Pedro Giovâni da Silva a, Renato P. Salomão b, Daniel González-Tokman c, d, Frederico S. Neves a, e, Mario E. Favila c, *
a Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas, Programa de Pós-Graduação em Ecologia, Conservação e Manejo da Vida Silvestre, Av. Presidente Antônio Carlos 6627, Pampulha, Belo Horizonte, 31270-901, Minas Gerais, Brazil
b Universidad Nacional Autónoma de México, Instituto de Ecología, Tercer circuito s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
c Instituto de Ecología A.C., Red de Ecoetología, Carretera antigua a Coatepec 351, Col. El Haya, 91073 Xalapa, Veracruz, Mexico
d Consejo Nacional de Ciencia y Tecnología, Av. de los Insurgentes Sur 1582, Col. Crédito Constructor, Benito Juárez, 03940 Ciudad de México, Mexico
e Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas, Departamento de Genética, Ecologia e Evolução, Programa de Pós-Graduação em Ecologia, Conservação e Manejo da Vida Silvestre, Av. Presidente Antônio Carlos 6627, Pampulha, Belo Horizonte, 31270-901, Minas Gerais, Brazil
*Autor para correspondencia: mario.favila@inecol.mx (M.E. Favila)
Recibido: 15 junio 2022; aceptado: 8 marzo 2023
Abstract
We evaluated short- (among months within-years) and long-term (between 1999-2000 and 2016-2017) temporal patterns of taxonomic and functional β-diversity (and its components of substitution and gain/loss) of dung beetle assemblages in forest fragments and pastures in the Los Tuxtlas Biosphere Reserve (LTBR). Habitat type affected the taxonomic dissimilarity and the richness difference component, with average values being respectively 1.42 and 1.56 times higher in pastures than in forest fragments. Only habitat type was important for functional richness, being 1.93 and 1.69 higher in forest fragments than pastures in 1999-2000 and 2016-2017, respectively. Pastures were taxonomically and functionally poorer but were also more temporally dynamic than forest fragments both within-year and between-years. Habitat type is a determining factor for temporal dynamics, with forest fragments presenting more stable dung beetle assemblages than cattle pastures.
Keywords: Anthropogenic landscape; Bioindicators; Habitat modification; Scarabaeinae; Temporal patterns
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Cambios temporales de la diversidad taxonómica y funcional en los escarabajos del estiércol que habitan fragmentos de bosque y pastizales en la Reserva de la Biosfera Los Tuxtlas, México
Resumen
Evaluamos los efectos temporales a corto (entre meses de cada año) y largo (1999-2000 y 2016-2017) plazo sobre la diversidad β taxonómica y funcional (y sus componentes de sustitución y pérdida/ganancia) de los ensambles de escarabajos del estiércol en fragmentos forestales y potreros en la Reserva de la Biosfera Los Tuxtlas (RBLT). El tipo de hábitat afectó la disimilitud taxonómica y el componente de diferencia de riqueza de especies (diversidad β), con valores promedios de 1.42 y 1.56 veces más altos en los potreros que en los fragmentos forestales, respectivamente. Solamente el tipo de hábitat fue determinante para la riqueza funcional, siendo 1.93 y 1.69 veces más alto en los fragmentos forestales que en los potreros en el 1999-2000 y en el 2016-2017, respectivamente. Los potreros fueron taxonómica y funcionalmente más pobres, aunque también fueron temporalmente más dinámicos que en los fragmentos forestales, tanto dentro de cada período anual, como entre años. El tipo de hábitat es un factor determinante para las dinámicas temporales y se nota que las selvas son ambientes más estables que los potreros para los ensambles de los escarabajos del estiércol.
Palabras clave: Paisajes antrópicos; Bioindicadores; Cambios de hábitat; Scarabaeinae; Patrones temporales
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Biodiversity is constantly changing throughout time (Dornelas et al., 2014). Depending on the temporal scale analysed, different processes drive the maintenance of the ecological communities (Castillo-Escrivà et al., 2020). When analysing changes in species diversity throughout a year (i.e., short-term scale), it is expected that the interaction between seasonal variation and the surrounding environment determines how species diversity changes (Tonkin et al., 2017). For example, in a study performed with dung beetles in the Amazon region it was observed that the environment type modulated the seasonal variation of these insects’ diversity (Noriega et al., 2021). On the other hand, when analysing biodiversity changes at long-term scale (e.g., changes in diversity between different decades), environmental characteristics related to ecological stability seems to be the strongest driving force in this temporal scale (Lindholm et al., 2020). In environments with unstable conditions for native biota, as pasturelands created in tropical rainforest biomes, there is a tendency towards more heterogeneous communities at long-term scales when compared to more stable ecosystems, as the native vegetation of a region (Salomão et al., 2020). Nonetheless, trends regarding different temporal scales are still incipient. By understanding how diversity changes behave at short- and long-term scales, we may comprehend and compare temporal contexts and depict if certain changes are related to seasonal variations (i.e., short-term scale) or to landscape stability contexts (i.e., long-term scale).
Anthropogenic activities are causing global biodiversity declines over time (Barlow et al., 2016; Newbold et al., 2015, 2020) and the replacement of native forests by agricultural land, such as pastures, is one of the main causes of the global biodiversity crisis (Laurance et al., 2014). This process reduces species richness and tends to homogenize the species composition in anthropogenic land uses, with severe consequences to taxonomic (Newbold et al., 2015; Solar et al., 2015), functional (Flynn et al., 2009; Rivera et al., 2021), and phylogenetic diversity (Edwards et al., 2015; Rivera et al., 2022). Especially in the tropics, agricultural practices have led to marked landscape transformations, with the replacement of more than half of native forest cover of many tropical forest ecosystems (Myers et al., 2000; Ribeiro et al., 2009; Vega-Vela et al., 2018). This phenomenon has risen major concerns because tropical forests are key ecosystems that harbour at least two-thirds of the global terrestrial biodiversity and provide important economic goods and ecosystem functions (Gardner et al., 2009). Therefore, anthropogenic land-use changes are now considered one of the most important environmental threats for biodiversity in the Anthropocene (Dirzo et al., 2014).
There are several different approaches to understand how community heterogeneity (i.e., β-diversity) is established across short- and long-term temporal scales (Legendre, 2019; Magurran et al., 2019; Tatsumi et al., 2021). By partitioning β-diversity into its 2 mainly studied components (i.e., richness difference/nestedness and replacement) it is possible to understand how community heterogeneity is being structured (Legendre, 2014). Previous studies suggest that an equilibrium between replacement and nestedness components is more related to well-preserved areas (Solar et al., 2015), while an increase in species substitution (i.e., turnover or replacement) is associated to more unstable environmental conditions (Salomão et al., 2020). There are scarce studies comprising the effects of habitat type and different time-scale studies on ecological communities from a β-diversity perspective. This is particularly alarming if we consider more than one facet of diversity (e.g., taxonomic and functional). Considering the ongoing scenario of native forest loss in tropical ecosystems (Hansen et al., 2020; Laurance, 2007), it is urgent to present different approaches that allow us to understand how biodiversity changes throughout years in the Anthropocene. Through the assessment of β-diversity approach, it is possible to understand how different environments may maintain biodiversity and stability of the ecological communities throughout years.
In both forest and pasture areas, dung beetles (Coleoptera: Scarabaeinae) are key components because they use dung for feeding and nesting. Due to this activity the dung beetles have meaningful roles in important ecosystem functions, such as nutrient recycling, bioturbation, secondary seed dispersal, plant growth enhancement, parasite control, and reduction of greenhouse gas emissions (Nichols et al., 2008; Slade et al., 2016). The loss of these functions and services is expected to have important effects on the ecosystem functioning (Braga et al., 2013; Slade et al., 2007). Besides, dung beetles are a diverse group of insects (taxonomically and functionally) that have been widely used to evaluate the effects of anthropogenic changes caused to natural environments, especially in tropical forests where they are cost-effective, responsive study models and biodiversity bioindicators (Barlow et al., 2010; Solar et al., 2015). These beetles also show a markedly seasonal pattern in response to climate conditions (temperature and precipitation) and availability of food resources (Hernández & Vaz-de Mello, 2009; Neves et al., 2010). Besides, few studies have evaluated dung beetle response to disturbance and habitat change over large time scales. Such studies present clear shifts in community structure throughout time (Escobar et al., 2008; Noriega et al., 2021), as well as an increase in dung beetle diversity with the increase of vegetation cover (Cuesta & Lobo, 2019). Therefore, both short- and long-term evaluations of dung beetle assemblages throughout time are helpful to understand how functional groups have changed among habitats and the underlying implications to conservation and management caused by habitat changes in ecosystem functioning.
Here, we evaluated the same dung beetle assemblages, sampled monthly in forest fragments and pastures in 1999-2000 and 2016-2017, aiming to unveil short- and long-term changes in functional diversity and taxonomic and functional composition in the Los Tuxtlas Biosphere Reserve (LTBR). We hypothesize that long-term effects on dung beetle assemblages (“between year β-diversity”) will be different than those assemblages analysed under short-term time scales (“within year β-diversity”). Moreover, we predict greater changes in pastures than forest fragments due to higher temporal microclimatic stability (da Silva et al., 2019; Vega-Vela et al., 2018). Specifically, we expect: i) higher temporal taxonomic and functional changes in pastures than forest fragments driven by species/traits substitution (the same number of species/traits being gained and lost) rather than species/traits gain/loss (species/traits only being gained or only being lost in a nested way); and ii) higher taxonomic than functional β-diversity between sampling years (between year β-diversity) than among months of each sampling year (within year β-diversity).
Table 1
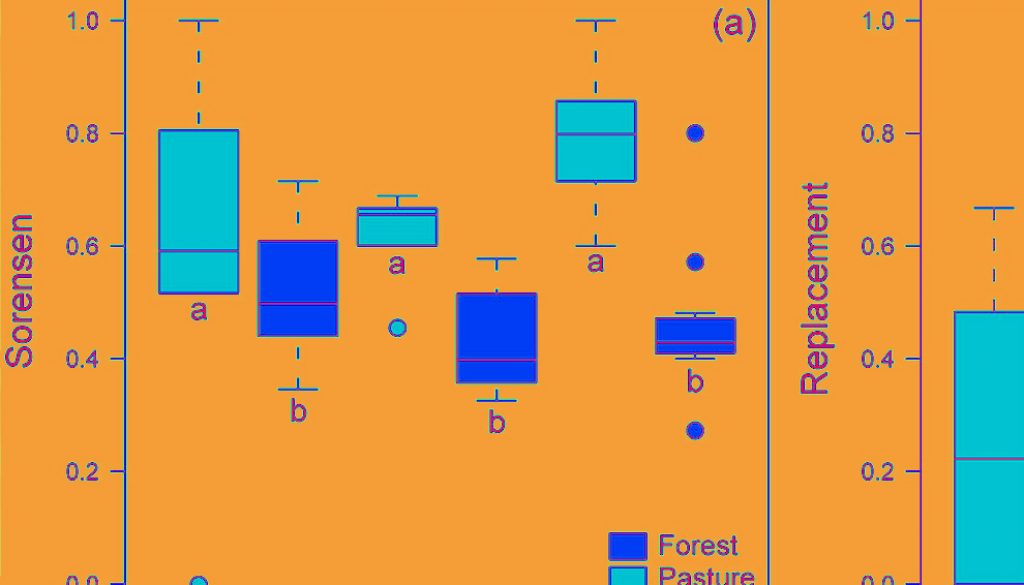
Results of generalized linear models estimating the effect of habitat (forest fragments or pasture), period (1999-2000, 2016-2017, and between sampling periods), and their interaction on dung beetles’ taxonomic β-diversity (Sorensen dissimilarity) and its components of replacement and richness difference (Podani’s approach). Temporal β-diversity values were calculated among months of each sampling period and between sampling periods. Sum Sq = sum of squares; Df = degrees of freedom; p < 0.05 are in bold.
| Response variable | Predictor variable | Sum Sq | Df | F-value | p |
| Sorensen | Habitat | 1.8244 | 1 | 17.6044 | 0.0001 |
| Period | 0.2438 | 2 | 1.1764 | 0.3177 | |
| Habitat: period | 0.6414 | 2 | 3.0943 | 0.0551 | |
| Residuals | 4.6636 | 45 | |||
| Replacement | Habitat | 0.3777 | 1 | 2.4295 | 0.1261 |
| Period | 0.6018 | 2 | 1.9356 | 0.1562 | |
| Habitat: period | 0.1785 | 2 | 0.5742 | 0.5672 | |
| Residuals | 6.9955 | 45 | |||
| Richness difference | Habitat | 0.7994 | 1 | 6.436 | 0.0147 |
| Period | 0.2378 | 2 | 0.9572 | 0.3917 | |
| Habitat: period | 0.4816 | 2 | 1.9385 | 0.1557 | |
| Residuals | 5.5895 | 45 |
Table 2
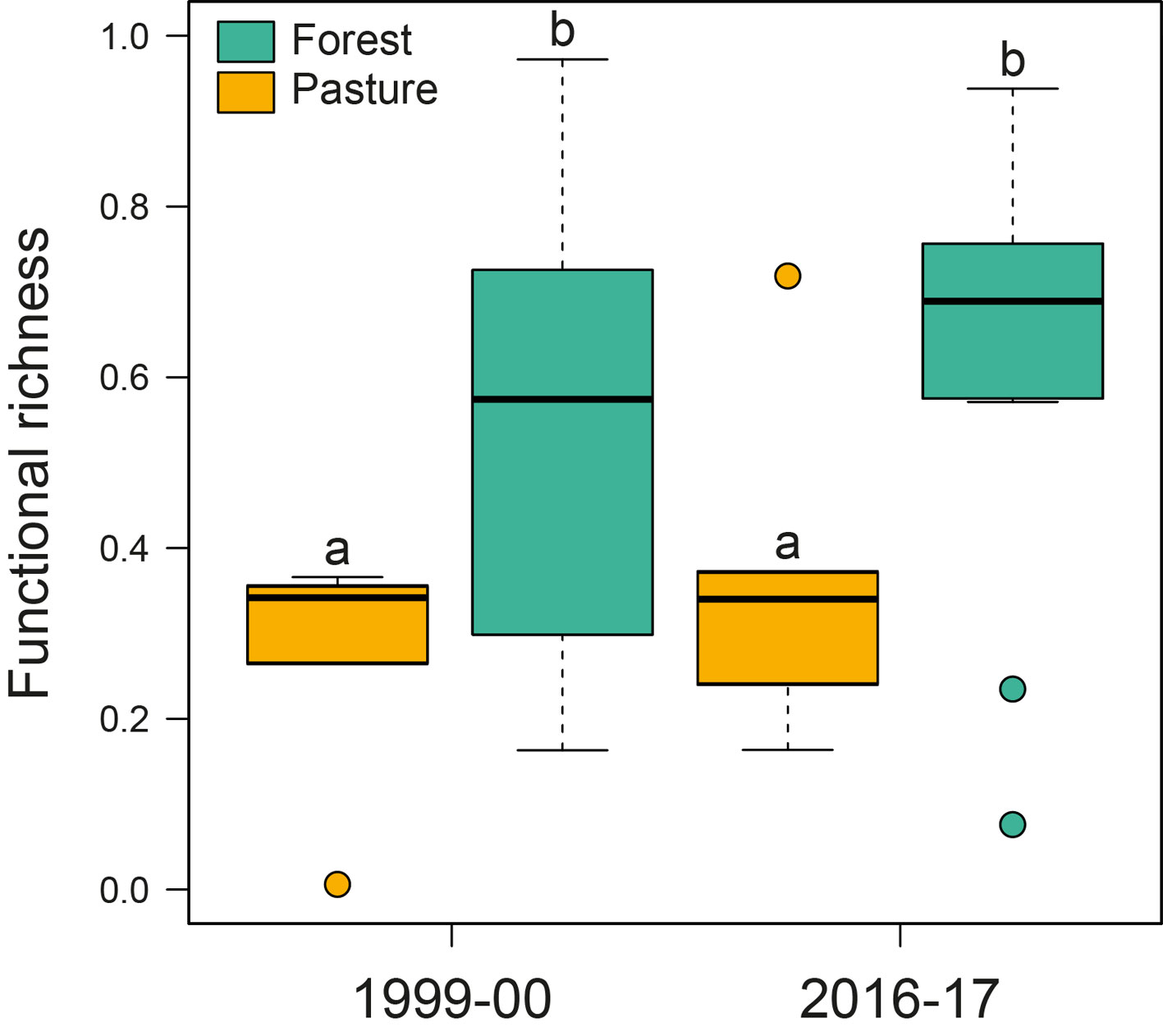
Results of generalized linear models estimating the effect of period (1999-2000, 2016-2017, and between sampling periods) on dung beetles’ functional richness of the entire assemblage and functional β-diversity (Sorensen dissimilarity) and its components of substitution and nestedness (Baselga’s approach) inhabiting forest fragments. Temporal β-diversity values were calculated over months of each sampling period and between sampling periods. We were unable to calculate functional metrics for pastures due to low site richness. Sum Sq = sum of squares; Df = degrees of freedom; p < 0.05 are in bold.
| Response variable | Predictor variable | Sum Sq | Df | F-value | p |
| Functional richness | Habitat | 1.7565 | 1 | 6.9908 | 0.0133 |
| Period | 0.3614 | 1 | 1.4384 | 0.2405 | |
| Habitat: period | 0.0006 | 1 | 0.0022 | 0.9629 | |
| Residuals | 7.0352 | 28 | |||
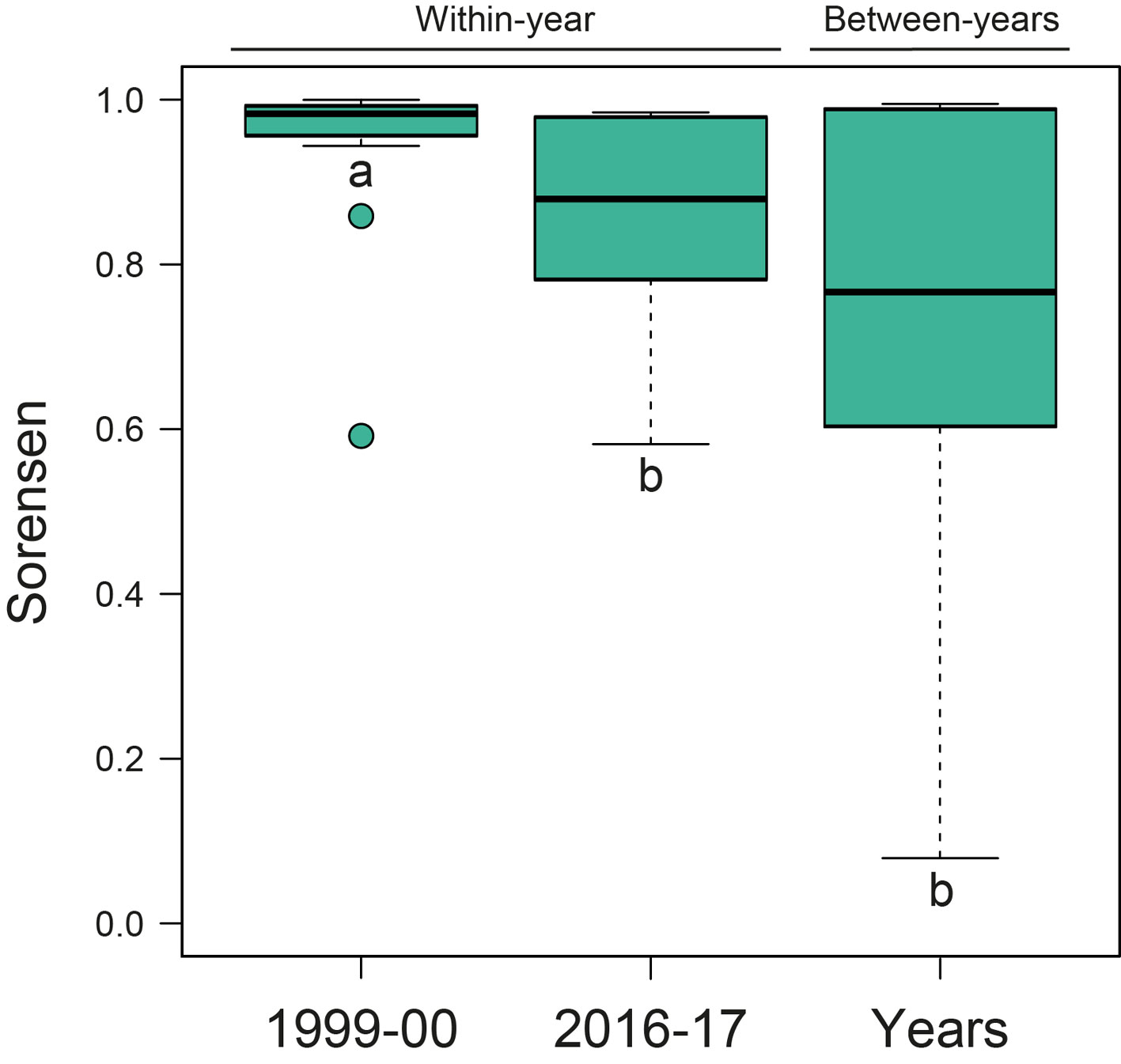
| Sorensen | Period | 1.8929 | 2 | 3.3632 | 0.0486 |
| Residuals | 8.1609 | 29 | |||
| Substitution | Period | 2.3620 | 2 | 2.7201 | 0.0827 |
| Residuals | 12.5910 | 29 | |||
| Nestedness | Period | 0.3466 | 2 | 0.5375 | 0.5899 |
| Residuals | 9.3497 | 29 |
Material and methods
The study was carried out in the Los Tuxtlas Biosphere Reserve (LTBR), located in the state of Veracruz, Mexico, within the municipalities of Catemaco, Hueyapan de Ocampo, San Andrés Tuxtla, and Santiago Tuxtla (18º20’-18º43’ N, 95º07’-95º25’ W; see Favila [2005] and Salomão et al. [2020] for maps of the study area and geographic coordinates of sites). The LTBR harbours some of the last rainforest remnants of the northernmost Neotropical region (UNESCO, 2020). Similar to other tropical regions, Los Tuxtlas has suffered high rates of deforestation (Guevara et al., 2000). Created in 1998, the LTBR ranges from coastal to higher elevations of the Sierra de Los Tuxtlas (from zero to 1,720 m asl) and spans across an area of 155,122 ha, which comprises 3 volcanoes: San Martín Pajapan, San Martín Tuxtla, and Santa Marta (Guevara et al., 2004). The mean annual temperature is 25 °C and precipitation ranges from 1,200 to 4,200 mm, directly related to elevation (Gutiérrez-García & Ricker, 2011). The landscape of LTBR was originally covered by tropical rainforest in lowlands and montane cloud forest in highlands (Favila, 2004; Guevara et al., 2000). The current landscape matrix in the LTBR comprises mainly agricultural land (pastures and fruticulture) (Guevara et al., 2000; Vega-Vela et al., 2018).
The forest fragments and pastures sampled in this study are located between the San Martín Tuxtla Volcano and the Sierra de Santa Marta Volcano (i.e., 2 nuclei areas). The elevation of the sites sampled ranges between 150-870 m asl (average ± SD = 554.7 ± 239.9 m), comprising both forest fragments and pastures. Temperature and precipitation were similar in both sampling years during the sampled months (Supplementary material: Fig. S1). For more details see Favila (2005) and Salomão et al. (2020).
We sampled the same 11 forest fragments and 6 pastures in 1999-2000 and 2016-2017. The data used in this study were obtained by Favila (2005) (data from 1999-2000) and Salomão et al. (2020) (data from 2016-2017). Both habitats were sampled monthly at each period almost at the same time, allowing us to compare the dung beetle assemblages between sampling periods and among months. We used baited pitfall traps to collect dung beetles in forest fragments and pastures. Pitfall traps (11 cm diameter × 7.5 cm depth) were baited with ca. 25 g of human faeces and carrion (i.e., fish meat) separately. Each trap was filled with ca. 200 mL of 70% alcohol to catch and preserve the sampled specimens and covered with a plastic plate to prevent rainfall and leaf litter from falling into the trap. We installed 2 sets of 4 traps per site, with traps spaced out 20 m from each other within each group, alternately baited with faeces and carrion. Each set of traps was spaced out 100 m apart. Traps were placed at a distance of at least 20 m from the edge of the habitat surveyed (i.e., forest fragment or pasture), which is sufficient to observe clear differences in the dung beetle assemblage of forest fragments and pastures within the LTBR (Favila, 2005). Traps were left in the field for 48 h in every sampling period. At each period (i.e., 1999-2000 and 2016-2017), a total of 816 traps was set (8 traps per site × 17 sites × 6 months): 528 in forest fragments and 288 in pastures. Specimens were identified by comparison with the entomological collection of the Instituto de Ecología, A.C. in Xalapa, Mexico, where they were also deposited. A detailed description of the sampling design can be found in Salomão et al. (2020).
Dung beetle species were characterized in terms of 4 biological attributes used to calculate the functional diversity metrics: food relocation behaviour (rollers, tunnellers or dwellers), diet (coprophagous, necrophagous, saprophagous or generalists), body length (clypeus to pygidium, in millimetres), and habitat use (forest specialists, grassland specialists, habitat generalists or too rare to classify) (see Chazdon et al., 2011) (Supplementary material: Table S1). These attributes are widely used to identify the functional groups of dung beetle species and each one has a particular impact on the ecosystem functioning (Barragan et al., 2011; Nichols et al., 2013). We classified dung beetle species into functional groups according to food relocation behaviour following the literature (Halffter & Edmonds, 1982; Scholtz et al., 2009). Dung beetle body length was mostly obtained from the literature (Díaz & Favila, 2009; Howden & Gill, 1993; Howden & Young, 1981; Zunino & Halffter, 1997) because we were unable to calculate the length from the old material. For the species that we could calculate body length, we obtained this variable by measuring dung beetles with a digital calliper (to the nearest 0.01 mm), using 1 to 10 individuals per species, depending on number of available individuals. We determined food preference for species with n ≥ 5 recorded individuals following Halffter and Arellano (2002): species in which more than 80% of their individuals were collected in faeces or carrion were considered coprophagous or necrophagous, and species in which less than 80% of their individuals were collected in one of the resource types was classified as generalist. Furthermore, we used literature to determine food preference from species that were rarely recorded in this study (n < 4) (Bourg et al., 2016; Navarrete & Halffter, 2008). We classified species according to their habitat use using the Multinomial Classification Model (CLAM) proposed by Chazdon et al. (2011). This model uses the relative species abundances in 2 distinct habitat categories (i.e., forest fragments and pastures), thus minimizing potential biases attributable to differences in sampling intensities between the 2 habitat categories and insufficient sampling of rare species in each habitat (Chazdon et al., 2011). We applied the liberal threshold of habitat specialization (K or specialization threshold value to assign shared species as habitat specialists), using the “simple majority” rule, with a cut-off point K = 1/2, which is highly sensitive for determining the habitat specificity of a given species. This analysis was carried out using the CLAM software version 1.0 (Chao & Lin, 2011). This method allowed classification of species as forest specialists, pasture specialists or habitat generalists. Species with insufficient sampling were grouped as “too rare”.
Based on 4 dung beetle traits important for ecosystem functioning, we calculated the “trait distances” between species based on Gower distance coefficient using the function “gowdis” from the R package FD (Laliberté et al., 2014). We then ran a principal coordinates analysis (PCoA; based on the Gower distances between species) using the function “cmdscale” from the R package stats (R Core Team, 2021) to provide trait vectors for subsequent β-diversity partitioning analyses and functional richness calculation. Contrary to the original trait variables, these trait vectors are continuous variables that can be used to describe trait differences between species. In practice, Euclidean distances between species based on the first 4 PCoA axes and original Gower distances between species were very strongly correlated (Mantel r = 0.943, p = 0.001), indicating that the 4 trait vectors reproduce well the information in the original species-by-species trait distances (da Silva et al., 2018). Besides, the number of dimensions (i.e., PCoA axes) was chosen based on the quality of the functional space, i.e., the extent to which it accurately represents the initial functional distances between species pairs, quantified by the mean squared-deviation index (Maire et al., 2015). We kept the minimum number of axes (i.e., 4 axes) that provides a high-quality functional space to minimize the number of sites or months we had to exclude to attain computation requirements (i.e., higher number of taxa than PCoA axes) (Villéger et al., 2008). Differences in quality between 4 and more dimensional spaces were relatively low, allowing us to construct a faithful representation of the initial functional trait values (Castro et al., 2020).
For each site per habitat type and period, we calculated dung beetle functional richness (FRic; amount of niche space occupied by the species within a community; Villéger et al. [2008]) and both taxonomic and functional β-diversity based on Sorensen dissimilarity. We calculated β-diversity using 2 approaches: “within year β-diversity” (between months for 1999-2000 and 2016-2017 separately) and “between year β-diversity” (between sampling periods of 1999-2000 and 2016-2017, after pooling the monthly data per period). We then partitioned β-diversity into its components of substitution and gain/loss (Legendre, 2014; Schmera et al., 2020) using the functions “beta.multi” (for taxonomic β-diversity) and “functional.beta.multi” (for functional β-diversity) from the R package BAT (Cardoso et al., 2020) and betapart (Baselga & Orme, 2012), respectively. These functions compute 3 multiple-time dissimilarities accounting for the substitution and the gain/loss components of taxonomic and functional β-diversity, and the sum of both values (Legendre, 2014; Schmera et al., 2020). The taxonomic partitioning is based on the species-by-site table (either species-by-forest sites or species-by-pasture sites), while the functional partitioning is based on the first 4 PCoA axes. We used different approaches to calculate taxonomic and functional β-diversity values and its components because the Podani’s approach is more robust to disentangle substitution and gain/loss of species than Baselga’s approach (Schmera et al., 2020). Podani’s approach calculates substitution and richness difference components and but not nestedness, which is more adequate for our data. On the other hand, Baselga’s approach is more robust than Podani’s approach to evaluate functional β-diversity metrics because it produces higher quality of functional spaces and functional substitution values independent of differences in functional richness (Loiseau et al., 2017). Therefore, we used Podani’s approach for the taxonomic calculations and Baselga’s approach for the functional calculations. Due to the low values of species richness found in most pasture sites (samples with < 5 species: 89.5% in 1999-2000 dataset, 73.7% in 2016-2017 dataset, 33.3% in between-years dataset; Supplementary material: Table S1, S2), we were unable to calculate functional values for most pasture sites because of computational restrictions; this analysis does not run with N > 2 sites and if sites have fewer than 5 species (Baselga et al., 2018) (Supplementary material: Table S2). Therefore, we used functional values only for forest fragments (those with ≥ 5 species) in further analyses.
We used generalized linear models (GLMs) in the R software (R Core Team, 2021) to test the effect of habitat type (forest fragments and pasture), sampling year (1999-2000, 2016-2017, and ‘between-years’), and the interaction between habitat type and sampling year on the temporal patterns of taxonomic dung beetle β-diversity and its substitution and gain/loss components (i.e., Sorensen dissimilarity and its components of substitution and richness difference were the response variables). Using GLMs, we also tested the effect of sampling year on functional β-diversity and its substitution and gain/loss components of dung beetles inhabiting forest fragments only. The binomial distribution corrected for overdispersion (quasi-binomial) for total dissimilarity (Sorensen) and its components were used for both taxonomic and functional values after checking the data dispersion and the resulting residuals (Crawley, 2013).
Results
A total of 4,563 individuals of 43 species were sampled (Supplementary material: Table S1). We found that habitat type had an effect on taxonomic β-diversity and on its richness difference component when analysing dung beetles’ temporal β-diversity (Table 1). Pastures had a taxonomic β-diversity and its richness difference component respectively 1.42 and 1.56 times higher than forest fragments, regardless the sampling year (Fig. 1a, c). However, there was no effect of habitat type (forest fragments or pasture), sampling year, or their interaction on species replacement (Fig. 1b).
Regarding functional diversity, we found that pastures had a lower functional richness than forest fragments in both sampling periods, being 1.93 times lower in 1999-2000 and 1.69 times lower in 2016-2017 (Table 2, Fig. 2). We also found that sampling year had effect on temporal values of functional β-diversity of forest fragments (Table 2). Within-year values were higher than between-year values (Fig. 3). There was no difference in temporal values of the components of turnover and nestedness of functional β-diversity in forest fragments (Table 2).
Discussion
Here, we evaluated short- and long-term changes in dung beetle taxonomic and functional diversity in forest fragments and surrounding cattle pastures. According to our first prediction, pastures suffered more important compositional changes than forest fragments over time, both within-years and between-years. The observed monthly changes in pastures were similar across sampling years and were explained by species gains/losses (richness differences) through time. This result is explained by the low species richness and functional richness found in pastures when compared to neighbouring forest fragments. On the other hand, contrary to our second prediction, taxonomic β-diversity was not higher between sampling years than within years, despite there was a general gain of species from 1999-2000 to 2016-2017 (Salomão et al., 2020). Within-year functional β-diversity was higher than between-years in forest fragments, although we were unable to evaluate this pattern for pastures. Also, contrary to our second prediction, dung beetle assemblages in forest fragments were more stable between-years than within-years, besides being functionally richer than pastures.

Pastures encompassed a higher taxonomic β-diversity of dung beetles than forest fragments at LTBR, which may be associated to the more unstable conditions of pastures and higher environmental complexity and stability of forest fragments (Filgueiras et al., 2019; Salomão et al., 2020). In open habitats, dung is exposed to high heat and desiccation rate, which implies in shorter periods of dung beetle activity on dung during the day (Horgan, 2005) and loss of attractiveness due to the creation of a hard dung crusts that reduce the scent emitted or are avoided by some species (Frank et al., 2018; Horgan, 2008). This can lead to a low reproductive success and decreased rates of dung decomposition (Horgan, 2005). Besides, extreme temperatures and higher temperature variation found in open habitats can cause physiological intolerance of dung beetle species to inhabit or constantly use these habitats (Giménez-Gómez et al., 2020). Pastures also have low heterogeneity regarding resources and habitat structure compared to forest fragments, which provide a high diversity of food resources that can fulfil the nutritional requirements of different dung beetle species (Gill, 1991). Herbivorous dung is the dominant food resource in pastures and has intermediate to low nutritional values compared to carnivorous and omnivorous dung (Frank et al., 2017; Horgan, 2008). The diversity of food resources in forest fragments can enhance both taxonomic and functional diversity of dung beetles, which can show many specialized species (Larsen et al., 2006). Besides, both dung beetles and detritus-feeding flies use mammal dung, carrion, and decaying fruits for feeding and/or breeding (Hanski, 1991). When they occur, both carrion and decaying fruits can be rapidly consumed by flies and other insects in open habitats, reducing the colonization chance by dung beetles (Horgan, 2005, 2008). These pasture-associated conditions can make them a more dynamic environment in terms of dung beetle composition.
Neotropical pastures harbour less species richness and abundance than forest fragments (Braga et al., 2013; Escobar et al., 2007; Estrada et al., 1998; Horgan, 2008), and they have been acknowledged to have more spatially homogeneous dung beetle assemblages when compared to forest fragments (Escobar et al., 2007; Solar et al., 2015). Therefore, temporal changes in agricultural land-uses in LTBR over 17 years (i.e., long term effects) due to increased forest area, decrease of livestock activity, and change of vegetation successional stage in open habitats may have caused a higher compositional change in pastures than in forest fragments, represented by species gains/losses. Another possibility is the way in which the compositional change of assemblages over time has been evaluated, since the approaches to partition β-diversity into its components of replacement and gain/loss are relatively recent (Legendre, 2014). Noriega et al. (2021) also found a nested gain/loss component as primary driver of the temporal β-diversity of dung beetles across a long-term successional recovery of the Amazon Forest from human-induced disturbances. Forest fragments and pastures also have different dung beetle species compositions due to the distinct environmental requirements of these species (da Silva et al., 2019). For instance, Canthidium centrale (Boucomont, 1928), Deltochilum pseudoparile Paulian, 1938, Canthidium sp., Uroxys bonetti Pereira & Halffter, 1961, Uroxys platypiga (Howden & Young, 1981), Dichotomius satanas (Harold, 1867), Canthidium pseudoperceptibile Kohlmann & Solís, 2006, Canthidium ardens Bates, 1887, Eurysternus maya Génier, 2009, Canthon subhyalinus Harold, 1867, Canthon morsei Howden, 1966, Canthon vazquezae Martinez, Halffter & Halffter, 1964, Coprophanaeus corytus (Harold, 1863), Bdelyropsis newtoni Howden, 1971, Canthon eurycelis Bates, 1887, Canthon femoralis Chevrolat, 1834, Deltochilum carrilloi González-Alvarado & Vaz-de-Mello, 2014, Onthophagus asperodorsatus Howden & Gill, 1993, and Onthophagus violetae Zunino & Halffter, 1997 were exclusively found in forest sites. Canthon indigaceus chiapas Robinson, 1948, Canthidium pseudopuncticolle Solís & Kohlmann, 2004, Phanaeus tridens Castelnau 1840, and Phanaeus mexicanus Harold, 1863 were found exclusively in pasture sites. Dung beetles are also affected by differences in climatic conditions during a year (Andresen & Feer, 2005; Ferreira et al., 2019; Neves et al., 2010), but forest fragments can buffer harsh climatic conditions both below and aboveground when compared to pastures (da Silva et al., 2019; Suggitt et al., 2011). This would lead to a high temporal microclimatic stability in forest fragments that allows different species to occur throughout the year, while species in pastures would be restricted to periods of more favourable conditions, causing high compositional changes. Under this rationale, the high changes of environmental conditions and resource availability expected to occur in pastures over the short- and long-term may cause the observed high compositional changes of dung beetle assemblages in these habitats.
The high values of temporal taxonomic β-diversity found in pastures (both within-years and between-years) must be taken with caution. This pattern might have emerged because of the species- and individual-poor assemblages sampled in pastures (Salomão et al., 2020), which resulted in low functional richness in both sampling years when compared to forest fragments. The replacement or loss of few species in pastures can have a considerable impact on the estimates of temporal taxonomic β-diversity. Dominant functional groups, which can maintain a similar functional structure over space and time, may cause a pattern of low functional β-diversity despite being more taxonomically dissimilar (Villéger et al., 2012). In our case, although richer, forest fragments were dominated by both rollers and tunnellers, with gains of dwellers through time (Supplementary material: Table S1). On the other hand, the low species richness, plus the gain of dwellers, the spatial dynamic of rollers, and a positive balance between disappearing and new appearances of some tunnellers (Supplementary material: Table S1), probably caused a high taxonomic β-diversity in pastures between sampling years and within-years. We also expect that these factors would cause a high functional β-diversity in pastures over time, although we were unable to measure these patterns. Besides, our results may suggest that increased species richness observed in forest fragments does not necessarily result in temporal changes of functional diversity. Barragan et al. (2011) found that both fragments and continuous forest in Mexican Montes Azules Biosphere Reserve included more functionally redundant dung beetle species, while all the species found in pastures were functionally different, despite pastures having low species richness and functional richness. On the other hand, forest fragments harbour more redundant species and high functional richness that ensure the functioning of the ecosystem even with the loss of some species with similar functions (Rosenfeld, 2002). In a meta-analysis, Biggs et al. (2020) showed that functional redundancy is correlated with ecological resilience and stability of communities and ecosystems, as ecosystem function of communities with more redundant species is buffered against the loss of individual species.
As a whole, the deforestation of primary and secondary forests to expand agricultural area decreased in Mexico from 1980 to 2000 (Gibbs et al., 2010) and stabilized to ca. 500,000 ha loss per year between 1993 and 2007 (Rosete-Vergés et al., 2014). In Mexico, there are few studies at the national level to understand the processes of change in land-use and vegetation. Nonetheless, at local and regional scales, there is a trend of forest loss and increase of agriculture and human settlements (Monjardín-Armenta et al., 2017). Besides, there are currently 182 protected natural areas in Mexico, covering 90.8 million hectares in total (Conanp, 2019).The establishment of the LTBR has led to the recovery of native vegetation, involving the increase of forested area and the reduction of the agricultural matrix (Vega-Vela et al., 2018). In the LTBR, there have been gains of dung beetle species and we are able to show that dung beetle assemblages are more stable in forest fragments than in pastures (Salomão et al., 2020). However, this recovering process can be slower than we expect in other regions (Audino et al., 2014; Escobar et al., 2008). For instance, Audino et al. (2014) found that 18 years since active restoration has not been long enough to recover a stable and diverse dung beetle assemblage in sites of the Atlantic Forest. Factors such as habitat loss in the surrounding landscape and increasing isolation of sites can contribute to the temporal changes of dung beetle assemblages (Escobar et al., 2008). In this sense, long-term studies in different ecosystems would be highly informative, as they are useful to inform about conservation success or failure and ways to carry out better management practices that should take into account integrative approaches at landscape-to-regional scales (Halffter, 2005).
In general, Neotropical pastures have lower taxonomic and functional diversity of dung beetles than adjacent forest fragments (Barragan et al., 2011; da Silva et al., 2019; Horgan, 2008; Solar et al., 2015). Removal and burial of dung and seeds are also higher in forest fragments than in pastures, which is associated to dung beetle richness, abundance, and biomass (Andresen, 2003; Horgan, 2005). Losses of dung beetle species or functional groups could leave unsaturated niche spaces or be replaced by other organisms, such as flies and termites in open habitats (Herrick & Lal, 1996; Horgan, 2008). On the other hand, these species- and individual-poor assemblages found in pastures of the LTBR are important to provide their ecosystem functions, even in a reduced way. For instance, dung beetles can reduce the survival and availability of gastrointestinal parasites in pastures, besides their essential role in dung degradation and nutrient cycling (Sands & Wall, 2017). However, they can be severely impacted by the common use of pesticides in livestock systems that disrupt their life cycle and behaviour (Alvarado et al., 2018; Correa et al., 2022; Verdú et al., 2018). This issue is particularly important considering the low taxonomic and functional richness estimates found in pasture sites.
Considering the general gain of species over the 17 years (Salomão et al., 2020), forest fragments have been maintaining and accumulating their taxonomic and functional diversity, suggesting a coupled conservation and recovery of dung beetle assemblages in the LTBR. Pastures harbour poor dung beetle assemblages with high temporal dynamics both within-years and between-years spaced 17 years apart. We showed that using taxonomic and functional approaches, interpreted in the light of accurate biological responses as we have shown for temporal patterns of taxonomic diversity of dung beetles, can be a suitable way for the long-term evaluation of biodiversity patterns. Evaluating short- and long-term changes of biological communities intrinsically related to ecosystem processes of forest regeneration, such as dung beetles, can be considered as an important tool to better inform society and decision-makers regarding effective conservation of biodiversity and ecosystem functioning in protected areas.
Acknowledgements
To A. Díaz, F. Caselín, F. Armas, and A. Jacome for support during fieldwork. We thank A. Díaz for the identification of collected materials; A. Córdoba-Aguilar and O. Ríos-Cárdenas for reviewing the project and making valuable suggestions. To the staff from Los Tuxtlas Biological Station (UNAM) for logistic support during fieldwork. PGdS thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for post-doctoral grant (Process 88882.316025/2019-01, Code 001). To Consejo Nacional de Ciencia y Tecnologia (Conacyt) for a scholarship granted to RPS over the course of the study and project CONACYT-Ciencia Básica No. 257894. RPS was also supported by DGAPA post-doc fellowship from Universidad Nacional Autónoma de México.
References
Alvarado, F., Escobar, F., Williams, D. R., Arroyo-Rodríguez, V., & Escobar-Hernández, F. (2018). The role of livestock intensification and landscape structure in maintaining tropical biodiversity. Journal of Applied Ecology, 55, 185–194. https://doi.org/10.1111/1365-2664.12957
Andresen, E. (2003). Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography, 26, 87–97. https://doi.org/10.1034/j.1600-0587.2003.03362.x
Andresen, E., & Feer, F. (2005). The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In P. M. Forget, J. E. Lambert, P. E. Hulme, & S. B. Vander-Walls (Eds.), Seed fate: predation, dispersal, and seedling establishment (pp. 331–349). CAB International: Wallingford.
Audino, L. D., Louzada, J., & Comita, L. (2014). Dung beetles as indicators of tropical forest restoration success: Is it possible to recover species and functional diversity? Biological Conservation, 169, 248–257. https://doi.org/10.1016/j.biocon.2013.11.023
Barlow, J., Gardner, T. A., Louzada, J., & Peres, C. A. (2010). Measuring the conservation value of tropical primary forests: the effect of occasional species on estimates of biodiversity uniqueness. Plos One, 5, e9609. https://doi.org/10.1371/journal.pone.0009609
Barlow, J., Lennox, G. D., Ferreira, J., Berenguer, E., Lees, A. C., Mac Nally, R. et al. (2016). Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature, 535, 144–147. https://doi.org/10.1038/nature18326
Barragan, F., Moreno, C. E., Escobar, F., Halffter, G., & Navarrete, D. (2011). Negative impacts of human land use on dung beetle functional diversity. Plos One, 6, e17976. https://doi.org/10.1371/journal.pone.0017976
Baselga, A., & Orme, C. D. L. (2012). betapart: an R package for the study of beta diversity. Methods in Ecology
and Evolution, 3, 808–812. https://doi.org/10.1111/j.204114210X.2012.00224.x
Baselga, A., Orme, D., Villéger, S., De Bortoli, J., & Leprieur, F. (2018). betapart: Partitioning beta diversity into turnover and nestedness components. R package version 1.5.0. https://CRAN.R-project.org/package=betapart.
Biggs, C. R., Yeager, L. A., Bolser, D. G., Bonsell, C., Dichiera, A. M., Hou, Z. et al. (2020). Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere, 11, e03184. https://doi.org/10.1002/ecs2.3184
Bourg, A., Escobar, F., MacGregor-Fors, I., & Moreno, C. E. (2016). Got dung? Resource selection by dung beetles in neotropical forest fragments and cattle pastures. Neotropical Entomology, 45, 490–498. https://doi.org/10.1007/s13744-016-0397-7
Braga, R. F., Korasaki, V., Andresen, E., & Louzada, J. (2013). Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: a rapid assessment of ecological functions associated to biodiversity. Plos One, 8, e57786. https://doi.org/10.1371/journal.pone.0057786.g001
Cardoso, P., Mammola, S., Rigal, F., & Carvalho, J. C. (2020). Package ‘BAT’: Biodiversity Assessment Tools. R package version 2.1.0. https://CRAN.R-project.org/package=BAT.
Castillo-Escrivà, A., Mesquita-Joanes, F., & Rueda, J. (2020). Effects of the temporal scale of observation on the analysis of aquatic invertebrate metacommunities. Frontiers in Ecology and Evolution, 8, 561838. https://doi.org/10.3389/fevo.2020.561838
Castro, D. M. P., da Silva, P. G., Solar, R., & Callisto, M. (2020). Unveiling patterns of taxonomic and functional diversities of stream insects across four spatial scales in the neotropical savanna. Ecological Indicators, 118, 106769. https://doi.org/10.1016/j.ecolind.2020.106769
Chao, A., & Lin, S. Y. (2011). Program CLAM (Classification method). Program and user’s guide. http://purl.oclc.org/clam. Assessed on: 20 October 2018.
Chazdon, R. L., Chao, A., Colwell, R. K., Lin, S.-Y., Norden, N., Letcher, S. G. et al. (2011). A novel statistical method for classifying habitat generalists and specialists. Ecology, 92, 1332–1343. https://doi.org/10.1890/10-1345.1
Conanp (Comisión Nacional de Áreas Naturales Protegidas). (2019). Áreas Naturales Protegidas decretadas. Gobierno de México. Recuperado el 16 noviembre, 2020 de: https://www.gob.mx/conanp/documentos/areas-naturales-protegidas-278226?idiom=es
Correa, C. M. A., Ferreira, K. R., Abot, A. R., Louzada, J., & Vaz-de-Mello, F. Z. (2022). Ivermectin impacts on dung beetle diversity and their ecological functions in two distinct Brazilian ecosystems. Ecological Entomology, 47, 736–748. https://doi.org/10.1111/een.13158
Crawley, M. J. (2013). The R Book. Chichester: John Wiley.
Cuesta, E., & Lobo, J. M. (2019). A comparison of dung beetle assemblages (Coleoptera, Scarabaeoidea) collected 34 years apart in an Iberian mountain locality. Journal of Insect Conservation, 23, 101–110. https://doi.org/10.1007/s10841-018-00119-5
da Silva, P. G., Hernández, M. I. M., & Heino, J. (2018). Disentangling the correlates of species and site contributions to beta diversity in dung beetle assemblages. Diversity and Distributions, 24, 1674–1686. https://doi.org/10.1111/ddi.12785
da Silva, P. G., Lobo, J. M., & Hernández, M. I. M. (2019). The role of habitat and daily activity patterns in explaining the diversity of mountain Neotropical dung beetle assemblages. Austral Ecology, 44, 300–312. https://doi.org/10.1111/aec.12675
Díaz, A., & Favila, M. E. (2009). Escarabajos coprófagos y necrofagos (Scarabaeidae, Trogidae y Silphidae) de al Reserva de la Biosfera Los Tuxtlas, México. In V. Hernández-Ortiz, C. Deloya, & P. Reyes-Castillos (Eds.), Memorias VIII Reunión Latinoamericana de Scarabaeoidología (pp. 1–4). Los Tuxtlas, Veracruz: Instituto de Ecología, A.C.
Dirzo, R., Young, H. S., Galetti, M., Ceballos, G., Isaac, N. J. B., & Collen, B. (2014). Defaunation in the Anthropocene. Science, 345, 401–406. https://doi.org/10.1126/science.1251817
Dornelas, M., Gotelli, N. J., McGill, B., Shimadzu, H., Moyes, F., Sievers, C. et al. (2014). Assemblage time series reveal biodiversity change but not systematic loss. Science, 344, 296–299. https://doi.org/10.1126/science.1248484
Edwards, D. P., Gilroy, J. J., Thomas, G. H., Uribe, C. A. M., & Haugaasen, T. (2015). Land-sparing agriculture best protects avian phylogenetic diversity. Current Biology, 25, 2384–2391. https://doi.org/10.1016/j.cub.2015.07.063
Escobar, F., Halffter, G., & Arellano, L. (2007). From forest to pasture: an evaluation of the influence of environment and biogeography on the structure of beetle (Scarabaeinae) assemblages along three altitudinal gradients in the Neotropical region. Ecography, 30, 193–208. https://doi.org/10.1111/j.2007.0906-7590.04818.x
Escobar, F., Halffter, G., Solís, Á., Halffter, V., & Navarrete, D. (2008). Temporal shifts in dung beetle community structure within a protected area of tropical wet forest: a 35-year study and its implications for long-term conservation. Journal of Applied Ecology, 45, 1584–1592. https://doi.org/10.1111/j.1365-2664.2008.01551.x
Estrada, A., Coates-Estrada, R., Dadda, A. A., & Cammarano, P. (1998). Dung and carrion beetles in tropical rain forest fragments and agricultural habitats at Los Tuxtlas, Mexico. Journal of Tropical Ecology, 14, 577–593. https://doi.org/10.1017/S0266467498000418
Favila, M. E. (2004). Los escarabajos y la fragmentación. In S. Guevara, J. Laborde, & G. Sánchez-Ríoss (Eds.), Los Tuxtlas, el paisaje de la sierra (pp. 135–157). Xalapa: Instituto de Ecología A.C.
Favila, M. E. (2005). Diversidad alfa y beta de los escarabajos del estiércol (Scarabaeinae) en Los Tuxtlas, México. In G. Halffter, J. Soberón, P. Koleff, & A. Melics (Eds.), Sobre diversidad biológica: el significado de las diversidades alfa, beta y gamma (pp. 209–219). Zaragoza: Sociedad Entomológica Aragonesa.
Ferreira, S. C., da Silva, P. G., Paladini, A., & Di Mare, R. A. (2019). Climatic variables drive temporal patterns of α and β diversities of dung beetles. Bulletin of Entomological Research, 109, 390–397. https://doi.org/10.1017/S0007485318000676
Filgueiras, B. K. C., Melo, D. H. A., Andersen, A. N., Tabarelli, M., & Leal, I. R. (2019). Cross-taxon congruence in insect responses to fragmentation of Brazilian Atlantic forest. Ecological Indicators, 98, 523–530. https://doi.org/10.1016/j.ecolind.2018.11.036
Flynn, D. F., Gogol-Prokurat, M., Nogeire, T., Molinari, N., Richers, B. T., Lin, B. B. et al. (2009). Loss of functional diversity under land use intensification across multiple taxa. Ecology Letters, 12, 22–33. https://doi.org/10.1111/j.1461.0248.2008.01255.x
Frank, K., Brückner, A., Blüthgen, N., & Schmitt, T. (2018). In search of cues: dung beetle attraction and the significance of volatile composition of dung. Chemoecology, 28, 145–152. https://doi.org/10.1007/s00049-018-0266-4
Frank, K., Bruckner, A., Hilpert, A., Heethoff, M., & Bluthgen, N. (2017). Nutrient quality of vertebrate dung as a diet for dung beetles. Scientific Reports, 7, 12141. https://doi.org/10.1038/s41598-017-12265-y
Gardner, T. A., Barlow, J., Chazdon, R., Ewers, R. M., Harvey, C. A., Peres, C. A. et al. (2009). Prospects for tropical forest biodiversity in a human-modified world. Ecology Letters, 12, 561–582. https://doi.org/10.1111/j.1461-0248.2009.01294.x
Gibbs, H. K., Ruesch, A. S., Achard, F., Clayton, M. K., Holmgren, P., Ramankutty, N. et al. (2010). Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proceedings of the National Academy of Sciences, 107, 16732–16737. https://doi.org/10.1073/pnas.0910275107
Gill, B. (1991). Dung beetles in tropical American forests. In I. Hanski, & Y. Cambeforts (Eds.), Dung beetle ecology (pp. 211–229). Princeton University Press: Princeton.
Giménez-Gómez, V. C., Verdú, J. R., & Zurita, G. A. (2020). Thermal niche helps to explain the ability of dung beetles to exploit disturbed habitats. Scientific Reports, 10, 13364. https://doi.org/10.1038/s41598-020-70284-8
Guevara, S., Laborde-Dovalí, J., & Sánchez-Ríos, G. (2000). La Reserva de la Biosfera Los Tuxtlas. Paris: UNESCO.
Guevara, S., Laborde, J., & Sánchez, G. (2004). Los Tuxtlas: el paisaje de la sierra. Mexico D.F.: Instituto de Ecología.
Gutiérrez-García, G., & Ricker, M. (2011). Climate and climate change in the region of Los Tuxtlas (Veracruz, Mexico): a statistical analysis. Atmósfera, 24, 347–373.
Halffter, G. (2005). Towards a culture of biodiversity conservation. Acta Zoológica Mexicana Nueva Serie, 21, 133–153. https://doi.org/10.21829/azm.2005.2121991
Halffter, G., & Arellano, L. (2002). Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica, 34, 144–154. https://doi.org/10.1111/j.1744-7429.2002.tb00250.x
Halffter, G., & Edmonds, W. D. (1982). Nesting behavior of dung beetles (Scarabaeinae). Mexico D.F.: Man and Biosphere Program – UNESCO.
Hansen, M. C., Wang, L., Song, X. P., Tyukavina, A., Turubanova, S., Potapov, P. V. et al. (2020). The fate of tropical forest fragments. Science Advances, 6, eaax8574. https://doi.org/10.1126/sciadv.aax8574
Hanski, I. (1991). The dung insect community. In I. Hanski, & Y. Cambeforts (Eds.), Dung beetle ecology (pp. 5–21). Princeton: Princeton University Press.
Hernández, M. I. M., & Vaz-de Mello, F. Z. (2009). Seasonal and spatial species richness variation of dung beetle (Coleoptera, Scarabaeidae s. str.) in the Atlantic Forest of southeastern Brazil. Revista Brasileira de Entomologia, 53, 607–613. https://doi.org/10.1590/S0085-56262009000400010
Herrick, J. E., & Lal, R. (1996). Dung decomposition and pedoturbation in a seasonally dry tropical pasture. Biology and Fertility of Soils, 23, 177–181. https://doi.org/10.1007/BF00336060
Horgan, F. G. (2005). Effects of deforestation on diversity, biomass and function of dung beetles on the eastern slopes of the Peruvian Andes. Forest Ecology and Management, 216, 117–133. https://doi.org/10.1016/j.foreco.2005.05.049
Horgan, F. G. (2008). Dung beetle assemblages in forests and pastures of El Salvador: a functional comparison. Biodiversity and Conservation, 17, 2961–2978. https://doi.org/10.1007/s10531-008-9408-2
Howden, H., & Gill, B. (1993). Mesoamerican Onthophagus Latreille in the dicranius and mirabilis species groups (Coleoptera: Scarabaeidae). The Canadian Entomologist, 125, 1091–1114. https://doi.org/10.4039/Ent1251091-6
Howden, H. F., & Young, O. P. (1981). Panamanian Scarabaeinae: Taxonomy, distribution, and habits (Coleoptera, Scarabaeidae). Contributions American Entomological Institute, 18, 1–204.
Laliberté, E., Legendre, P., & Shipley, B. (2014). FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12. https://cran.r-project.org/web/packages/FD/index.html.
Larsen, T., Lopera, A., & Forsyth, A. (2006). Extreme trophic and habitat specialization by Peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 60, 315–324. https://doi.org/10.1649/0010-065X(2006)60[315:ETAHSB]2.0.CO;2
Laurance, W. F. (2007). Have we overstated the tropical biodiversity crisis? Trends in Ecology & Evolution, 22, 65–70. https://doi.org/10.1016/j.tree.2006.09.014
Laurance, W. F., Sayer, J., & Cassman, K. G. (2014). Agricultural expansion and its impacts on tropical nature. Trends in Ecology & Evolution, 29, 107–116. https://doi.org/10.1016/j.tree.2013.12.001
Legendre, P. (2014). Interpreting the replacement and richness difference components of beta diversity. Global Ecology and Biogeography, 23, 1324–1334. https://doi.org/10.1111/geb.12207
Legendre, P. (2019). A temporal beta-diversity index to identify sites that have changed in exceptional ways in space–time surveys. Ecology and Evolution, 9, 3500–3514. https://doi.org/10.1002/ece3.4984
Lindholm, M., Alahuhta, J., Heino, J., & Toivonen, H. (2020). No biotic homogenisation across decades but consistent effects of landscape position and pH on macrophyte communities in boreal lakes. Ecography, 43, 294–305. https://doi.org/10.1111/ecog.04757
Loiseau, N., Legras, G., Gaertner, J.-C., Verley, P., Chabanet, P., & Mérigot, B. (2017). Performance of partitioning functional beta-diversity indices: Influence of functional representation and partitioning methods. Global Ecology and Biogeography, 26, 753–762. https://doi.org/10.1111/geb.12581
Magurran, A. E., Dornelas, M., Moyes, F., Henderson, P. A., & Storch, D. (2019). Temporal β diversity —a macroecological perspective. Global Ecology and Biogeography, 28, 1949–1960. https://doi.org/10.1111/geb.13026
Maire, E., Grenouillet, G., Brosse, S., & Villéger, S. (2015). How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Global Ecology and Biogeography, 24, 728–740. https://doi.org/10.1111/geb.12299
Monjardín-Armenta, S. A., Pacheco-Angulo, C. E., Plata-Roch, W., & Corrales-Barraza, G. (2017). La deforestación y sus factores causales en el estado de Sinaloa, México. Madera y Bosques, 23, 7–22. https://doi.org/10.21829/myb.2017.2311482
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–845. https://doi.org/10.1038/35002501
Navarrete, D., & Halffter, G. (2008). Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: the effects of anthropogenic changes. Biodiversity and Conservation, 17, 2869–2898. https://doi.org/10.1007/s10531-008-9402-8
Neves, F. d. S., Oliveira, V. H. F., Espírito-Santo, M. M., Vaz-de-Mello, F. Z., Louzada, J., Sanchez-Azofeifa, A. et al. (2010). Successional and seasonal changes in a community of dung beetles (Coleoptera: Scarabaeinae) in a Brazilian tropical dry forest. Natureza & Conservacao, 8, 160–164. https://doi.org/10.4322/natcon.00802009
Newbold, T., Bentley, L. F., Hill, S. L. L., Edgar, M. J., Horton, M., Su, G. et al. (2020). Global effects of land use on biodiversity differ among functional groups. Functional Ecology, 34, 684–693. https://doi.org/10.1111/1365-2435.13500
Newbold, T., Hudson, L. N., Hill, S. L., Contu, S., Lysenko, I., Senior, R. A. et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520, 45–50. https://doi.org/10.1038/nature14324
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., Favila, M. E. et al. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation, 141, 1461–1474. https://doi.org/10.1016/j.biocon.2008.04.011
Nichols, E., Uriarte, M., Bunker, D. E., Favila, M. E., Slade, E. M., Vulinec, K. et al. (2013). Trait-dependent response of dung beetle populations to tropical forest conversion at local and regional scales. Ecology, 94, 180–189. https://doi.org/10.1890/12-0251.1
Noriega, J. A., Santos, A. M. C., Calatayud, J., Chozas, S., & Hortal, J. (2021). Short- and long-term temporal changes in the assemblage structure of Amazonian dung beetles. Oecologia, 195, 719–736. https://doi.org/10.1007/s00442-020-04831-5
R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing: Austria, Vienna.
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J., & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142, 1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Rivera, J. D., da Silva, P. G., & Favila, M. E. (2021). Landscape effects on taxonomic and functional diversity of dung beetle assemblages in a highly fragmented tropical forest. Forest Ecology and Management, 496, 119390. https://doi.org/10.1016/j.foreco.2021.119390
Rivera, J. D., Espinosa-de los Monteros, A., da Silva, P. G., & Favila, M. E. (2022). Dung beetles maintain phylogenetic divergence but functional convergence across a highly fragmented tropical landscape. Journal of Applied Ecology, 59, 1781–1791. https://doi.org/10.1111/1365-2664.14185
Rosenfeld, J. S. (2002). Functional redundancy in ecology and conservation. Oikos, 98, 156–162. https://doi.org/10.1034/j.1600-0706.2002.980116.x
Rosete-Vergés, F. A., Pérez-Damián, J. L., Villalobos-Delgado, M., Navarro-Salas, E. N., Salinas-Chávez, E., & Remond-Noa, R. (2014). El avance de la deforestación en México 1976-2007. Madera y Bosques, 20, 21–35. https://doi.org/10.21829/myb.2014.201173
Salomão, R. P., Favila, M. E., & González-Tokman, D. (2020). Spatial and temporal changes in the dung beetle diversity of a protected, but fragmented, landscape of the northernmost Neotropical rainforest. Ecological Indicators, 111, 105968. https://doi.org/10.1016/j.ecolind.2019.105968
Sands, B., & Wall, R. (2017). Dung beetles reduce livestock gastrointestinal parasite availability on pasture. Journal of Applied Ecology, 54, 1180–1189. https://doi.org/10.1111/1365-2664.12821
Schmera, D., Podani, J., & Legendre, P. (2020). What do beta diversity components reveal from presence-absence community data? Let us connect every indicator to an indicandum! Ecological Indicators, 117, 106540. https://doi.org/10.1016/j.ecolind.2020.106540
Scholtz, C. H., Davis, A. L. V., & Kryger, U. (2009). Evolutionary biology and conservation of dung beetles. Sofia, Bulgary: Pensoft Publishers.
Slade, E. M., Mann, D. J., Villanueva, J. F., & Lewis, O. T. (2007). Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. Journal of Animal Ecology, 76, 1094–1104. https://doi.org/10.1111/j.1365-2656.2007.01296.x
Slade, E. M., Riutta, T., Roslin, T., & Tuomisto, H. L. (2016). The role of dung beetles in reducing greenhouse gas emissions from cattle farming. Scientific Reports, 6, 18140. https://doi.org/10.1038/srep18140
Solar, R. R., Barlow, J., Ferreira, J., Berenguer, E., Lees, A. C., Thomson, J. R. et al. (2015). How pervasive is biotic homogenization in human-modified tropical forest landscapes? Ecology Letters, 18, 1108–1118. https://doi.org/10.1111/ele.12494
Suggitt, A. J., Gillingham, P. K., Hill, J. K., Huntley, B., Kunin, W. E., Roy, D. B. et al. (2011). Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos, 120, 1–8. https://doi.org/10.1111/j.1600-0706.2010.18270.x
Tatsumi, S., Iritani, R., & Cadotte, M. W. (2021). Temporal changes in spatial variation: partitioning the extinction and colonisation components of beta diversity. Ecology Letters, 24, 1063–1072. https://doi.org/10.1111/ele.13720
Tonkin, J. D., Bogan, M. T., Bonada, N., Rios-Touma, B., & Lytle, D. A. (2017). Seasonality and predictability shape temporal species diversity. Ecology, 98, 1201–1216. https://doi.org/10.1002/ecy.1761
UNESCO (United Nations Educational, Scientific and Cultural Organization). (2020). Los Tuxtlas Biosphere Reserve, Mexico. Retrieved on August 17, 2020. Available at: https://en.unesco.org/biosphere/lac/los-tuxtlas
Vega-Vela, V., Muñoz-Robles, C. A., Rodríguez-Luna, E., López-Acosta, J. C., & Serna-Lagunes, R. (2018). Analysis of landscape fragmentation in the Los Tuxtlas Biosphere Reserve, Veracruz, Mexico. Ecosistemas y Recursos Agropecuarios, 5, 227–238. https://doi.org/10.19136/era.a5n14.1442
Verdú, J. R., Lobo, J. M., Sánchez-Piñero, F., Gallego, B., Numa, C., Lumaret, J. P. et al. (2018). Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Science of the Total Environment, 618, 219–228. https://doi.org/10.1016/j.scitotenv.2017.10.331
Villéger, S., Mason, N. W. H., & Mouillot, D. (2008). New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology, 89, 2290–2301. https://doi.org/10.1890/07-1206.1
Villéger, S., Miranda, J. R., Hernandez, D. F., & Mouillot, D. (2012). Low functional β-diversity despite high taxonomic β-diversity among tropical estuarine fish communities. Plos One, 7, e40679. https://doi.org/10.1371/journal.pone.0040679
Zunino, M., & Halffter, G. (1997). Sobre Onthophagus Latreille, 1802 americanos (Coleoptera: Scarabaeidae: Scarabaeinae). Elytron, 11, 157–178.