Eduardo Manolo Medrano-Zapata a, Jorge Luis Becerra-López b, Pedro Almaguer-Sierra a, Cristina García-De la Peña b, *, Ludivina Barrientos-Lozano a, Juan Flores-Gracia a,
Karen Berenice Lara-Rodríguez a, Felipe Vaca-Paniagua c,
Clara E. Díaz-Velásquez c, Aldo H. De la Cruz-Montoya c
a Tecnológico Nacional de México, Instituto Tecnológico Ciudad Victoria, División de Estudios de Posgrado e Investigación, Boulevard Emilio Portes Gil No. 1301, 87010 Ciudad Victoria, Tamaulipas, Mexico
b Universidad Juárez del Estado de Durango, Facultad de Ciencias Biológicas, Av. Universidad s/n, Fracc. Filadelfia, 35010 Gómez Palacio, Durango, Mexico
c Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Laboratorio Nacional en Salud: Diagnóstico molecular y efecto ambiental en enfermedades crónico-degenerativas, Av. De Los Barrios 1, Los Reyes Iztacala, 54090 Tlalnepantla, Estado de México, Mexico
*Corresponding author: cristina.garcia@ujed.mx (C. García-De la Peña)
Received: 26 September 2023; accepted: 16 December 2023
Abstract
The blood bacterial microbiota of the Texas tortoise (Gopherus berlandieri) in Tamaulipas, Mexico, was characterized by next-generation sequencing. In 2019, blood was collected from 6 free-living tortoises. DNA was extracted, the V3-V4 region of the 16S rRNA gene was amplified, and Illumina sequencing was performed. The results showed 9 phyla, 20 classes, 42 orders, 81 families, 176 genera and 299 bacterial species. Firmicutes was the most abundant phylum in the blood of G. berlandieri; this taxon has been recorded as predominant in the intestine, excrement, nasal exudates and saliva of other species of the genus Gopherus. The dominant bacterial genera were Caldalkalibacillus, Anaerobacillus, Nesterenkonia and Bacillus. These taxa have been recorded in alkaline and halophilic soils, such as those found in G. berlandieri burrows. All of these bacterial taxa can enter the bloodstream of G. berlandieri via intestinal, oral and nasal translocation. Likewise, 3 bacterial taxa (Coxiella sp., Ehrlichia sp. and Anaplasma phagocytophilum) that are transmitted by arthropod vectors, as well as the potentially pathogenic Salmonella enterica were recorded. This information is the first bacteriological reference for the blood of G. berlandieri, and is expected to be useful for health and conservation programs.
Keywords: Sequencing; Coxiella; Ehrlichia; Anaplasma phagocytophilum; Salmonella enterica
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Microbiota bacteriana sanguínea de la tortuga texana Gopherus berlandieri en Tamaulipas, México
Resumen
La microbiota bacteriana sanguínea de la tortuga texana (Gopherus berlandieri) en Tamaulipas, México, fue caracterizada mediante secuenciación de siguiente generación. En 2019 se colectó sangre de 6 tortugas silvestres. Se extrajo el DNA, se amplificó la región V3-V4 del gen 16S rRNA y se realizó secuenciación con Illumina. Los resultados mostraron 9 phyla, 20 clases, 42 órdenes, 81 familias, 176 géneros y 299 especies bacterianas. Firmicutes fue el phylum más abundante en la sangre de G. berlandieri; este taxón ha sido registrado como predominante en intestino, excremento, exudados nasales y saliva de otras especies de Gopherus. Los géneros bacterianos dominantes fueron Caldalkalibacillus, Anaerobacillus, Nesterenkonia y Bacillus. Estos taxa se encuentran en suelos alcalinos y halófilos como los que se presentan en las madrigueras de G. berlandieri. Todos estos taxa bacterianos podrían ingresar al torrente sanguíneo vía translocación intestinal, oral y nasal. En la sangre de esta tortuga se registraron 3 taxa (Coxiella sp., Ehrlichia sp. y Anaplasma phagocytophilum) que se trasmiten por artrópodos y el patógeno potencial Salmonella enterica. Esta información es una primera referencia bacteriológica de la sangre de G. berlandieri y se espera que sea de utilidad en programas de salud y conservación.
Palabras clave: Secuenciación; Coxiella, Ehrlichia; Anaplasma phagocytophilum; Salmonella enterica
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
Bacteria form countless diverse and complex communities that reside within each metazoan species, forming what is known as microbiota (Colston & Jackson, 2016; Lee & Mazmanian, 2010). The microbiota is defined as a set of microorganisms and their genomes that inhabit a particular environment, including animals and humans (Bäckhed et al., 2005). Recent advances in molecular biology have provided the opportunity to characterize entire bacterial communities, including those that cannot be isolated in vitro (Bahrndorff et al., 2016). Currently, the 16S rRNA gene is considered an excellent marker for analyzing bacterial communities from any type of sample (Caporaso et al., 2012).
Microbiota studies have provided ecological and evolutionary information showing a strong link with health and disease (Costa et al., 2012). Recently, studies on bacterial microbiota in wild animals have been of great importance since they help to understand the role that these microorganisms play in the health of populations (Santoro et al., 2006). Reptiles carry a great diversity of gram-negative pathogens and are part of their normal microbiota (Ebani et al., 2005). Different epithelia of the body show dissimilar microbial communities. Each bacterial community within the body varies in population structure depending on its anatomical location (Martín et al., 2014). Bacterial communities have been documented on the mucosal surfaces of reptiles, such as the oral, nasal and cloacal cavities, and the intestine (García-De la Peña, Garduño-Niño et al., 2019; García-De la Peña, Rojas-Domínguez et al., 2019; Mackie et al., 2004; Price et al., 2017; Santoro et al., 2006). However, the presence of a blood bacterial microbiota in healthy reptiles has not yet been documented, which, as in domestic animals, would help to better understand the relationship between these bacteria and the health of their host (Peña-Cearra et al., 2021).
The circulatory system is closed, and for a long time, blood was considered a sterile environment in healthy organisms (Drennan, 1942; Proal et al., 2014). Recently, the presence of bacteria in the blood of domestic animals (Mandal et al., 2016; Scarsella et al., 2020; Vientós-Plotts et al., 2017) and healthy humans (Nikkari et al., 2001) has been documented. This perspective of the existence of a “healthy blood microbiota” has sparked much interest in the scientific community (Païssé et al., 2016), with a growing number of studies exploring the idea that the presence of “foreign” microorganisms in vertebrate blood does not necessarily indicate an infection or a disease state (Castillo et al., 2019).
Desert tortoises (genus Gopherus) play an important ecological role, as their burrows provide shelter for various species of animals that inhabit their ecosystems (Witz et al., 1991). In particular, the Texas tortoise (Gopherus berlandieri) is distributed from southeast Texas and northeast Mexico to the states of Coahuila, Nuevo León, San Luis Potosí and Tamaulipas (Bury & Germano, 1994; Judd & Rose, 1983). Its status is considered threatened according to the Official Mexican Norm 059 (Semarnat, 2010), as Least Concern by the International Union for Conservation of Nature (IUCN), and with a high environmental vulnerability score (EVS) according to Terán-Juárez et al. (2016). As like any other animal species, this tortoise is susceptible to bacterial diseases that affect the health of its populations. Because it is necessary to start with a healthy state for reference to diagnose a disease state, in the present study, the composition and abundance of bacteria in the blood of apparently healthy individuals of wild G. berlandieri were determined using next-generation sequencing (16S rRNA gene) in Tamaulipas, Mexico. It is expected that this information might contribute to the development of health, conservation and management programs that consider this microbiological aspect for the survival of this reptile.
Materials and methods
Tamaulipas is located in the northeastern region of Mexico. This state shares borders with Nuevo León, San Luis Potosí, Veracruz, and Texas in the United States of America. Tamaulipas is the sixth largest state in Mexico, with a territorial area of 80,249 km2 (27°40’45” – 22°12’25” N, 97°08’39” – 100°08’42” W) (INEGI, 2017). Due to its geographical location, this region has a high richness of species and temperate and tropical ecosystems (Toledo-Manzur, 1998). The predominant vegetation is the Tamaulipas Thorny Scrub, covering a large part of its surface (Romero, 1999).
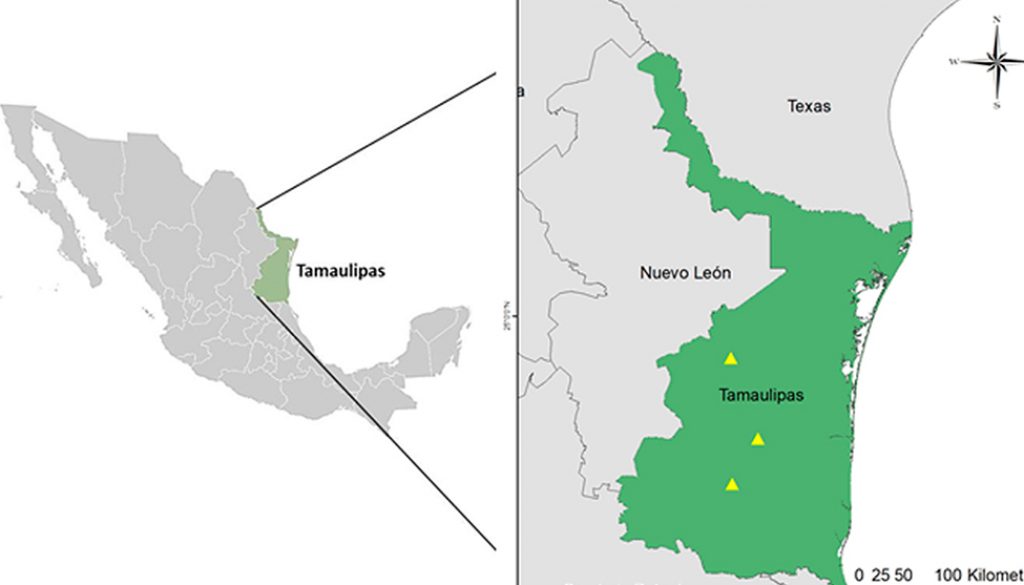
Individuals of G. berlandieri were captured manually from August to November 2019 in 3 localities, San Carlos, Casas, and Llera de Canales (Fig. 1). The soil that occurs in these localities is clay-type with a moderately alkaline pH (Zavala et al., 2015). For each tortoise, the following data were recorded: sex (females have a flat plastron, short tail and absence of ventral chin glands; males have a concave plastron, longer tail and presence of chin glands), length and width of the carapace (using a tape measure) and weight (with a hook scale) (Swann et al., 2002). The physical state of each tortoise was determined following the protocols proposed by Mader (2006).
With a syringe of 1 ml (25-27 G), a total of 0.5 ml of blood was extracted from each tortoise by puncture in the jugular vein considering the corresponding antiseptic measures (Mader, 2006). Next, 10 drops of blood were deposited into Bashing-bead tubes containing 750 μl of Xpedition™ Zymo Research™ lysing/stabilizing buffer. Each tube was processed in a TerraLyzer cell disruptor (Zymo Research Corporation) for DNA conservation according to the manufacturer. The tubes were placed in a cooler at 4 °C for transportation. When data and blood collection were complete, the tortoises were released at the site of their capture.
Laboratory work. DNA from the blood samples was extracted using the Zymo Research™ DNA Zymobiomics kit. The quantity and quality of DNA per sample was quantified in a ThermoScientific® nanodrop. Amplification of the V3-V4 region of the 16S rRNA gene was developed using the primers suggested by Klindworth et al. (2013):
S-D-Bact-0341-b-S-17, 5´-CCTACGGGNGGCWGCAG-
3´ and S-D-Bact-0785-a-A-21, 5´-GACTACHVGGGT
ATCTAATCC-3´, which produced an amplicon of ~460 bp. These sequences were synthesized with the “overhang” adapters of the Illumina protocol (Illumina, 2019a) as follows:

5´TCGTCGGCAGCGTCAGATGTGTATAAGAGACA
GCCTACGGGNGGCWGCAG-3´ and 5´-GTCTCGTGG
GCTCGGAGATGTGTATAAGAG ACAGGACTACHV
GGGTATCTAATCC-3´ (~550 bp amplicon). The Illumina PCR protocol (2017a) was implemented using 12.5 μl of MyTaqTM Ready Mix 1X (Bioline®), 1 μl of each primer (10 µM), 5 μl of DNA (50 ng total) and 5.5 μl of molecular grade H2O. The following cycle was used: 95 °C for 3 minutes; 25 cycles of 95 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 30 seconds; and 72 °C for 5 minutes in a Labnet MultigeneTM Gradient PCR thermocycler. One microliter of the PCR products was placed on a Bioanalyzer DNA 1000 chip to verify the amplicon size (~550 bp). Amplicon purification was performed using 0.8% Agentcourt® AMPure® XP beads. Subsequently, the amplicons were labeled using Nextera (Illumina, 2019b) following this cycle: 95 °C for 3 min; 10 cycles of 95 °C for 30 sec, 55 °C for 30 sec, and 72 °C for 30 sec; and 72 °C for 5 min. Purification was performed again with 1.2% Agencourt® AMPure® XP beads. One microliter of the library was placed on a Bioanalyzer DNA 1,000 chip to verify the amplicon size, expecting a size of ~ 630 bp. Finally, quantification, normalization (equimolarity) and next-generation sequencing (MiSeq Illumina® of 2 × 250 paired-end reads) were performed, following the protocol for 16S metagenomics (Illumina, 2019a).
Bioinformatic analysis. Sequence analysis was performed in an Oracle VM VirtualBox 5.1.14 virtual machine using Quantitative Insights into Microbial Ecology (QIIME) v.1.9.0 bioinformatics software (Caporaso et al., 2010). Sequence processing included quality review using FASTQC and removal of chimeras using USEARCH (Edgar, 2010). Operational taxonomic units (OTUs) were obtained at 97% similarity using the UCLUST method (Edgar, 2010); a random representative sequence was obtained for each OTU, and the taxonomy was assigned using the EzBioCloud database as a reference (Yoon et al., 2017). Afterward, the absolute abundance of OTUs was obtained to construct the rarefaction curve graph to observe the coverage depth; this graph was made in PAST 4.0 (Hammer et al., 2001). Tables of the relative abundance of OTUs were generated at the phylum, class, order, family, genus, and species levels. For the phylum level, a stacked bar graph was created using RStudio; for class to genus levels, a heatmap was constructed to visualize the relative abundance of the most abundant taxa using the Morpheus program (https://software.broadinstitute.org/morpheus/). Finally, a bibliographic search was conducted to review the available literature of all registered genera and species to determine potential pathogens for tortoises, as well as possible zoonotic agents.
Results
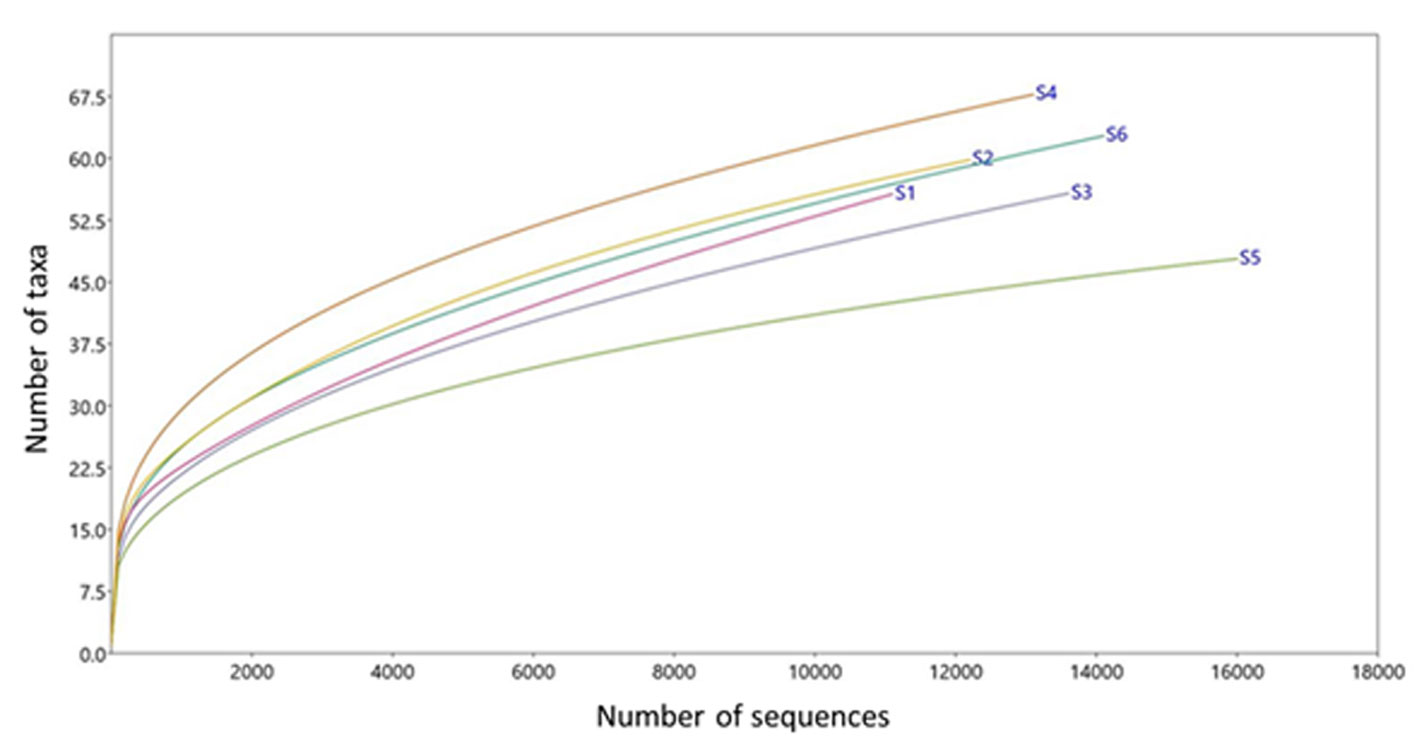
The results are shown for 6 G. berlandieri tortoises (1 female and 5 males), tagged as S1 (female from San Carlos), S2 and S3 (males from San Carlos), S4 and S5 (males from Casas), and S6 (male from Llera de Canales). These individuals showed the following morphological means: shell length = 18.5 cm, shell width = 13.93 cm, and weight = 1.2 kg. The mean altitude where tortoises were captured was 274.7 m asl. The mean for the assembled sequences was 28,822.1, the mean for the bacterial sequences was 13,499.3 and for OTUs was 10071.8 (Table 1). An acceptable coverage depth was observed since all rarefaction curves tended to reach an asymptote near 11,000 sequences (Fig. 2).
Nine bacterial phyla were recorded, from which Firmicutes (x = 73.5%), Proteobacteria (x = 13.2%) and Actinobacteria (x = 11.6%) were the most abundant (Fig. 3). A total of 20 classes of bacteria were identified, from which Bacilli (x = 70.0%), an unknown class from the phylum Actinobacteria (x = 11.6%), Gammaproteobacteria (x = 7.8%) and Betaproteobacteria (x = 5.0%) showed the highest abundances. Additionally, 42 orders were identified, from which Bacillales (x = 70.0%), Micrococcales (x = 10.9%), Oceanospirillales (x = 5.0%) and Burkholderiales (x = 5.0%) predominated. A total of 81 families were recorded; those showing the highest abundances were Bacillaceae (x = 69.9%), Micrococcaceae (x = 10.5%), Halomonadaceae (x = 5.0%), and Oxalobacteraceae (x = 4.9%). A total of 176 genera were identified, and those with the highest abundances were Caldalkalibacillus (x = 36.2%), Anaerobacillus (x = 14.3%), Nesterenkonia (x = 10.3%) and Bacillus (x = 9.3%) (Fig. 4).

Notably, the potentially zoonotic genera Coxiella and Ehrlichia were present in 1 individual (S1). Finally, 299 taxa at species level were recorded, of which only 95 showed taxonomic names (Table 2), and the rest were unknown. The presence of Anaplasma phagocytophilum in 1 tortoise (S2) is highlighted.
Discussion
It has been proposed that the first bacteria that reach the blood of a given vertebrate species come from the mother during pregnancy, when the sibling acquires part of the mother’s bacterial microbiota.
Subsequently, other bacteria will reach the blood primarily by physiological translocation, a phenomenon in which live bacteria or their products cross the intestinal barrier, the mucosa of the oral, nasal and urogenital cavities, or the skin of the host into the bloodstream (Blekhman et al., 2015; Emery et al., 2021; Gosiewski et al., 2017; Lloyd-Price et al., 2016; Peña-Cearra et al., 2021). Likewise, some particular bacteria from the microbiota of hematophagous insect vectors (fleas, mosquitoes, and ticks, among others) can enter the circulatory system of vertebrates, sometimes causing diseases in the host (Boulanger et al., 2019; Márquez-Jiménez et al., 2005). In the present study, the phylum Firmicutes was the most abundant in the blood of Gopherus berlandieri. This phylum has been reported to be predominant in the intestine and excrement of G. flavomarginatus (García-De la Peña, Garduño-Niño et al., 2019) and G. polyphemus (Yuan et al., 2015), where they perform sugar fermentation functions (hemicellulose and cellulose) (Sharmin et al., 2013). On the other hand, Firmicutes has also been reported in nasal exudates of G. agassizii, G. berlandieri, G. morafkai and G. polyphemus (Weitzman et al., 2018), as well as in saliva of G. flavomarginatus (García-De la Peña, Rojas-Domínguez et al., 2019). It is possible that bacteria from this phylum enter the bloodstream of G. berlandieri by translocation from the intestinal, nasal, and oral membranes, as proposed by Gosiewski et al. (2017). Likewise, the family Bacillaceae and the genera Caldalkalibacillus, Anaerobacillus, Nesterenkonia, and Bacillus were the most abundant in the blood samples of G. berlandieri. The first 3 genera are common inhabitants of alkaline soils where halophilic vegetation occurs (Bassil & Lloyd, 2019; Borsodi et al., 2015; De Jong, 2020; Edouard et al., 2014). Bacillus is characterized by living in different natural soils (Martin & Travers, 1989; Ohba & Aizawa, 1986), although it has also been reported as an intestinal symbiont in herbivorous vertebrates (Siegel, 2001). Although the bacteria in this soil were not analyzed, it is possible that these 4 genera were part of the substrate where G. berlandieri is active. Since this is a tortoise that digs burrows underground, with a daily contact that this reptile maintains with the soil and, therefore, with its bacteria must be considerably high. It has also been reported that species such as armadillos, badgers, rodents, among other vertebrates and invertebrates can live for short or long periods of time in the burrows of G. berlandieri (Kazmaier et al., 2001). It is likely, that the fecal bacteria of these species remain in the burrows where G. berlandieri could later have contact and acquire them via the oronasal route. Subsequently, it is probable that these soil and fecal bacteria infiltrate the blood through the oral capillaries or even enter through microwounds that the tortoise suffers while feeding on herbs and grasses, which are usually very dry and hard (Forner et al., 2006). Either way, these are environmental bacteria that until now have not been recorded as a possible health risk to tortoises.
Some bacterial taxa recorded in G. berlandieri blood that represent important findings are Coxiella sp., Ehrlichia sp., Anaplasma phagocytophilum, and Salmonella enterica. The first 3 are potential pathogens transmitted by arthropod vectors (ticks, lice, fleas, and mosquitoes) that can infect a wide range of vertebrate species, including reptiles (Jin et al., 2012). Coxiella has been documented as an endosymbiont of several tick species (Daveu et al., 2021). To date, there is only 1 described species (Coxiella burnetii) that is the causal agent of zoonotic Q fever (Široký et al., 2010). Some species of turtles have been found to harbor this bacterium in the oral cavity and cloaca (Emydoidea blandingii, Chrysemys picta, and Terrapene ornata in the United States; Sander et al., 2021). In other chelonians, such as Testudo graeca in Iran, C. burnetii has been recorded in blood, where the tick Hyalomma aegyptium is the vector of this bacterium (Khademi et al., 2023). Likewise, Ehrlichia has been reported in Amblyomma sparsum ticks parasitizing Geochelone pardalis in Zambia, as well as in H. aegyptium ticks parasitizing G. elegans and T. graeca in Jordan (Andoh et al., 2015). Finally, A. phagocytophilum (recorded in the present study in only 1 individual of G. berlandieri) has been previously documented in lizards, snakes, and alligators (Nieto et al., 2009), as well as in 2 wild individuals of G. polyphemus (Raskin et al., 2020). During the manipulation of the G. berlandieri individuals in the present study, no ticks were observed on their bodies. However, it is likely that this tortoise is eventually parasitized by ticks that transmit these bacteria, as in the case of G. agassizii – O. turicata – O. parkeri (Grover & DeFalco, 1995), G. polyphemus – O. turicata (Díaz-Figueroa, 2005), G. polyphemus – A. tuberculatum (Budachetri et al., 2016) and G. flavomarginatus – Ornithodoros turicata (Barraza-Guerrero et al., 2020). Therefore, it is important to determine the species of ticks that parasitize G. berlandieri in the study sites of Tamaulipas, as well as their bacterial microbiota. As a result, the origin of the vector-transmitted bacteria that were recorded in the blood of this tortoise would be established. Additionally, Salmonella enterica is a facultative bacillus that inhabits the intestines of reptiles mostly without causing disease (Lecis et al., 2011). Generally, reptiles acquire these microorganisms orally through contact with contaminated feces, food, water or soil (Pees et al., 2023). This bacterial species has been found in cloacal swabs of G. polyphemus (Lockhart et al., 2008), and in the oral cavity of G. flavomarginatus (García-De la Peña, Rojas-Domínguez et al., 2019); however, this is the first time it has been reported circulating in the bloodstream of G. berlandieri. It can be inferred that S. enterica can possibly enter the blood of this tortoise orally as previously described, and remain there without causing apparent illness.
Finally, it is important to emphasize that the information generated in the present study on the composition and abundance of bacteria in the blood of G. berlandieri joins a list of scientific works that have reported bacteria in the blood of healthy animals, including mice, birds, cats, dogs, and humans (Castillo et al., 2019; Mandal et al., 2016; Sze et al., 2014; Vientós-Plotts et al., 2017). Expectedly, this knowledge will be useful for the conservation and veterinary diagnosis of this chelonian, since it represents the first bacteriological reference in the blood of this tortoise in apparently good health. However, it will be important to periodically evaluate G. berlandieri individuals, not only in Tamaulipas but throughout its geographic distribution, to detect possible signs of microbiological diseases and analyze their blood microbiota during illness. In this way, significant progress could be made regarding wildlife health in northeastern Mexico.
Acknowledgements
Data and blood samples were taken under the permit SGPA/DGVS/11452/19 granted by SEMARNAT. All the methods and activities of this study were in strict accordance with accepted guidelines for ethical use, care and welfare of animals in research at international and national levels, with institutional approval reference number UJED-FCB-2019-04. The sequences used in
this study were deposited into the NCBI database (Bio
Sample accessions SAMN37501304, SAMN37501305, SAMN37501306, SAMN37501307, SAMN37501308, and SAMN37501309).
References
Andoh, M., Sakata, A., Takano, A., Kawabata, H., Fujita, H., Une, Y. et al. (2015). Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and amphibians imported into Japan. Plos One, 10, e0133700 https://doi.org/10.1371/journal.pone.0133700
Bäckhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A., & Gordon, J. I. (2005). Host-bacterial mutualism in the human intestine. Science, 307, 1915–1920. https://doi.org/10.1126/science.1104816
Bahrndorff, S., Alemu, T., Alemneh, T., & Lund-Nielsen, J. (2016). The microbiome of animals: implications for conservation biology. International Journal of Genomics, 2016, 1–8. https://doi.org/10.1155/2016/5304028
Barraza-Guerrero, S. I., Meza-Herrera, C. A., García-De la Peña, C., González-Álvarez, V. H., Vaca-Paniagua, F., Díaz-Velásquez, C. E. et al. (2020). General microbiota of the soft tick Ornithodoros turicata parasitizing the bolson tortoise (Gopherus flavomarginatus) in the Mapimi Biosphere Reserve, Mexico. Biology, 9, 275. https://doi.org/10.3390/biology9090275
Bassil, N. M., & Lloyd, J. R. (2019). Anaerobacillus isosaccharinicus sp. nov., an alkaliphilic bacterium which degrades isosaccharinic acid. International Journal of Systematic and Evolutionary Microbiology, 69, 3666–3671. https://doi.org/10.1099/ijsem.0.002721
Blekhman, R., Goodrich, J. K., Huang, K., Sun, Q., Bukowski, R., Bell, J. T. et al. (2015). Host genetic variation impacts microbiome composition across human body sites. Genome Biology, 16, 1–12. https://doi.org/10.1186/s13059-015-0759-1
Borsodi, A. K., Barany, A., Krett, G., Marialigeti, K., & Szili-Kovacs, T. (2015). Diversity and ecological tolerance of bacteria isolated from the rhizosphere of halophyton plants living nearby Kiskunság soda ponds, Hungary. Acta Microbiologica et Immunologica Hungarica, 62, 183–197. https://doi.org/10.1556/030.62.2015.2.8
Boulanger, N., Boyer, P., Talagrand-Reboul, E., & Hansmann, Y. (2019). Ticks and tick-borne diseases Tiques et maladies vectorielles à tiques. Medecine et Maladies Infectieuses, 49, 87–97. https://doi.org/10.1016/j.medmal.2019.01.007
Budachetri, K., Gaillard, D., Williams, J., Mukherjee, N., & Karim, S. (2016). A snapshot of the microbiome of Amblyomma tuberculatum ticks infesting the gopher tortoise, an endangered species. Ticks and Tick-borne Diseases, 7, 1225–1229. https://doi.org/10.1016/j.ttbdis.2016.07.010
Bury, R. B., & Germano, D. (1994). Aspects of the ecology and management of the tortoise Gopherus berlandieri at Laguna Atascosa, Texas. The Southwetern Naturalist, 31, 387–394. https://doi.org/10.2307/3671844
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K. et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7, 335–336. https://doi.org/10.1038/nmeth.f.303
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N. et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal, 6, 1621–1624. https://doi.org/10.1038/ismej.2012.8
Castillo, D. J., Rifkin, R. F., Cowan, D. A., & Potgieter, M. (2019). The healthy human blood microbiome: fact or fiction? Frontiers in Cellular and Infection Microbiology, 9, 449041. https://doi.org/10.3389/fcimb.2019.00148
Colston, T. J., & Jackson, C. R. (2016). Microbiome evolution along divergent branches of the vertebrate tree of life: what is known and unknown. Molecular Ecology, 25, 3776–3800. https://doi.org/10.1111/mec.13730
Costa, M. C., Arroyo, L. G., Allen-Vercoe, E., Stämpfli, H. R., Kim, P. T., Sturgeon, A. et al. (2012). Comparison of the Fecal Microbiota of Healthy Horses and Horses with colitis by High Throughput Sequencing of the V3-V5 Region of the 16S rRNA gene. Plos One, 7, e41484. https://doi.org/10.1371/journal.pone.0041484
Daveu, R., Laurence, C., Bouju-Albert, A., Sassera, D., & Plantard, O. (2021). Symbiont dynamics during the blood meal of Ixodes ricinus nymphs differ according to their sex. Ticks and Tick-borne Diseases, 12, 101707. https://doi.org/10.1016/j.ttbdis.2021.101707
De Jong, S. I., van den Broek, M. A., Merkel, A. Y., de la Torre-Cortés, P., Kalamorz, F., Cook, G. M. et al. (2020). Genomic analysis of Caldalkalibacillus thermarum TA2. A1 reveals aerobic alkaliphilic metabolism and evolutionary hallmarks linking alkaliphilic bacteria and plant life. Extremophiles, 24, 923–935. https://doi.org/10.1007/s00792-020-01205-w
Díaz-Figueroa, O. (2005). Characterizing the health status of the Louisiana gopher tortoise (Gopherus polyphemus). Baton Rouge: Louisiana State University/ Agricultural & Mechanical College.
Drennan M. (1942). What is ‘Sterile Blood’? British Medical Journal, 2, 526.
Ebani, V. V., Cerri, D., Fratini, F., Meille, N., Valentini, P., & Andreani, E. (2005). Salmonella enterica isolates from faeces of domestic reptiles and a study of their antimicrobial in vitro sensitivity. Research in Veterinary Science, 78, 117–121. https://doi.org/10.1016/j.rvsc.2004.08.002
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26, 2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Edouard, S., Sankar, S., Dangui, N. P. M., Lagier, J. C., Michelle, C., Raoult, D. et al. (2014). Genome sequence and description of Nesterenkonia massiliensis sp. nov. strain NP1 T. Standards in Genomic Sciences, 9, 866–882. https://doi.org/10.4056/sigs.5631022
Emery, D. C., Cerajewska, T. L., Seong, J., Davies, M., Paterson, A., Allen-Birt, S. J. et al. (2021). Comparison of blood bacterial communities in periodontal health and periodontal disease. Frontiers in Cellular and Infection Microbiology, 10, 577485. https://doi.org/10.3389/fcimb.2020.577485
Forner, L., Larsen, T., Kilian, M., & Holmstrup, P. (2006). Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. Journal of Clinical Periodontology, 33, 401–407. https://doi.org/10.1111/j.1600-051X.2006.00924.x
García-De la Peña, C., Garduño-Niño, E., Vaca-Paniagua, F., Díaz-Velásquez, C., Barrows, C. W., Gómez-Gil, B. et al. (2019). Comparison of the fecal bacterial microbiota composition between wild and captive Bolson tortoises (Gopherus flavomarginatus). Herpetological Conservation and Biology, 14, 587–600.
García-De la Peña, C., Rojas-Domínguez, M., Ramírez-Bautista, A., Vaca-Paniagua, F., Díaz-Velásquez, C., Ávila-Rodríguez, V. et al. (2019). Microbiota bacteriana oral de la tortuga del bolsón Gopherus flavomarginatus en la Reserva de la Biosfera Mapimí, México. Revista Mexicana de Biodiversidad, 90, e902683. https://doi.org/10.22201/ib.20078706e.2019.90.2683
Gosiewski, T., Ludwig-Galezowska, A. H., Huminska, K., Sroka-Oleksiak, A., Radkowski, P., Salamon, D. et al. (2017). Comprehensive detection and identification of bacterial DNA in the blood of patients with sepsis and healthy volunteers using next-generation sequencing method-the observation of DNAemia. European Journal of Clinical Microbiology & Infectious Diseases, 36, 329–336. https://doi.org/10.1007/s10096-016-2805-7
Grover, M. C., & DeFalco, L. A. (1995). Desert tortoise (Gopherus agassizii): status-of knowledge outline with references. Intermountain Research Station, US Forest Service, General Tech. Rpt. INT-GTR-316.
Hammer, Ø., Harper, D., & Ryan, P. (2001). PAST: paquete de programas de estadística paleontológica para enseñanza y análisis de datos. Palaeontological Electrón, 4, 4.
Illumina (2019a). 16S Metagenomic sequencing library preparation, preparing 16S ribosomal RNA gene amplicons for the Illumina MiSeq system. Recuperado el 29 de septiembre 2019 de: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf
Illumina (2019b). Nextera XT DNA library prep kit reference guide. Recuperado el 29 de septiembre 2019, de: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/samplepreps_nextera/nexteradna/nextera-dna-library-prep-reference-guide-15027987-01.pdf
INEGI (Instituto Nacional de Estadística y Geografía). (2017). Anuario estadístico y geográfico de Tamaulipas 2017. Aguascalientes: Instituto Nacional de Estadística y Geografía.
Jin, H., Wei, F., Liu, Q., y Qian, J. (2012). Epidemiology and control of human granulocytic anaplasmosis: a systematic review. Vector-Borne and Zoonotic Diseases, 12, 269–274. https://doi.org/10.1089/vbz.2011.0753
Judd, F. W., & Rose, F. L. (1983). Population stucture, density and movements of the Texas tortoise Gopherus berlandieri. The Southwestern Naturalist, 28, 387–398. https://doi.org/10.2307/3670817
Kazmaier, R. T., Hellgren, E. C., & Synatzske, D. R. (2001). Patterns of behavior in the Texas tortoise, Gopherus berlandieri: a multivariate ordination approach. Canadian Journal of Zoology, 79, 1363–1371. https://doi.org/10.1139/z01-092
Khademi, P., Tukmechi, A., Ownagh, A., Enferadi, A., & Hadian, M. (2023). The first molecular detection of Coxiella burnetii in blood samples of turtles (T. graeca) and their associated ticks. Preprint from Research Square. PPR691830. https://doi.org/10.21203/rs.3.rs-3155593/v1
Klindworth, A., Pruesse, E., Schweer, T., Peplies, J., Quast, C., Horn, M. et al. (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Research, 41, 1–11 https://doi.org/10.1093/nar/gks808
Lecis R., Paglietti, B., Rubino, S., Are, B. M., Muzzeddu, M., Berlinguer, F. et al. (2011). Detection and characterization of Mycoplasma spp. and Salmonella spp. in free-living European tortoises (Testudo hermanni, Testudo graeca, and Testudo marginata) Journal of Wildlife Diseases, 47, 717–724. https://doi.org/10.7589/0090-3558-47.3.717
Lee, Y. K., & Mazmanian, S. K. (2010). Has the microbiota played a critical role in the evolution of the adaptive immune system? Science, 330, 1768–1773. https://doi.org/10.1126/science.1195568
Lloyd-Price, J., Abu-Ali, G., & Huttenhower, C. (2016). The healthy human microbiome. Genome Medicine, 8, 1–11. https://doi.org/10.1186/s13073-016-0307-y
Lockhart, J. M., Lee, G., Turco, J., & Chamberlin, L. (2008). Salmonella from gopher tortoises (Gopherus polyphemus) in South Georgia. Journal of Wildlife Diseases, 44, 988–991. https://doi.org/10.7589/0090-3558-44.4.988
Mackie, R. I., Rycyk, M., Ruemmler, R. L., Aminov, R. I., & Wikelski, M. (2004). Biochemical and microbiological evidence for fermentative digestion in free-living land iguanas (Conolophus pallidus) and marine iguanas (Amblyrhynchus cristatus) on the Galápagos Archipelago. Physiological and Biochemical Zoology, 77, 127–138. https://doi.org/10.1086/383498
Mader, D. R. (2006). Reptile medicine and surgery. St. Louis, Missouri: Saunders Elsevier.
Mandal, R. K., Jiang, T., Al-Rubaye, A. A., Rhoads, D. D., Wideman, R. F., Zhao, J. et al. (2016). An investigation into blood microbiota and its potential association with Bacterial Chondronecrosis with Osteomyelitis (BCO) in Broilers. Scientific Reports, 6, 1–11. https://doi.org/10.1038/srep25882
Márquez-Jiménez, F. J., Hidalgo-Pontiveros, A., Contreras-Chova, F., Rodríguez-Liébana, J. J., & Muniain-Ezcurra, M. A. (2005). Ticks (Acarina: Ixodidae) as vectors and reservoirs of pathogen microorganisms in Spain. Enfermedades Infecciosas y Microbiología Clínica, 23, 94–102. https://doi.org/10.1157/13071613
Martin, P. A., & Travers, R. S. (1989). Worldwide abundance and distribution of Bacillus thuringiensis isolates. Applied and Environmental Microbiology, 55, 2437–2442. https://doi.org/10.1128/aem.55.10.2437-2442.1989
Martín, R., Miquel, S., Langella, P., & Bermúdez-Humarán, L. G. (2014). The role of metagenomics in understanding the human microbiome in health and disease. Virulence, 5, 413–423. https://doi.org/10.4161/viru.27864
Nieto, N. C., Foley, J. E., Bettaso, J., & Lane, R. S. (2009). Reptile infection with Anaplasma phagocytophilum, the causative agent of granulocytic anaplasmosis. Journal of Parasitology, 95, 1165–1170. https://doi.org/10.1645/GE-1983.1
Nikkari, S., McLaughlin, I. J., Bi, W., Dodge, D. E., & Relman, D. A. (2001). Does blood of healthy subjects contain bacterial ribosomal DNA?. Journal of Clinical Microbiology, 39, 1956–1959. https://doi.org/10.1128/jcm.39.5.1956-1959.2001
Ohba, M., & Aizawa, K. (1986). Distribution of Bacillus thuringiensis in soils of Japan. Journal of Invertebrate Pathology, 47, 277–282. https://doi.org/10.1016/0022-2011(86)90097-2
Païssé, S., Valle, C., Servant, F., Courtney, M., Burcelin, R., & Amar, J. (2016). Comprehensive description of blood microbiome from healthy donors assessed by 16 S targeted metagenomic sequencing. Transfusion, 56, 1138–1147. https://doi.org/10.1111/trf.13477
Pees, M., Brockmann, M., Steiner, N., & Marschang, R. E. (2023). Salmonella in reptiles: a review of occurrence, interactions, shedding and risk factors for human infections. Frontiers in Cell and Developmental Biology, 11, 1251036. https://doi.org/10.3389/fcell.2023.1251036
Peña-Cearra, A., Belanche, A., González-López, M., Lavín, J. L., Pascual-Itoiz, M. Á., Jiménez, E. et al. (2021). Peripheral blood mononuclear cells (PBMC) microbiome is not affected by colon microbiota in healthy goats. Animal Microbiome, 3, 1–11. https://doi.org/10.1186/s42523-021-00091-7
Price, J. T., Paladino, F. V., Lamont, M. M., Witherington, B. E., Bates, S. T., & Soule, T. (2017). Characterization of the juvenile green turtle (Chelonia mydas) microbiome throughout an ontogenetic shift from pelagic to neritic habitats. Plos One, 12, e0177642. https://doi.org/10.1371/journal.pone.0177642
Proal, A. D., Albert, P. J., & Marshall, T. G. (2014). Inflammatory disease and the human microbiome. Discovery Medicine, 17, 257–265.
Raskin, R. E., Crosby, F. L., & Jacobson, E. R. (2020). Newly recognized Anaplasma sp. in erythrocytes from gopher tortoises (Gopherus polyphemus). Veterinary Clinical Pathology, 49, 17–22. https://doi.org/10.1111/vcp.12823
Romero, F. G. (1999). Caracterización ecológica y definición de esquemas de muestreo en el matorral espinoso tamaulipeco del noreste de México (Master’s Thesis). Universidad Autónoma de Nuevo León. Nvo. León, México.
Sander, W. E., King, R., Graser, W., Kapfer, J. M., Engel, A. I., Adamovicz, L. et al. (2021). Coxiella burnetii in 3 species of turtles in the upper midwest, United States. Emerging Infectious Diseases, 27, 3199–3202. https://doi.org/10.3201/eid2712.211278
Santoro, M., Orrego, C. M., & Hernández-Gómez, G. (2006). Flora bacteriana cloacal y nasal de Lepidochelys olivacea (Testudines: Cheloniidae) en el Pacífico norte de Costa Rica. Revista de Biología Tropical, 54, 43–48. https://doi.org/10.15517/rbt.v54i1.13990
Scarsella, E., Sandri, M., Monego, S. D., Licastro, D., & Stefanon, B. (2020). Blood microbiome: a new marker of gut microbial population in dogs? Veterinary Sciences, 7, 198. https://doi.org/10.3390/vetsci7040198
Semarnat (Secretaría de Medio Ambiente y Recursos Naturales). (2010). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías en riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación. 30 diciembre 2010, Segunda Sección. México, D.F.
Sharmin, F., Wakelin, S., Huygens, F., & Hargreaves, M. (2013). Firmicutes dominate the bacterial taxa within sugar-cane processing plants. Scientific Reports, 3, 1–7. https://doi.org/10.1038/srep03107
Siegel, J. P. (2001). The mammalian safety of Bacillus thuringiensis-based insecticides. Journal of Invertebrate Pathology, 77, 13–21. https://doi.org/10.1006/jipa.2000.5000
Široký, P., Kubelová, M., Modrý, D., Erhart, J., Literák, I., Špitalská, E. et al. (2010). Tortoise tick Hyalomma aegyptium as long term carrier of Q fever agent Coxiella burnetii—evidence from experimental infection. Parasitology Research, 107, 1515–1520. https://doi.org/10.1007/s00436-010-2037-1
Swann, D. E., Averill-Murray, R. C., & Schwalbe, C. R. (2002). Distance sampling for Sonoran desert tortoises. The Journal of Wildlife Management, 66, 969–975. https://doi.org/10.2307/3802929
Sze, M. A., Tsuruta, M., Yang, S. W. J., Oh, Y., Man, S. P., Hogg, J. C. et al. (2014). Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. Plos One, 9, e111228. https://doi.org/10.1371/journal.pone.0111228
Terán-Juárez, S. A., García-Padilla, E., Mata-Silva, V., Johnson, J. D., & Wilson, L. D. (2016). The herpetofauna of Tamaulipas, Mexico: composition, distribution, and conservation. Mesoamerican Herpetology, 3, 43–113.
Toledo-Manzur, V. M. (1998). Diagnóstico de los escenarios de la biodiversidad de México a través de un sistema de información ecográfica. Centro de Investigaciones en Ecosistemas-Universidad Nacional Autónoma de México. Informe final SNIB-Conabio, proyecto Núm. A006, México D.F.
Vientós-Plotts, A. I., Ericsson, A. C., Rindt, H., Grobman, M. E., Graham, A., Bishop, K. et al. (2017). Dynamic changes of the respiratory microbiota and its relationship to fecal and blood microbiota in healthy young cats. Plos One, 12, e0173818. https://doi.org/10.1371/journal.pone.0173818
Weitzman, C. L., Sandmeier, F. C., & Tracy, C. R. (2018). Host species, pathogens and disease associated with divergent nasal microbial communities in tortoises. Royal Society Open Science, 5, 181068. https://doi.org/10.1098/rsos.181068
Witz, B. W., Wilson, D. S., & Palmer, M. D. (1991). Distribution of Gopherus polyphemus and its vertebrate symbionts in three burrow categories. American Midland Naturalist, 126, 152–158.
Yoon, S. H., Ha, S. M., Kwon, S., Lim, J., Kim, Y., Seo, H. et al. (2017). Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. International Journal of Systematic and Evolutionary Microbiology, 67, 1613. https://doi.org/
10.1099/ijsem.0.001755
Yuan, M. L., Dean, S. H., Longo, A. V., Rothermel, B. B., Tuberville, T. D., & Zamudio, K. R. (2015). Kinship, inbreeding and fine-scale spatial structure influence gut microbiota in a hindgut-fermenting tortoise. Molecular Ecology, 24, 2521–2536. https://doi.org/10.1111/mec.
13169
Zavala, M. E. E. H., Salas, C. C., Diguero, P. N., Pérez, R., Álvarez, M. A. V. M., & Briseño, P. R. (2015). Tipos de suelos caracterizados por pH y textura en la zona sur de Tamaulipas. Encuentro Nacional de Investigación Científica y Tecnológica del Golfo de México, 41. Tampico, Tamaulipas, Enero 2015.