazas son los altos niveles de contaminación del agua y de eutrofización, además de las especies invasoras dentro del hábitat del achoque. Por estas razones, un importante esfuerzo de conservación se ha enfocado en la crianza del achoque en cautiverio. Sin embargo, el cautiverio es un estresor derivado de las condiciones no óptimas que pueden tener importantes consecuencias fisiológicas que se reflejan en las condiciones corporales. Por tanto, nuestro objetivo fue evaluar la condición de individuos de A. dumerilii a través del análisis de la morfología utilizando diferentes parámetros como tallas, morfometría geométrica, asimetría fluctuante y alometría, en individuos del lago de Pátzcuaro y en cautiverio. Encontramos que casi todos los rasgos tienen una relación alométrica negativa con el tamaño corporal en individuos de ambas condiciones. Nuestros resultados mostraron que los individuos del lago presentaron mayores tamaños en los caracteres morfológicos, cuerpos más delgados y mayores niveles de asimetría fluctuante que los individuos en cautiverio, todos estos resultados son consistentes en el contexto del desempeño con mayores adaptaciones potenciales para aumentar el rendimiento del nado que los individuos en cautiverio.
Palabras clave: Ambystoma; Achoque; Patrones alométricos; Asimetría fluctuante; Morfometría geométrica; Perturbación del hábitat
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The family Ambystomatidae is composed of the genus Ambystoma, with 35 species distributed from southern Canada and Alaska to the southern limit of the Mexican highlands (Casas-Andreu et al., 2004). In Mexico, there are 17 species of which 15 are classified in some risk category according to the NOM-059-Semarnat-2010 (Ortega, 1999). Specifically, A. dumerilii, known as “achoque”, is a micro-endemic salamander from Lake Pátzcuaro, whose population is close to extinction (Zambrano et al., 2011), and even considered a critically endangered species according to the IUCN (2020), as well as being under special protection by Semarnat (2010). Like other members of the Ambystomatidae family, this species is very sensitive to anthropogenic activities (Soto-Rojas et al., 2017). For example, in Ambystoma ordinarium an increase in the frequency of morphological abnormalities related to habitat degradation has been described (Soto-Rojas et al., 2017). In the same species, a high parasitic infection was found associated with disturbed streams (Ramírez-Hernández et al., 2019). The conditions of Lake Pátzcuaro have deteriorated due to the high disturbance derived from anthropogenic factors such as changes in land use, contamination by wastewater, herbicides and pesticides (Zambrano et al., 2011). It is in a eutrophic state due to high levels of nitrogen and phosphorous, it has decreased in depth by 6 m since 1939 and has sedimentation rates of around 100,000 m3 each year (Ramírez-Herrejón et al., 2014; Tomasini-Ortiz et al., 2016). According to Aguilar-Miguel (2005) Ambystoma dumerilii has a restricted area of occupancy of less than 10 km2.
The endemic nature of A. dumerilii and the critical status of the habitat has led to breeding this species in captivity as a conservation strategy (Huacuz-Elías, 2002; IUCN SSC Amphibian Specialist Group, 2020). Therefore, the maintenance of “achoques” is vital to enhance conservation efforts. However, captive amphibian populations can present morphological and physiological problems associated with long-term stress derived from non-optimal conditions (Assis et al., 2015; Michaels et al., 2014; Titon et al., 2017). One of the main problems is the adaptability of organisms to captive conditions, since there are species of salamanders that have highly specialized microclimate and microhabitat requirements, therefore replicating these conditions in captivity is complicated, as is the case of Ambystoma cingulatum. The maintenance of the appropriate temperature for organisms is also important to avoid infectious diseases caused by bacteria, parasites and fungi. If the water quality is poor due to inadequate filtering and continuous replacement, it can contain dissolved substances such as ammonia, urea or toxins (de Vosjoli, 1999). An inadequate diet with low nutritional intake also has negative effects on development (Slight et al., 2015).
Regardless of the stress source, amphibian response underlies shaping an organism’s phenotype (Denver, 2009). A permanent stress source induces an overproduction of glucocorticoids, the stress hormones, that negatively affect amphibian growth and induce changes in their morphology (Davis & Maerz, 2010; Davis & Maney, 2018; Davis et al., 2020; Gangenova et al., 2020), leading to permanent alterations in morphology (Brunson et al., 2001; Matthews, 2002; Hu et al., 2008). Morphological changes in amphibians occur during their ontogenetic development, from the larval phase to the adult stage (Shi et al., 1996; Steinicke et al., 2015). Hence, amphibians of the same species show different morphological forms, depending on the degree of stress suffered during their development (Tejedo et al., 2010). Morphological changes derived from environmental stress involve different morphological parameters, such as size and shape of morphological traits, allometric patterns, and fluctuating asymmetry.
Environmental stress decreases growth with smaller size at metamorphosis (Cayuela et al., 2017; Delgado-Acevedo & Restrepo, 2008; Iglesias-Carrasco et al., 2017). Changes in morphology (Denver et al., 1998; Relyea & Hoverman, 2003) and the shape and size of morphological traits also vary in predictable ways in response to environmental stress (Morrison et al., 2004; Phillips et al., 2006). For example, amphibians can develop smaller hindlimbs in individuals present in fragmented habitats (Delgado-Acevedo & Restrepo, 2008; Steinicke et al., 2015).
Variation in morphological traits often scales with overall body size, defined as morphological allometry (Fairbairn, 1997, Shaffer, 1984). However, the degree of such correspondence can range from nearly perfect covariance of a trait with body size (i.e., isometry) to highly uncorrelated, where specific morphological traits change at a different rate than the body size (i.e., allometry). Positive allometry occurs when morphological characters have greater growth than body size, while negative allometry is associated with lower growth of morphological characters than body size (Fairbairn, 1997; Fox et al., 2015). Changes in allometric patterns can influence amphibian fitness (Delgado-Acevedo & Restrepo, 2008; Tejedo et al., 2010). For example, in salamanders, the scaling relationships of head shape with body size have been related to larval diet and predation risk (Shaffery & Relyea, 2015; Van Buskirk, 2011). In addition, positive allometric relationships between head and body size improve the vocal efficiency of frogs in a sexual selection context (Riva-Tonini et al., 2020).
Stressful conditions induce changes during development that result in morphological asymmetry (Lens et al., 2002; Wright & Zamudio, 2002), such as fluctuating asymmetry (FA) that measure slight (Zhelev et al., 2014), random deviations of bilateral symmetrical traits, reflecting developmental instability of the organisms. In disturbed or high stress environments, metamorphosis and growth can be accelerated (Lowe et al., 2006), leading to higher FA (Møller & Manning, 2003). For example, Pelophylax ridibundus and Pseudepidalea viridis showed higher FA in sites with high levels of anthropogenic disturbance (Zhelev et al., 2014). In a similar study, Pelophylax ridibundus in highly contaminated sites, presented high levels of FA where they evaluated the levels of FA in 10 morphological traits while individuals in uncontaminated sites presented FA in 3 morphological characters (Zhelev et al., 2015, 2019).
Understanding how amphibians respond phenotypically to environmental changes resulting from habitat disturbance is an important challenge to detecting the degree of susceptibility to new stressful environments and then proposing conservation strategies (Lomolino et al., 2001). Amphibians show morphological plasticity to adjust to a changing environment such as predator presence, habitat quality, competitors, and stressful conditions (Johansson et al., 2010; Relyea, 2001; Relyea & Hoverman, 2003; Stoler & Relyea, 2013). Due to the deterioration of Lake Pátzcuaro and the stress induced in captivity, we expected to find physiological stress in individuals of A. dumerilii that can be reflected in their morphological traits.
Therefore, our objective was to evaluate the condition of A. dumerilii individuals through the analysis of the morphology of A. dumerilii, using different parameters such as morphological character sizes, geometric morphometrics, fluctuating asymmetry and allometry, in individuals from Lake Pátzcuaro and captivity. We hypothesized that the anthropogenic disturbance present in the lake and the maintenance of sub-optimal conditions in captivity would cause stress on the organisms, therefore, we expect to detect this using FA, geometric morphometrics and allometry patterns, with an increase under the most stressful condition. Our results will allow us to know the conditions of organisms in both populations, as well as provide useful information for future conservation and management plans of this species in its habitat, and for the improvement of captivity conditions.
Materials and methods
We analyzed 107 individuals, 60 individuals from the lake (males and females) and 47 from captivity (males and females). All salamanders sampled were individuals classified as adults with a minimum snout-vent length (SVL) of 60 mm (Anderson & Worthington, 1971).
The captive salamanders were sampled at a management unit for wildlife conservation and sustainable use. At this center, A. dumerilii is bred in captivity. Captive individuals were born and raised in captivity. The salamanders are kept in a covered area that protects them from direct sunlight. Between 6 to 8 individuals are kept in oval recycled plastic tubs of approximately 180 L, with artificial shelters, natural aquatic plants and hiding places made of PVC. The containers are shallow, allowing them to move around mainly using their limbs. Temperatures were maintained around 17 °C ± 1°C. The “achoques” were fed with a mixed diet of brine shrimp, fish fillets, tubifex (Tubificidae) and acociles (Procambarus sp.). The pH oscillated between 7.5 and 7.8.
The wild specimens were sampled in Lake Pátzcuaro that is located in the western portion of the Transmexican Volcanic Belt (09°32’-19°42’ N, 101°32’-101°42’ W.) Sampling was carried out monthly from March 2019 to December 2020, using 180 metallic cylinder traps with a conical inlet, set at the bottom of the lake. To identify the “achoques” collected in the lake, we used a microchip marking system, to avoid resampling. The microchip marking system was initiated in 2018.
For all salamanders, we registered snout-vent length (SVL, mm) and total length (mm), using a digital caliper. The sex of each individual was determined based on the cloacal bulge. We used the SVL as a standard measure of body size (Gangenova et al., 2020). To analyze all morphological traits, we obtained a digital image from the dorsal part of each organism with a high-resolution camera (Sony α350), ensuring that all pictures were taken with the same objective and at the same distance (30 cm) with a scale. All pictures had a resolution of 14.2 megapixels. All images were used for the morphometric measurements, FA and allometry (Alarcón-Ríos et al., 2017; Soto-Rojas et al., 2017).
To determine differences in body shape between individuals from captivity and lake conditions, we used the photographs to measure the following morphometric traits: eye to eye distance (EED), head width (HW), head length (HL), body width (BW), total length (TL), tail width (TW), tail length (TLe), femur length right and left (FLR, FLL), tibia-fibula length right and left (TFLR, TFLL), radius-ulna length right and left (RULR, RULL), humerus length right and left (HLR, HLL) (Fig. 1A). The sizes were measured with the Image J 1.44 software. To analyze differences in morphological characters between both conditions, we used a one-way ANOVA test the lake and captivity effect. Individuals from the lake and captivity were considered as independent variables and morphological characters as dependent ones.
For allometric relationships between body size and all morphological traits, we used the SLV as standard measure of body size. We compared captivity and lake conditions with normalized data to remove allometric effects, following the method developed by Lleonart et al. (2000). The theoretical equation adjusts the shape considering allometry and scales all individuals to the same size, and the absolute values of morphometric characters are standardized as follows: (Yi* = Yi (SVL0/SVLi)b. Where Yi*: size-adjusted proportion character of specimen i; Yi: body character; SVL0: mean value of SVL; SVLi: SVL of specimen i; b: within-habitat treatment regression slope of log (Y) against log (SVL). We transformed all variables to log. We evaluated the effect of lake and captivity on SVL and its allometric relationship with morphological characters, applying an ordinary least squares regression between snout-vent length (x-axis: mm) and size of morphological characters (y-axis: mm) for individuals from the lake and captivity. We calculated the allometric slope for each regression. For those variables in which a correlation was identified, we applied an analysis of covariance (ANCOVA) to test the difference in regression slopes between individuals from the lake and captivity.
With the digital image obtained for each individual, we measured FA of abdomen and head (Fig. 1B). Fluctuating asymmetry was calculated as the absolute value of the difference among the distances from the middle to the left and right margins of the body part (|Ai – Bi|), divided by the average distance (Ai + Bi / 2), to correct for the fact that asymmetry may be size-dependent. Additionally, 10 individuals were blindly re-measured, without reference to previous measurements to control the measurement error in FA. We then evaluated the degree of significance of FA relative to measurement error using two-way mixed-model ANOVA. The significance of the interaction (individual × body part × side) indicated that variation in FA was greater than expected by measurement error: (F9,25 = 22.4; p < 0.002).
Fluctuating asymmetry is found when the right-minus-left (R-minus-L) differences are normally distributed with a mean value = 0, unlike directional asymmetry that is found when the R-minus-L differences are also normally distributed, but with a mean significantly different from 0, and antisymmetry, characterized by a platykurtic or bimodal distribution of R-minus-L differences about a mean of 0 (Palmer & Strobeck, 1986). To determine whether our data fitted only FA and no other types of asymmetry, we performed a Student’s t test and Lilliefors’ normality test to evaluate whether mean values of signed R-minus-L values differed significantly from 0. We found that R-minus-L measurements did not differ from 0 (t = 1.1; p > 0.05), and therefore, we discarded the presence of directional asymmetry in our data. In the same way, we also reject the presence of antisymmetry because our data (R-minus-L) exhibited a normal distribution (p > 0.05). Once determined that our data fitted only in FA criterium, we used an analysis of variance (ANOVA) to determine the differences in FA levels between individuals that occur in the lake and captivity condition. In all cases, the normality was tested after suitable transformations.
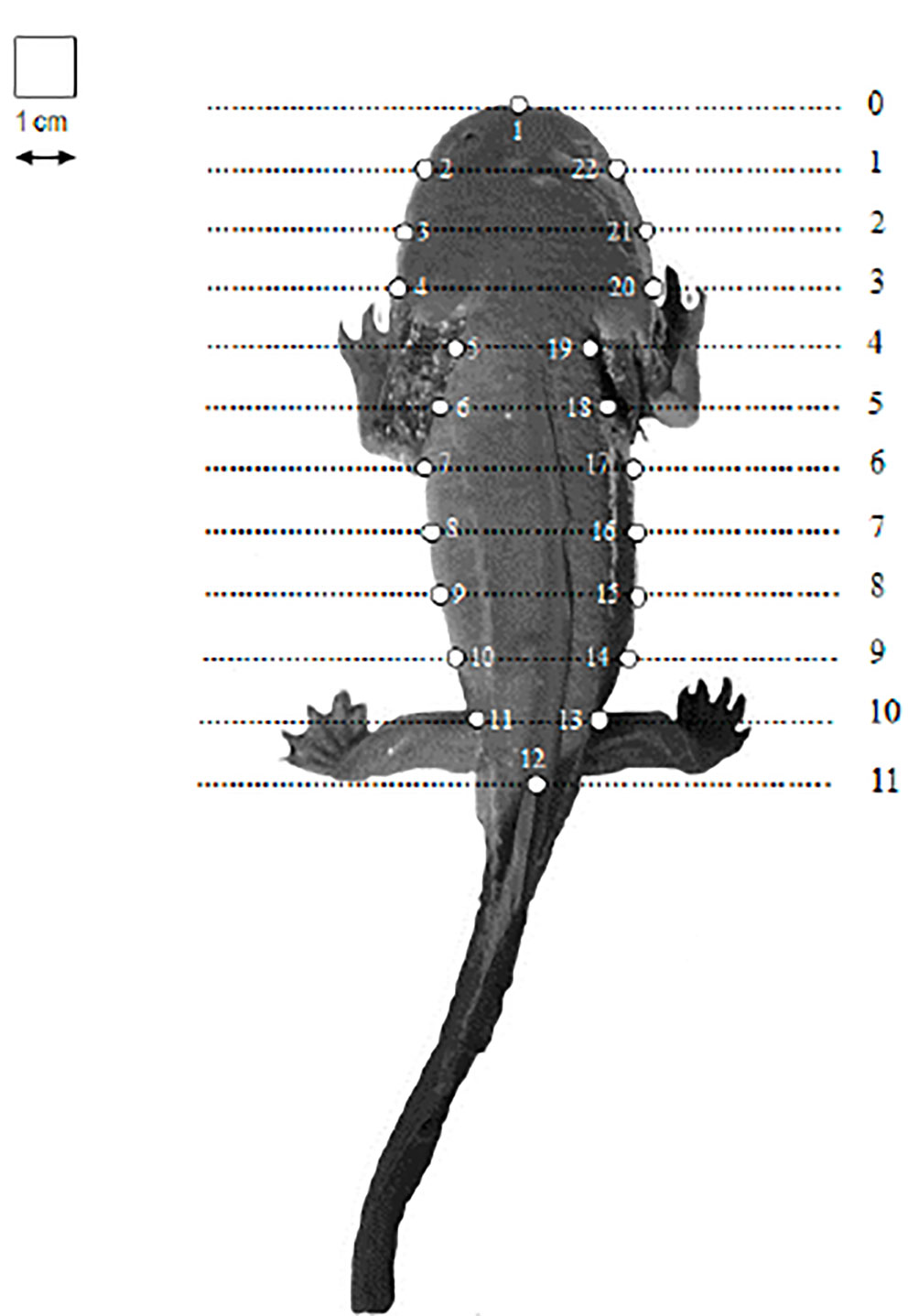
Differences in body morphology between individuals that occur in the lake and captivity conditions, were analyzed using geometric morphometric techniques (Vega-Trejo et al., 2014). Each individual was photographed separately. We used grid lines as guides in order to obtain the maximum vertical image. In each image, 22 landmarks type II landmarks were placed along the corporal shape of the salamanders and 2 additional landmarks over the centimeter as size reference to record the coordinates (x, y) of the 22 landmarks in each salamander image (Cuevas-Reyes et al., 2018) (Fig. 2). The type of landmarks used are classified as homologous type II landmarks, since they represented pairs of points in the places with greater curvature from the body shape (sensu Bookstein, 1997). For the application of landmarks, the TPS software package was used (Rohlf, 2015). Then, a Procrustes superimposition analysis was performed using the Integrated Morphometrics Package (IMP series: http://www.canisius.edu/~sheets/morphsoft.html) to align the landmark coordinates and eliminate size effect (Vega-Trejo et al., 2014). This Procrustes superimposition analysis rescales, translates, and rotates (using a least-squares criterion) the raw landmark coordinates in order to eliminate any non-shape variation (Bookstein, 1997; Klingenberg, 2003). The mean configuration of all individuals for this condition was considered as reference of shape variables (Procrustes distances) and calculated by a superimposition coordinates analysis (Cuevas-Reyes et al., 2018). We applied a principal component analysis (PCA) to determine shape differences between lake and captivity conditions (Cuevas-Reyes et al., 2018). The PCA test produces ordination plots indicating the differences in the shape of the salamanders. These analyses were performed in MorphoJ software v1.07a (Klingenberg, 2011).

Results
We found differences in morphological characters between both populations, with individuals from the lake with a larger head width, head length, total length, tail width, tail length, femur length of left side, tibia-fibula length of left side, radius-ulna length of right and left sides, humerus length of right and left sides, radius-ulna length of left and right side and SVL than individuals raised in captivity. In the case of body width, femur length right, tibia-fibula length right we did not find any differences (Table 1).
We observed significant allometric relationships in most of the traits in individuals of A. dumerilii from the lake and captivity. We found that almost all traits showed a negative allometric relationship with body size in individuals from the lake. In the case of tail width, radius-ulna length of left side, fibula length of left size showed an isometric relationship with body size in captive individuals. Femur length of left size showed a positive relationship (Table 2).
ANCOVA analyses showed significant differences between slopes in some allometric relationships of both habitat conditions. In individuals from the lake, the slopes of the allometric equations between body size and humerus length of right size and radius-ulna length of right side were significantly higher than in individuals from captivity (HLR: F = 77.6; p = 0.0001. RULR: F = 105.9; p = 0.0001). In the rest of traits, the slopes of the allometric equations were higher in individuals from captivity
(HW: F = 10.68; p = 0.0001. HL: F = 4.1; p = 0.0001.
BW: F = 112.7; p = 0.0001. TL: F = 47.6; p = 0.0001.
TW: F = 62.23; p = 0.0001. TLe: F = 115.5; p = 0.0001. FLR: F = 41.02; p = 0.0001. TFLR: F = 28.7; p = 0.0001. FLL: F = 53.29; p = 0.0001. TFLL: F = 40.89; p = 0.0001).
We found differences in FA between salamanders from the lake and those from captive conditions. Our results show higher FA in the body (F = 27.9, d. f. = 1, p = 0.0001) and head (F = 47.1, d. f. = 1, p = 0.067) of individuals sampled in the lake.
Table 1
ANOVA of morphological characters in individuals of A. dumerilii from captivity and wildlife. Eye to eye distance (EED), head width (HW), head length (HL), body width (BW), total length (TL), tail width (TW), tail length (TLe), femur length right and left (FLR, FLL), tibia-fibula length right and left (TFLR, TFLL), radius-ulna length right and left (RULR, RULL), humerus length right and left (HLR, HLL). Numbers in bold indicate statistically significant differences.
| Character | Lake | Captivity | d. f. | F | p |
| HW | 4.678 ± 0.389 | 4.033 ± 0.228 | 1 | 102.17 | 0.0001 |
| HL | 4.496 ± 0.494 | 3.47 ± 0.528 | 1 | 106.96 | 0.0001 |
| BW | 3.529 ± 0.388 | 3.624 ± 0.357 | 1 | 1.6966 | 0.1956 |
| TL | 24.9 ± 1.747 | 21.4 ± 1.002 | 1 | 142.14 | 0.0001 |
| TW | 1.418 ± 0.183 | 1.3064 ± 0.178 | 1 | 9.9329 | 0.0021 |
| TLe | 10.532 ± 1.445 | 8.482 ± 0.995 | 1 | 68.819 | 0.0001 |
| FLR | 1.465 ± 0.279 | 1.406 ± 0.227 | 1 | 1.3783 | 0.2431 |
| FLL | 1.4567 ± 0.259 | 1.332 ± 0.206 | 1 | 7.1695 | 0.0086 |
| TFLR | 1.3385 ± 0.272 | 1.2991 ± 0.2219 | 1 | 0.6458 | 0.4234 |
| TFLL | 1.4035 ± 0.278 | 1.2977 ± 0.182 | 1 | 4.084 | 0.04584 |
| HLR | 1.553 ± 0.206 | 1.3826 ± 0.225 | 1 | 7.9356 | 0.0069 |
| HLL | 1.555 ± 0.203 | 1.3028 ± 0.231 | 1 | 17.071 | 0.0001 |
| RULR | 1.515 ± 0.155 | 1.2717 ± 0.165 | 1 | 29.847 | 0.0001 |
| RULL | 1.499 ± 0.211 | 1.248 ± 0.177 | 1 | 23.017 | 0.0001 |
| SVL | 14.32 ± 1.393 | 13.01 ± 2.386 | 1 | 12.47 | 0.0001 |
Table 2
Allometric patterns of morphological characters in individuals of A. dumerilii from the lake and captivity in relation to standard body size SLV. Eye to eye distance (EED), head width (HW), head length (HL), body width (BW), total length (TL), tail width (TW), tail length (TLe), femur length right and left (FLR, FLL), tibia-fibula length right and left (TFLR, TFLL), radius-ulna Length right and left (RULR, RULL), humerus length right and left (HLR, HLL) . Numbers in bold indicate statistically significant differences.
| Character (Log) | Lake | Captivity | ||||
| Slope b (95% CI) | r2 | p | Slope b (95% CI) | r2 | p | |
| HW | 0.6 (0.39, 0.8) | 0.36 | 0.0001 | 0.73 (0.05, 0.81) | 0.88 | 0.0001 |
| HL | 0.58 (0.3, 0.85) | 0.23 | 0.0001 | 0.71 (0.49, 0.9) | 0.49 | 0.0001 |
| BW | 0.86 (0.59, 1.1) | 0.41 | 0.0001 | 0.97 (0.83, 1.1) | 0.82 | 0.0001 |
| TL | 0.89 (0.72 1.05) | 0.67 | 0.0001 | 1 (0.91, 1.04) | 0.95 | 0.0001 |
| TW | 0.73 (0.41, 1.05) | 0.26 | 0.0001 | 0.86 (0.67, 1.05) | 0.65 | 0.0001 |
| TLe | 0.83 (0.49, 1.16) | 0.3 | 0.0001 | 0.95 (0.79, 1.1) | 0.75 | 0.0001 |
| FLR | 0.53 (0.07, 1) | 0.08 | 0.025 | 1 (0.85, 1.27) | 0.69 | 0.0001 |
| FLL | 0.44 (-0.04, 0.9) | 0.06 | 0.06 | 1.12 (1.05, 1.49) | 0.75 | 0.0001 |
| TFLR | 0.9 (0.36, 1.4) | 0.16 | 0.002 | 0.91(0.66, 1.15) | 0.55 | 0.0001 |
| TFLL | 0.8 (0.29, 1.3) | 0.15 | 0.002 | 1 (0.79, 1.19) | 0.68 | 0.0001 |
| HLL | 0.68 (0.24, 1.1) | 0.35 | 0.004 | 0.94 (0.62, 1.2) | 0.71 | 0.0001 |
| HLR | 0.91 (0.4, 1.4) | 0.44 | 0.001 | 0.82 (0.51, 1.12) | 0.44 | 0.0001 |
| RULR | 0.86 (0.49, 1.22) | 0.58 | 0.0001 | 0.59 (0.34, 0.84) | 0.38 | 0.0001 |
| RULL | 0.78 (0.3, 1.25) | 0.38 | 0.001 | 0.83 (0.57, 1.09) | 0.55 | 0.0001 |
| EED | 0.3 (-0.08, 0.7) | 0.04 | 0.12 | 0.67 (0.57, 0.77) | 0.83 | 0.0001 |

Based on a coordinate superimposition analysis, we found differences in body shape between individuals of the lake and captivity (Fig. 3A), where the PC1 and PC2 explained 58.8% and 13.3%, respectively. Two well-segregated groups were formed between lake and captive individuals in our PCA (Fig. 3A). The wireframe graph based on Procrustes coordinates showed that body shape of individuals from the lake were slimmer than individuals from captivity (Fig. 3B). This difference in body shape between individuals from captivity and lake is supported by discriminant analysis, where both distances (values) of Mahalanobis (3.95) and Procrustes (0.046) were significant (p = 0.0001).
Discussion
Anthropogenic threats have led to the deterioration of the “achoque” (Zambrano et al., 2011), leading it to live under stressful conditions. The conservation alternative, captivity, necessary to face the imminent extinction of this endangered species, also represents stressful conditions derived from non-optimal environment (Michaels et al., 2014). Morphological changes in amphibians occur during their ontogenetic development, from the larval phase to the adult stage (Shi et al., 1996; Steinicke et al., 2015), hence amphibians of the same species can show different morphological forms, depending on the degree of stress suffered during their development (Tejedo et al., 2010). Our results showed that individuals from the lake are larger, have higher FA and the slopes of allometric correlations of almost all traits were lower, suggesting that SVL increases faster according to morphological characters than those individuals in captivity. Conversely, individuals from captivity present shorter morphological traits and narrower bodies than individuals from the lake.
In our study, individuals from the lake are larger than individuals from captivity. This result is the opposite to what would be expected if we hypothesize that the conditions in the lake were more stressful than those in captivity, and that amphibian exposure to environmental stressors decreased the growth rate of the main morphological traits (Delgado-Acevedo & Restrepo, 2008; Tejedo et al., 2010). However, A. dumerilii does not show a decrease in size, since the larger sizes and higher FA occur in individuals from the lake. To explain the relationship between growth and FA, it has been hypothesized that a favorable environment, such as greater availability of food items with a higher nutrient content (Milligan et al., 2008), allows for rapid growth of organisms, prompting higher developmental instability and FA levels (Lempa et al., 2000; Martel et al., 1999). The main reason is that there are trade-offs between growth rate and life-history traits such as developmental stability (Sibly & Calow, 1984), and within species, different genotypes in specific environments need to change from optimizing growth rate to optimizing developmental quality. Therefore, we can expect higher FA in individuals of A. dumerilii that reach an optimal growth rate for their development in lake conditions, showing that fluctuating asymmetry might not be an unequivocal indicator of environmentally induced stress, since other factors can be involved, such as genetic stress or growth rate (Milligan et al., 2008; Velickovic & Perisic, 2006).
An important factor may be the resource availability that can lead to larger body size (Jessop et al., 2006; Wu et al., 2006). Ambystoma dumerilii in the natural habitat has a high degree of trophic specialization, and consumes mainly crayfish (Cambarellus sp.), an abundant resource in Lake Pátzcuaro (Huacuz-Elías, 2008), although sometimes it consumes other crustaceans, insects, worms, small fish and tadpoles too (Aguilar-Miguel, 2005; Velarde-Mendoza, 2012; Semarnat, 2018). In captivity, A. dumerilii had a mixed diet of fish fillet, earthworm, tubifex and acociles. According to some authors a mixed diet results in a slower growth rate than a bloodworm-only diet, and it seems that for captive aquatic species such as A. dumerilii, an invariant but good-quality diet is a better option, indicating that mixed diets being best is not universally true (Slight et al., 2015).
Morphology of amphibians is directly related to movement, locomotion ability and individual performance (Aubret & Shine, 2008; Ijspeert & Cabelguen, 2006; Irschick & Garland, 2001). In our study, it was expected that individuals from the lake presented traits that are correlated with the fast-swimming performance in salamanders, necessary to escape from predation but also to capture prey (Urban, 2010; Van Buskirk & Schmidt, 2000). For example, tail morphology is known to have an impact on the locomotor performance while swimming (Van Buskirk & Schmidt, 2000; Vorndran et al., 2002), large tails are adaptations for rapid acceleration (Duellman & Trueb, 1986), and are associated to the response to chemical predator cues (Van Buskirk & McCollum, 2000). In our study, we found larger and wider tails in individuals from the lake, suggesting that these individuals have developed a greater capacity of movement. A larger tail surface area can also generate greater thrust, so a greater stride can be achieved (Aubret & Shine, 2008). The morphology of Ambystoma is adapted to have larger tails but also larger heads. In Ambystoma larvae head size is associated with a greater acceleration ability, high acceleration bursts, and high swimming velocity (Hoff et al., 1989), as well as head width is positively related to propulsive performance and may serve an important stabilizing function (Fitzpatrick et al., 2003). In adult salamanders, head size is associated with food intake and level of aggression (Adams, 2000, 2004; Adams & Rohlf, 2000).
Aquatic species have elongated bodies because these species may use their whole trunk for swimming using a posterior traveling wave along their bodies (Deban & Schilling, 2009). Aquatic salamanders have an undulatory swimming gait with limbs tucked against the body (Frolich & Biewener, 1992). However, this species also uses its limbs for aquatic walking on the substrate (Azizi & Horton, 2004). In the case of A. dumerilii it can move towards the water column, however, by being considered epibenthic, it can swim or use its limbs (Aguilar-Miguel & Casas-Andreu, 2005; Montes-Calderón et al., 2011). Our results show that lacustrine organisms have larger limbs, as in other species of the genus Ambystoma where it has been reported that A. ordinarium have a daily displacement between 4 and 20 m (Aguilar-Miguel & Casas-Andreu, 2005; Montes-Calderón et al., 2011), while A. maculatum has a range of 3.3 to 29.4 m (Duellman & Trueb, 1994), which coincides with the displacements of other salamanders with an average distance of 10 m so that A. dumerilii could show a similar behavior (Duellman & Trueb, 1994). On the other hand, in captivity, individuals of A. dumerilii may not develop traits associated with swimming, as do individuals from the lake due to the effect of the size of the enclosure, and the limitations of artificial conditions (Álvarez & Nicieza, 2002; Altwegg & Reyer, 2003; Relyea & Hoverman, 2003), where there are no competitors or predators of other species. Therefore, it is not easy to replicate natural abiotic conditions (Essner & Suffian, 2010). In this way, Ambystoma salamanders perceive captivity as “mildly stressful” (Davis & Maerz, 2011), however they show smaller sizes due to suboptimal environments and present less swimming capacity due to the limited space available for movement, greater susceptibility to hunger, and higher mortality when raised in conditions where resources are limited (Álvarez & Nicieza, 2002; Altwegg & Reyer, 2003; Relyea & Hoverman, 2003).
Variation in morphological traits scales with body size, ranging from the perfect covariance of a trait with body size (isometry) to highly uncorrelated, where morphological traits grow more or less slowly than body size (Fairbairn, 1997). The ontogenetic allometry is the source of morphological variation during the growth process (Murta-Fonseca et al., 2020). In our study, the allometric relationships between SVL and morphological traits were highly consistent, with most of the morphological characters showing negative allometric patterns or hypoallometry in many traits and the slopes of allometric correlation of many traits were lower in salamanders from the lake than captivity. We suggest that stressful conditions in the lake, such as disturbance derived from anthropogenic factors, change in land use, contamination by wastewater, herbicides and pesticides (Zambrano et al., 2011), promote lower development rates of these traits than body growth.
It is important to mention that although we did not measure individual fitness and performance, all variations in phenotypic traits such as body size and body proportions, could affect performance. Our results are consistent in the context of performance, since individuals in the lake with slimmer bodies, reduced limbs, and higher FA suggest potential adaptations toward increased swimming performance and the opposite for those in captivity. Amphibians with smaller body sizes are associated with reduced survival (Altwegg & Reyer, 2003), and may dehydrate quicker when compared with larger individuals (Gray & Smith, 2005). It is important to ask if individuals in captivity are functionally similar to their wild counterparts (Calisi & Bentley, 2009).
Suboptimal environmental conditions can lead to stress that affects the health of the organisms and modifies their morphology, as well as their behavioral and physiological performance (Denver, 1997). The captivity conditions are designed to replicate the wild environmental parameters, in order to maintain animals in good long-term health and potentially improve their fitness, and on occasions promote their reintroduction into suitable environments (Davis & Maerz, 2011), therefore our study could help to rethink the conditions in which the organisms are found, since negative allometric patterns were found in relation to the extremities, which plays an important role for their fitness.
Since different populations of the same species are not found under the same conditions, the inclusion of morphological diversity data in biodiversity conservation seems to be an important developing strategy for reducing biodiversity losses under global change (Des Roches et al., 2018).
Acknowledgements
This study was supported by The Mohamed bin Zayed Species Conservation Fund, with the project number: 182518929, “Evaluating populations of the Michoacan axolot (A. dumerilii) in Lake Pátzcuaro for the recovery of local management and fisheries”, and Chester Zoo with the project “Conservation and long-term management of the “achoque” (Ambystoma dumerilii) and its habitat”. We thank the Regional Center for Fisheries Research in Pátzcuaro for the facilities, and two anonymous reviewers whose comments helped us to improve the manuscript.
References
Adams, D. C. (2000). Divergence of trophic morphology and resource use among populations of Plethodon cinereus and P. hoffmani in Pennsylvania – a possible case of character displacement. In R. C. Bruce, R. G. Jaeger, & L. D. Houck (Eds.), Biology of plethodontid salamanders (pp. 383–395). Boston, MA: Springer Science. https://doi.org/10.1007/978-1-4615-4255-1_19
Adams, D. C. (2004). Character displacement via aggressive interference in Appalachian salamanders. Ecology, 85, 2664–2670. https://doi.org/10.1890/04-0648
Adams, D. C., & Rohlf, F. J. (2000). Ecological character displacement in Plethodon: biomechanical differences found from a geometric morphometric study. Proceedings of the National Academy of Sciences, 97, 4106–4111. https://doi.org/10.1073/pnas.97.8.4106
Aguilar-Miguel, X. (2005). Ambystoma dumerilii. Algunas especies de anfibios y reptiles contenidos en el Proyecto de Norma Oficial Mexicana PROY-NOM-059-ECOL-2000. Facultad de Ciencias, Centro de Investigación en Recursos Bióticos, Universidad Autónoma del Estado de México. Bases de datos SNIBCONABIO. Proyecto W035. México. D.F. Disponible en: http://www.conabio.gob.mx/conocimiento/ise/fichasnom/Ambystomadumerilii00.pdf
Aguilar-Miguel, X., & Casas Andreu, G. (2005). Ficha técnica de Ambystoma dumerilii. In Aguilar-Miguel, X. Algunas especies de anfibios y reptiles contenidos en el Proyecto de Norma Oficial Mexicana PROY-NOM-059-ECOL-2000. Facultad de Ciencias, Centro de Investigación en Recursos Bióticos, Universidad Autónoma del Estado de México. Bases de datos SNIB-CONABIO. Proyecto No. W035. México, D.F. Retrieved on November 28th, 2021 from: https://www.naturalista.mx/taxa/26782-Ambystoma-dumerilii
Alarcón-Ríos, L., Velo-Antón, G., & Kaliontzopoulou, A. (2017). A non-invasive geometric morphometrics method for exploring variation in dorsal head shape in urodeles: sexual dimorphism and geographic variation in Salamandra salamandra. Journal of Morphology, 278, 475–485. https://doi.org/10.1002/jmor.20643
Altwegg, R., & Reyer, H. U. (2003). Patterns of natural selection on size at metamorphosis in water frogs. Evolution, 57, 872–882. https://doi.org//10.1111/j.0014-3820.2003.tb00298.x
Álvarez, D., & Nicieza, A. G. (2002). Effects of induced variation in anuran larval development on postmetamorphic energy reserves and locomotion. Oecologia, 131, 186–195. https://doi.org//10.1007/s00442-002-0876-x
Anderson, J. D., & Worthington, R. D. (1971). The life history of the Mexican salamander Ambystoma ordinarium Taylor. Herpetologica, 27, 165–176.
Assis, V. R., Titon, S. C. M., Barsotti, A. M. G., Titon, B. Jr., & Gomes, F. R. (2015). Effects of acute restraint stress, prolonged captivity stress and transdermal corticosterone application on immunocompetence and plasma levels of corticosterone on the cururu toad (Rhinella icterica). Plos One, 10, e0121005. https://doi.org/10.1371/journal.pone.0121005
Aubret, F., & R. Shine. (2008). The origin of evolutionary innovations: locomotor consequences of tail shape in aquatic snakes. Functional Ecology, 22, 312–322. https://doi.org/10.1111/j.1365-2435.2007.01359.x
Azizi, E., & Horton, J. M. (2004). Pattern of axial and appendicular movements during aquatic walking in the salamander Siren lacertina. Zoology, 107, 111–120. https://doi.org/10.1016/j.zool.2004.03.002
Bookstein, F. L. (1997). Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Medical Image Analysis, 1, 225–243. https://doi.org/10.1016/S1361-8415(97)85012-8
Brunson, K. L., Avishai-Eliner, S., Hatalski, C. G., & Baram, T. Z. (2001). Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Molecular Psychiatry, 6, 647–656. https://doi.org/10.1038/sj.mp.4000942
Calisi, R. M., & Bentley, G. E. (2009). Lab and field experiments: are they the same animal? Hormones and Behavior, 56, 1–10. https://doi.org/10.1016/j.yhbeh.2009.02.010
Casas-Andreu, G., Cruz-Aviña, R., & Aguilar-Miguel, X. (2004). Un regalo poco conocido de México al mundo: el ajolote o axolotl (Ambystoma: Caudata: Amphibia). Con algunas notas sobre la crítica situación de sus poblaciones. Ciencia Ergo Sum, 10, 304–308.
Cayuela, H., Quay, L., Dumet, A., Léna, J. P., Miaud, C., & Rivière, V. (2017). Intensive vehicle traffic impacts morphology and endocrine stress response in a threatened amphibian. Oryx, 51, 182–188. https://doi.org/10.1017/S0030605315000812
Cuevas-Reyes, P., Canché-Delgado, A., Maldonado-López, Y., Fernandes, G. W., Oyama, K., & González-Rodríguez, A. (2018). Patterns of herbivory and leaf morphology in two Mexican hybrid oak complexes: importance of fluctuating asymmetry as indicator of environmental stress in hybrid plants. Ecological Indicators, 90, 164–170. https://doi.org/10.1016/j.ecolind.2018.03.009
Davis, A. K., & Maerz, J. C. (2010). Effects of exogenous corticosterone on circulating leukocytes of a salamander (Ambystoma talpoideum) with unusually abundant eosinophils. International Journal of Zoology, 2010, 735937. https://doi.org/10.1371/journal.pone.0163736
Davis, A. K., & Maerz, J. C. (2011). Assessing stress levels of captive-reared amphibians with hematological data: implications for conservation. Initiatives Journal of Herpetology, 45, 40–44. https://doi.org/10.1670/10-180.1
Davis, A. K., & Maney, D. L. (2018). The use of glucocor-
ticoid hormones or leucocyte profiles to measure stress in vertebrates: What’s the difference? Methods in Ecology and Evolution, 9, 1556–1568. https://doi.org/10.1111/2041-210X.13020
Davis, D. R., Ferguson, K. J., Schwarz, M. S., & Kerby, J. L. (2020). Effects of agricultural pollutants on stress hormones and viral infection in larval salamanders. Wetlands, 40, 577–586. https://doi.org/10.1007/s13157-019-01207-1
de Vosjoli, P. (1999). Designing environments for captive amphibians and reptiles. Veterinary clinics of North America: Exotic Animal Practice, 2, 43–68.
Deban, S. M., & Schilling, N. (2009). Activity of trunk muscles during aquatic and terrestrial locomotion in Ambystoma maculatum. Journal of Experimental Biology, 212, 2949–2959. https://doi.org/10.1242/jeb.032961
Delgado-Acevedo, J., & Restrepo, C. (2008). The contribution of habitat loss to changes in body size, allometry, and bilateral asymmetry in two Eleutherodactylus frogs from Puerto Rico. Conservation Biology, 22, 773–782. https://doi.org/10.1111/j.1523-1739.2008.00930.x
Denver, R. J. (1997). Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Hormones and Behavior, 31, 169–179. https://doi.org/10.1006/hbeh.1997.1383
Denver, R. J. (2009). Stress hormones mediate environment-genotype interactions during amphibian development. General and Comparative Endocrinology, 164, 20–31. https://doi.org/10.1016/j.ygcen.2009.04.016
Denver, R. J., Mirhadi, N., & Phillips, M. (1998). Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology, 79, 1859–1872. https://doi.org/10.2307/176694
Des Roches, S., Post, D. M., Turley, N. E., Bailey, J. K., Hendry, A. P., Kinnison, M. T. et al. (2018). The ecological importance of intraspecific variation. Nature Ecology & Evolution, 2, 57–64. https://doi.org/10.1038/s41559-017-0402-5
Duellman, W. E., & Trueb, L. (1986). Biology of amphibians. New York: McGraw-Hill Book. https://doi.org/10.2307/1445022
Duellman, W. E., & Trueb, L. (1994). Biology of amphibians. Baltimore: The Johns Hopkins University Press.
Essner, Jr., R. L., & Suffian, D. J. (2010). Captive husbandry in the Rocky Mountain Tailed Frog, Ascaphus montanus. Herpetological Review, 41, 181–184. https://doi.org/10.1126/sciadv.abn1104
Fairbairn, D. J. (1997). Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in
males and females. Annual Review of Ecology and Systematics, 28, 659–687. https://doi.org/10.1146/annurev.ecolsys.28.1.659
Fitzpatrick, B. M., Benard, M. F., & Fordyce, J. A. (2003). Morphology and escape performance of tiger salamander larvae (Ambystoma tigrinum mavortium). Journal of Experimental Zoology, 297A, 147–159. https://doi.org/
10.1002/JEZ.A.10254
Fox, G. A., Cooper, A. M., & Hayes, W. K. (2015). The dilemma of choosing a reference character for measuring sexual size dimorphism, sexual body component dimorphism, and character scaling: cryptic dimorphism and allometry in the scorpion Hadrurus arizonensis. Plos One, 10, e0120392. https://doi.org/10.1371/journal.pone.0120392
Frolich, L. M., & Biewener, A. A. (1992). Analysis of the functional role of the body axis during terrestrial and aquatic locomotion in the salamander Ambystoma tigrinum. Journal of Experimental Biology, 162, 107–130. https://doi.org/10.1242/jeb.162.1.107
Gangenova, E., Giombini, M. I., Zurita, G. A., & Marangoni, F. (2020). Morphological responses of three persistent native anuran species after forest conversion into monoculture pine plantations: tolerance or prosperity? Integrative Zoology, 15, 428–440. https://doi.org/10.1111/1749-4877.12440
Gray, M. J., & L. M. Smith. (2005). Influence of land use on postmetamorphic body size of playa lake amphibians. Journal of Wildlife Management, 69, 515–524.
Hoff, K., Huq, N., King, V. A., & Wassersug, R. J. (1989). The kinematics of larval salamander swimming. Canadian Journal of Zoology, 67, 2756–2761. https://doi.org/10.1139/z89-391
Hu, F., Crespi, E. J., & Denver, R. J. (2008). Programming neuroendocrine stress axis activity by exposure to glucocorticoids during postembryonic development of the frog Xenopus laevis. Endocrinology, 149, 5470–5481. https://doi.org/10.1210/en.2008-0767
Huacuz-Elías, D. C. (2002). Programa de conservación y manejo de Ambystoma dumerilii: el A. dumerilii del Lago de Pátzcuaro. Morelia, Mich: UMSNH.
Huacuz-Elías, D. C. (2008). Biología y conservación del género Ambystoma en Michoacán, México (Ph.D. Thesis). Universidad de Salamanca, Salamanca, España.
Huerto-Delgadillo, R. I., Vargas-Velázquez, S., & Ortiz-Paniagua, C. F. (2011). Estudio ecosistémico del lago de Pátzcuaro: aportes en gestión ambiental para el fomento del desarrollo sustentable. Jiutepec, Morelos: Instituto Mexicano de Tecnología del Agua.
Iglesias-Carrasco, M., Martín, J., & Cabido, C. (2017). Urban habitats can affect body size and body condition but not immune response in amphibians. Urban Ecosystems, 20, 1331–1338. https://doi.org/10.1007/s11252-017-0685-y
Ijspeert, A. J., & Cabelguen, J. M. (2006). Gait transition from swimming to walking: investigation of salamander locomotion control using nonlinear oscillators. In H. Kimura, K. Tsuchiya, A. Ishiguro, & H. Witte (Eds.), Adaptive motion of animals and machines (pp. 177–188). Tokio, Japan: Springer. https://doi.org/10.1007/4-431-31381-8_16
Irschick, D. J., & Garland, T. J. (2001). Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Annual Review of Ecology and Systematics, 32, 367–396. https://doi.org/10.1146/annurev.ecolsys.32.081501.114048
IUCN (International Union for Conservation of Nature) SSC (Species Survival Commission) Amphibian Specialist Group. (2020). Ambystoma dumerilii. The IUCN Red List of Threatened Species: e.T59055A53973725. Retrieved on November 28th 2020, from: https://dx.doi.org/10.2305/IUCN.UK.2020-1.RLTS.T59055A53973725.en.
Jessop, T. M., Madsen, T., Sumner, J., Rudiharto, H., Phillips, J. A., & Ciofi, C. (2006). Maximum body Size among insular Komodo dragon populations covaries with large prey density. Oikos, 112, 422–429. https://doi.org/10.1111/j.0030-1299.2006.14371.x
Johansson, F., Lederer, B., & Lind, M. I. (2010). Trait performance correlations across life stages under environmental stress conditions in the common frog, Rana temporaria. Plos One, 5, e11680. https://doi.org/10.1371/journal.pone.0011680
Klingenberg, C. P. (2003). Developmental instability as a research tool: using patterns of fluctuating asymmetry to infer the developmental origins of morphological integration. In M. Polak (Ed.), Developmental instability: causes and consequences (pp.427–442). New York: Oxford University Press.
Klingenberg, C. P. (2011). MorphoJ: an integrated software pack-age for geometric morphometrics. Molecular Ecology Resources, 11, 353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x
Lempa, K., Martel, J., Koricheva, J., Haukioja, K., Ossipov, V., Ossipova, S. et al. (2000). Covariation of fluctuating asymmetry, herbivory and chemistry during birch leaf expansion. Oecologia, 122, 354–360. https://doi.org/10.
1007/s004420050041
Lens, L., von Dongen, S., & Matthysen, E. (2002). Fluctuating asymmetry as an early warning system in the critically endangered Taita Thrush. Conservation Biology, 16, 479–487. https://doi.org/10.1046/j.1523-1739.2002.00516.x
Lleonart, J., Salat, J., & Torres, G. J. (2000). Removing allometric effects of body size in morphological analysis. Journal of Theoretical Biology, 205, 85–93. https://doi.org/10.1006/jtbi.2000.2043
Lomolino, M. V., Channell, R., Perault, D. R., & Smith, G. A. (2001). Down- sizing nature: anthropogenic dwarfing of species and ecosystems. In J. L. Lockwood, & M. L. McKinney (Eds.), Biotic homogenization (pp. 223–243). New York: Kluwer Academic/ Plenum. https://doi.org/10.1007/978-1-4615-1261-5_11
Lowe, W. H., Likens, G. E., & Cosentino, B. J. (2006). Self-organization in streams: the relationship between movement behaviour and body condition in a headwater salamander. Freshwater Biology, 51, 2052–2062. https://
doi.org/10.1111/j.1365-2427.2006.01635.x
Martel, J., Lempa, K., & Haukioja, E. (1999). Effect of stress and rapid growth on fluctuating asymmetry and insect damage in birch leaves. Oikos, 86, 208–216. https://doi.org/10.2307/3546439
Matthews, S. G. (2002). Early programming of the hypothalamo–pituitary–adrenal axis. Trends in Endocrinology and Metabolism, 13, 373–380. https://doi.org/10.1016/s1043-2760(02)00690-2
Michaels, C. J., Gini, B., & Preziosi, R. F. (2014). The importance of natural history and species-specific approaches in amphibian ex-situ conservation. The Herpetological Journal, 24, 135–145.
Milligan, J. R., Krebs, R. A., & Mal, T. K. (2008). Separating developmental and environmental effects on fluctuating asymmetry in Lythrum salicaria and Penthorum sedoides. International Journal of Plant Sciences, 169, 625–630. https://doi.org/10.1086/533600
Møller, P. A., & Manning, J. (2003). Growth and developmental instability. The Veterinary Journal, 166, 19–27. https://doi.org/10.1016/s1090-0233(02)00262-9
Montes-Calderón, M. A., Alvarado-Díaz, J., & Suazo-Ortuño, I. (2011). Abundancia, actividad espacial y crecimiento de Ambystoma ordinarium Taylor 1940 (Caudata: Ambysto-matidae) en Michoacán, México. Biológicas, 13, 50–53.
Morrison, C., Hero, J. M., & Browning, J. (2004). Altitudinal variation in the age at maturity, longevity, and reproductive lifespan of anuran in subtropical Queensland. Herpetologica, 60, 34–44. https://doi.org/10.1655/02-68
Murta-Fonseca, R. A., Folly, M., Carmo, L. F., & Martins, A. (2020). Growing towards disparity: geometric morphometrics reveals sexual and allometric differences in Aparasphenodon brunoi (Anura: Hylidae: Lophyohylinae) head shape. Cuadernos de Herpetología, 34, 1–11. https://doi.org/10.31017/CdH.2020.(2019-032)
Ortega, A. J. (1999). El ajolote. Elementos: Ciencia y Cultura, 036, 55–57.
Palmer, A. R., & Strobeck, C. (1986). Fluctuating asymmetry: measurement, analysis, patterns. Annual Review of Ecology Systematics, 17, 391–421. https://doi.org/10.1146/ANNUREV.ES.17.110186.002135
Phillips, B. L., Brown, G. P., Webb, J. K., & Shine, R. (2006). Invasion and the evolution of speed in toads. Nature,
439, 803. https://doi.org/10.1038/439803a
Ramírez-Hernández, G., Suazo-Ortuño, I., Alvarado-Díaz, J., Escalera-Vázquez, L. H., Maldonado-López, Y., & Tafolla-Venegas, D. (2019). Effects of habitat disturbance on parasite infection and stress of the endangered Mexican stream salamander Ambystoma ordinarium. Salamandra, 55, 160–172.
Ramírez-Herrejón, J. P., Zambrano, L., Mercado-Silva, N., Torres-Téllez, A., Pineda-García, F., Caraveo-Patiño, J. et al. (2014). Long term changes in the fish fauna of Lago de Pátzcuaro in Central México. Latin American Journal of Aquatic Research, 42, 137–149. https://doi.org/10.3856/vol42-issue1-fulltext-11
Relyea, R. A. (2001). Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology, 82, 523–540. https://doi.org/10.2307/2679877
Relyea, R. A., & Hoverman, J. T. (2003). The impact of larval predators and competitors on the morphology and fitness of juvenile treefrogs. Oecologia, 134, 596–604. https://doi.org/10.1007/s00442-002-1161-8
Riva-Tonini, J. F., Provete, D. B., Maciel, N. M., Morais, A. R., Goutte, S., Toledo, L. F. et al. (2020). Allometric escape from acoustic constraints is rare for frog calls. Ecology and Evolution, 10, 3686–3695. https://doi.org/10.1002/
ece3.6155
Rohlf, F. J. (2015). The tps series of software. Hystrix the Italian Journal of Mammalogy, 26, 9–12. https://doi.org/10.4404/hystrix-26.1-11264
Semarnat (Secretaría del Medio Ambiente y Recursos Naturales). (2010). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental – Especies nativas de México de flora y fauna silvestres – Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio – Lista de especies en riesgo. Diario Oficial de la Federación. 30 de diciembre de 2010, Segunda Sección, México.
Semarnat (Secretaría de Medio Ambiente y Recursos Naturales) (2018). Programa de Acción para la Conservación de las Especies Ambystoma spp. Ciudad de México: Semarnat/ Conanp.
Shaffer, H. B. (1984). Evolution in a paedomorphic lineage. II. Allometry and form in the Mexican ambystomatid salamanders. Evolution, 38, 1207–1218. https://doi.org/10.2307/2408629
Shaffery, H. M., & Relyea, R. A. (2015). Predator-induced defenses in five species of larval Ambystoma. Copeia, 103, 552–562. https://doi.org/10.1643/CE-14-043
Shi, Y., Puzianowska-Kuznicka, M., & Stolow, M. A. (1996). Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: roles of thryoid hormone and its receptors. Bioessays, 18, 391–399. https://doi.org/10.1002/bies.950180509
Sibly, R., & Calow, P. (1984). Direct and absorption costing in the evolution of life cycles. Journal of Theoretical Biology, 111, 463–473. https://doi.org/10.1016/S0022-5193(84)80234-9
Slight, D. J., Nichols, H. J., & Arbuckle, K. (2015). Are mixed diets beneficial for the welfare of captive axolotls (Ambystoma mexicanum)? Effects of feeding regimes on growth and behavior. Journal of Veterinary Behavior, 10, 185–190. https://doi.org/10.1016/j.jveb.2014.09.004
Soto-Rojas, C., Suazo-Ortuño, I., Montoya-Laos, J. A., & Alvarado-Díaz, J. (2017). Habitat quality affects the incidence of morphological abnormalities in the endangered salamander Ambystoma ordinarium. Plos One, 12, e0183573. https://doi.org/10.1371/journal.pone.0183573
Steinicke, H., Gruber, B., Grimm, A., Grosse, W. R., & Henle, K. (2015). Morphological shifts in populations of generalist and specialist amphibians in response to fragmentation of the Brazilian Atlantic forests. Nature Conservation, 13, 47–59. https://doi.org/10.3897/natureconservation.13.7428
Stoler, A. B., & Relyea, R. A. (2013). Leaf litter quality induces morphological and developmental changes in larval amphibians. Ecology, 94, 1594–1603. https://doi.org/10.1890/12-2087.1
Tejedo, M., Marangoni, F., Pertoldi, C., Richter-Boix, A., Laurila, A., Orizaola, G. et al. (2010). Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Climate Research, 43, 31–39. https://doi.org/10.3354/cr00878
Titon, S. C. M., Assis, V. R., Titon, Jr. B., Cassettari, B. O., Fernandes, P. A. C. M., & Gomes, F. R. (2017). Captivity effects on immune response and steroid plasma levels of a Brazilian toad (Rhinella schneideri). Journal of Experimental Zoology A, 327, 127–138. https://doi.org/10.1002/jez.2078
Tomasini-Ortiz, C., Bravo-Inclán, L., Sánchez-Chávez, J., & Moeller-Chávez, G. (2016). Monitoreo de descargas de aguas residuales y su impacto en el lago de Pátzcuaro, México (2006-2011). Revista AIDIS de Ingeniería y Ciencias Ambientales: Investigación, Desarrollo y Práctica, 9, 61–74. Retrieved from https://revistas.unam.mx/index.php/aidis/article/view/50113
Urban, M. C. (2010). Microgeographic adaptations of spotted salamander morphological defenses in response to a predaceous salamander and beetle. Oikos, 119, 646–658. https://doi.org/10.1111/j.1600-0706.2009.17970.x
Van Buskirk, J. (2011). Amphibian phenotypic variation along a gradient in canopy cover: species differences and plasticity. Oikos, 120, 906–914. https://doi.org/10.1111/j.1600-0706.2010.18845.x
Van Buskirk, J., & McCollum, S. A. (2000). Functional mechanisms of an inducible defence in tadpoles: morphology and behaviour influence mortality risk from predation. Journal of Evolutionary Biology, 13, 336–347. https://doi.org/10.1046/j.1420-9101.2000.00173.x
Van Buskirk, J., & Schmidt, B. R. (2000). Predator-induced phenotypic plasticity in larval newts: trade-offs, selection, and variation in nature. Ecology, 81, 3009–3028. https://doi.org/10.2307/177397
Vega-Trejo, R., Zúñiga-Vega, J. J., & Langerhans, R. B. (2014). Morphological differentiation among populations of Rhinella marina (Amphibia: Anura) in western Mexico. Evolutionary Ecology, 28, 69–88. https://doi.org/10.1007/s10682-013-9667-6
Velarde-Mendoza, T. (2012). Importancia ecológica y cultural de una especie endémica de ajolote (Ambystoma dumerilii) del lago de Pátzcuaro, Michoacán. Etnobiología, 10, 40–49.
Velickovic, M., & Perisic, S. (2006). Leaf fluctuating asymmetry of common plantain as an indicator of habitat quality. Plant Biosystems, 140, 138–145. https://doi.org/10.1080/11263500600756322
Vorndran, I. C., Reichwaldt, E., & Nürnberger, B. (2002). Does differential susceptibility to predation in tadpoles stabilize the Bombina hybrid zone? Ecology, 83, 1648–1659. https://doi.org/10.2307/3071985
Wright, A. N., & Zamudio, K. R. (2002). Color pattern asymmetry as a correlate of habitat disturbance in spotted salamanders (Ambystoma maculatum). Journal of Herpetology,
36, 129–133. https://doi.org/10.1670/0022-1511(2002)036[0129:CPAAAC]2.0.CO;2
Wu, Z., Li, Y., & Murray, B. R. (2006). Insular shifts in body size of rice frogs in the Zhoushan archipelago, China. Journal of Animal Ecology, 75, 1071–1080. https://doi.org/10.1111/j.1365-2656.2006.01126.x
Zambrano, L., Cordoval-Tapia, F., Ramírez-Herrejón, J. P., Mar-Silva, V., Bustamante, L., Camargo, T. et al. (2011). Las especies exóticas en el lago de Pátzcuaro, Michoacán, México. In R. Huerto-Delgadillo, S. Vargas-Velázquez, & C. Ortiz-Paniagua, (Eds.). Estudio ecosistémico del lago de Pátzcuaro. Jiutepec, Morelos: Instituto Mexicano de Tecnología del Agua. https://doi.org/10.13140/RG.2.1.3619.0242
Zhelev, Z. M., Popgeorgiev, G. S., & Georgieva, Z. K. (2014). Fluctuating asymmetry in the populations of Pelophylax ridibundus and Pseudepidalea viridis (Amphibia: Anura) in the region of the lead and zinc plant “Kardzhali” (South Bulgaria). Acta Zoologica Bulgarica, 66, 83–87.
Zhelev, Z. M., Popgeorgiev, G. S., Arnaudov, A. D., Georgieva, K. N., & Mehterov, N. H. (2015). Fluctuating asymmetry in Pelophylax ridibundus (Amphibia: Ranidae) as a response to anthropogenic pollution in south Bulgaria. Archives of Biological Sciences, 67, 1009–1023. https://doi.org/10.2298/ABS141210064Z
Zhelev, Z. M., Tsonev, S. V., & Angelov, M. V. (2019). Fluctuating asymmetry in Pelophylax ridibundus meristic morphological traits and their importance in assessing environmental health. Ecological Indicators, 107, 105589. https://doi.org/10.1016/j.ecolind.2019.105589




